Designing Dual-Effect Nanohybrids for Removing Heavy Metals and Different Kinds of Anions from the Natural Water
Abstract
1. Introduction
2. Materials and Methods
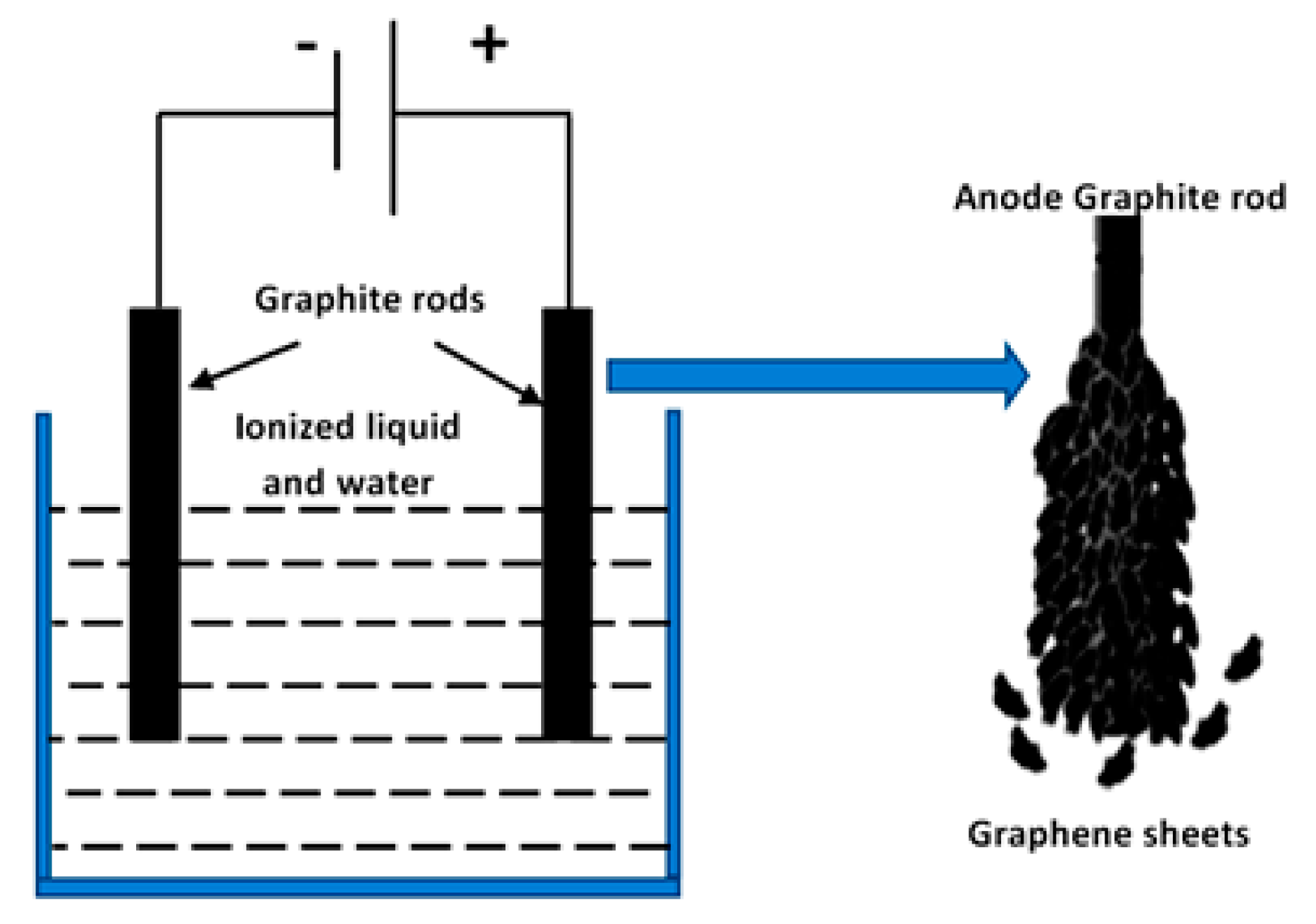
2.1. Preparation of Graphene Sheets
2.2. Preparation of Nanohybrids
2.3. Characterization
2.4. Adsorption Experiment
3. Results
3.1. Powder X-Ray Diffraction
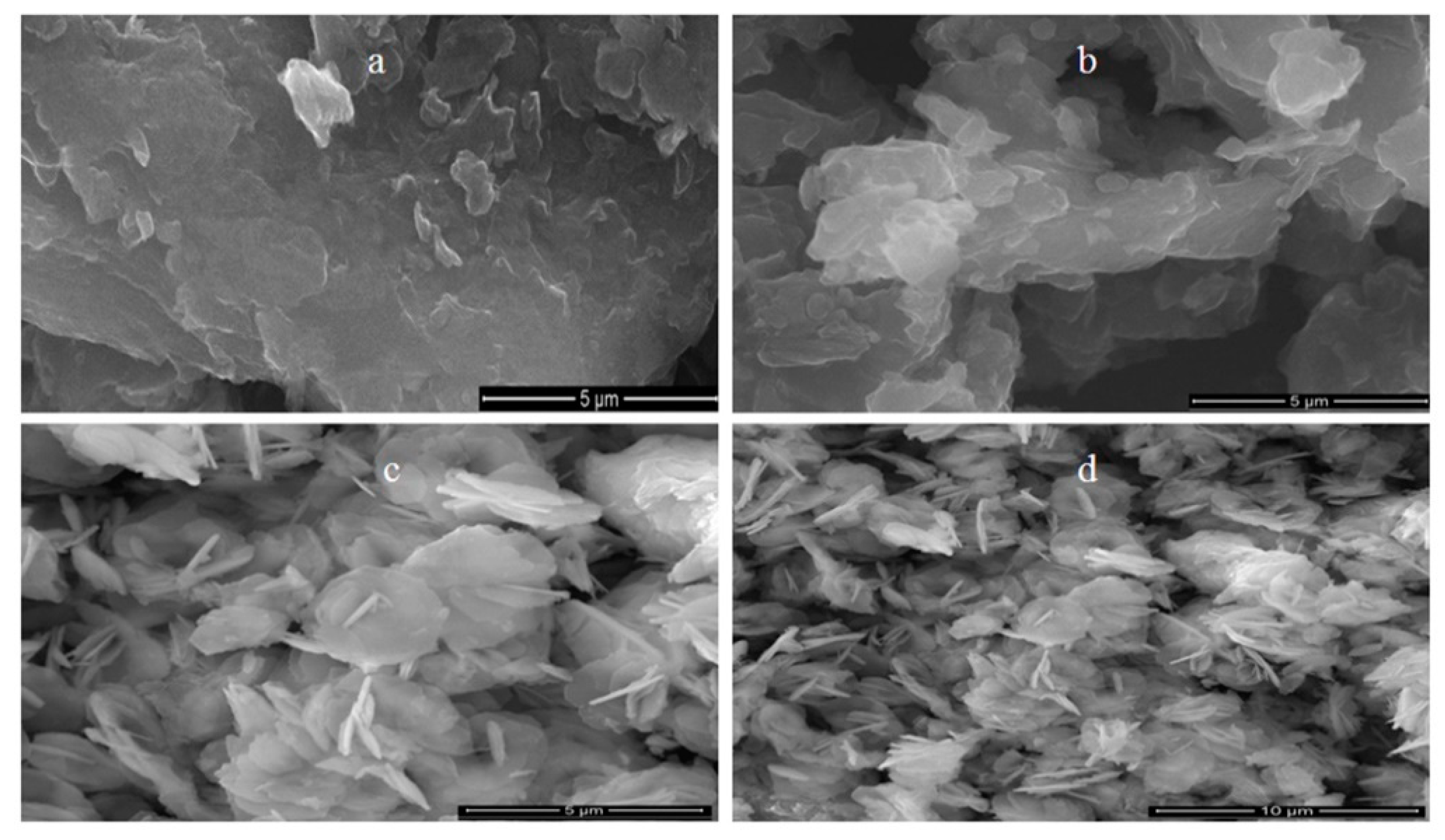
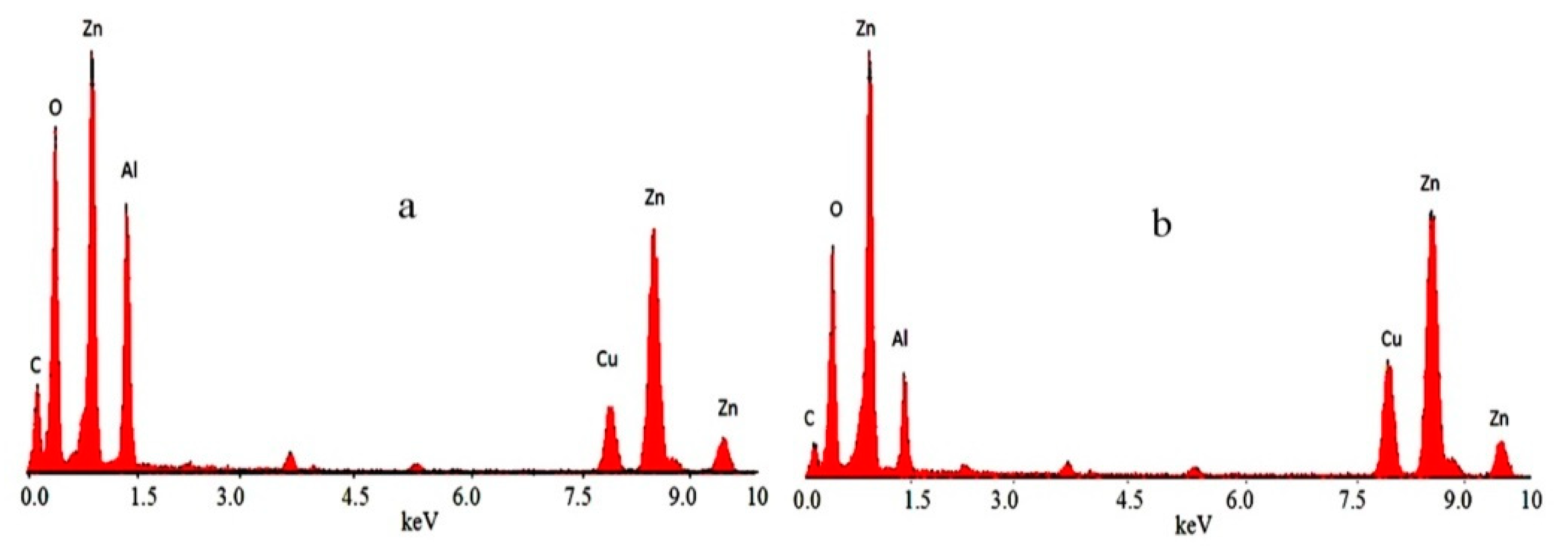
3.2. Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectrometry
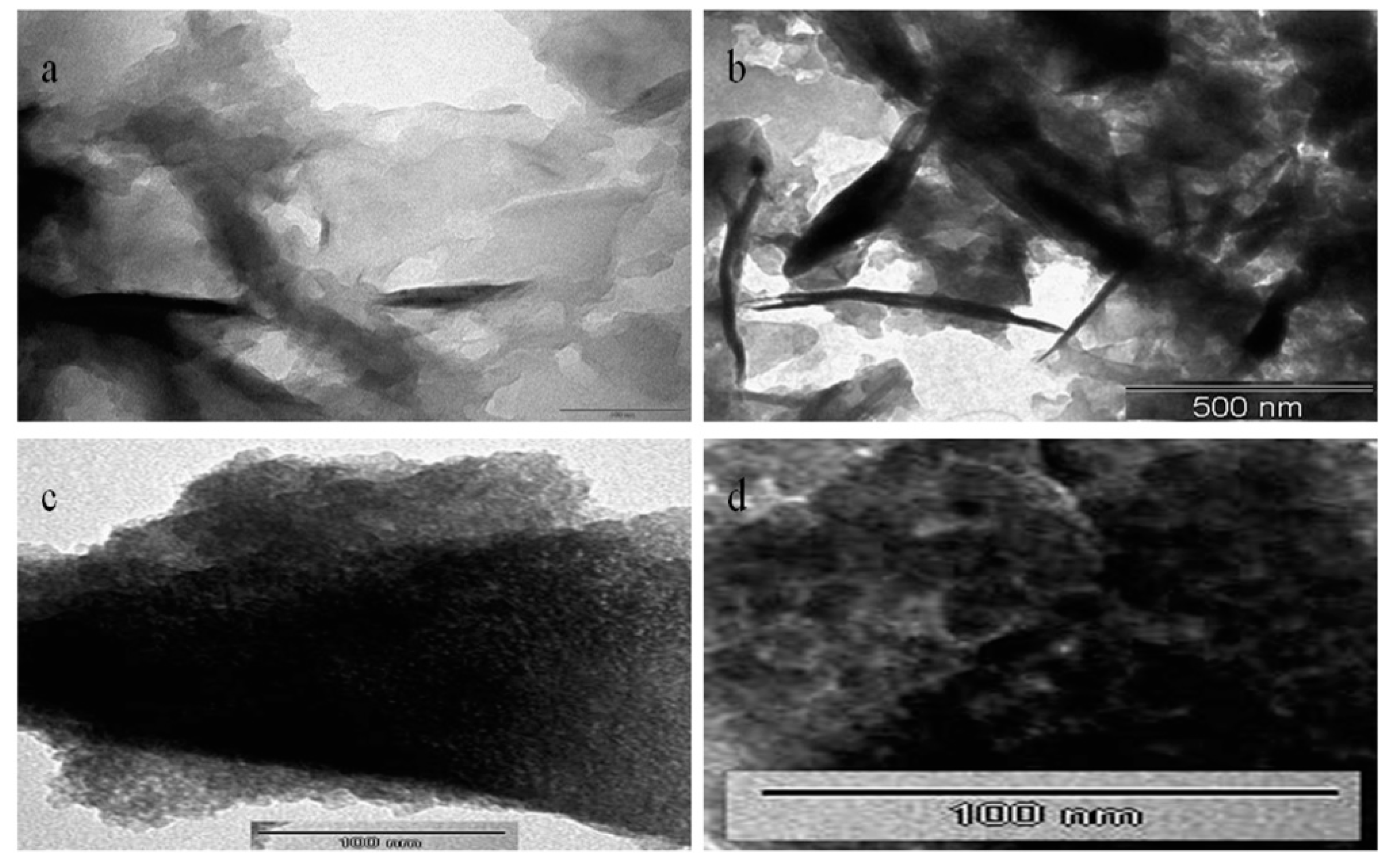
3.3. Transmission Electron Microscopy
3.4. Raman Spectroscopy
3.5. Fourier Transform Infrared Spectroscopy
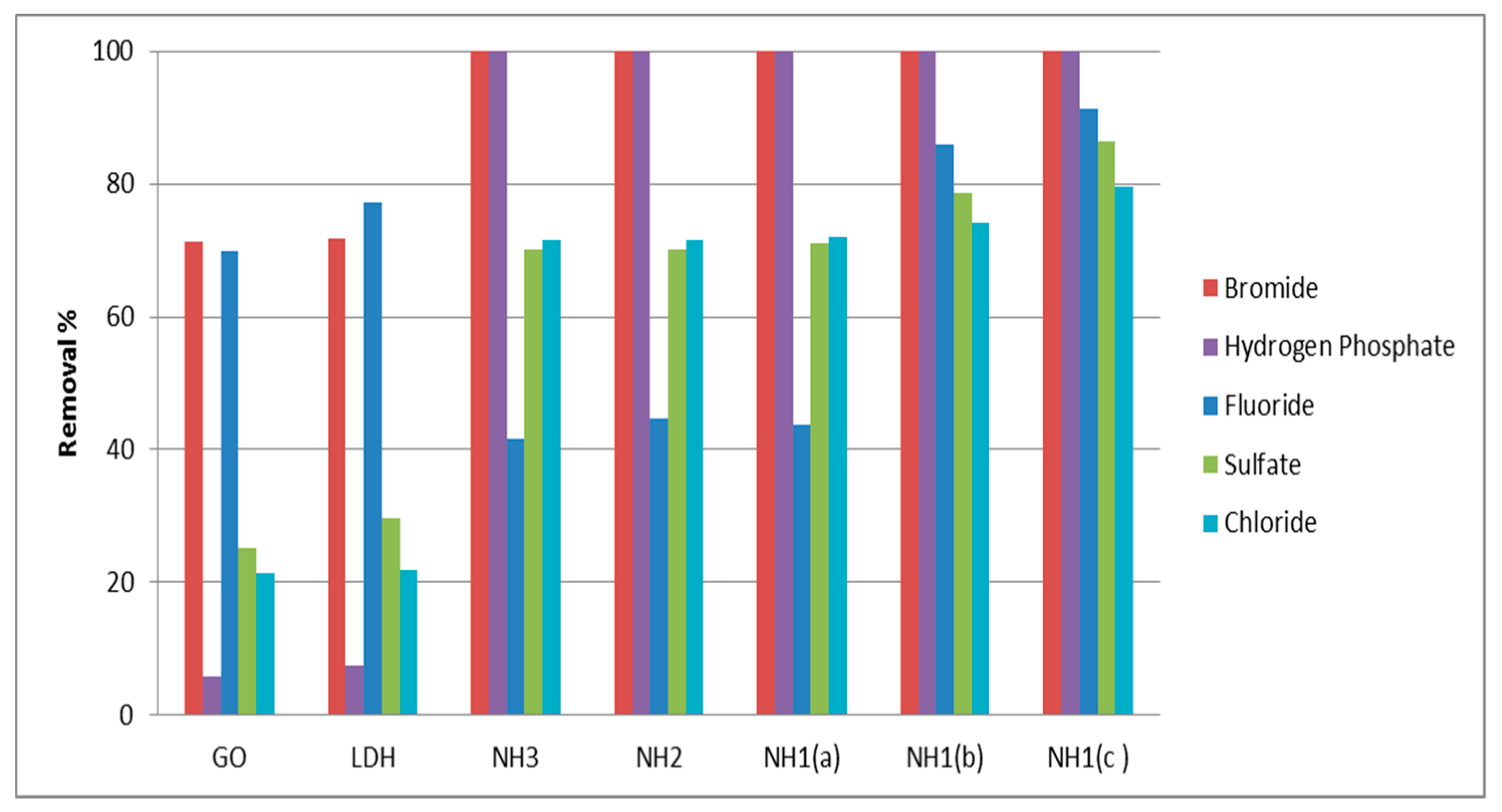
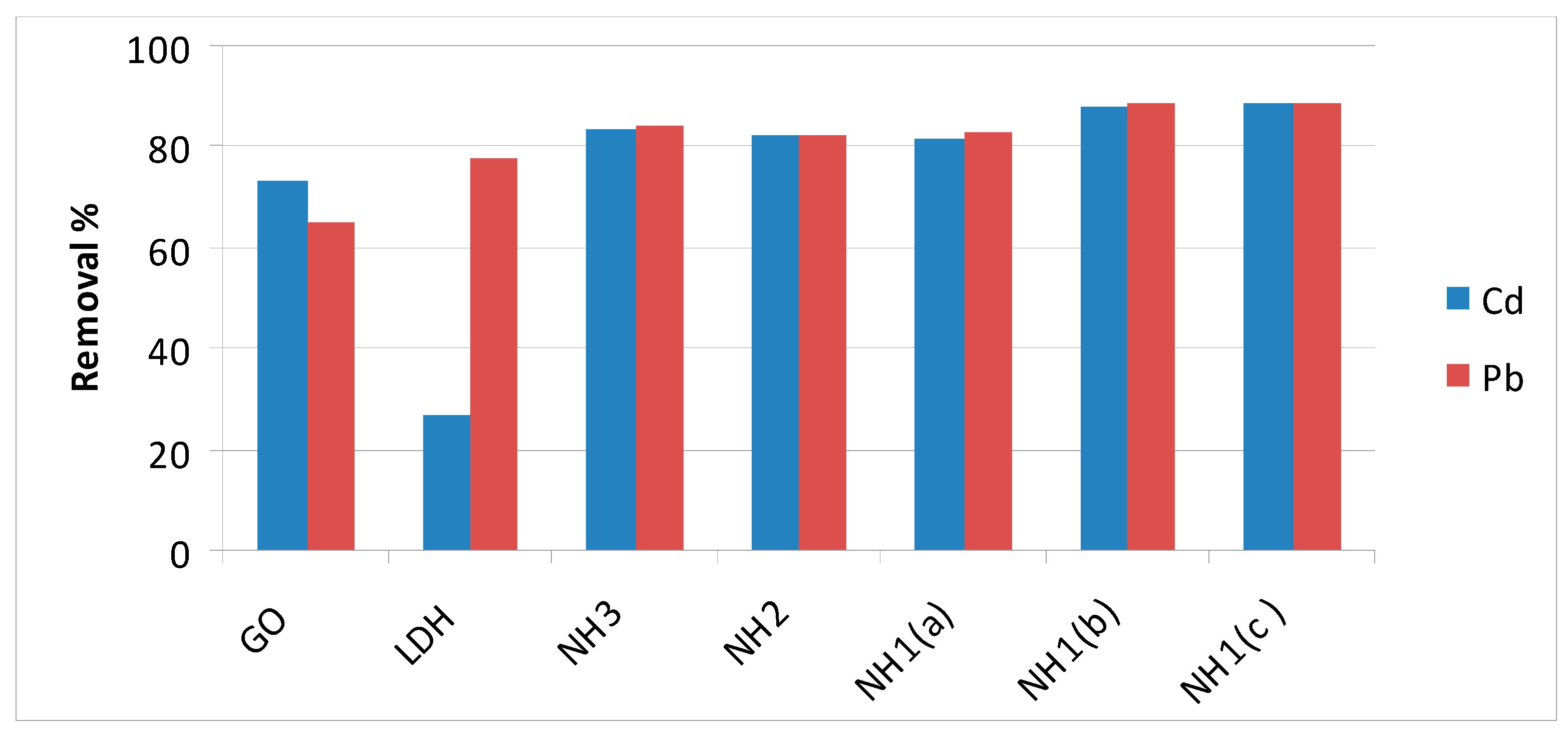
3.6. Purification of Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Guidelines for drinking-water quality. In Chemical Fact Sheet; World Health Organization: Geneva, Switzerland, 2004; pp. 296–459. [Google Scholar]
- Strobl, R.O.; Robillard, P.D. Network design for water quality monitoring of surface freshwaters: A review. J. Environ. Manag. 2008, 87, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Kawarada, K.; Haneishi, K.; Iida, T. Pore structure and performance for drinking water treatment of activated carbon prepared from sugi thinning from water source forest in Tokyo. Wood Ind. 2005, 60, 398–401. [Google Scholar]
