Complementary SEM-AFM of Swelling Bi-Fe-O Film on HOPG Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Characterization Techniques
3. Results
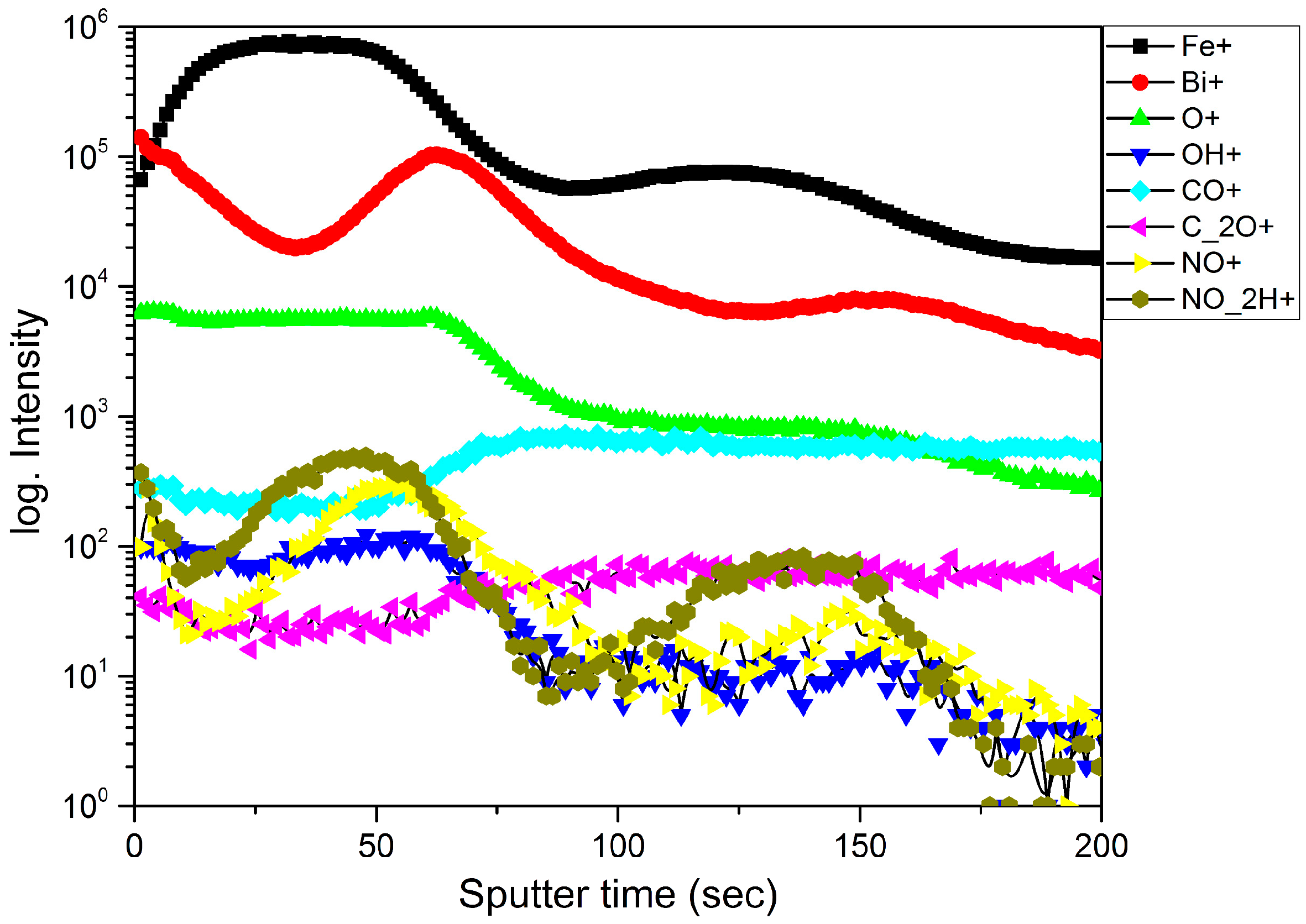
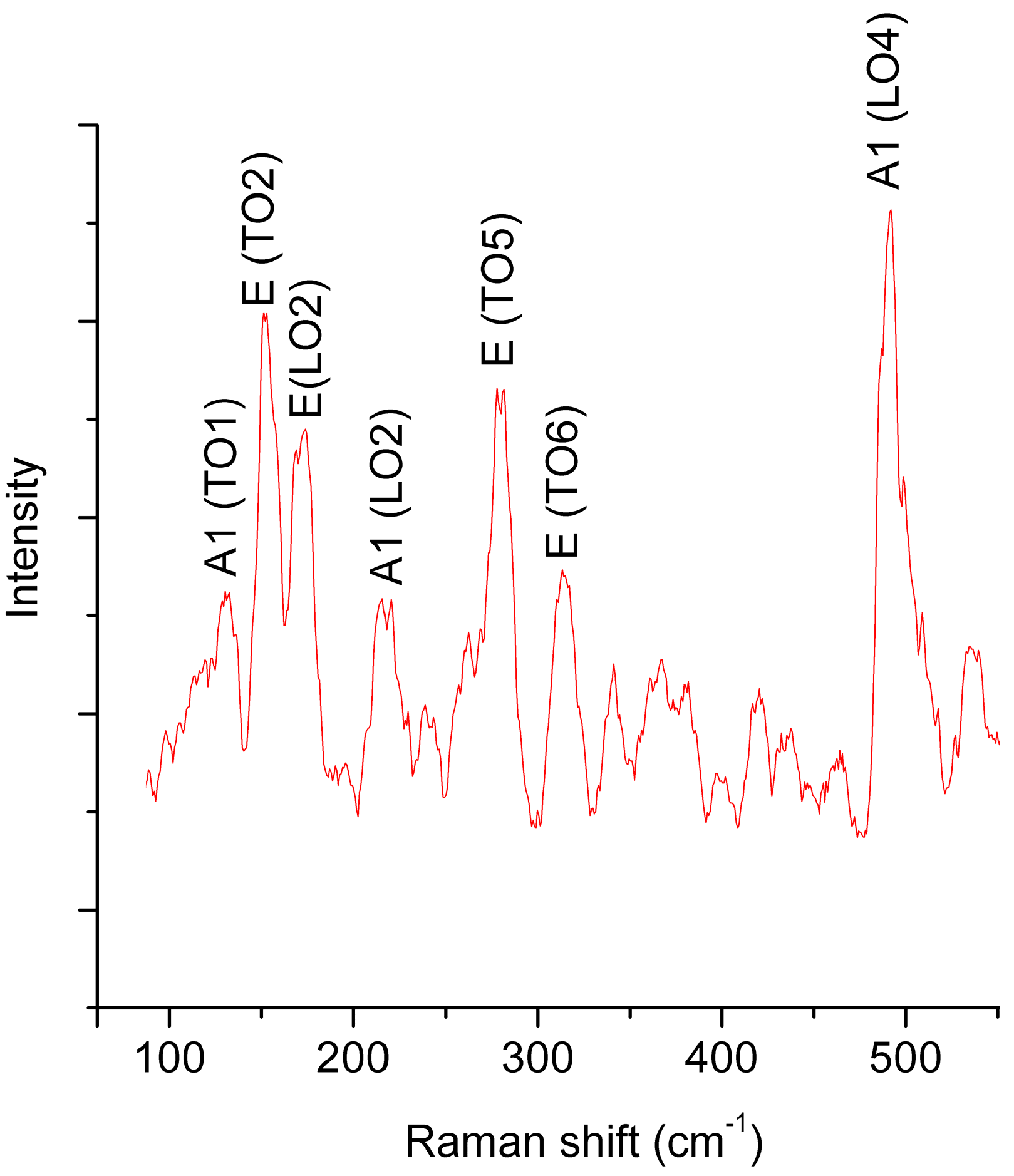
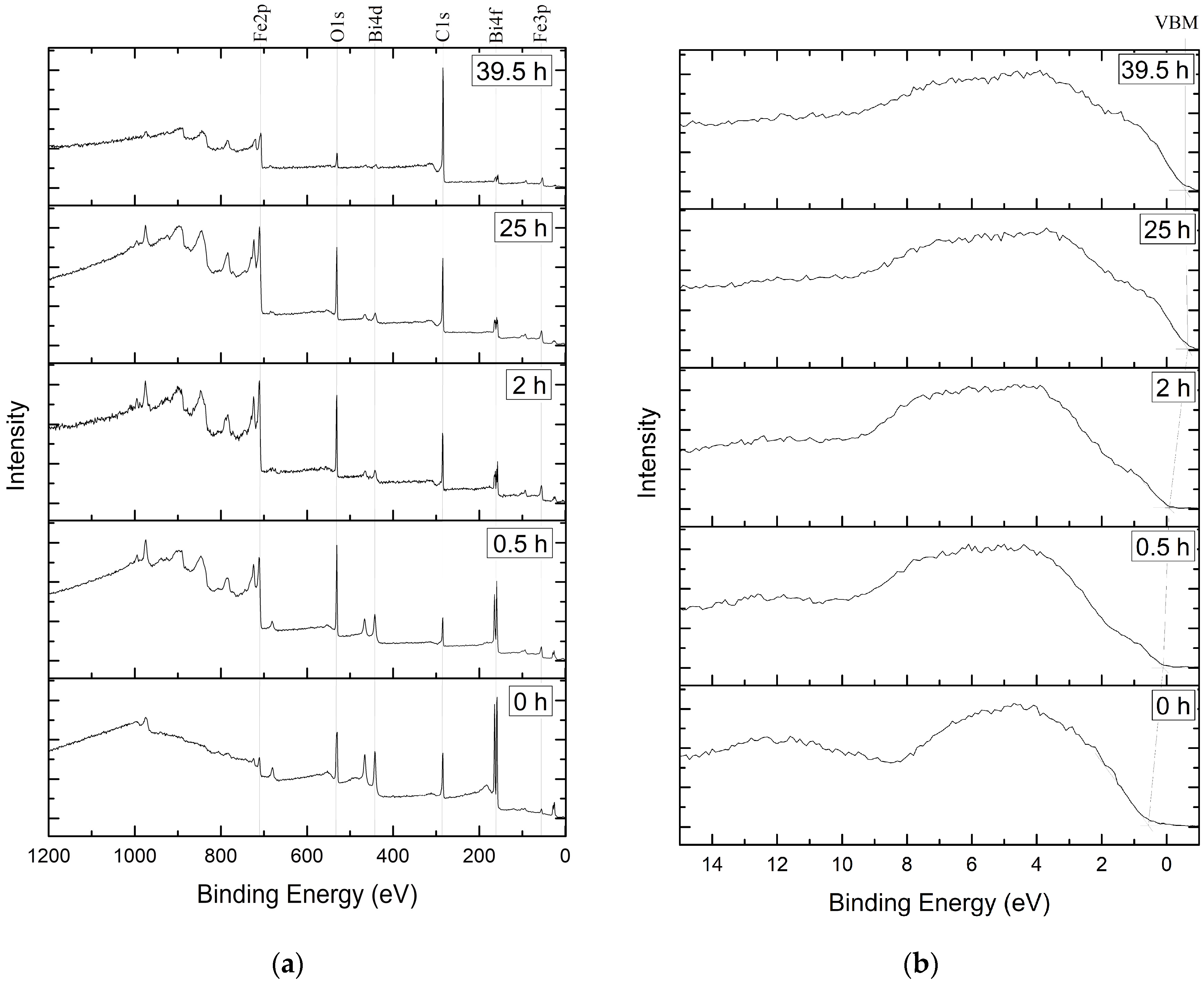
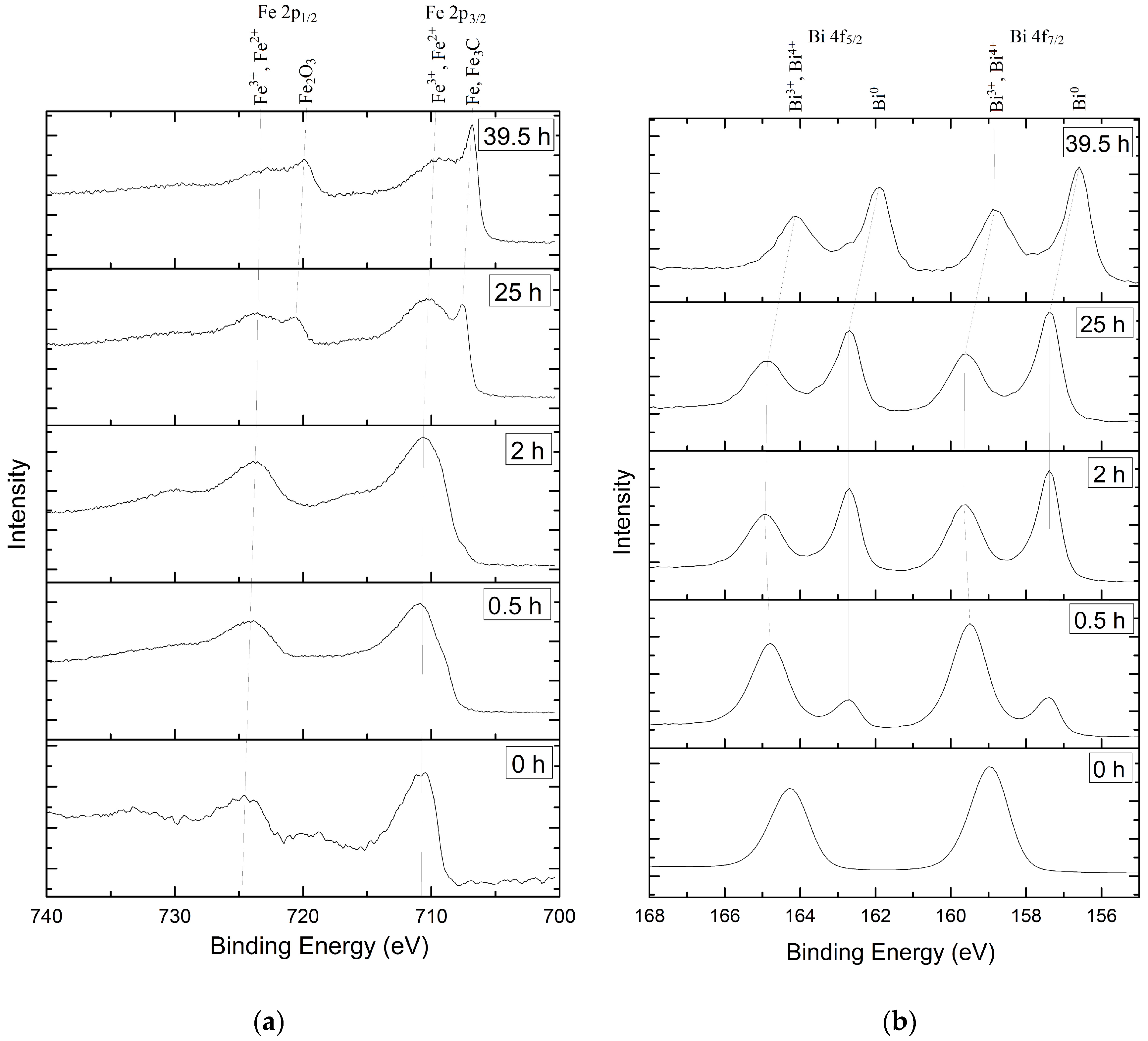
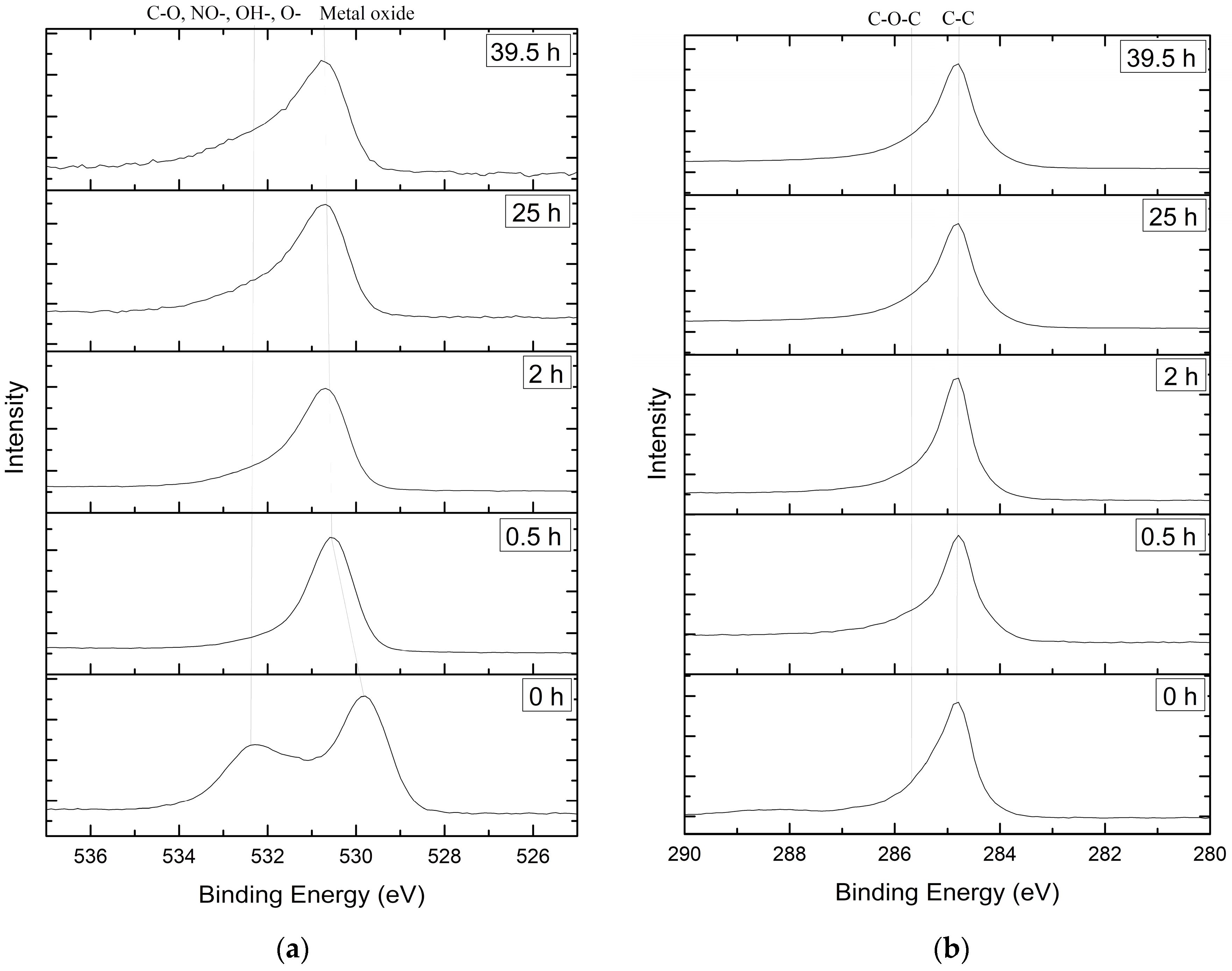
3.1. Samples Comosition
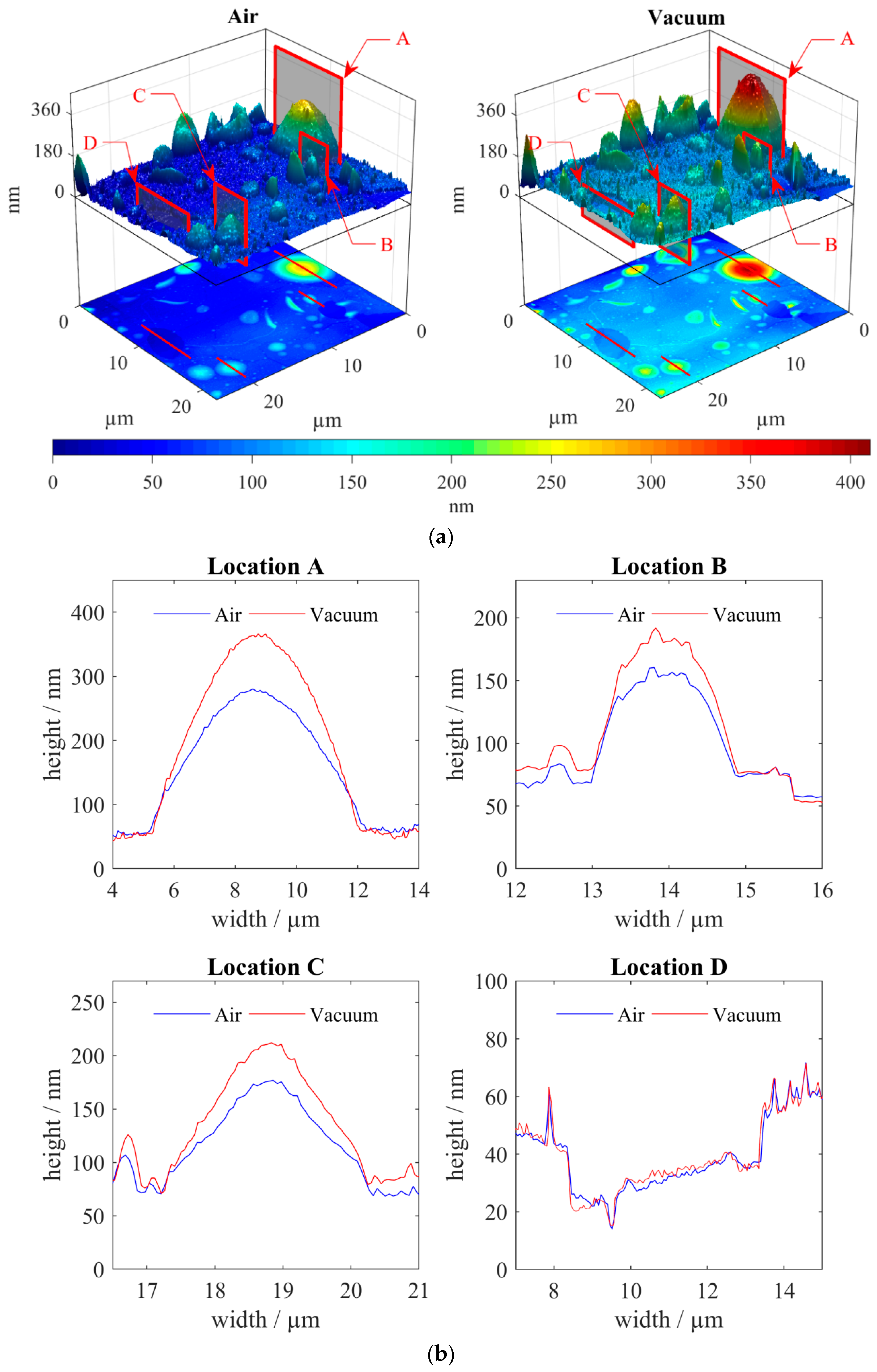
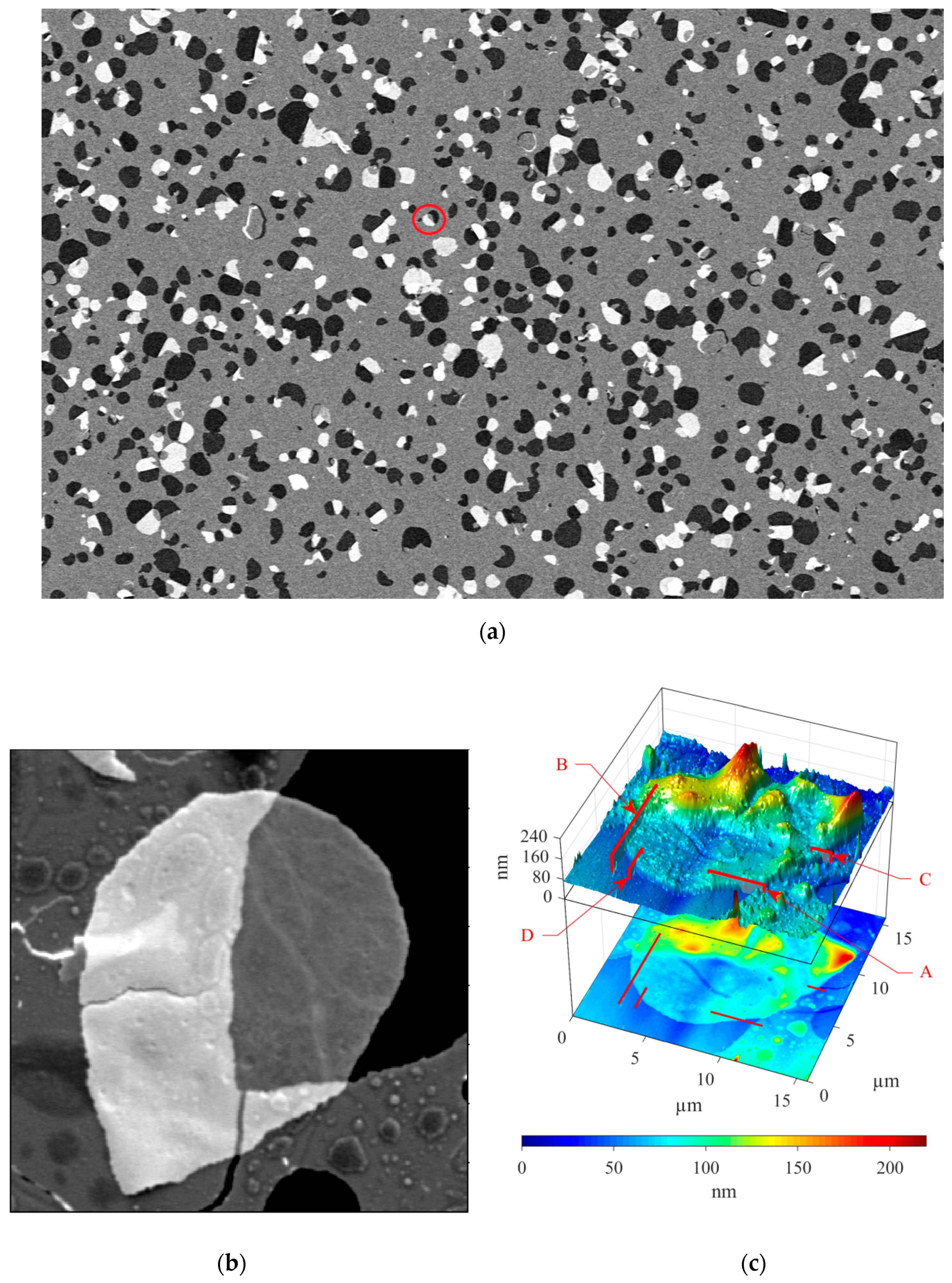
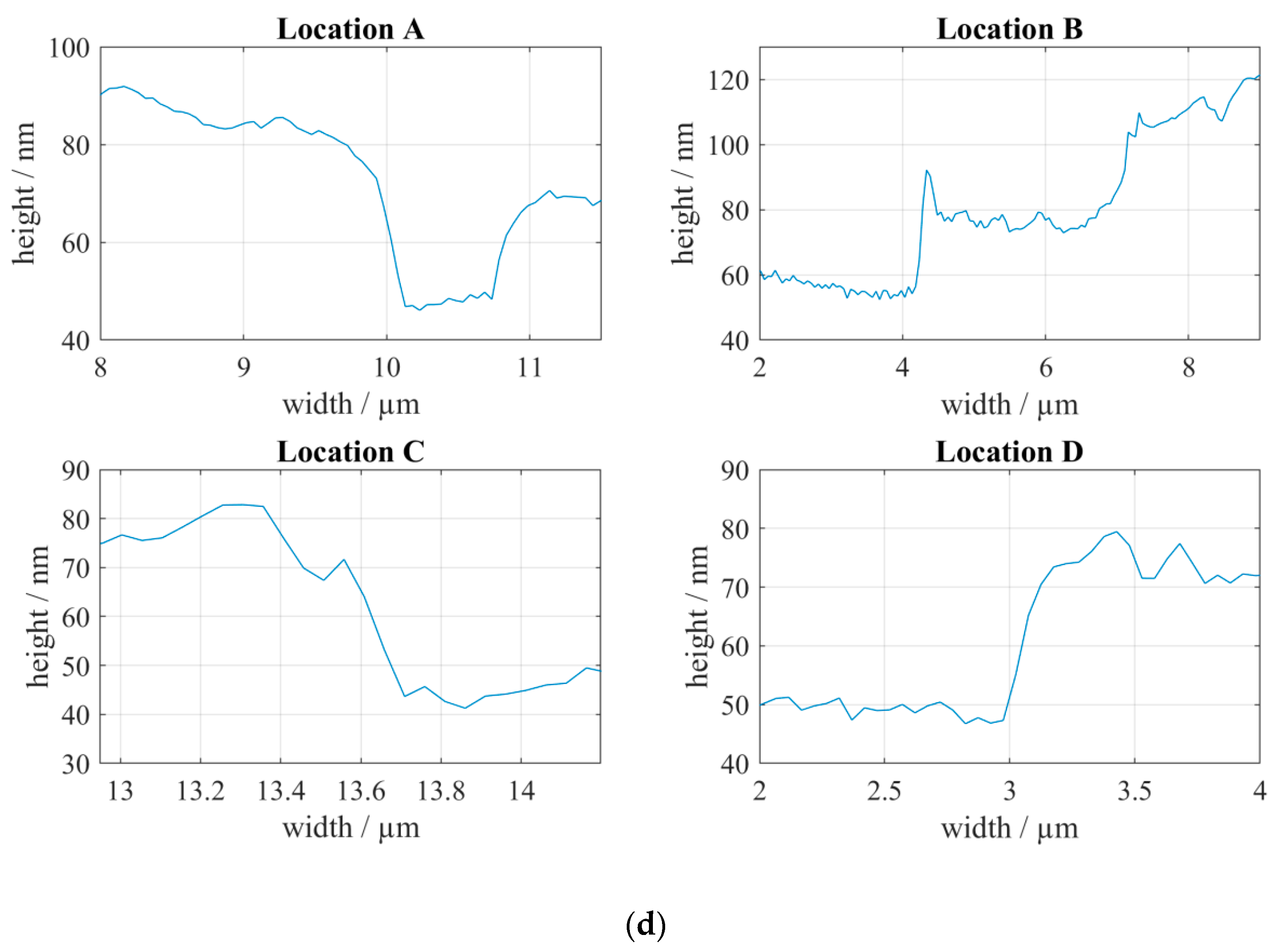
3.2. Correlative SEM-AFM
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bajpai, O.P.; Mandal, S.; Ananthakrishnan, R.; Mandal, P.; Khastgir, D.; Chattopadhyay, S. Structural features, magnetic properties and photocatalytic activity of bismuth ferrite nanoparticles grafted on graphene nanosheets. New J. Chem. 2018, 42, 10712–10723. [Google Scholar] [CrossRef]
- Ibrahim, F.; Hallal, A.; Lerma, D.S.; Waintal, X.; Tsymbal, E.Y.; Chshiev, M. Unveiling multiferroic proximity effect in graphene. 2D Mater. 2019, 7, 015020. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Qi, J.; Tian, Y.; Sun, M.; Zhang, J.; Hu, T.; Wei, M.; Liu, Y.; Yang, J. Enhanced magnetic properties of BiFeO3 thin films by doping: Analysis of structure and morphology. Nanomaterials 2018, 8, 711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Jang, H.M.; Ryu, S.; Jo, M.-H. Polarized Raman scattering of multiferroic BiFeO3 epitaxial films with rhombohedral R3c symmetry. Appl. Phys. Lett. 2006, 88, 042907. [Google Scholar] [CrossRef]
