Photoelectrochromic Devices with Enhanced Power Conversion Efficiency
Abstract
1. Introduction
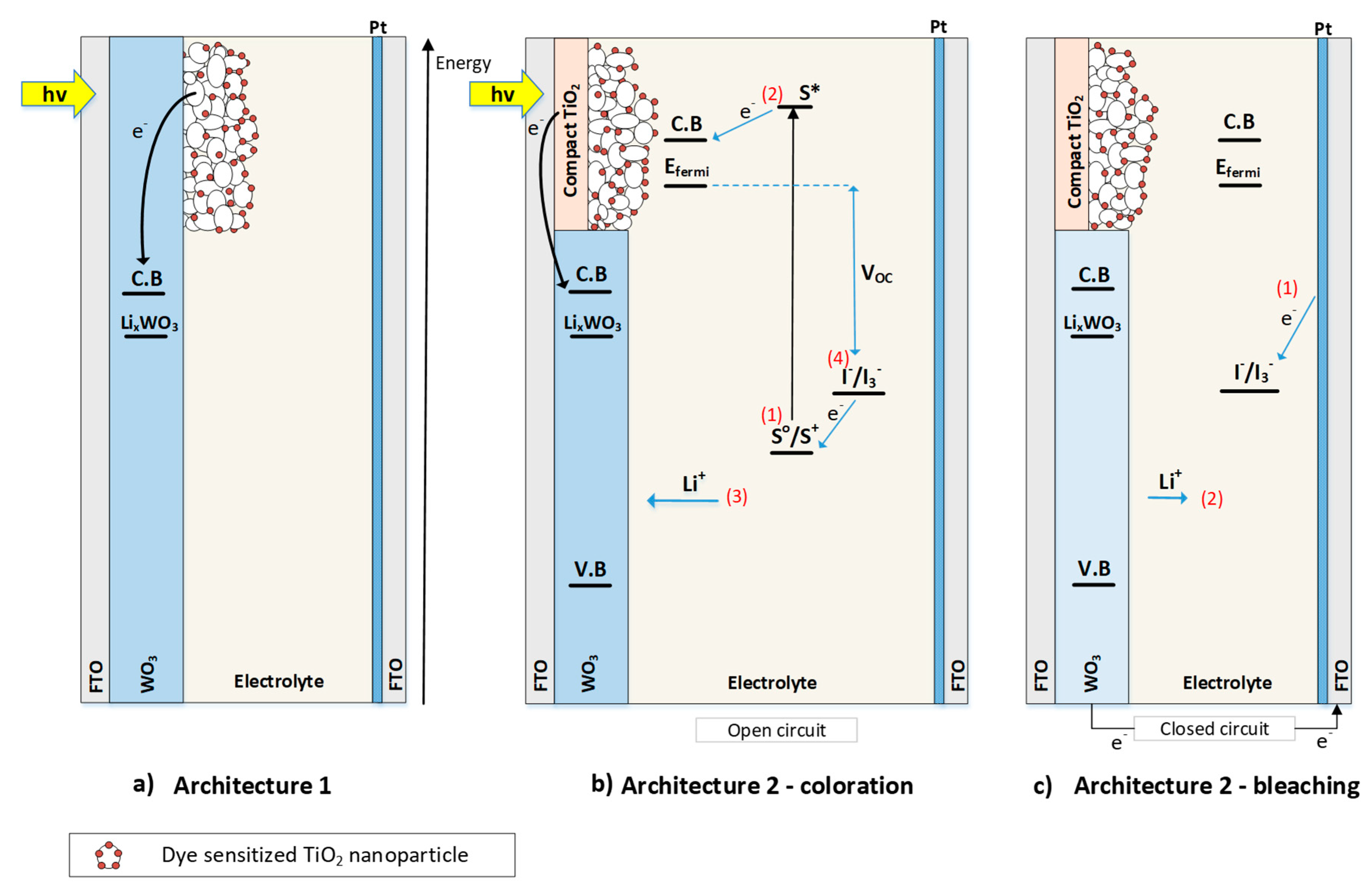
1.1. Layout and Operation of the Partly Covered PECs
- An electrochromic thin film (WO3 in this work), which is the active part of the device, as it changes its transparency;
- A wide band gap mesoporous semiconductor film (TiO2 in this work) covering different percentages of the total surface area of the device. The film is sensitized with an appropriate dye (N719 in this work) in order to absorb a larger portion of the solar spectrum;
- An electrolyte that contains a redox couple (usually I−/I3−) and lithium ions (Li+);
- A second transparent FTO glass substrate with an ultra-thin platinum (Pt) coating.
