Removal Mechanism of Oxide Layer on the Surface of Sn-0.4Ti Alloy for Quartz Glass Sealing
Abstract
1. Introduction
2. Materials and Methods
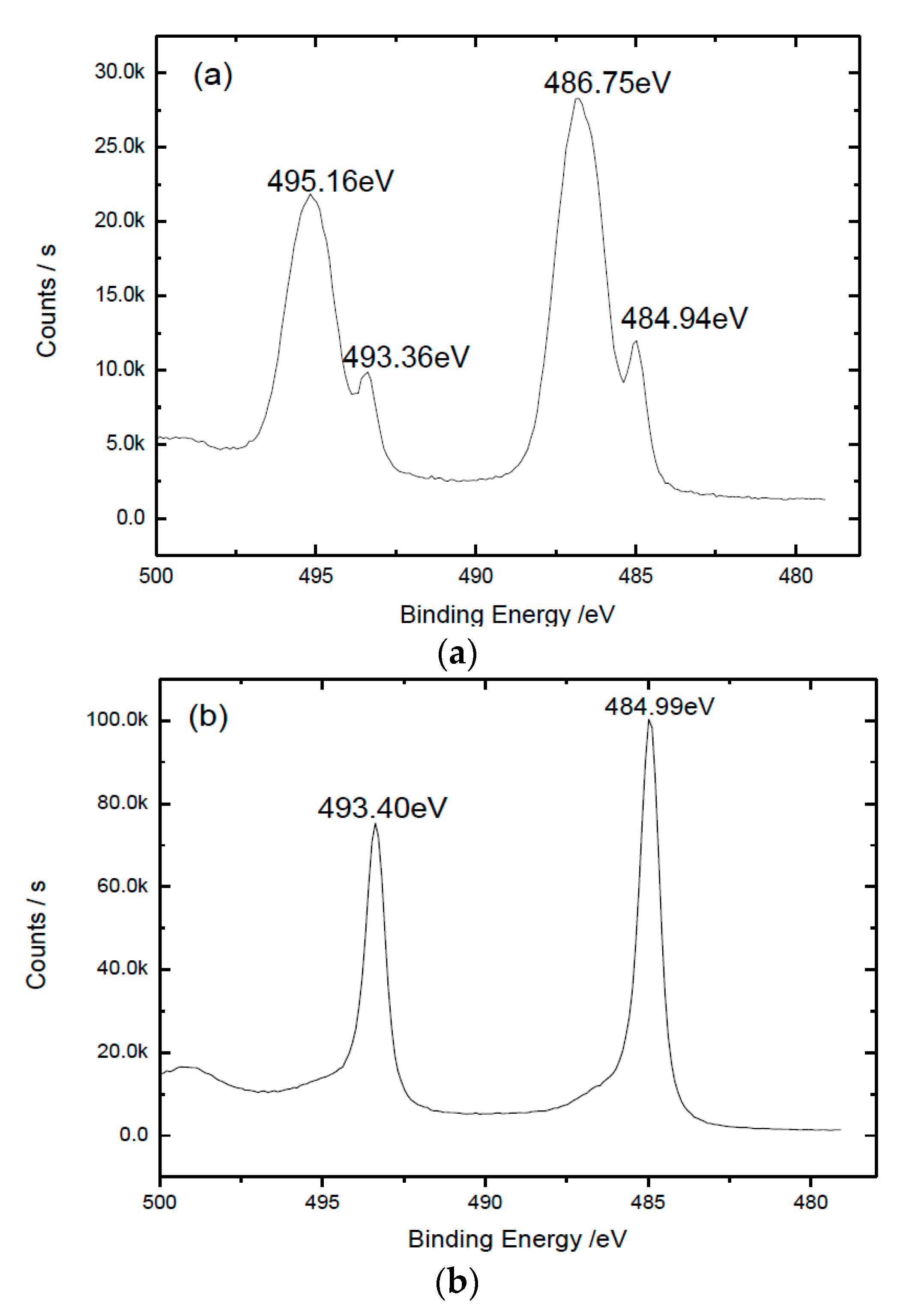
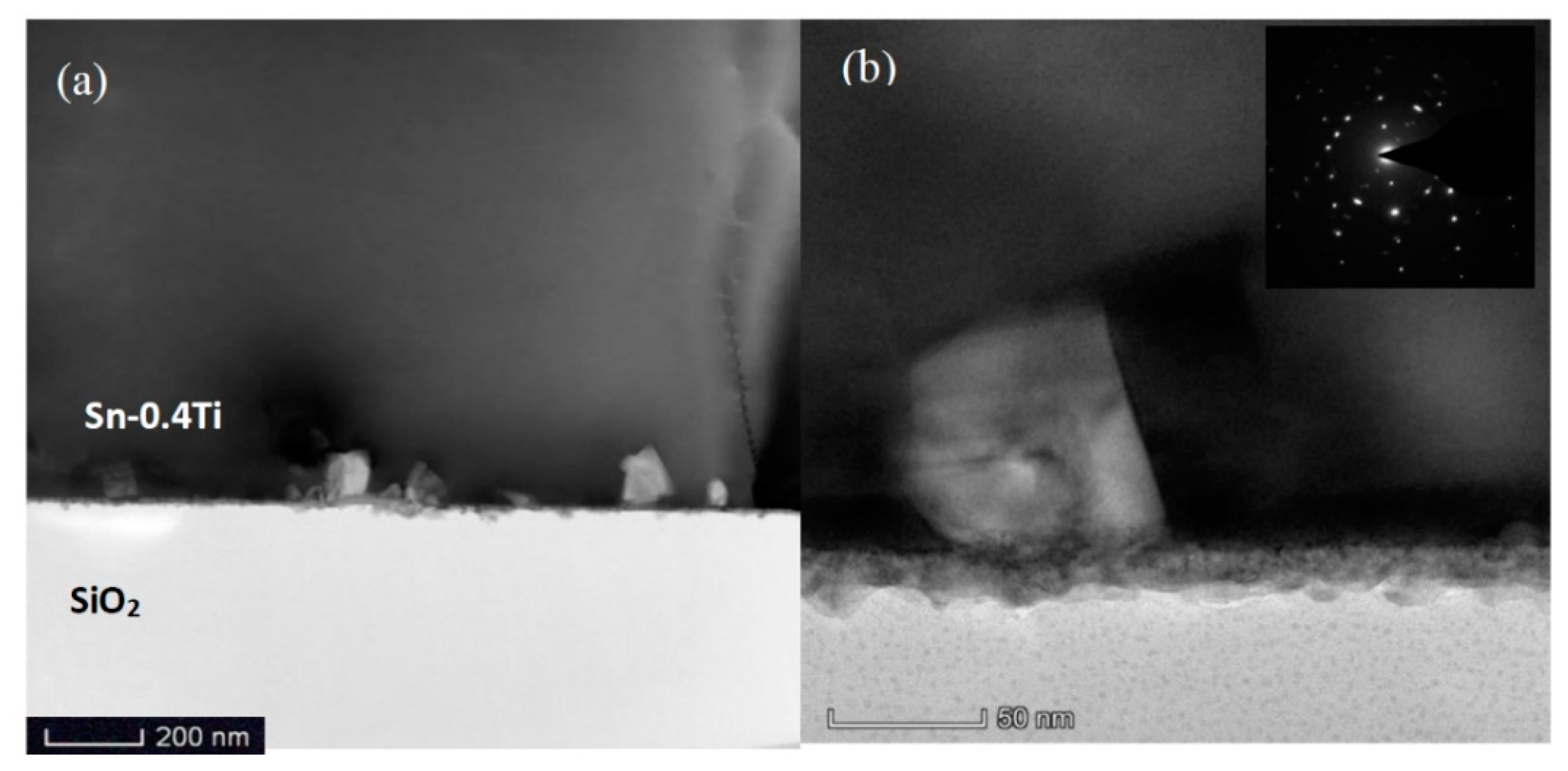
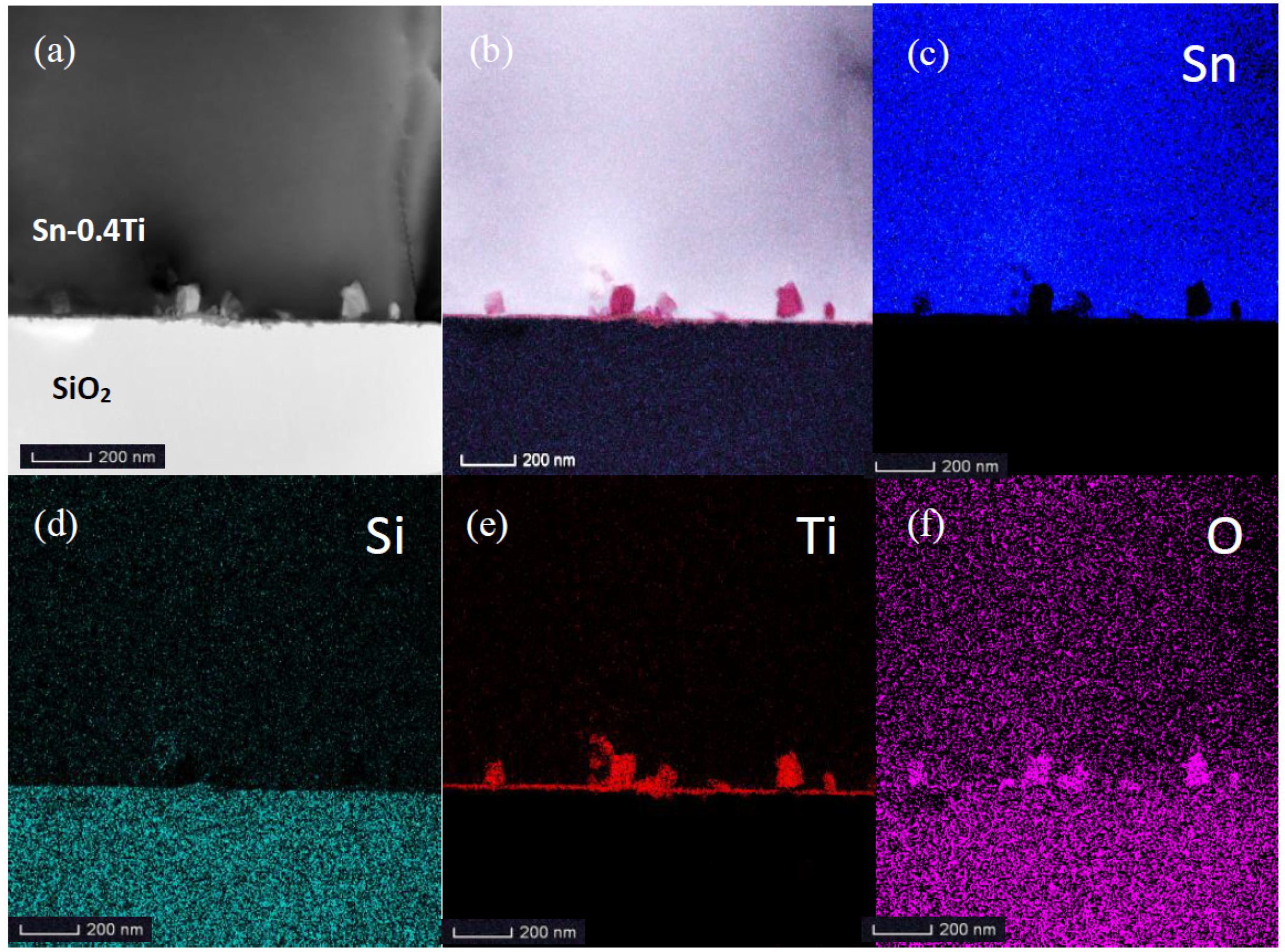
3. Results
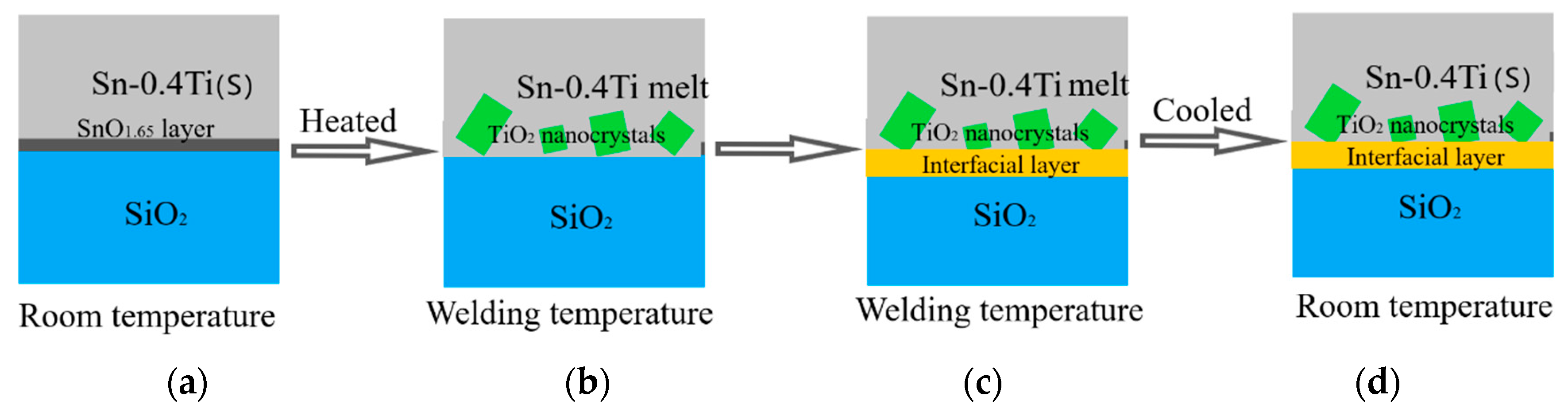
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lei, D.; Wang, Z.; Li, J. The analysis of residual stress in glass-to-metal seals for solar receiver tube. Mater. Des. 2010, 31, 1813–1820. [Google Scholar] [CrossRef]
- Mantel, M. Effect of double oxide layer on metal-glass sealing. J. Non-Cryst. Solids 2000, 273, 294–301. [Google Scholar] [CrossRef]
- Gao, L.; Shen, Z. Influence of A1203 adding on properties of glass bead and wettabilitybetween glass and Kovar alloy. Electron. Compon. Mater. 2011, 30, 52–54. [Google Scholar]
- Chanmuang, C.; Naksata, M.; Chairuangsri, T.; Jain, H.; Lyman, C.E. Microscopy and strength of borosilicate glass-to-Kovar alloy joints. Mater. Sci. Eng. A 2008, 474, 218–224. [Google Scholar] [CrossRef]