- Wei, Y.C.; Yang, Z.S.; Alsaedi, A.H.; Wang, X. Understanding the adsorption mechanism of Ni(II) on graphene oxides by batch experiments and density functional theory studies. Sci. China Chem. 2016, 59, 412–419. [Google Scholar]
- Soyluoglu, M.; Ersan, M.S.; Ateia, M.; Karanfil, T. Removal of bromide from natural waters: Bromide-selective vs. conventional ion exchange resins. Chemosphere 2020, in press. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.H.; Ok, Y.S.; Tsang, D.C.W.; Tsange, Y.F.; Giria, B.S.; Singha, R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616–617, 1242–1260. [Google Scholar] [CrossRef]
- Ashane, W.; Fernando, M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishettyb, M. Challenges and opportunities in the removal of sulphate ions in contaminated mine water: A review. Miner. Eng. 2018, 117, 74–90. [Google Scholar]
- Angst, U.; Elsener, B. Mechanism of electrochemical chloride removal. Corros. Sci. 2007, 49, 4504–4522. [Google Scholar]
- Costantino, U.; Nocchetti, M.; Sisani, M.; Vivani, R. Recent progress in the synthesis and application of organically modified hydrotalcites. Z. Kristallogr. 2009, 224, 273–281. [Google Scholar] [CrossRef]
