Influence of Fly Ash on Mechanical Properties and Hydration of Calcium Sulfoaluminate-Activated Supersulfated Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Mechanical Properties
2.2.2. Isothermal Conduction Calorimetry
2.2.3. Sample Preparation for X-ray Diffraction (XRD) and Thermogravimetric Analysis (TGA)
2.2.4. X-Ray Diffraction
2.2.5. Thermogravimetric Analysis
3. Results
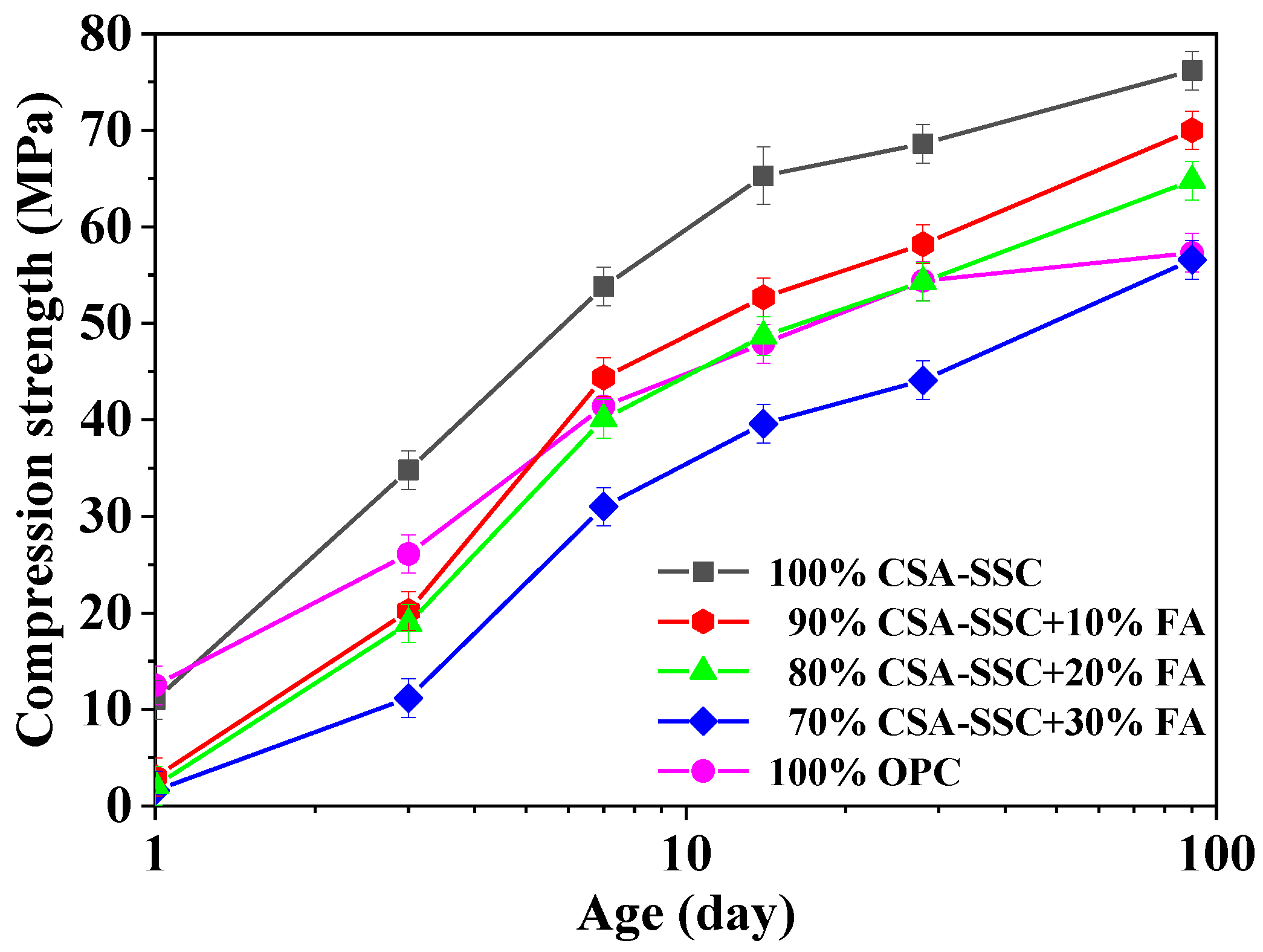
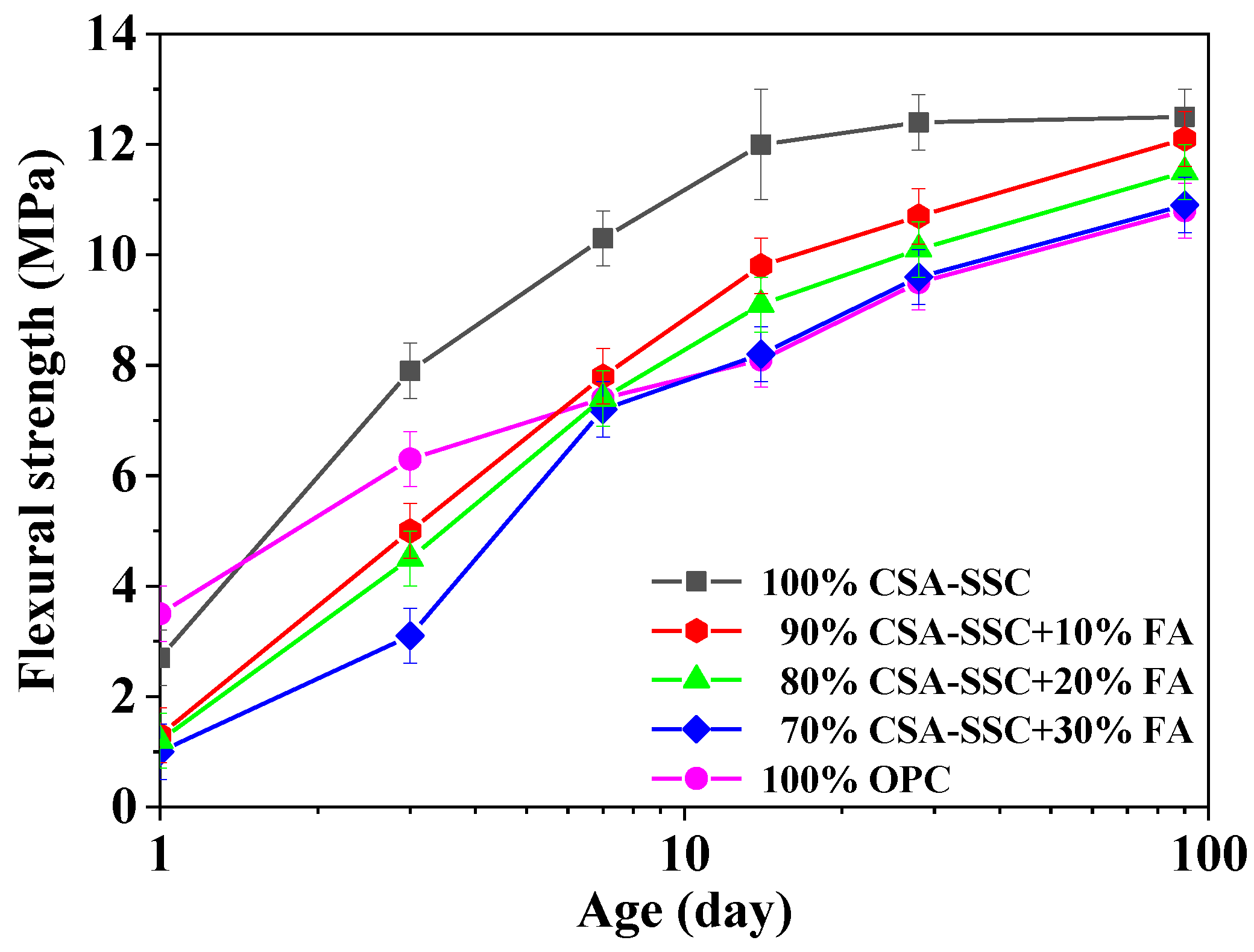
3.1. Strength Development
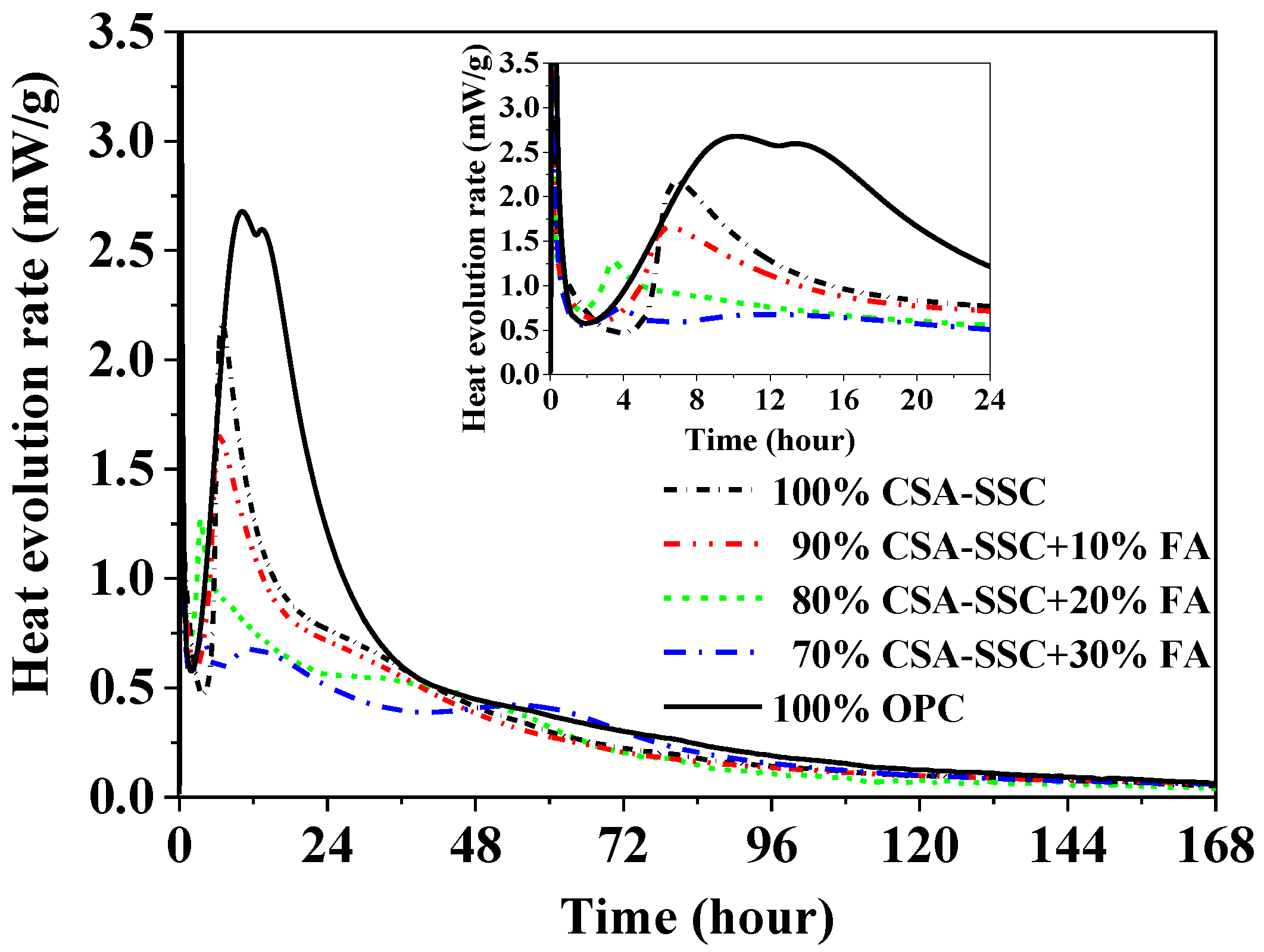
