Effect of Exclusive Primer and Adhesive on Microtensile Bond Strength of Self-Adhesive Resin Cement to Dentin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
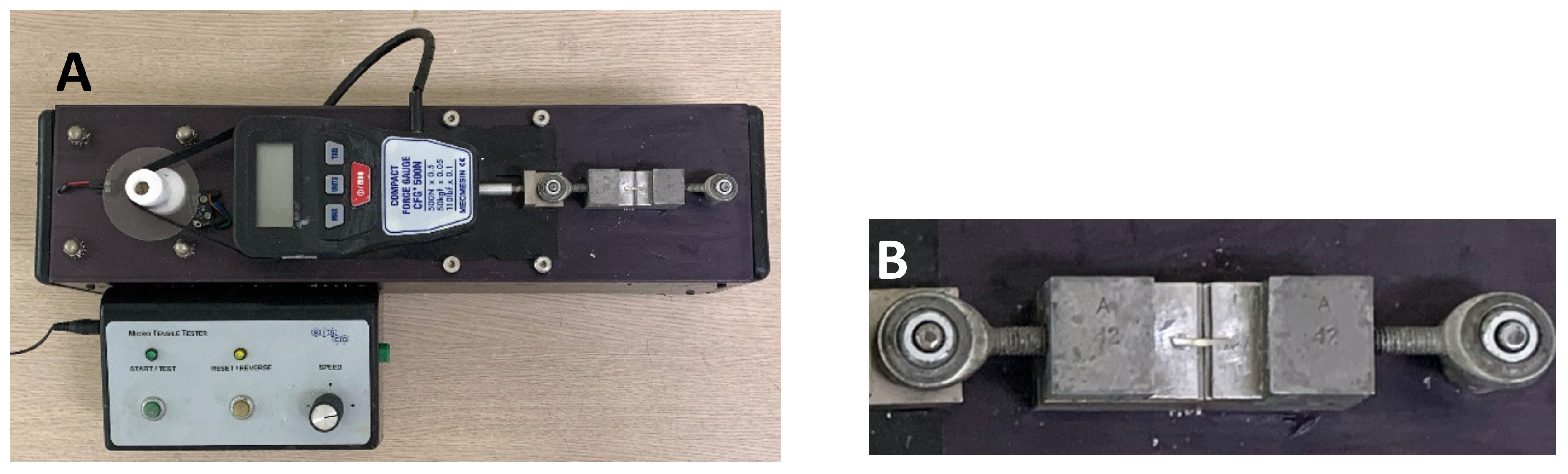
2.2. Microtensile Bond Strength (µTBS) Testing
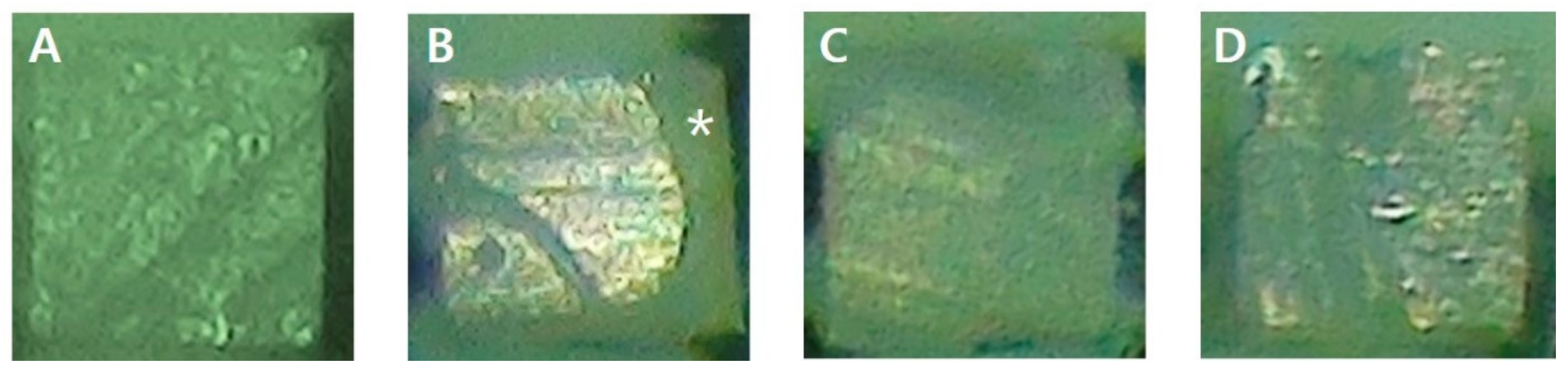
2.3. Analysis of Failure Mode
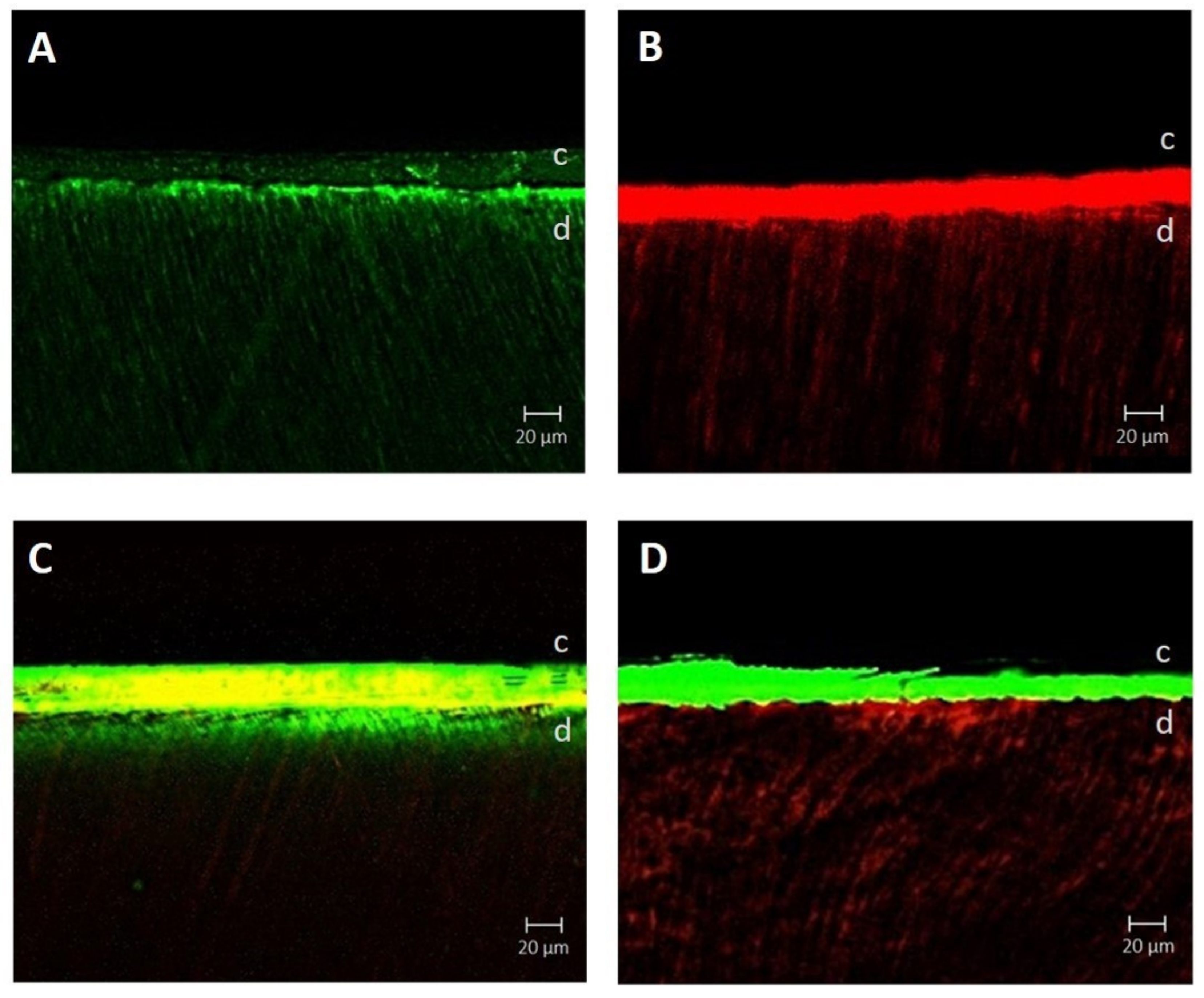
2.4. Confocal Laser Scanning
2.5. Statistical Analysis
3. Results
3.1. μTBS
3.2. Analysis of Failure Mode
3.3. Confocal Laser Scanning
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenstiel, S.F.; Land, M.F.; Crispin, B.J. Dental luting agents: A review of the current literature. J. Prosthet. Dent. 1998, 80, 280–301. [Google Scholar] [CrossRef]
- Radovic, I.; Monticelli, F.; Goracci, C.; Vulicevic, Z.R.; Ferrari, M. Self-adhesive resin cements: A literature review. J. Adhes. Dent. 2008, 10, 251–258. [Google Scholar]
- van Dijken, J.W.; Sunnegårdh-Grönberg, K.; Lindberg, A. Clinical long-term retention of etch-and-rinse and self-etch adhesive systems in non-carious cervical lesions: A 13 years evaluation. Dent. Mater. 2007, 23, 1101–1107. [Google Scholar] [CrossRef]
- De Munck, J.; Vargas, M.; Van Landuyt, K.; Hikita, K.; Lambrechts, P.; Van Meerbeek, B. Bonding of an auto-adhesive luting material to enamel and dentin. Dent. Mater. 2004, 20, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Krämer, N.; Petschelt, A. Technique sensitivity of dentin bonding: Effect of application mistakes on bond strength and marginal adaptation. Oper. Dent. 2000, 25, 324–330. [Google Scholar] [PubMed]
- Poggio, C.; Pigozzo, M.; Ceci, M.; Scribante, A.; Beltrami, R.; Chiesa, M. Influence of different luting protocols on shear bond strength of computer aided design/computer aided manufacturing resin nanoceramic material to dentin. Dent. Res. J. 2016, 13, 91–97. [Google Scholar] [CrossRef]
- Belli, R.; Pelka, M.; Petschelt, A.; Lohbauer, U. In vitro wear gap formation of self-adhesive rein cements: A CLSM evaluation. J. Dent. 2009, 37, 984–993. [Google Scholar] [CrossRef]