- Parida, K.M.; Dash, S.K. Adsorption of Cu2+ on spherical Fe-MCM-41 and its application for oxidation of adamantane. J. Hazard. Mater. 2010, 179, 642–649. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, Z.; Cabreraa, C.R.; Chen, Z. Graphene, inorganic graphene analogs and their composites for lithium ion batteries. J. Mater. Chem. A 2014, 2, 12104–12122. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Wei, F. Hierarchical Nanocomposites Derived from Nanocarbons and Layered Double Hydroxides-Properties, Synthesis, and Applications. Adv. Funct. Mater. 2012, 22, 675–694. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z.L. Removal of arsenate from aqueous solution by nanocrystalline Mg/Al layered double hydroxide: Sorption characteristics, prospects, and challenges. Water Sci. Technol. 2010, 61, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, J.E.; Zhang, L.; Chen, X.; Zhang, H.; Zhang, F.; Xu, S.; Evans, D.G. Facile synthesis of NiAl-layered double hydroxide/graphene hybrid with enhanced electrochemical properties for detection of dopamine. Nanoscale 2011, 3, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L. Green and facile production of high-quality graphene from graphite by the combination of hydroxyl radical and electrical exfoliation. RSC Adv. 2018, 8, 40621–40631. [Google Scholar] [CrossRef]