2. Materials and Methods
2.1. Development of Materials
2.1.1. FTO Substrates
2.1.2. TiO2 Compact Layer
2.1.3. Dye Sensitized TiO2 Films
2.1.4. WO3 Films
2.1.5. Electrolyte
2.1.6. Counter Electrode
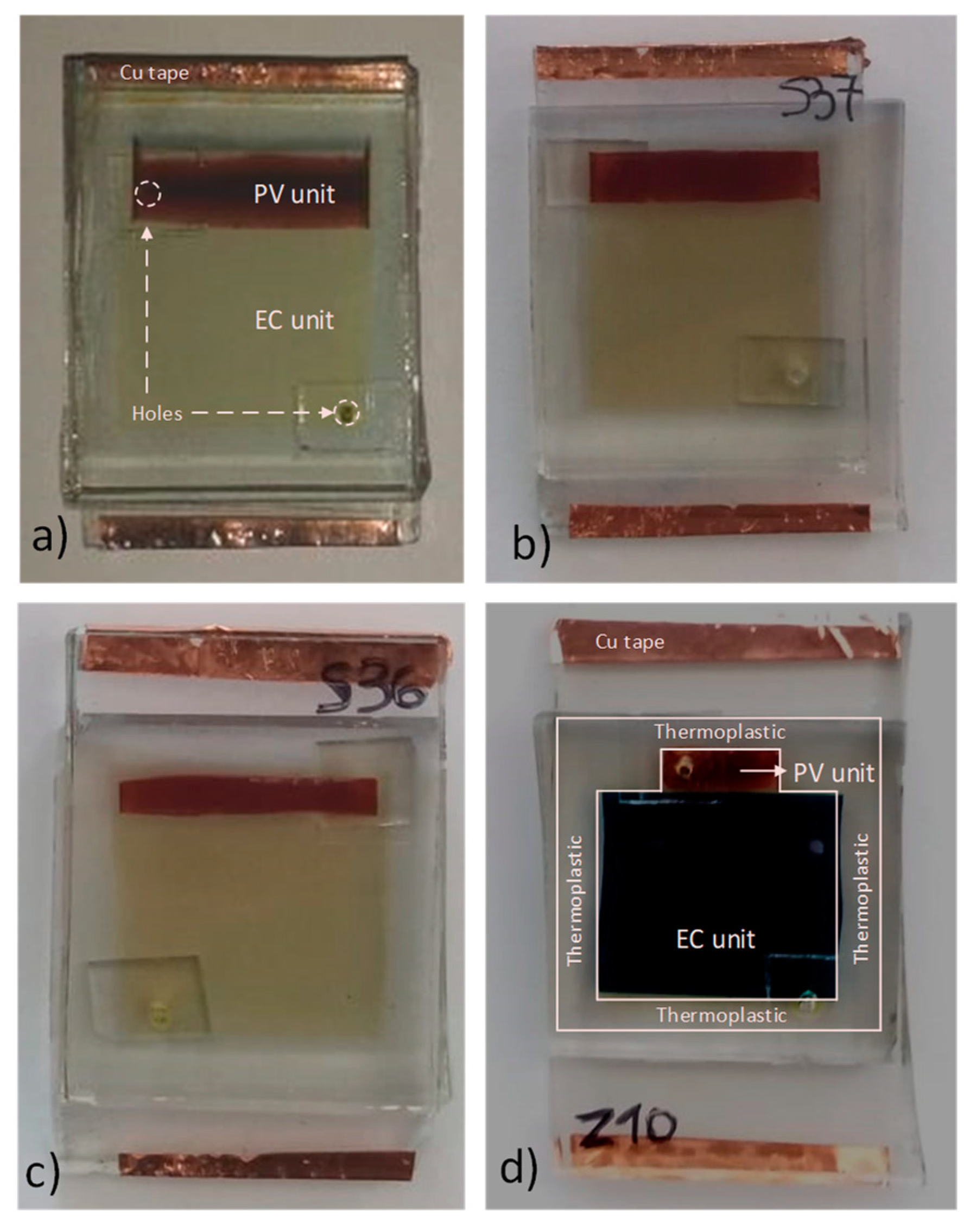
2.1.7. Fabrication of the Devices
2.2. Characterization Methods
2.2.1. Optical Measurements
2.2.2. Electrical Characteristics
2.2.3. Extended Testing
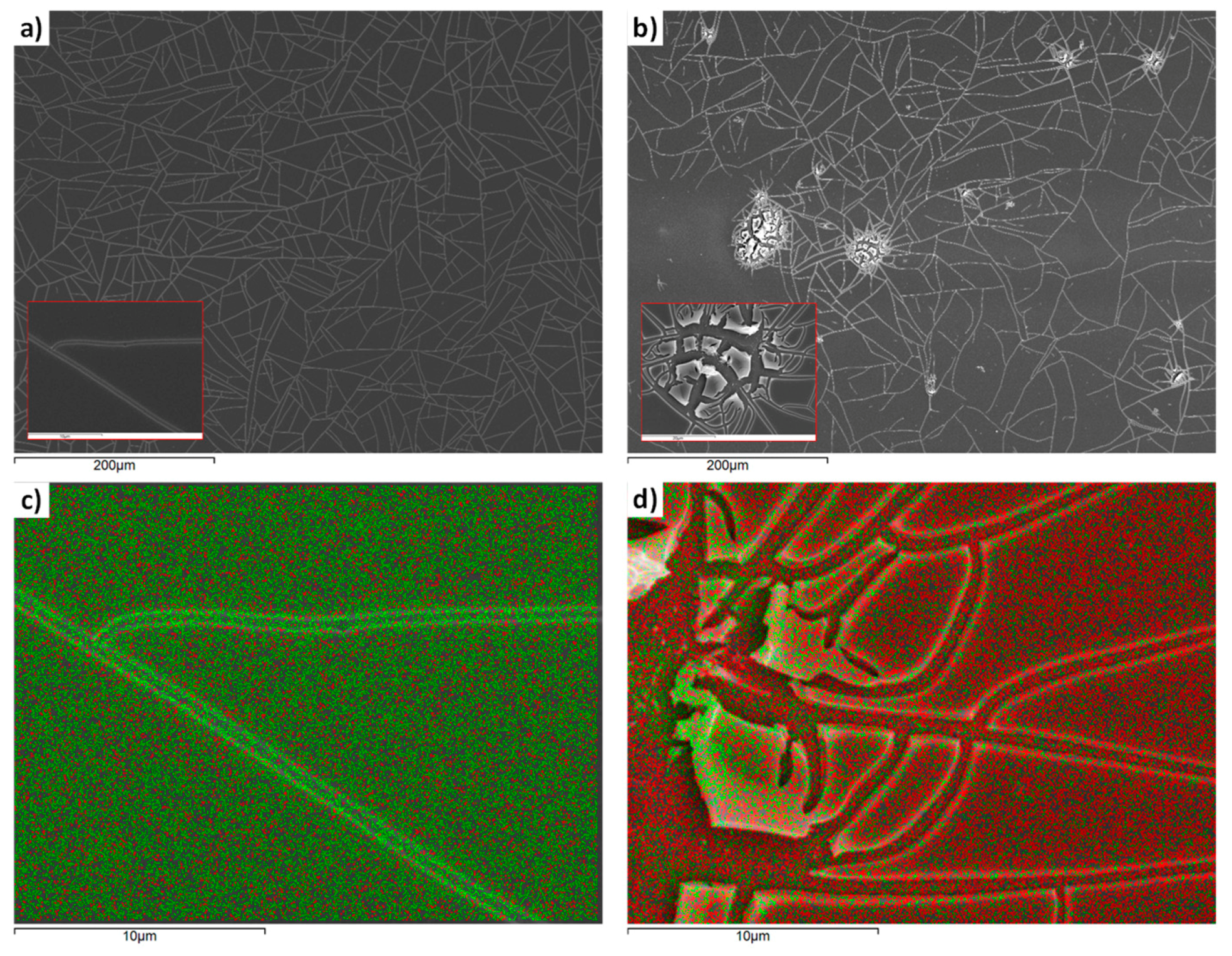
2.2.4. Morphological and Structural Characterization
2.2.5. Thickness Measurements
3. Results
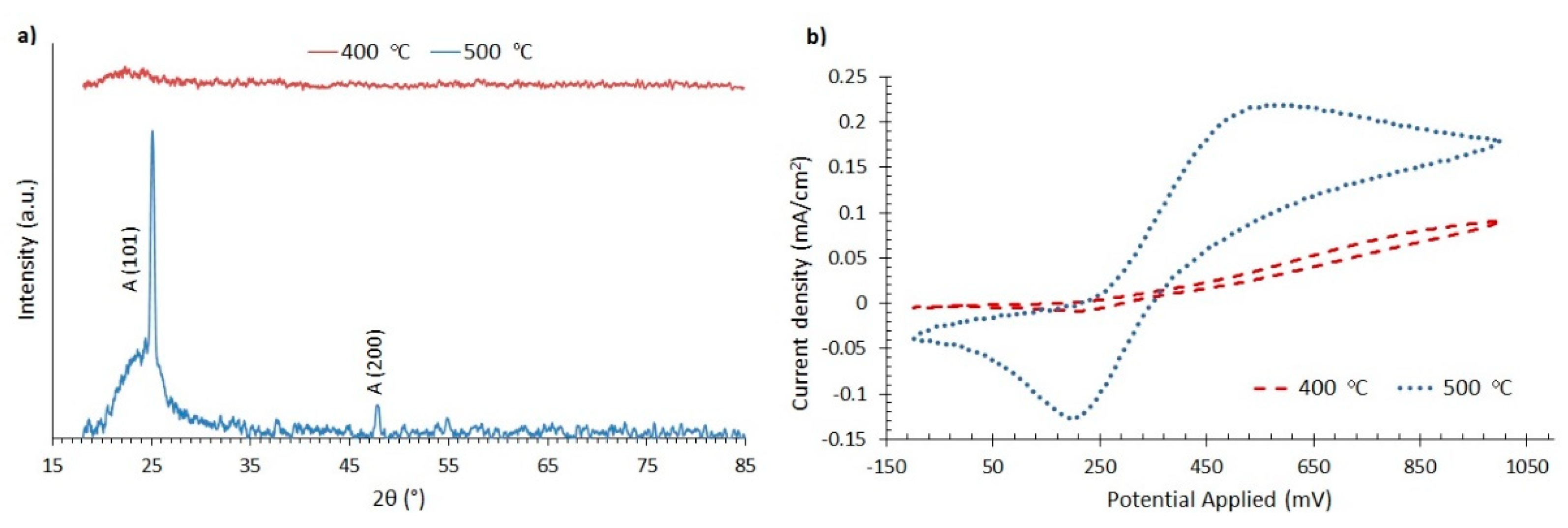
3.1. Morphology, Structure and Electrochemical Properties of the TiO2 Compact Layer
3.2. Optical and Electrical Characteristics
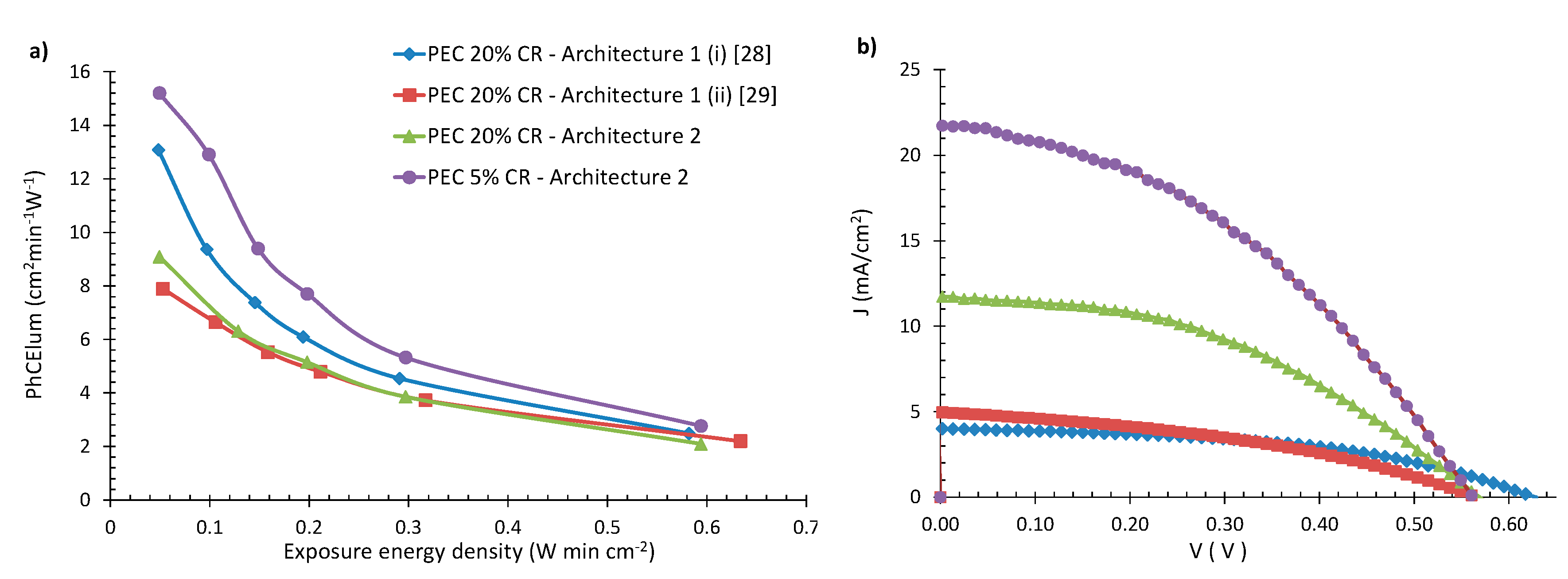
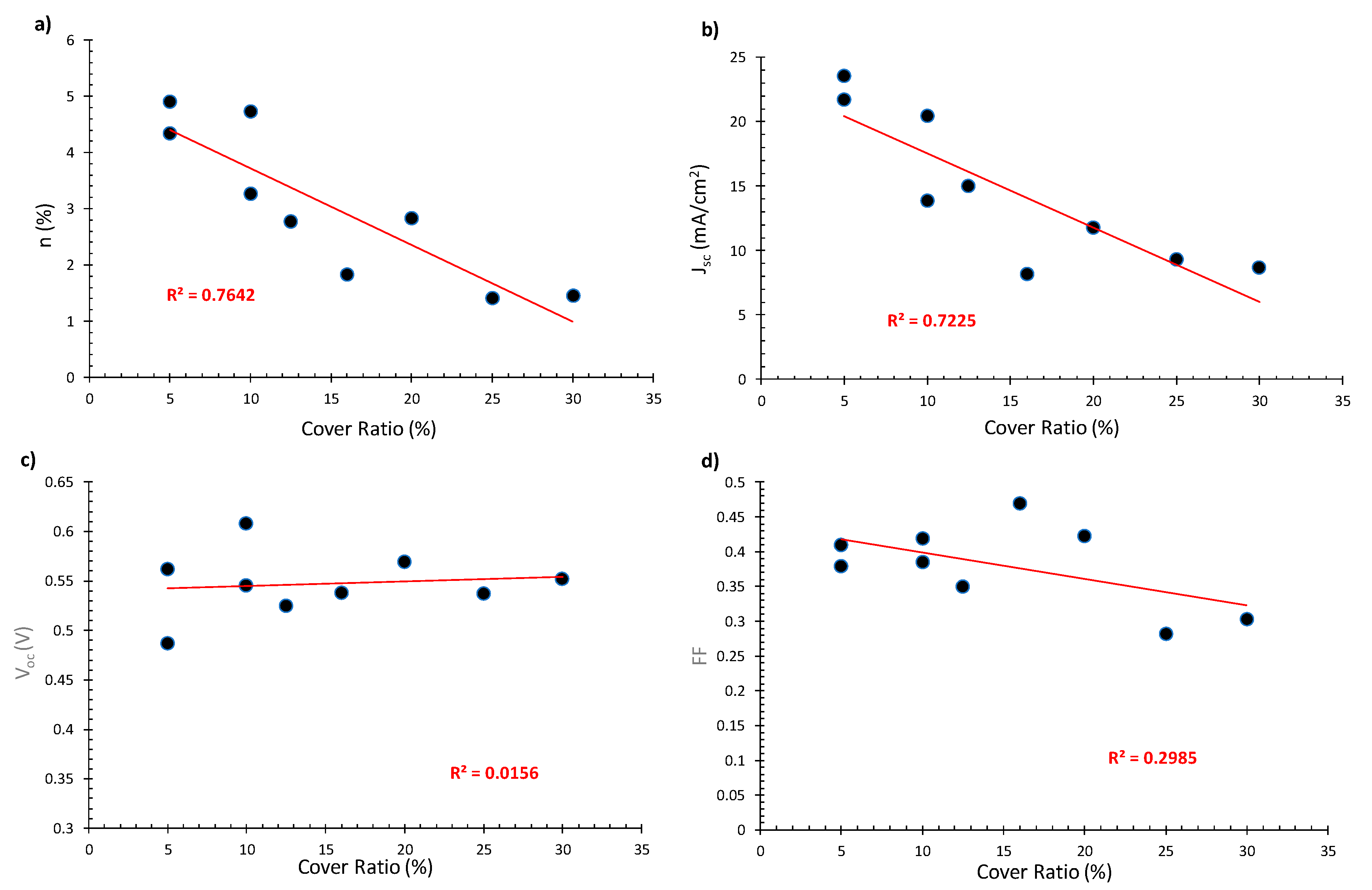
3.3. Effects of the Cover Ratio
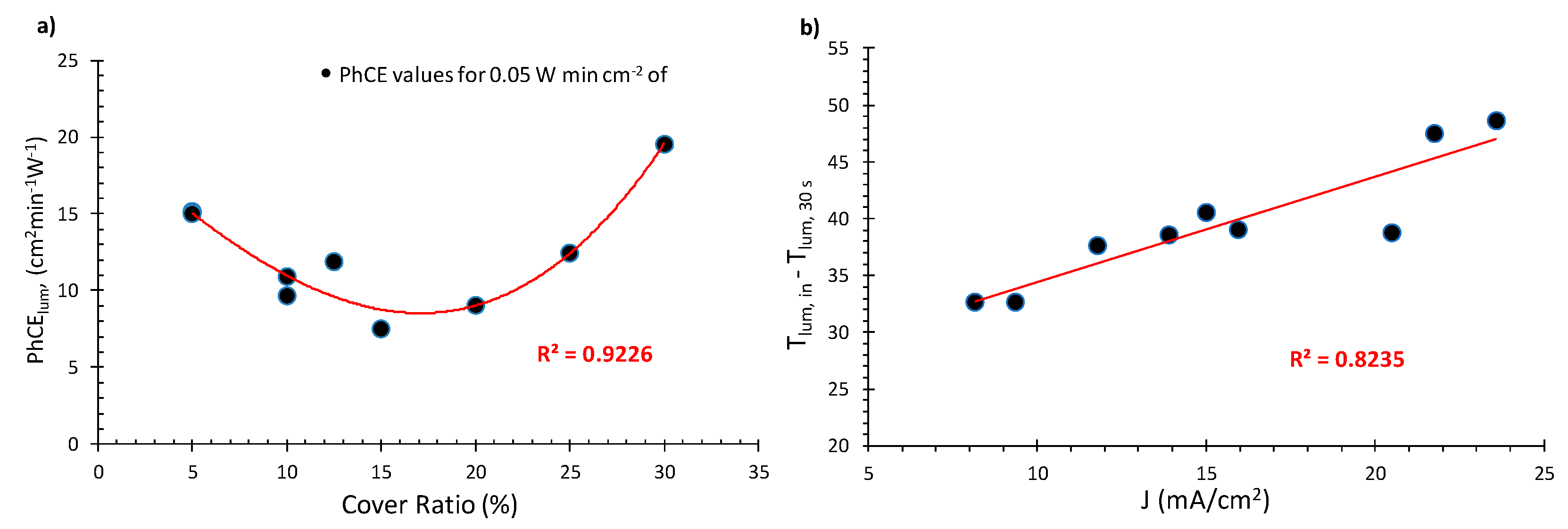
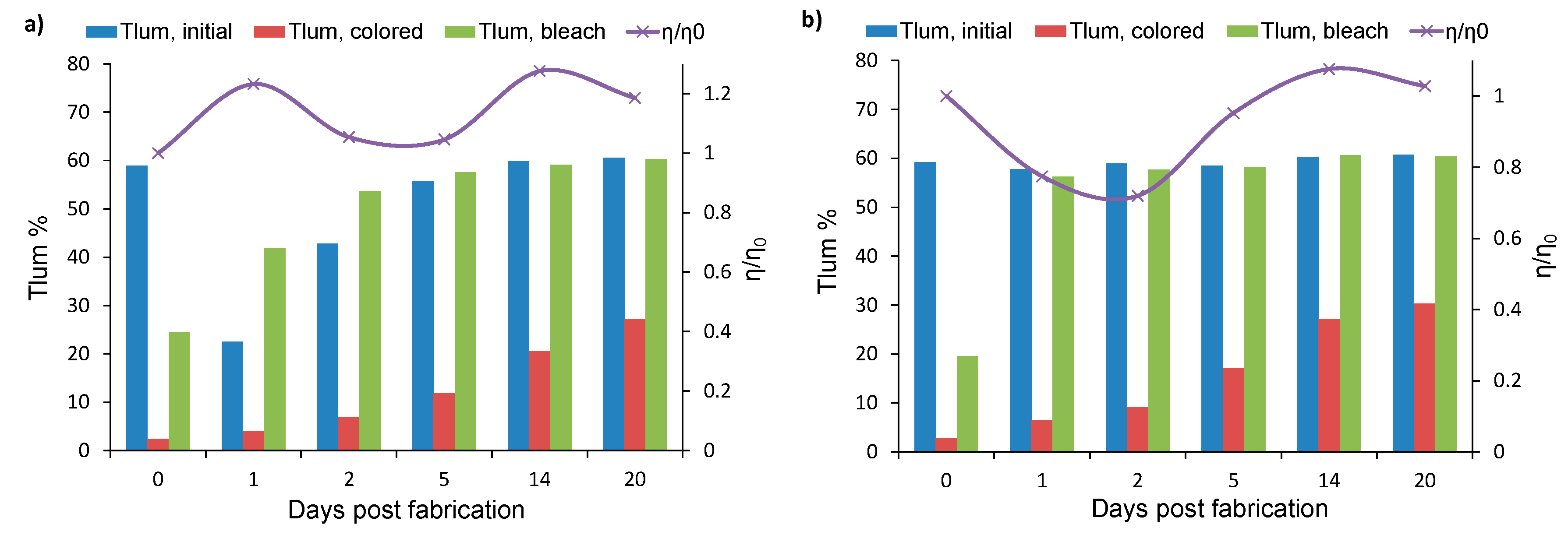
3.3.1. Extended Testing
4. Conclusions
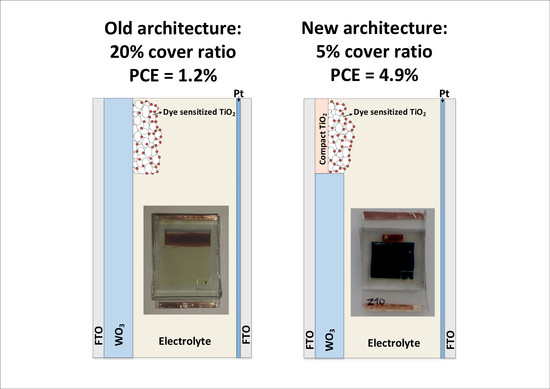
- This work constitutes an experimental study of partly covered photoelectrochromic devices fabricated with a new architecture aimed to improve power conversion efficiency;
- In the new architecture 2, the TiO2 film is deposited first on the substrate, covering a small part of its surface, followed by the WO3 film that covers the remaining device area. This allows proper treatment of the TiO2 layer without affecting the morphology of the WO3, and thus the EC film retains its initial electrochromical capabilities;
- PEC devices of the new architecture 2 and 20% CR exhibit 2.4 times higher PCE than their architecture 1 counterparts. The highest PCE (namely 4.9%) was measured in a device of 5% CR and architecture 2, resulting in an overall increase in PCE between architecture 1 and 2 of 415%;