- Solon-de-Mello, M.; da Silva Fidalgo, T.K.; dos Santos Letieri, A.; Masterson, D.; Granjeiro, J.M.; Monte Alto, R.V.; Maia, L.C. Longevity of indirect restorations cemented with self-adhesive resin luting with and without selective enamel etching. A Systematic review and meta-analysis. J. Esthet. Restor. Dent. 2019, 31, 327–337. [Google Scholar] [CrossRef]
- Gerth, H.U.; Dammaschke, T.; Züchner, H.; Schäfer, E. Chemical analysis and bonding reaction of Relyx Unicem and Bifix composites-a comparative study. Dent. Mater. 2006, 22, 934–941. [Google Scholar] [CrossRef]
- Lührs, A.K.; Guhr, S.; Schilke, R.; Borchers, L.; Geurtsen, W.; Günay, H. Shear bond strength of self-etch hesives to enamel with additional phosphoric acid etching. Oper. Dent. 2008, 33, 155–162. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef]
- Turp, V.; Sen, D.; Tuncelli, B.; Ozcan, M. Adhesion of 10-MDP containing resin cements to dentin with and without the etch-and-rinse technique. J. Adv. Prosthodont. 2013, 5, 226–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Shinya, A.; Gomi, H.; Shinya, A. Bonding of self-adhesive resin cements to enamel using different surface treatments: Bond strength and etching pattern evaluations. Dent. Mater. J. 2010, 29, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, I.H.; Son, S.A.; Park, J.K. The effects of deproteinization and primer treatment on microtensile bond strength of self-adhesive resin cement to dentin. Korean J. Dent. Mater. 2019, 46, 99–108. [Google Scholar] [CrossRef]
- Lim, G.E.; Son, S.A.; Park, J.K. Effect of dentin surface treatment and exclusive primer on bond strength of a self-adhesive resin cement. Korean J. Dent. Mater. 2019, 46, 195–203. [Google Scholar] [CrossRef]
- Suh, B.I.; Feng, L.; Pashley, D.H.; Tay, F.R. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part III. Effect of acidic resin monomers. J. Adhes. Dent. 2003, 5, 267–282. [Google Scholar]