- Gonazlez, M.A.; Pavlovic, I.; Rojas-Delgado, R.; Barriga, C. Removal of Cu2+, Pb2+ and Cd2+ by layered double hydroxide-humate hybrid. Sorbate and sorbent comparative studies. Chem. Eng. J. 2014, 254, 605–611. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Wang, J.; Zhou, C.; Tang, Q.; Rao, X. Calcined graphene/MgAl-layered double hydroxides for enhanced Cr (VI) removal. Chem. Eng. J. 2013, 221, 204–213. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, J.; Zhao, J.; Hu, G.; Dang, Z.A. hybrid Mg–Al layered double hydroxide/graphene nanostructure obtained via hydrothermal synthesis. Chem. Phys. Lett. 2014, 605–606, 77–80. [Google Scholar] [CrossRef]
- Qiu, T.; Yang, J.; Bai, X.; Wang, Y. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef]
- Saber, O.; Aljaafari, A.; Alomair, H.A.; Alshoaibi, A. Novel Strategy for Producing Nanoplatelets to be Used as Building Blocks for Shaping Nanofibers through Layered Double Hydroxides and Poly Vinyl Alcohol. Chem. Sel. 2019, 4, 4293–4300. [Google Scholar] [CrossRef]
- Saber, O.; Aljaafari, A.; Alshoaibi, A.; Al-Yaari, M.; Osama, M. A Novel Route for Controlling and Improving the Texture of Porous Structures Through Dual Growth of Alumina Nanoparticles and Carbon Nanotubes Using Explosion Process of Solid Fuel. J. Mater. Res. Technol. 2020, 9, 67–75. [Google Scholar] [CrossRef]