- Wang, C.; Takahashi, M.; Fujino, H.; Zhao, X.; Kume, E.; Horiuchi, T.; Sakai, S. Leakage current of multiferroic (Bi0.6Tb0.3La0.1)FeO3 thin films grown at various oxygen pressures by pulsed laser deposition and annealing effect. J. Appl. Phys. 2006, 99, 054104. [Google Scholar] [CrossRef]
- Golovina, I.S.; Falmbigl, M.; Plokhikh, A.V.; Parker, T.C.; Johnson, C.; Spanier, J.E. Effect of annealing conditions on the electrical properties of ALD-grown polycrystalline BiFeO3 films. J. Mater. Chem. C 2018, 6, 5462–5472. [Google Scholar] [CrossRef]
- Plokhikh, A.V.; Falmbigl, M.; Golovina, I.S.; Akbashev, A.R.; Karateev, I.A.; Presnyakov, M.Y.; Vasiliev, A.L.; Spanier, J.E. Formation of BiFeO3 from a binary oxide superlattice grown by atomic layer deposition. ChemPhysChem 2017, 18, 1966–1970. [Google Scholar] [CrossRef]
- Afzal, A.; Javed, Y.; Hussain, S.; Ali, A.; Yaqoob, M.; Mumtaz, S. Enhancement in photovoltaic properties of bismuth ferrite/zinc oxide heterostructure solar cell device with graphene/indium tin oxide hybrid electrodes. Ceram. Int. 2020, 46, 9161–9169. [Google Scholar] [CrossRef]
- Pham, C.D.; Chang, J.; Zurbuchen, M.; Chang, J.P. Synthesis and characterization of BiFeO3 thin films for multiferroic applications by radical enhanced atomic layer deposition. Chem. Mater. 2015, 27, 7282–7288. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, G.; Zhao, W.; Wang, L.; Zheng, L.; Liu, S.; Liu, B.; Dong, L.; Liu, X.; Yan, G.; et al. Atomic layer deposition of BiFeO3 thin films using β-diketonates and H2O. J. Phys. Chem. C 2013, 117, 24579–24585. [Google Scholar] [CrossRef]