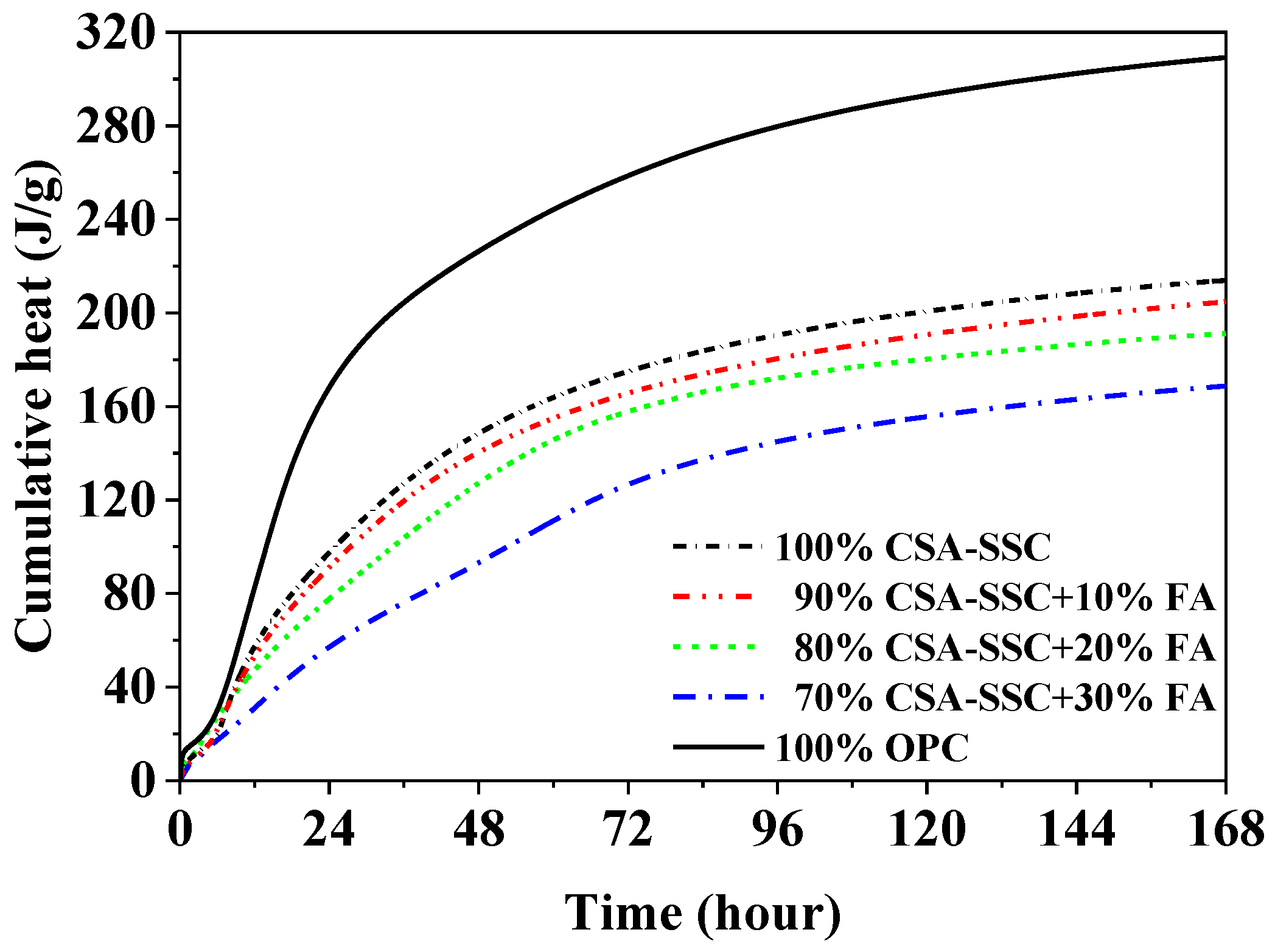
3.2. Heat Evolution
3.3. Hydration Products
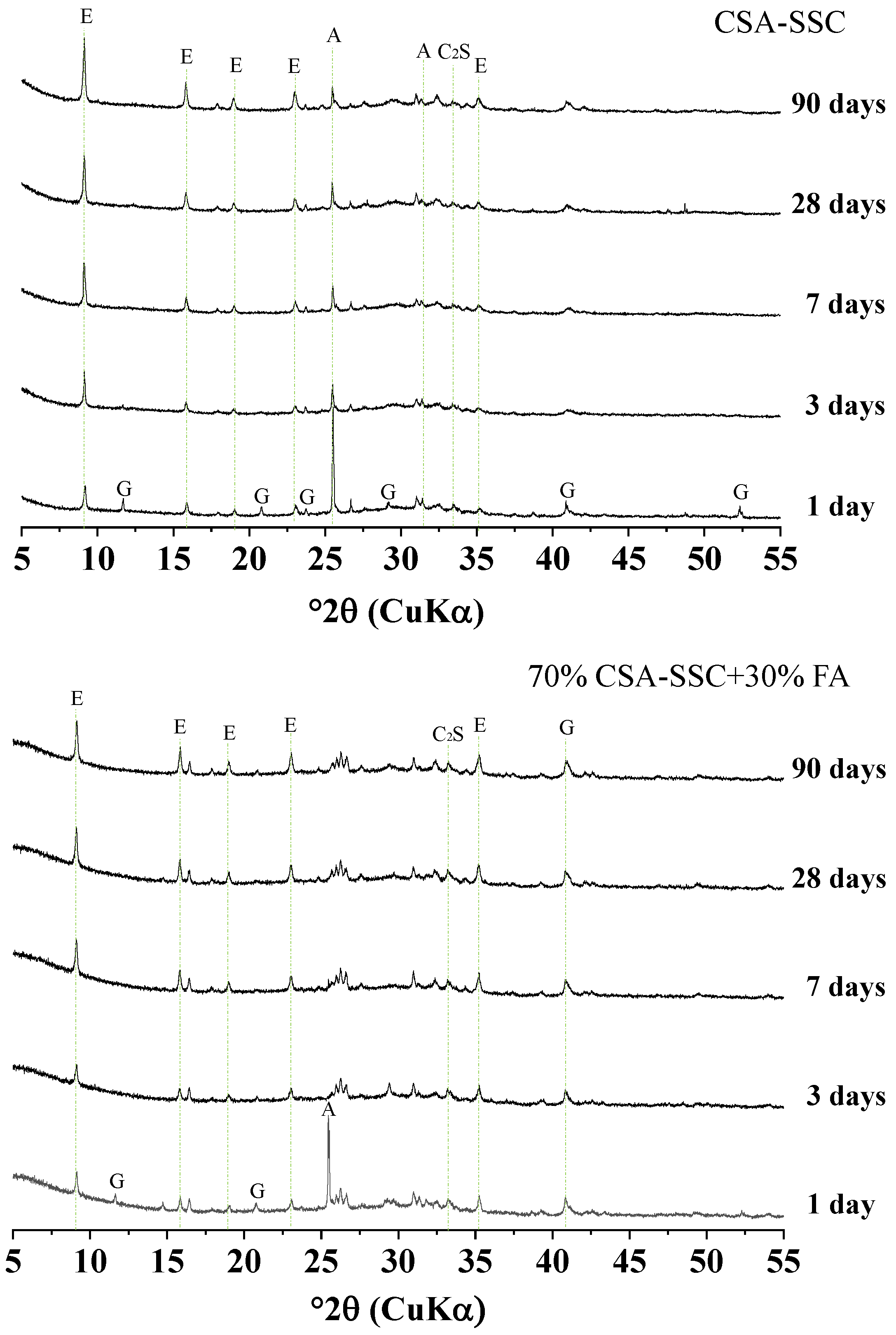
3.3.1. XRD
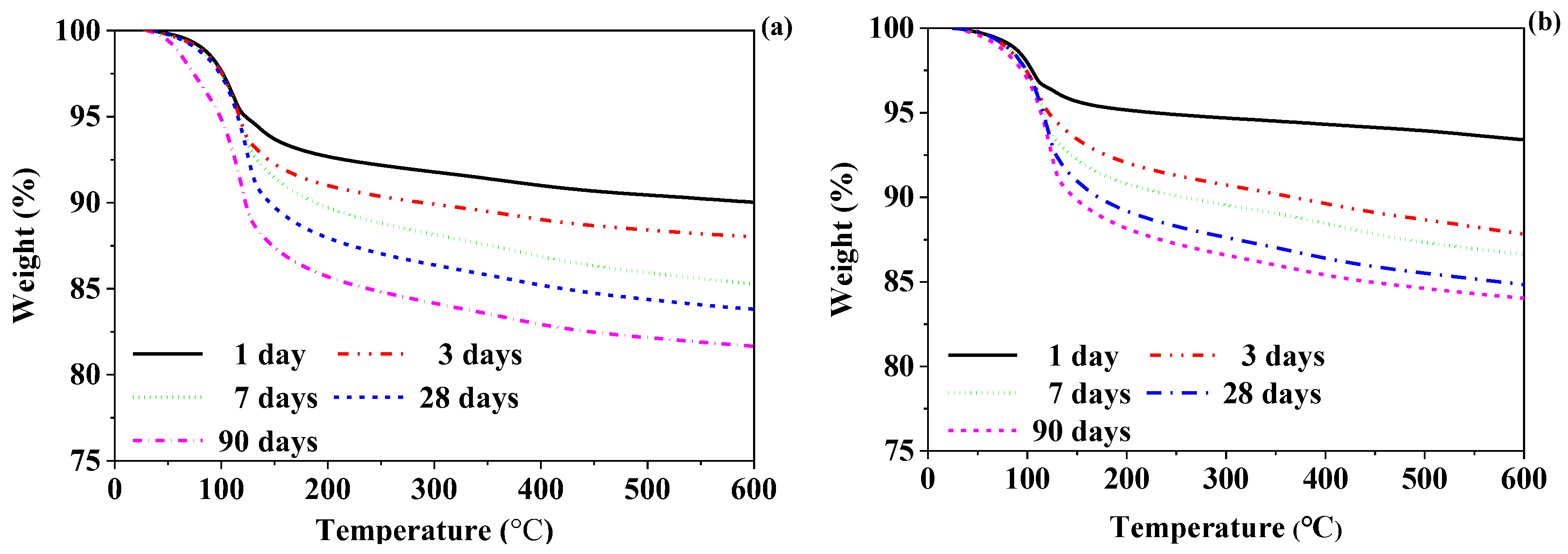
3.3.2. TGA
4. Discussion
5. Conclusions
- The addition of FA to CSA-SSC resulted in a decrease in compressive and flexural strengths proportionally.
- The CSA-SSC mixed with FA did not change the duration of the initial reaction but shortened the duration of the induction period. The acceleration period of the CSA-SSC and CSA-SSC with FA pastes was shorter compared with that of the OPC paste. The cumulative heat of the CSA-SSC was only 191 J/g after 168 h of hydration. As the content of FA was increased in the CSA-SSC system, the cumulative heat of the CSA-SSC with FA was further reduced.