- Wu, F.; Liang, J.; Peng, Z.; Liu, B. deposition and characterization of Zn–Al layered double hydroxides (LDHs) films on magnesium alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- Shamsayai, M.; Yamini, Y.; Asiabi, H. Fabrication of zwitterionic histidine/layered double hydroxide hybrid nanosheets for highly e_cient and fast removal of anionic dyes. J. Colloid Interface Sci. 2018, 529, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.L.; Yuan, X.L.; Zou, W.J.; Huang, X.J.; Mo, S.S.; Yuan, D.S. Synthesis of highly graphitic mesoporous carbon using Ni–Fe double-layered hydroxide as both template and catalyst precursor. Carbon 2013, 28, 563–564. [Google Scholar] [CrossRef]
- Atchudana, R.; Edison, T.N.J.I.; Perumal, S.; Shanmugam, M.; Lee, Y.R. Direct solvothermal synthesis of zinc oxide nanoparticle decorated graphene oxide nanocomposite for efficient photodegradation of azo-dyes. J. Photochem. Photobiol. A Chem. 2017, 337, 100–111. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xing, W.; Li, L.; Xue, Q.; Yan, Z. Sandwich-like graphene/polypyrrole/layered double hydroxide nanowires for high-performance supercapacitors. J. Power Sources 2016, 331, 67–75. [Google Scholar] [CrossRef]