- Coll, M.; Napari, M. Atomic layer deposition of functional multicomponent oxides. APL Mater. 2019, 7, 110901. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Schneider, J.R.; MacIsaac, C.; Baker, J.G.; Bent, S.F. Synthesis of doped, ternary, and quaternary materials by atomic layer deposition: A review. Chem. Mater. 2018, 31, 1142–1183. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Ngo, T.Q.; Hu, S.; Posadas, A.; Demkov, A.A.; Ekerdt, J.G. Atomic layer deposition of perovskite oxides and their epitaxial integration with Si, Ge, and other semiconductors. Appl. Phys. Rev. 2015, 2, 041301. [Google Scholar] [CrossRef]
- Coll, M.; Gazquez, J.; Fina, I.; Khayat, Z.; Quindeau, A.; Alexe, M.; Varela, M.; Trolier-McKinstry, S.; Obradors, X.; Puig, T. Nanocrystalline ferroelectric BiFeO3 thin films by low-temperature atomic layer deposition. Chem. Mater. 2015, 27, 6322–6328. [Google Scholar] [CrossRef]
- Sønsteby, H.H.; Fjellvåg, H.; Nilsen, O. Functional perovskites by atomic layer deposition—An overview. Adv. Mater. Interfaces 2017, 4, 1600903. [Google Scholar]
- Lai, S.; Ehrhardt, M.; Lorenz, P.; Hirsch, D.; Zajadacz, J.; Lu, J.; Han, B.; Zimmer, K. Submicron bubbles/voids formation in the subsurface region of soda-lime glass by single pulse fs laser-induced spallation. Appl. Surf. Sci. 2020, 502, 144134. [Google Scholar] [CrossRef]
- Andrianova, N.; Borisov, A.; Mashkova, E.; Shemukhin, A.; Shulga, V.; Virgiliev, Y. Relief evolution of HOPG under high-fluence 30 keV argon ion irradiation. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 2015, 354, 146–150. [Google Scholar] [CrossRef]