- The effect of cover ratio on PCE was evaluated. It was found that, with decreasing CR, the PCE values increase mostly due to the increase in photocurrent density;
- The effect of the cover ratio on the coloration speed of the devices was also tested. It was found that when starting at 30% CR, down to 15% with CR decreasing, the values of the PhCE (at 0.05 W min cm−2 of exposure) also decrease in accordance to our previous research. However, below 15% CR, an upward trend was observed, as devices with 5% CR exhibit similar PhCE values to their 25% CR counterparts;
- Devices with architecture 2 and 5% CR can combine fast coloration, high PCE and low optical obstruction;
- Storage of the devices in short circuit conditions was found to accelerate optical reversibility without affecting their photovoltaic and coloration performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casini, M. Active dynamic windows for buildings: A review. Renew. Energy 2018, 119, 923–934. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Arvizu, M.A.; Bayrak-Pehlivan, I.; Qu, H.Y.; Wen, R.T.; Niklasson, G.A. Electrochromic materials and devices for energy efficiency and human comfort in buildings: A critical review. Electrochim. Acta 2018, 259, 1170–1182. [Google Scholar] [CrossRef]
- Ghosh, A.; Norton, B. Advances in switchable and highly insulating autonomous (self-powered) glazing systems for adaptive low energy buildings. Renew. Energy 2018, 126, 1003–1031. [Google Scholar] [CrossRef]