- Perdigão, J.; Swift, E.J. Universal adhesives. J. Esthet. Restor. Dent. 2015, 27, 331–334. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Sutil, E.; Malaquias, P.; de Paris Matos, T.; de Souza, L.M.; Reis, A.; Perdigão, J.; Loguercio, A.D. Effect of self-curing activators and curing protocols on adhesive properties of universal adhesives bonded to dual-cured composites. Dent. Mater. 2017, 33, 775–787. [Google Scholar] [CrossRef]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP based dental adhesives: Adhesive interface characterization and adhesive stability—A systematic review. Materials 2019, 12, 790. [Google Scholar] [CrossRef] [Green Version]
- Fukegawa, D.; Hayakawa, S.; Yoshida, Y.; Suzuki, K.; Osaka, A.; Van Meerbeek, B. Chemical interaction of phosphoric acid ester with hydroxyapatite. J. Dent. Res. 2006, 85, 941–944. [Google Scholar] [CrossRef]
- Behr, M.; Rosentritt, M.; Regnet, T.; Lang, R.; Handel, G. Marginal adaptation in dentin of a self-adhesive universal resin cement compared with well-tried systems. Dent. Mater. 2004, 20, 191–197. [Google Scholar] [CrossRef]
- Tay, E.R.; Carvalho, R.M.; Pashley, D.H. Effect of smear layers on the bonding of a self-etching primer to dentin. J. Adhes. Dent.. 2000, 2, 99–116. [Google Scholar] [PubMed]
- Calixto, L.R.; Bandéca, M.C.; Clavijo, V.; Andrade, M.F.; Vaz, L.G.; Campos, E.A. Effect of resin cement system and root region on the push-out bond strength of a translucent fiber post. Oper. Dent. 2012, 37, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel-dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef]
- Fujita Nakajima, K.; Nikaido, T.; Francis Burrow, M.; Iwasaki, T.; Tanimoto, Y.; Hirayama, S.; Nishiyama, N. Effect of the demineralisation efficacy of MDP utilized on the bonding performance of MDP-based all-in-one adhesives. J. Dent. 2018, 77, 59–65. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, C.Y.; Zhang, L.; Zhang, Z.L.; Fu, B.P.; Hannig, M. Influence of priming time and primer’s concentrations on bovine enamel bond strengths. J. Adhes. Sci. Technol. 2013, 27, 2558–2570. [Google Scholar] [CrossRef]
