Mechanism of the Microstructural Evolution of 18Cr2Ni4WA Steel during Vacuum Low-Pressure Carburizing Heat Treatment and Its Effect on Case Hardness
Abstract
:1. Introduction
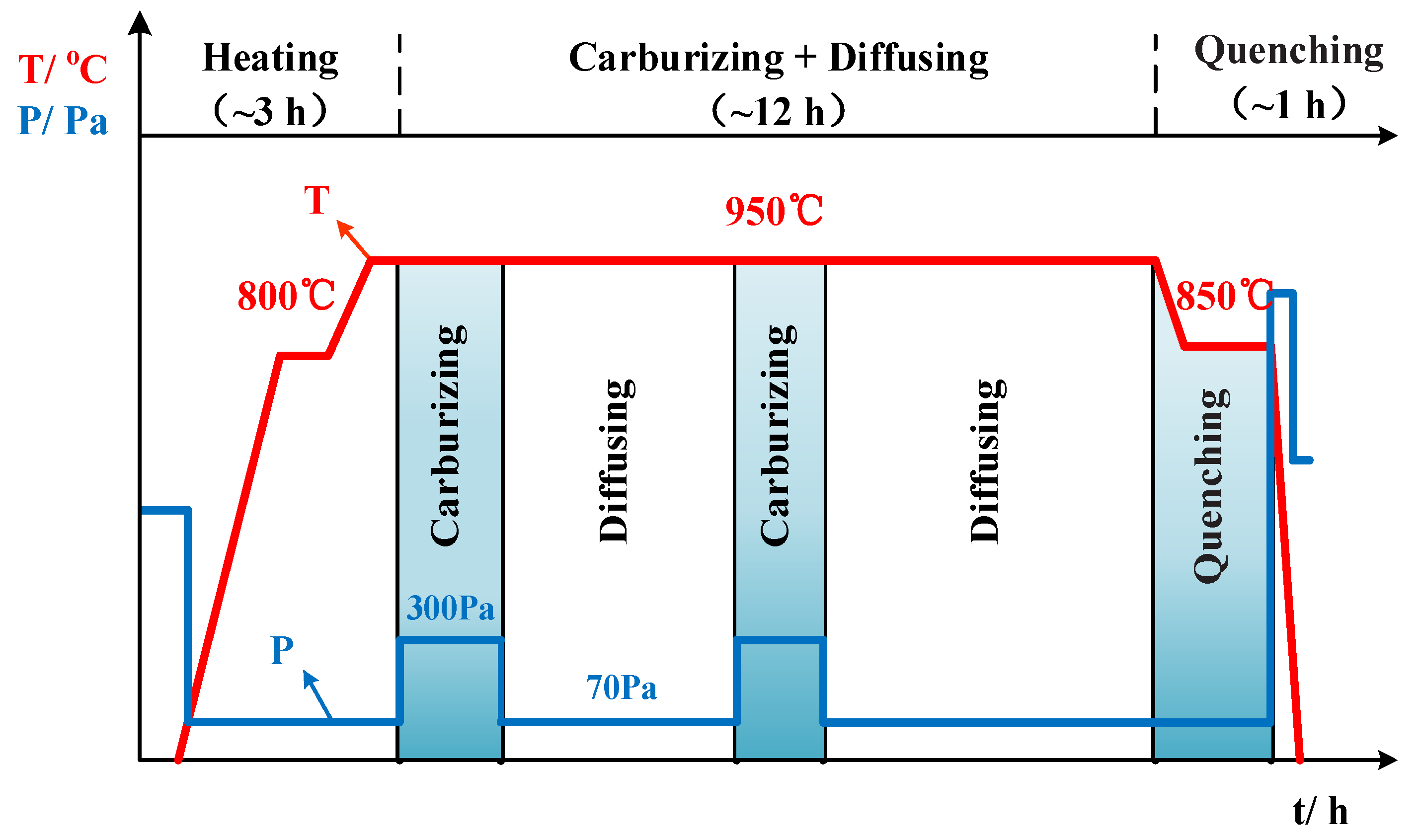
2. Experimental Materials and Methods of Vacuum Low-Pressure Carburization