- The CSA-SSC and CSA-SSC with FA mainly formed ettringite in the early stage, and C-S-H was formed in the late stage. The amount of hydration products of the CSA-SSC was more than that of the CSA-SSC with FA.
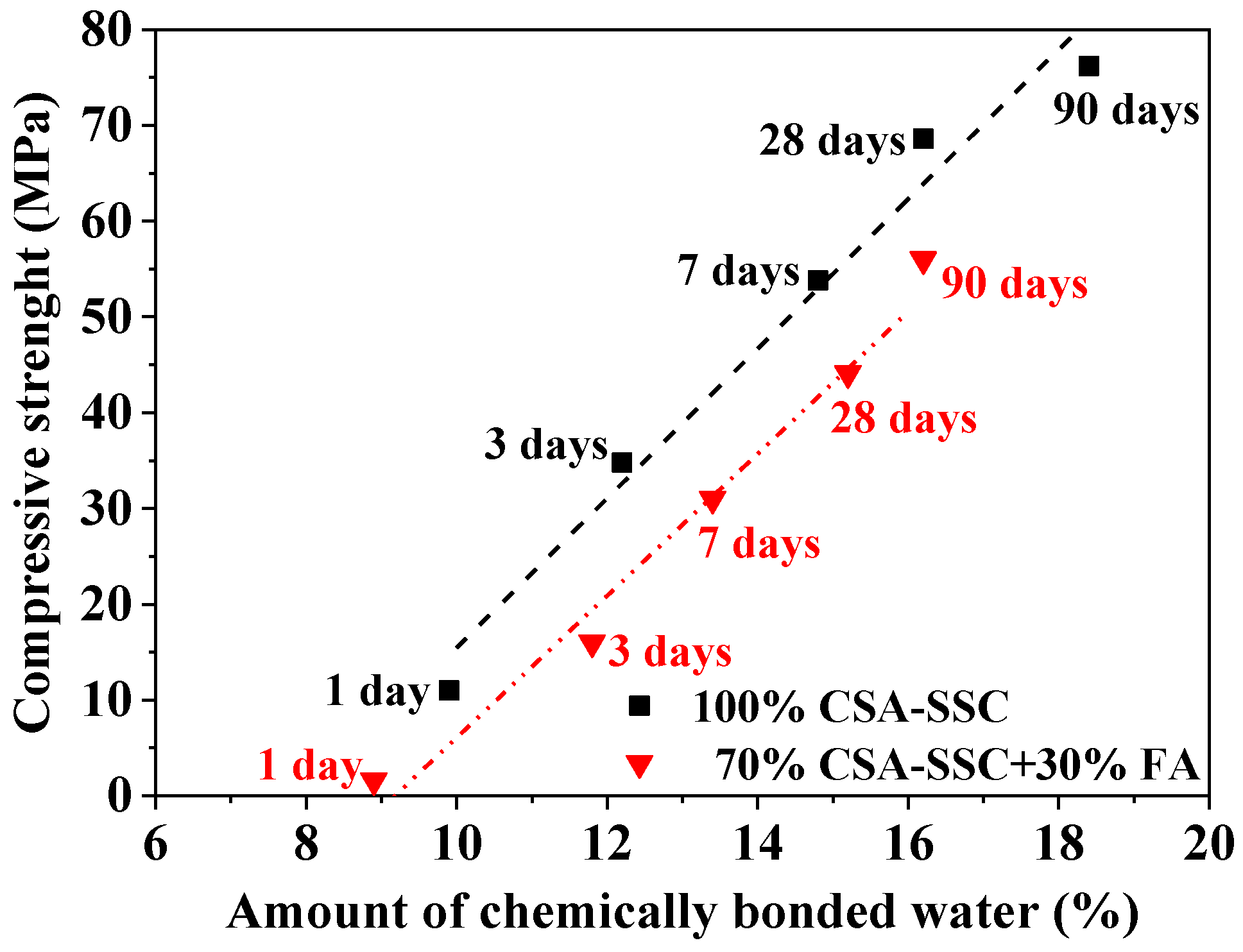
- The compressive strength of the CSA-SSC and 70% CSA-SSC + 30% FA had a linear growth relationship with the amount of chemically bound water.