- Khobragade, P.S.; Hansora, D.P.; Jitendra, N.; Njuguna, J.; Mishra, S. Preparation and analysis of multi-layered hybrid nanostructures. Appl. Clay Sci. 2016, 132–133, 668–674. [Google Scholar] [CrossRef]
- Hatami, H.; Fotovat, A.; Halajnia, A. Comparison of adsorption and desorption of phosphate on synthesized Zn-Al LDH by two methods in a simulated soil solution. Appl. Clay Sci. 2018, 152, 333–341. [Google Scholar] [CrossRef]
- Lv, L.; Sun, P.; Gu, Z.; Du, H.; Pang, X.; Tao, X.; Xu, R.; Xu, L. Removal of chloride ion from aqueous solution by ZnAl–NO3 layered double hydroxides as anion–exchanger. J. Hazard. Mater. 2009, 161, 1444–1449. [Google Scholar] [CrossRef]
- Elhalil, A.; Qourzal, S.; Mahjoubi, F.Z.; Elmoubarki, R.; Farnane, M.; Tounsadi, H.; Sadiq, M.; Abdennouri, M.; Barka, N. Defluoridation of groundwater by calcined Mg/Al layered double hydroxide. Emerg. Contam. 2016, 2, 42–48. [Google Scholar] [CrossRef]
- Kameda, T.; Oba, J.; Yoshioka, T. Recyclable Mg–Al layered double hydroxides for fluoride removal: Kinetic and equilibrium studies. J. Hazard. Mater. 2015, 300, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Q.; Xiao, H.; Lu, H.; Zhou, Y. Synthesis of Li–Al Layered Double Hydroxides (LDHs) for Efficient Fluoride Removal. Ind. Eng. Chem. Res. 2012, 51, 11490–11498. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Baruah, A.; Mondal, S.; Sahoo, L.; Gautam, U.K. Ni-Fe-layered double hydroxide/N-doped graphene oxide nanocomposite for the highly efficient removal of Pb(II) and Cd(II) ions from water. J. Solid State Chem. 2019, 280, 120963. [Google Scholar] [CrossRef]
- Pérez, M.R.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Hermosín, M.C.; Ulibarri, M.A. Uptake of Cu2+, Cd2+ and Pb2+ on Zn–Al layered double hydroxide intercalated with EDTA. Appl. Clay Sci. 2006, 32, 245–251. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.H. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]




| Assignment | Samples (Wavenumbers, cm−1) | ||||
|---|---|---|---|---|---|
| Zn-Al LDH | NH1 | NH2 | NH3 | Graphene | |
| νO–H | 3418 | 3407 | 3431 | 3404 | - |
| Absorption bands (C–H) or H–bond | 2988 | 2986 | 3048 | 2915 284 | 2916 2847 |
| δH2O | 1649 | 1606 | 1637 | 1637 | 1698 |
| Absorption bands (COO–) | 1429 1360 | 1355 | 1360 | 1434 1355 | 1562 |
| Anions | mg/L | Heavy Metal | mg/L |
|---|---|---|---|
| Bromide | 16.2 | Lead | 0.25 |
| Hydrogen phosphate | 0.35 | - | - |
| Fluoride | 0.98 | Cadmium | 0.23 |
| Chloride | 246 | - | - |
| Sulfate | 117 | - | - |
| Samples | Graphene Oxide (Wt.%) | SBET (m2/g) | SBJH (m2/g) | VpBJH (cm3/g) | RpBJH (nm) |
|---|---|---|---|---|---|