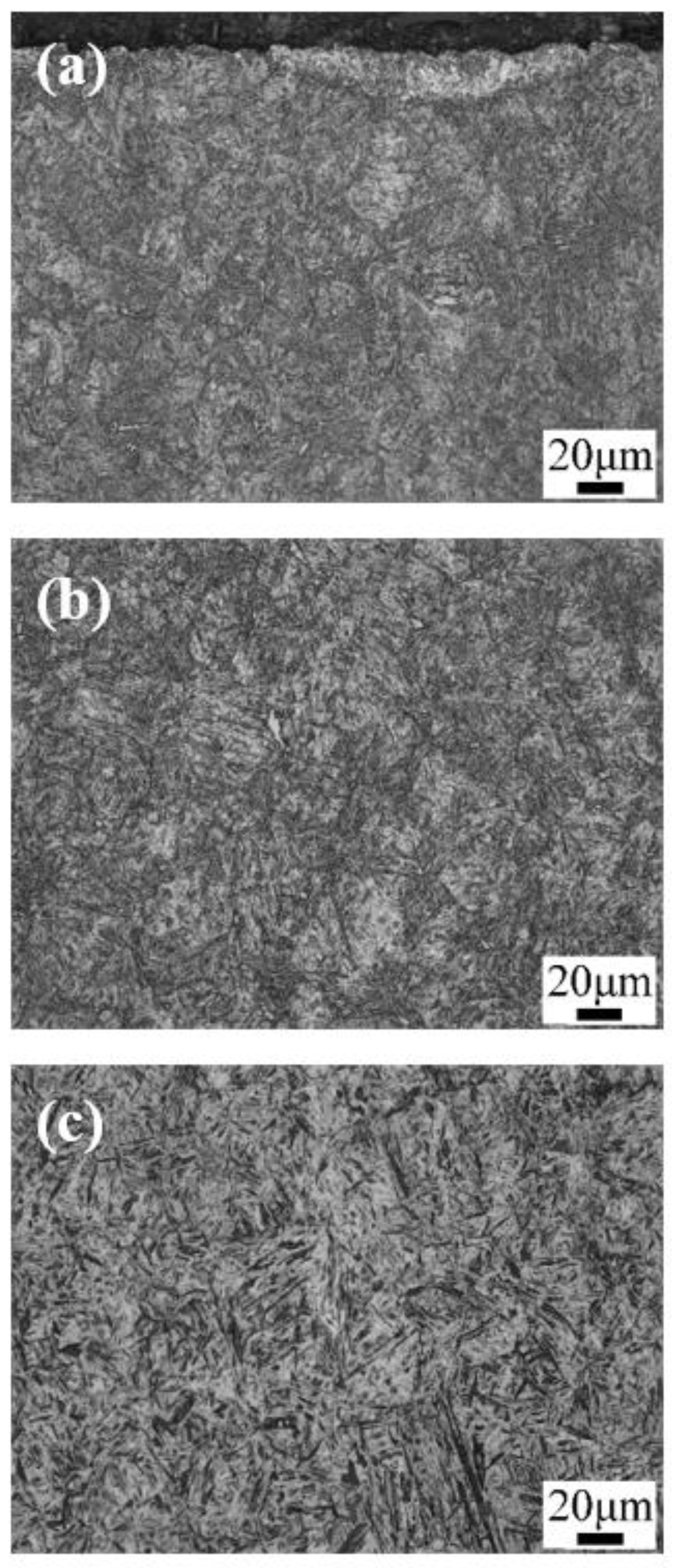
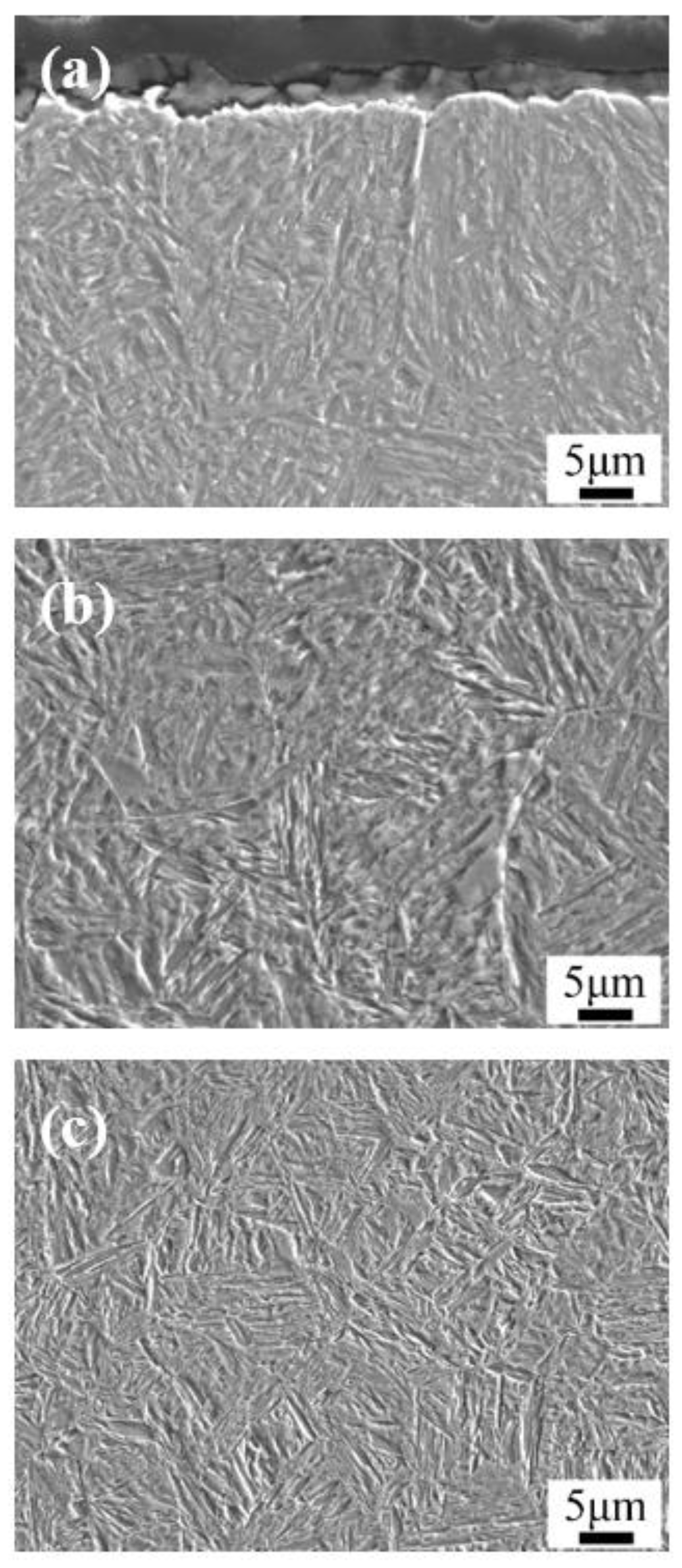
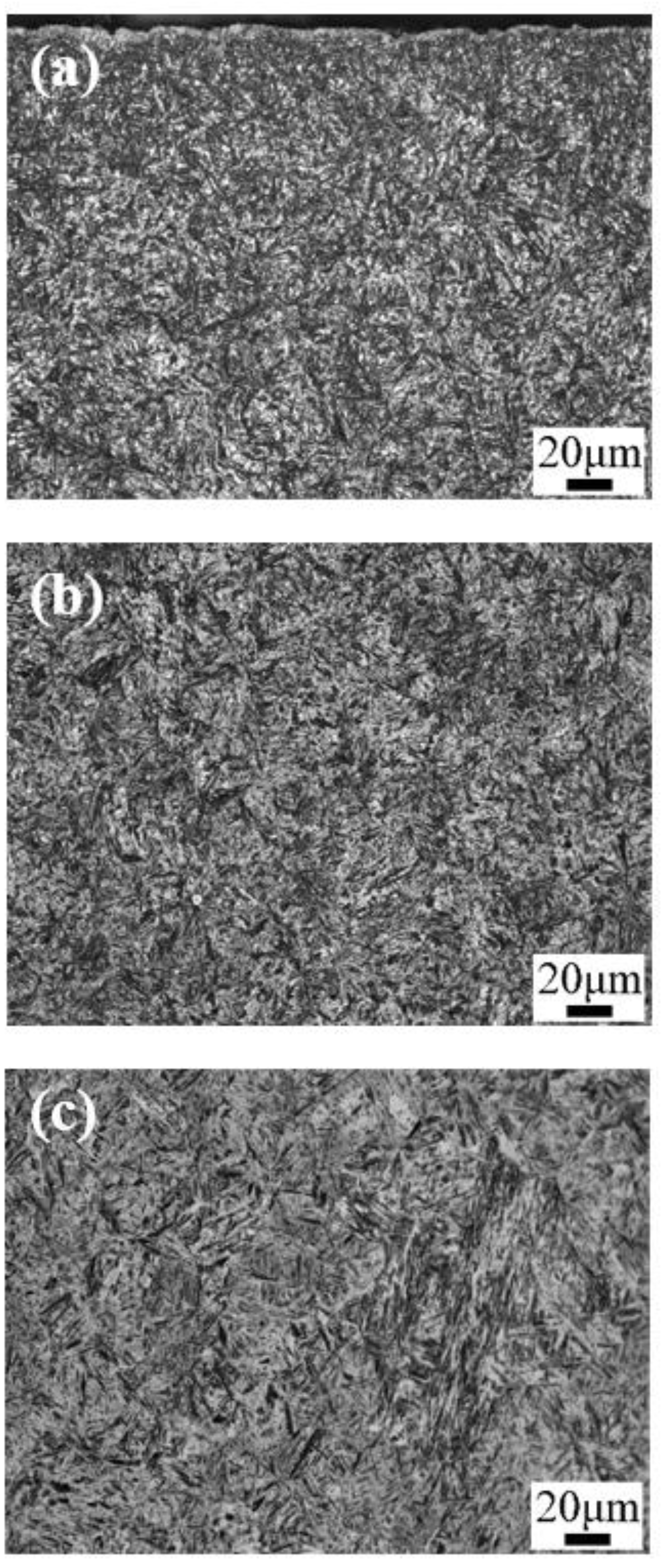