Author Contributions
Funding
Conflicts of Interest
References
- Ha, J.H.; Jung, Y.S.; Cho, Y.G. Thermal crack control in mass concrete structure using an automated curing system. Autom. Constr. 2014, 45, 16–24. [Google Scholar] [CrossRef]
- Bao, Y.; Meng, W.N.; Chen, Y.Z.; Chen, G.D.; Khayat, K.H. Measuring mortar shrinkage and cracking by pulse pre-pump Brillouin optical time domain analysis with a single optical fiber. Mater. Lett. 2015, 145, 344–346. [Google Scholar] [CrossRef]
- Rhee, I.K.; Kim, K.D.; Kim, T.O.; Lee, J.S. Heat of Hydration and Thermal Crack Control for Floating Concrete Mass Foundation. Appl. Phys. Lett. 2010, 14, 2639–2648. [Google Scholar]
- Klemczak, B.; Batog, M.; Pilch, M.; Zmij, A. Analysis of cracking risk in early age mass concrete with different aggregate types. Procedia Eng. 2017, 193, 234–241. [Google Scholar] [CrossRef]
- Lee, M.H.; Khil, B.S.; Yun, H.D. Influence of cement type on heat of hydration and temperature rise of the mass concrete. Indian J. Eng. Mater. Sci. 2014, 21, 536–542. [Google Scholar]
- Lee, M.H.; Khil, B.S.; Yun, H.D. Thermal analysis of hydration heat in mass concrete with different cement binder proportions. Appl. Mech. Mater. 2013, 372, 199–202. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F.; Münch, B.; Figi, R.; Ko, S.-C.; Adler, M.; Mäder, U. Quantification of hydration phases in supersulfated cements: Review and new approaches. Adv. Cem. Res. 2011, 23, 265–275. [Google Scholar] [CrossRef]
- Rubert, S.; Luz, C.A.D.; Varela, M.V.F.; Pereira Filho, J.I.; Hooton, R.D. Hydration mechanisms of supersulfated cement. J. Therm. Anal. Calorim. 2018, 134, 971–980. [Google Scholar] [CrossRef]
- Luz, C.A.D.; Hooton, R.D. Influence of curing temperature on the process of hydration of supersulfated cements at early age. Cem. Concr. Res. 2015, 77, 69–75. [Google Scholar]
- Liu, S.; Wang, L.; Gao, Y.; Yu, B.; Tang, W. Influence of fineness on hydration kinetics of supersulfated cement. Thermochim. Acta. 2015, 605, 37–42. [Google Scholar] [CrossRef]
- Kühl, H. Verfahren zur Herstellung von Zement aus Hochofenschlacke. German Patent No. 237777, 23 December 1908. [Google Scholar]
- Erdem, E.; Ölmez, H. The mechanical properties of supersulphated cement containing phosphogypsum. Cem. Concr. Res. 1993, 23, 115–121. [Google Scholar] [CrossRef]
- Ding, S.; Shui, Z.; Chen, W.; Lu, J.X.; Tian, S.F. Properties of supersulphated phosphogypsum slag cement (SSC) concrete. J. Wuhan Univ. Technol. Mat. 2014, 29, 109–113. [Google Scholar] [CrossRef]
- Neeraj, J.; Mridul, G. Formulation of Sulphate Resistant Super Sulphated Cement Using Fluorogypsum and Granulated Blast Furnace Slag. IOSR J. Mech. Civ. Eng. 2015, 12, 153–159. [Google Scholar]
- Liu, S.H.; Wang, L.; Yu, B.Y. Effect of modified phosphogypsum on the hydration properties of the phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2019, 214, 9–16. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Mehrotra, V.P.; Sai, A.S.R.; Kapur, P.C. Plaster of Paris activated supersulfated slag cement. Cem. Concr. Res. 1982, 12, 463–473. [Google Scholar] [CrossRef]
- Grounds, T.; Nowell, D.V.; Wilburn, F.W. Resistance of supersulfated cement to strong sulfate solutions. J. Therm. Anal. Calorim. 2003, 72, 181–190. [Google Scholar] [CrossRef]
- Cerulli, T.; Pistolesi, C.; Maltese, C.; Salvioni, D. Durability of traditional plasters with respect to blast furnace slag-based plaster. Cem. Concr. Res. 2003, 33, 1375–1383. [Google Scholar] [CrossRef]
- Aziz, M.A.; Aleem, S.A.; Heikal, M.; Didamony, H.E. Hydration and durability of sulphate-resisting and slag cement blends in Caron’s Lake water. Cem. Concr. Res. 2005, 35, 1592–1600. [Google Scholar] [CrossRef]
- Nguyen, H.-A.; Chang, T.-P.; Shih, J.-Y.; Chen, C.-T. Formulating for Innovative Self-Compacting Concrete with Low Energy Super-Sulfated Cement Used for Sustainability Development. J. Mater. Sci. Chem. Eng. 2016, 4, 22–28. [Google Scholar] [CrossRef]