- Cannavale, A.; Cossari, P.; Eperon, G.E.; Colella, S.; Fiorito, F.; Gigli, G.; Listorti, A.; Snaith, H.J. Forthcoming perspectives of photoelectrochromic devices: A critical review. Energy Environ. Sci. 2016, 9, 2682–2719. [Google Scholar] [CrossRef]
- Bechinger, C.; Ferrere, S.; Zaban, A.; Sprague, J.; Gregg, B.A. Photoelectrochromic windows and displays. Nature 1996, 383, 608–610. [Google Scholar] [CrossRef]
- Hauch, A.; Georg, A.; Baumgärtner, S.; Krašovec, U.O.; Orel, B. New photoelectrochromic device. Electrochim. Acta 2001, 46, 2131–2136. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. Development of photoelectrochromic devices for dynamic solar control in buildings. Sol. Energy Mater. Sol. Cells 2010, 94, 2304–2313. [Google Scholar] [CrossRef]
- Bella, F.; Leftheriotis, G.; Griffini, G.; Syrrokostas, G.; Turri, S.; Grätzel, M.; Gerbaldi, C. A New Design Paradigm for Smart Windows: Photocurable Polymers for Quasi-Solid Photoelectrochromic Devices with Excellent Long-Term Stability under Real Outdoor Operating Conditions. Adv. Funct. Mater. 2016, 26, 1127–1137. [Google Scholar] [CrossRef]
- Theodosiou, Κ.; Dokouzis, A.; Antoniou, I.; Leftheriotis, G. Gel electrolytes for partly covered photoelectrochromic devices. Sol. Energy Mater. Sol. Cells 2019, 202, 110124. [Google Scholar] [CrossRef]
- Bogati, S.; Georg, A.; Graf, W. Photoelectrochromic devices based on sputtered WO3 and TiO2 films. Sol. Energy Mater. Sol. Cells 2017, 163, 170–177. [Google Scholar] [CrossRef]
- Cannavale, A.; Manca, M.; Malara, F.; de Marco, L.; Cingolani, R.; Gigli, G. Highly efficient smart photovoltachromic devices with tailored electrolyte composition. Energy Environ. Sci. 2011, 4, 2567–2574. [Google Scholar] [CrossRef]