| Zn-Al LDH | 0.0 | 5.60 | 3.30 | 0.0120 | 1.80 |
| NH1 | 3.50 | 33.40 | 42.0 | 0.080 | 3.50 |
| NH2 | 2.30 | 61.50 | 75.30 | 0.130 | 3.20 |
| NH3 | 1.30 | 60.30 | 57.0 | 0.290 | 3.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, O.; Asiri, S.M.; Ezzeldin, M.F.; El-Azab, W.I.M.; Abu-Abdeen, M. Designing Dual-Effect Nanohybrids for Removing Heavy Metals and Different Kinds of Anions from the Natural Water. Materials 2020, 13, 2524. https://doi.org/10.3390/ma13112524
Saber O, Asiri SM, Ezzeldin MF, El-Azab WIM, Abu-Abdeen M. Designing Dual-Effect Nanohybrids for Removing Heavy Metals and Different Kinds of Anions from the Natural Water. Materials. 2020; 13(11):2524. https://doi.org/10.3390/ma13112524
Chicago/Turabian StyleSaber, Osama, Sarah Mousa Asiri, Mohamed Farouk Ezzeldin, Waleed I. M. El-Azab, and Mohammed Abu-Abdeen. 2020. "Designing Dual-Effect Nanohybrids for Removing Heavy Metals and Different Kinds of Anions from the Natural Water" Materials 13, no. 11: 2524. https://doi.org/10.3390/ma13112524
APA StyleSaber, O., Asiri, S. M., Ezzeldin, M. F., El-Azab, W. I. M., & Abu-Abdeen, M. (2020). Designing Dual-Effect Nanohybrids for Removing Heavy Metals and Different Kinds of Anions from the Natural Water. Materials, 13(11), 2524. https://doi.org/10.3390/ma13112524