- Akbashev, A.R.; Chen, G.; Spanier, J.E. A facile route for producing single-crystalline epitaxial perovskite oxide thin films. Nano Lett. 2013, 14, 44–49. [Google Scholar] [CrossRef]
- Elliott, S.D.; Scarel, G.; Wiemer, C.; Fanciulli, M.; Pavia, G. Ozone-based atomic layer deposition of alumina from TMA: Growth, morphology, and reaction mechanism. Chem. Mater. 2006, 18, 3764–3773. [Google Scholar] [CrossRef]
- Guerra-Nuñez, C.; Döbeli, M.; Michler, J.; Utke, I. Reaction and growth mechanisms in Al2O3 deposited via atomic layer deposition: Elucidating the hydrogen source. Chem. Mater. 2017, 29, 8690–8703. [Google Scholar] [CrossRef]
- Anderson, J.S.; Rittle, J.; Peters, J.C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 2013, 501, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Arashiba, K.; Nakajima, K.; Tanaka, H.; Yoshizawa, K.; Nishibayashi, Y. Nitrogen fixation catalyzed by ferrocene-substituted dinitrogen-bridged dimolybdenum–dinitrogen complexes: Unique behavior of ferrocene moiety as redox active site. Chem. Sci. 2015, 6, 3940–3951. [Google Scholar] [CrossRef] [PubMed]
- Porporati, A.A.; Tsuji, K.; Valant, M.; Axelsson, A.-K.; Pezzotti, G. Raman tensor elements for multiferroic BiFeO3 with rhombohedral R3c symmetry. J. Raman Spectrosc. 2010, 41, 84–87. [Google Scholar] [CrossRef]