- Cannavale, A.; Manca, M.; de Marco, L.; Grisorio, R.; Carallo, S.; Suranna, G.P.; Gigli, G. Photovoltachromic device with a micropatterned bifunctional counter electrode. ACS Appl. Mater. Interfaces 2014, 6, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Cannavale, A.; Eperon, G.E.; Cossari, P.; Abate, A.; Snaith, H.J.; Gigli, G. Perovskite photovoltachromic cells for building integration. Energy Environ. Sci. 2015, 8, 1578–1584. [Google Scholar] [CrossRef]
- Syrrokostas, G.; Dokouzis, A.; Yannopoulos, S.N.; Leftheriotis, G. Novel Photoelectrochromic Devices Incorporating Carbon-Based Perovskite Solar Cells. 2020; in press, 1st revision pending. [Google Scholar]
- Zhou, F.; Ren, Z.; Zhao, Y.; Shen, X.; Wang, A.; Li, Y.Y.; Surya, C.; Chai, Y. Perovskite Photovoltachromic Supercapacitor with All-Transparent Electrodes. ACS Nano 2016, 10, 5900–5908. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, B.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C.; Zhang, W.; Zhang, J. A solar-driven flexible electrochromic supercapacitor. Materials 2020, 13, 1206. [Google Scholar] [CrossRef]
- Syrrokostas, G.; Leftheriotis, G.; Yianoulis, P. Effect of acidic additives on the structure and performance of TiO2 films prepared by a commercial nanopowder for dye-sensitized solar cells, Renew. Energy 2014, 72, 164–173. [Google Scholar] [CrossRef]
- Giannouli, M.; Syrrokostas, G.; Yianoulis, P. Effects of using multi-component electrolytes on the stability and properties of solar cells sensitized with simple organic dyes, Prog. Photovoltaics Res. Appl. 2010, 18, 128–136. [Google Scholar] [CrossRef]