- Pech, J.; Braccini, M.; Mortensen, A.; Eustathopoulos, N. Wetting, interfacial interactions and sticking in glass/steel systems. Mater. Sci. Eng. A 2004, 384, 117–128. [Google Scholar] [CrossRef]
- Widgeon, S.J.; Corral, E.L.; Spilde, M.N.; Loehman, R.E. Glass-to-Metal Seal Interfacial Analysis using Electron Probe Microscopy for Reliable Solid Oxide Fuel Cells. J. Am. Ceram. Soc. 2009, 92, 781–786. [Google Scholar] [CrossRef]
- Zhu, S. The Molybdenum Flake of High Strength Gas Discharge Lamp-Siles Seal. Light Light. 2002, 26, 17–18. [Google Scholar]
- Xiang, Z.; Wang, Y.; Sui, M. Improvement of Quartz-Tungsten Intermediate Sealing Glass and Research of Sealing Technology. Light Light. 2006, 30, 54–56. [Google Scholar]
- Hao, W.; Li, F.; Ma, Y.; Zhang, W.; Shi, L. Nano-layered-structure Interface between Sn-Ti alloy and Quartz glass for hermetic seals. Mater. Lett. 2019, 236, 506–509. [Google Scholar] [CrossRef]
- Kuper, C.; Peng, W.Q.; Pisch, A.; Goesmann, F.; Schmid-Fetzer, R. Phase formation and reaction kinetics in the system Ti-Sn. Z. Met. 1998, 89, 855–862. [Google Scholar]
- Okamoto, H. Sn-Ti (Tin-Tinium). J. Phase Equilibria Diffus. 2010, 31, 202–203. [Google Scholar] [CrossRef]