- Hermet, P.; Goffinet, M.; Kreisel, J.; Ghosez, P. Raman and infrared spectra of multiferroic bismuth ferrite from first principles. Phys. Rev. B 2007, 75. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Dallaev, R.; Ramazanov, S.; Nebojsa, A.; Rezaee, S.; Grmela, L. Characterization of Fe2O3 thin film on highly oriented pyrolytic graphite by AFM, ellipsometry and XPS. Appl. Surf. Sci. 2019, 493, 673–678. [Google Scholar] [CrossRef]
- Collar, K.N.; Li, J.; Jiao, W.; Guan, Y.; Losurdo, M.; Humlicek, J.; Brown, A.S. Determination of the impact of Bi content on the valence band energy of GaAsBi using x-ray photoelectron spectroscopy. AIP Adv. 2017, 7, 075016. [Google Scholar] [CrossRef]
- Fujii, T.; De Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2 -assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef]
- Schafranek, R.; Baniecki, J.D.; Ishii, M.; Kotaka, Y.; Kurihara, K. The SrTiO3/BiFeO3(001) interface: Commutativity of energy band discontinuities. New J. Phys. 2013, 15, 053014. [Google Scholar] [CrossRef]
- Chaitongrat, B.; Chaisitsak, S. Fabrication of SWNTs/α-Fe2O3 as room-temperature LPG sensor. 2015 IEEE SENSORS 2015, 1–4. [Google Scholar] [CrossRef]
- Li, Y.; Sritharan, T.; Zhang, S.; He, X.; Liu, Y.; Chen, T. Multiferroic properties of sputtered BiFeO3 thin films. Appl. Phys. Lett. 2008, 92, 132908. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Gai, L.; Gong, Y.; Guan, H.; He, R.; Lyu, H. Different approaches for preparing a novel thiol-functionalized graphene oxide/Fe-Mn and its application for aqueous methylmercury removal. Chem. Eng. J. 2017, 319, 229–239. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Sun, F.; Weng, Y.; You, S.; Shao, Y. Temperature-induced phase changes in bismuth oxides and efficient photodegradation of phenol and p -chlorophenol. J. Hazard. Mater. 