- Giannopoulos, P.; Raptis, D.; Theodosiou, K.; Andreopoulou, A.K.; Anastasopoulos, C.; Dokouzis, A.; Leftheriotis, G.; Lianos, P.; Kallitsis, J.K. Organic dyes end-capped with perfluorophenyl anchors: Synthesis, electrochemical properties and assessment of sensitization capacity of titania photoanodes. Dyes Pigment. 2018, 148, 167–179. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Costa, C.; Ivanou, D.; Pinto, J.; Mendes, J.; Mendes, A. Impact of the architecture of dye sensitized solar cell-powered electrochromic devices on their photovoltaic performance and the ability to color change. Sol. Energy 2019, 182, 22–28. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Fan, R.; Wang, P.; Dong, Y. Enhanced photovoltaic performance of dye-sensitized solar cells using a new photoelectrode material: Upconversion YbF3-Ho/TiO2 nanoheterostructures. Nanoscale 2016, 8, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, M.; Kim, J.M.; Kim, J.J.; Lee, I.H. Enhanced photovoltaic performance of back-illuminated dye-sensitized solar cell based on TiO2 nanoparticle/nanowire composite film in cobalt redox system. J. Alloys Compd. 2016, 656, 568–572. [Google Scholar] [CrossRef]
- Kumara, N.T.R.N.; Lim, A.; Lim, C.M.; Petra, M.I.; Ekanayake, P. Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 78, 301–317. [Google Scholar] [CrossRef]
- Son, Y.J.; Kang, J.S.; Yoon, J.; Kim, J.; Jeong, J.; Kang, J.; Lee, M.J.; Park, H.S.; Sung, Y.E. Influence of TiO2 Particle Size on Dye-Sensitized Solar Cells Employing an Organic Sensitizer and a Cobalt(III/II) Redox Electrolyte. J. Phys. Chem. C 2018, 122, 7051–7060. [Google Scholar] [CrossRef]