- Hewlett, P.C.; Liska, M. Lea’s Chemistry of Cement and Concrete, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 499–502. [Google Scholar]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F.; Figi, R.; Ko, S.-C.; Adler, M.; Mäder, U. Hydration mechanisms of super sulphated slag cement. Cem. Concr. Res. 2008, 38, 983–992. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Holzer, L.; Figi, R.; Winnefeld, F. Hydration of alkali-activated slag Comparison with ordinary portland cement. Adv. Cem. Res. 2006, 18, 119–128. [Google Scholar] [CrossRef]
- Bijen, J.; Niël, E. Supersulphated cement from blastfurnace slag and chemical gypsum available in the Netherlands and neighbouring countries. Cem. Concr. Res. 1981, 11, 307–322. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997; pp. 271–272. [Google Scholar]
- Zhang, Z.Q. Rapid-Setting and Hardening, High-Belite Sulfoaluminate Cement Clinker as well as Application and Production Process Thereof. U.S. Patent NO. 9822036 B2, 23 February 2017. [Google Scholar]
- European Committee for Standardization (CEN). DIN EN 196-1, Method of Testing Cement—Part 1—Determination of Strength; CEN: Brussels, Belgium, 2016. [Google Scholar]
- Snellings, R.; Salze, A.; Scrivener, K.L. Use of X-ray diffraction to quantify amorphous supplementary cementitious materials in anhydrous and hydrated blended cements. Cem. Concr. Res. 2014, 64, 89–98. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016; pp. 178–208. [Google Scholar]
- European Committee for Standardization (CEN). EN 15743, Supersulfated Cement-Composition, Specification and Conformity Criteria; CEN: Brussels, Belgium, 2015. [Google Scholar]

| Oxides/% | CaO | SiO2 | Al2O3 | MgO | SO3 | Fe2O3 | TiO2 | LOI | Total | Blaine Values (cm2/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| CSA-SSC | 39.25 | 26.95 | 13.21 | 8.51 | 7.84 | 0.41 | 0.53 | 0.31 | 97.01 | 4500 |
| FA | 3.05 | 48.63 | 37.37 | 1.6 | 0.84 | 3.78 | - | 3.61 | 98.88 | 4200 |
| OPC | 64.31 | 19.79 | 5.31 | 2.52 | 2.47 | 2.88 | 0.34 | 1.46 | 99.08 | 3500 |
| Mineralogical Composition/% | C4A3 | C2S | f-CaO | CaSO4 | Other |
|---|---|---|---|---|---|
| HB-CSA clinker | 28 | 45 | 4.6 | 16 | 6.4 |
| Sample | Water/Binders (wt./wt.) | Cement (wt.%) | Fly Ash (wt.%) | Binders/Sand (wt./wt.) |
|---|---|---|---|---|
| CSA-SSC | 1:2 | 100 | - | 1:3 |
| 70% CSA-SSC + 30% FA | 1:2 | 70 | 30 | 1:3 |
| OPC | 1:2 | 100 | - | 1:3 |
| Sample | Water/Binder (wt./wt.) | Cement (wt./%) | Fly Ash (wt./%) |
|---|---|---|---|
| CSA-SSC | 2:5 | 100 | - |
| 70% CSA-SSC + 30% FA | 2:5 | 70 | 30 |
| Hydration Products | Temperature of the Major Peak | Temperature Range of Weight Loss |
|---|---|---|
| Ettringite | 120 °C | 50–400 °C |
| C-S-H | 150 °C | 50–600 °C |
| Gypsum | 140 °C | 100–170 °C |
| OH-hydrotalcite | 250 °C and 400 °C | 50–500 °C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhou, J.; Qi, Q.; Li, H.; Zhang, N.; Mu, R. Influence of Fly Ash on Mechanical Properties and Hydration of Calcium Sulfoaluminate-Activated Supersulfated Cement. Materials 2020, 13, 2514. https://doi.org/10.3390/ma13112514
Sun Z, Zhou J, Qi Q, Li H, Zhang N, Mu R. Influence of Fly Ash on Mechanical Properties and Hydration of Calcium Sulfoaluminate-Activated Supersulfated Cement. Materials. 2020; 13(11):2514. https://doi.org/10.3390/ma13112514
Chicago/Turabian StyleSun, Zhengning, Jian Zhou, Qiulin Qi, Hui Li, Na Zhang, and Ru Mu. 2020. "Influence of Fly Ash on Mechanical Properties and Hydration of Calcium Sulfoaluminate-Activated Supersulfated Cement" Materials 13, no. 11: 2514. https://doi.org/10.3390/ma13112514
APA StyleSun, Z., Zhou, J., Qi, Q., Li, H., Zhang, N., & Mu, R. (2020). Influence of Fly Ash on Mechanical Properties and Hydration of Calcium Sulfoaluminate-Activated Supersulfated Cement. Materials, 13(11), 2514. https://doi.org/10.3390/ma13112514