2016, 301, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yu, X.; Zhu, Y.; Zhang, Y. Graphene oxide/BiOCl nanocomposite films as efficient visible light photocatalysts. Front. Chem. 2018, 6, 274. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, S.; Xu, B.; Xu, Y.; Cao, K.; Jin, Z.; Qiu, C.; Zhang, M.; Su, C.; Ohno, T. A facile approach to build Bi2O2CO3/PCN nanohybrid photocatalysts for gaseous acetaldehyde efficient removal. Catal. Today 2018, 315, 184–193. [Google Scholar] [CrossRef]
- Jaiswar, S.; Mandal, K.D. Evidence of enhanced oxygen vacancy defects inducing ferromagnetism in multiferroic CaMn7O12 manganite with sintering time. J. Phys. Chem. C 2017, 121, 19586–19601. [Google Scholar] [CrossRef]
- Shukla, K.K.; Shahi, P.; Gopal, S.; Kumar, A.; Ghosh, A.K.; Singh, R.; Sharma, N.; Das, A.; Sinha, A.K.; Joshi, A.G.; et al. Magnetic and optical properties of Fe doped crednerite CuMnO2. RSC Adv. 2015, 5, 83504–83511. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Hou, S.; Eidson, N.; Fan, X.; Liang, Y.; Deng, T.; Jiang, J.; Wang, C. Azo compounds derived from electrochemical reduction of nitro compounds for high performance Li-Ion batteries. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Elmahdy, A.M.; Abdel-Khalek, N.A.; El-Aref, M.; ElManawi, A.E.-H. XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore. Appl. Surf. Sci. 2015, 345, 127–140. [Google Scholar] [CrossRef]