- Park, S.K.; Bae, J.Y.; Kim, J.H.; Ahn, K.-S.; Lee, D.K.; Han, Y.S. Optimization of the TiO2-Surface Modification Temperature for Performance Enhancement of Dye-Sensitized Solar Cells. J. Nanosci. Nanotechnol. 2014, 14, 5828–5832. [Google Scholar] [CrossRef]
- Dokouzis, A.; Theodosiou, K.; Leftheriotis, G. Assessment of the long-term performance of partly covered photoelectrochromic devices under insolation and in storage. Sol. Energy Mater. Sol. Cells 2018, 182, 281–293. [Google Scholar] [CrossRef]
- Dokouzis, A.; Bella, F.; Theodosiou, K.; Gerbaldi, C.; Leftheriotis, G. Photoelectrochromic devices with cobalt redox electrolytes. Mater. Today Energy 2020, 15, 100365. [Google Scholar] [CrossRef]
- Syrrokostas, G.; Leftheriotis, G.; Yianoulis, P. Performance and stability of “partly covered” photoelectrochromic devices for energy saving and power production. Solid State Ion. 2015, 277, 11–22. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. Photocoloration efficiency and stability of photoelectrochromic devices. Solid State Ion. 2013, 231, 30–36. [Google Scholar] [CrossRef]
- Giribabu, L.; Bolligarla, R.; Panigrahi, M. Recent Advances of Cobalt(II/III) Redox Couples for Dye-Sensitized Solar Cell Applications. Chem. Rec. 2015, 15, 760–788. [Google Scholar] [CrossRef] [PubMed]
- Syrrokostas, G.; Siokou, A.; Leftheriotis, G.; Yianoulis, P. Degradation mechanisms of Pt counter electrodes for dye sensitized solar cells. Sol. Energy Mater. Sol. Cells 2012, 103, 119–127. [Google Scholar] [CrossRef]