- Hiraishi, N.; Tochio, N.; Kigawa, T.; Otsuki, M.; Tagami, J. Role of 2-hydroxyethyl methacrylate in the interaction of dental monomers with collagen studied by saturation transfer difference NMR. J. Dent. 2014, 42, 484–489. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Matsumoto, T.; Minagi, S.; Osaka, A.; Van Landuyt, K.; Van Meerbeek, B. HEMA inhibits interfacial nano-layering of the functional monomer MDP. J. Dent. Res. 2012, 91, 1060–1065. [Google Scholar] [CrossRef]
- Kirihara, M.; Inoue, G.; Nikaido, T.; Ikeda, M.; Sadr, A.; Tagami, J. Effect of fluoride concentration in adhesives on morphology of acid-base resistant zones. Dent. Mater. J. 2013, 32, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Araoka, D.; Hosaka, K.; Nakajima, M.; Foxton, R.; Thanatvarakorn, O.; Prasansuttiporn, T.; Chiba, A.; Sato, K.; Takahashi, M.; Otsuki, M.; et al. The strategies used for curing universal adhesives affect the micro-bond strength of resin cement used to lute indirect resin composites to human dentin. Dent. Mater. J. 2018, 37, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Arrais, C.A.; Kasaz Ade, C.; Albino, L.G.; Rodrigues, J.A.; Reis, A.F. Effect of curing mode on the hardness of dual-cured composite resin core build-up materials. Braz. Oral. Res. 2010, 24, 245–249. [Google Scholar] [CrossRef] [PubMed]
| Material | Manufacturer | Composition |
|---|---|---|
| G-CEM One Primer | GC Corp., Tokyo, Japan | ethanol, 10-MDP, 10-methacryloyloxydecyl dihydrogen thiophosphate, 4-META, 2-hydroxy-1,3 dimethoxypropane, vanadyl acetylacetonate, 2,6-di-tert-butyl-p-cresol |
| All-Bond Universal | Bisco, Schaumburg, IL, USA | 10-MDP, 2-HEMA, Bis-GMA, ethanol, water, photoinitiator |
| G-CEM One | GC Corp., Tokyo, Japan | Paste A: fluoroaluminosilicate glass, UDMA, dimethacrylate, initiator, stabilizer, pigment, silicon dioxide, MDP Paste B: SiO2, trimethoxysilane, UDMA, 2-hydroxy-1,3-dimethacryloxypropane, MDP, 6-tert-butyl-2,4-xylenol, 2,6-di-tert-butyl-p-cresol, EDTA disodium salt dehydrate, vanadyl acetylacetonate, TPO, ascorbic acid, camphorquinone, MgO |