- Cuharuc, A.S.; Zhang, G.; Unwin, P.R. Electrochemistry of ferrocene derivatives on highly oriented pyrolytic graphite (HOPG): Quantification and impacts of surface adsorption. Phys. Chem. Chem. Phys. 2016, 18, 4966–4977. [Google Scholar] [CrossRef]
- Jin, T.; Han, Q.; Wang, Y.; Jiao, L. 1D Nanomaterials: design, synthesis, and applications in sodium-ion batteries. Small 2017, 14, 1703086. [Google Scholar] [CrossRef] [PubMed]
- Teichert, C.; Beinik, I. Conductive atomic-force microscopy investigation of nanostructures in microelectronics. Nanowires 2010, 691–721. [Google Scholar] [CrossRef]
- Hartmann, U. Magnetic force microscopy. Annu. Rev. Mater. Res. 1999, 29, 53–87. [Google Scholar] [CrossRef]
- Zhong, J.; Yan, J. Seeing is believing: Atomic force microscopy imaging for nanomaterial research. RSC Adv. 2016, 6, 1103–1121. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y. Application of AFM in microbiology: A review. Scanning 2010, 32, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Abadias, G.; Chason, E.; Keckes, J.; Sebastiani, M.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.; Stoessel, C.H.; Martinu, L. Review article: Stress in thin films and coatings: Current status, challenges, and prospects. J. Vac. Sci. Technol. A 2018, 36, 020801. [Google Scholar] [CrossRef]
- Xu, T. Characterization of minienvironments in a clean room: Design characteristics and environmental performance. Build. Environ. 2007, 42, 2993–3000. [Google Scholar] [CrossRef]
- Frackers, Foes OK New Pact. Available online: https://www.wvgazettemail.com/business/frackers-foes-ok-new-pact/article_685cc916-7eb3-589f-9436-fa976fb24411.html (accessed on 22 May 2020).