- Syrrokostas, G.; Govatsi, K.; Leftheriotis, G.; Yannopoulos, S.N. Platinum decorated zinc oxide nanowires as an efficient counter electrode for dye sensitized solar cells. J. Electroanal. Chem. 2019, 835, 86–95. [Google Scholar] [CrossRef]
- Lee, W.J.; Ramasamy, E.; Lee, D.Y. Effect of electrode geometry on the photovoltaic performance of dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 1448–1451. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. Partly covered photoelectrochromic devices with enhanced coloration speed and efficiency. Sol. Energy Mater. Sol. Cells 2012, 96, 86–92. [Google Scholar] [CrossRef]

| PEC Architecture | CR (%) | Jm (mA/cm2) | Vm (mV) | JSC (mA/cm2) | VOC (mV) | FF | n (%) |
|---|---|---|---|---|---|---|---|
| 1(i) | 20 | 2.86 | 412 | 4.00 | 628 | 0.469 | 1.18 |
| 1(ii) | 20 | 2.78 | 344 | 5.05 | 566 | 0.384 | 1.08 |
| 2 2 | 20 5 | 8.50 14.26 | 332 344 | 11.74 21.75 | 568 561 | 0.423 0.401 | 2.83 4.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dokouzis, A.; Zoi, D.; Leftheriotis, G. Photoelectrochromic Devices with Enhanced Power Conversion Efficiency. Materials 2020, 13, 2565. https://doi.org/10.3390/ma13112565
Dokouzis A, Zoi D, Leftheriotis G. Photoelectrochromic Devices with Enhanced Power Conversion Efficiency. Materials. 2020; 13(11):2565. https://doi.org/10.3390/ma13112565
Chicago/Turabian StyleDokouzis, Alexandros, Dimitra Zoi, and George Leftheriotis. 2020. "Photoelectrochromic Devices with Enhanced Power Conversion Efficiency" Materials 13, no. 11: 2565. https://doi.org/10.3390/ma13112565
APA StyleDokouzis, A., Zoi, D., & Leftheriotis, G. (2020). Photoelectrochromic Devices with Enhanced Power Conversion Efficiency. Materials, 13(11), 2565. https://doi.org/10.3390/ma13112565