- Yin, F.; Tedenac, J.; Gascoin, F. Thermodynamic modelling of the Ti-Sn system and calculation of the Co–Ti–Sn system. Calphad 2007, 31, 370–379. [Google Scholar] [CrossRef]
- Liu, C.; Klotz, U.E.; Uggowitzer, P.J.; Löffler, J.F. Thermodynamic Assessment of the Sn-Ti System. Mon. Chem. 2005, 136, 1921–1930. [Google Scholar] [CrossRef]
- O’Brien, J.W.; Dunlap, R.A.; Dahn, J.R. A Mössbauer effect and X-ray diffraction investigation of Ti–Sn intermetallic compounds: I. Equilibrium phases. J. Alloy. Compd. 2003, 353, 65–73. [Google Scholar]
- O’Brien, J.W.; Dunlap, R.A.; Dahn, J.R. A Mössbauer effect and X-ray diffraction investigation of Ti–Sn intermetallic compounds: II. Nanostructured phases prepared by ball milling with Al2O3. J. Alloy. Compd. 2003, 353, 65–73. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, L.B.; Tuo, B.Y.; Bai, W.M.; Wang, X. Computational Study of Mobilities and Diffusion in Ti-Sn Alloy. J. Phase Equilibria Diffus. 2015, 36, 248–253. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Y.; Lu, Y. Measurement of Interdiffusivities and Calculation of Kinetics in bcc Ti–Sn and Ti-Ni Binary Systems. J. Xiamen Univ. 2017, 56, 25–32. [Google Scholar]