| Groups | Surface treatment | Application Procedure |
|---|---|---|
| Control | No surface treatment | |
| GCOP | G-CEM One Primer | Apply on dentin surface and rubbing with a microbrush for 10 s then air dry for 5 s |
| ABU | All-Bond Universal | Apply on dentin surface and air dry to remove excess solvent then light curing for 10 s |
| GCOP/ABU | G-CEM One Primer followed by All-Bond Universal | Apply G-CEM One Primer followed by All-Bond Universal in the same way above. |
| ABU/GCOP | All-Bond Universal followed by G-CEM One Primer | Apply All-Bond Universal followed by G-CEM One Primer in the same way above. |
| Groups | µTBS |
|---|---|
| Control | 9.2 (2.2)a |
| GCOP | 20.1 (5.5)b |
| ABU | 13.9 (1.7)c |
| GCOP/ABU | 18.1 (2.3)b |
| ABU/GCOP | 16.9 (4.4)b,c |
| Groups | A | M | RC | DC |
|---|---|---|---|---|
| Control | 11 (73.3) | 0 | 1 (6.7) | 3 (20) |
| GCOP | 6 (40) | 1 (6.7) | 2 (13.3) | 6 (40) |
| ABU | 9 (60) | 0 | 2 (13.3) | 4 (26.7) |
| GCOP/ABU | 9 (60) | 1 (6.7) | 0 | 5 (33.3) |
| ABU/GCOP | 10 (66.7) | 0 | 0 | 5 (33.3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-N.; Son, S.-A.; Park, J.-K. Effect of Exclusive Primer and Adhesive on Microtensile Bond Strength of Self-Adhesive Resin Cement to Dentin. Materials 2020, 13, 2353. https://doi.org/10.3390/ma13102353
Kim B-N, Son S-A, Park J-K. Effect of Exclusive Primer and Adhesive on Microtensile Bond Strength of Self-Adhesive Resin Cement to Dentin. Materials. 2020; 13(10):2353. https://doi.org/10.3390/ma13102353
Chicago/Turabian StyleKim, Bit-Na, Sung-Ae Son, and Jeong-Kil Park. 2020. "Effect of Exclusive Primer and Adhesive on Microtensile Bond Strength of Self-Adhesive Resin Cement to Dentin" Materials 13, no. 10: 2353. https://doi.org/10.3390/ma13102353
APA StyleKim, B.-N., Son, S.-A., & Park, J.-K. (2020). Effect of Exclusive Primer and Adhesive on Microtensile Bond Strength of Self-Adhesive Resin Cement to Dentin. Materials, 13(10), 2353. https://doi.org/10.3390/ma13102353