- Szirte, T.; Green, S. Applied dimensional analysis and modeling. J. Fluids Eng. 1998, 120, 637. [Google Scholar] [CrossRef]
- Ma, S.; Gao, T.; Chen, X.; Xiao, C.; Lu, T.; Jiang, X. Formation and behaviors of helium bubbles in Li4SiO4: A molecular dynamics simulation. Comput. Mater. Sci. 2019, 169, 169. [Google Scholar] [CrossRef]
- Zitzler, L.; Herminghaus, S.; Mugele, F. Capillary forces in tapping mode atomic force microscopy. Phys. Rev. B 2002, 66. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobola, D.; Ramazanov, S.; Konečný, M.; Orudzhev, F.; Kaspar, P.; Papež, N.; Knápek, A.; Potoček, M. Complementary SEM-AFM of Swelling Bi-Fe-O Film on HOPG Substrate. Materials 2020, 13, 2402. https://doi.org/10.3390/ma13102402
Sobola D, Ramazanov S, Konečný M, Orudzhev F, Kaspar P, Papež N, Knápek A, Potoček M. Complementary SEM-AFM of Swelling Bi-Fe-O Film on HOPG Substrate. Materials. 2020; 13(10):2402. https://doi.org/10.3390/ma13102402
Chicago/Turabian StyleSobola, Dinara, Shikhgasan Ramazanov, Martin Konečný, Farid Orudzhev, Pavel Kaspar, Nikola Papež, Alexandr Knápek, and Michal Potoček. 2020. "Complementary SEM-AFM of Swelling Bi-Fe-O Film on HOPG Substrate" Materials 13, no. 10: 2402. https://doi.org/10.3390/ma13102402
APA StyleSobola, D., Ramazanov, S., Konečný, M., Orudzhev, F., Kaspar, P., Papež, N., Knápek, A., & Potoček, M. (2020). Complementary SEM-AFM of Swelling Bi-Fe-O Film on HOPG Substrate. Materials, 13(10), 2402. https://doi.org/10.3390/ma13102402