- Mu, D.; Feng, K.; Lin, Q. Low-temperature wetting of sapphire using Sn-Ti active solder alloys. Ceram. Int. 2019, 45, 22175–22182. [Google Scholar] [CrossRef]
- Liao, X.; Mu, D.; Wang, J. Formation of TiC via interface reaction between diamond grits and Sn–Ti alloys at relatively low temperatures. Int. J. Refract. Met. Hard Mater. 2017, 66, 252–257. [Google Scholar] [CrossRef]
- Fu, W.; Passerone, A.; Bian, H. Wetting and interfacial behavior of Sn-Ti alloys on zirconia. J. Mater. Sci. 2019, 54, 812–822. [Google Scholar] [CrossRef]
- Fu, W.; Song, X.; Passerone, A. Interactions, joining and microstructure of Sn-Ti/ZrO2 system. J. Eur. Ceram. Soc. 2019, 39, 1525–1531. [Google Scholar] [CrossRef]
- Jiang, L.X.; Qi, H.Q.; Kui, M.D. Wettability of Sn-Ti Alloys on Poly-Crystalline CVD Diamond Plates. Solid State Phenom. 2018, 273, 181–186. [Google Scholar]
- Hao, W.; Li, F.; Ma, Y.; Zhang, W.; Shi, L. Microstructure and Properties of Sn-Ti Alloy for quartz glass sealing. Status 2020. manuscript in preparation. [Google Scholar]
- Oxide Layer on Common Metal Surface. Available online: https://www.douban.com/note/398604075/ (accessed on 4 December 2019).
- NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 4 December 2019).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.; Li, F.; Ma, Y.; Zhang, W.; Shi, L. Removal Mechanism of Oxide Layer on the Surface of Sn-0.4Ti Alloy for Quartz Glass Sealing. Materials 2020, 13, 2620. https://doi.org/10.3390/ma13112620
Hao W, Li F, Ma Y, Zhang W, Shi L. Removal Mechanism of Oxide Layer on the Surface of Sn-0.4Ti Alloy for Quartz Glass Sealing. Materials. 2020; 13(11):2620. https://doi.org/10.3390/ma13112620
Chicago/Turabian StyleHao, Wanli, Fangzi Li, Yongbo Ma, Weiguang Zhang, and Liqun Shi. 2020. "Removal Mechanism of Oxide Layer on the Surface of Sn-0.4Ti Alloy for Quartz Glass Sealing" Materials 13, no. 11: 2620. https://doi.org/10.3390/ma13112620
APA StyleHao, W., Li, F., Ma, Y., Zhang, W., & Shi, L. (2020). Removal Mechanism of Oxide Layer on the Surface of Sn-0.4Ti Alloy for Quartz Glass Sealing. Materials, 13(11), 2620. https://doi.org/10.3390/ma13112620