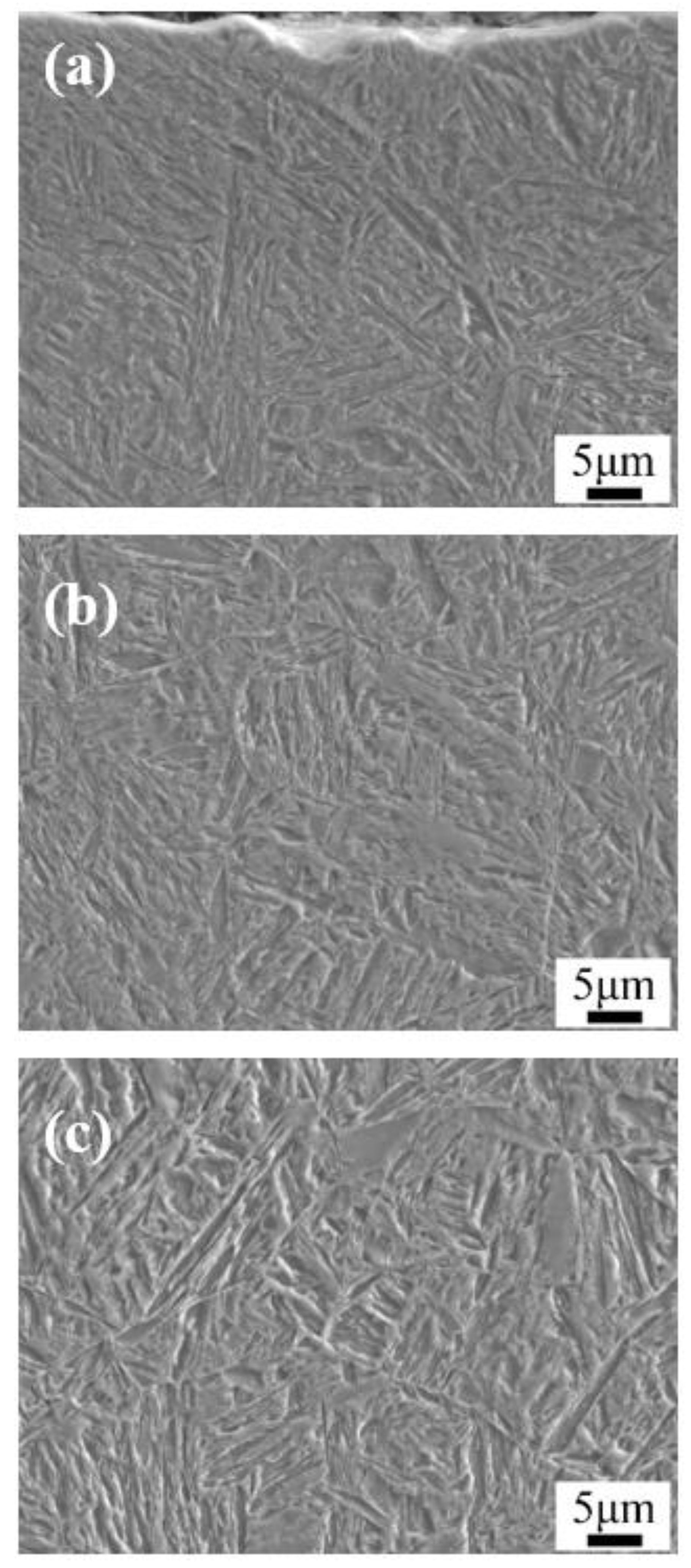
3. Results: Microstructure and Performance after Carburizing and Quenching
4. Discussion
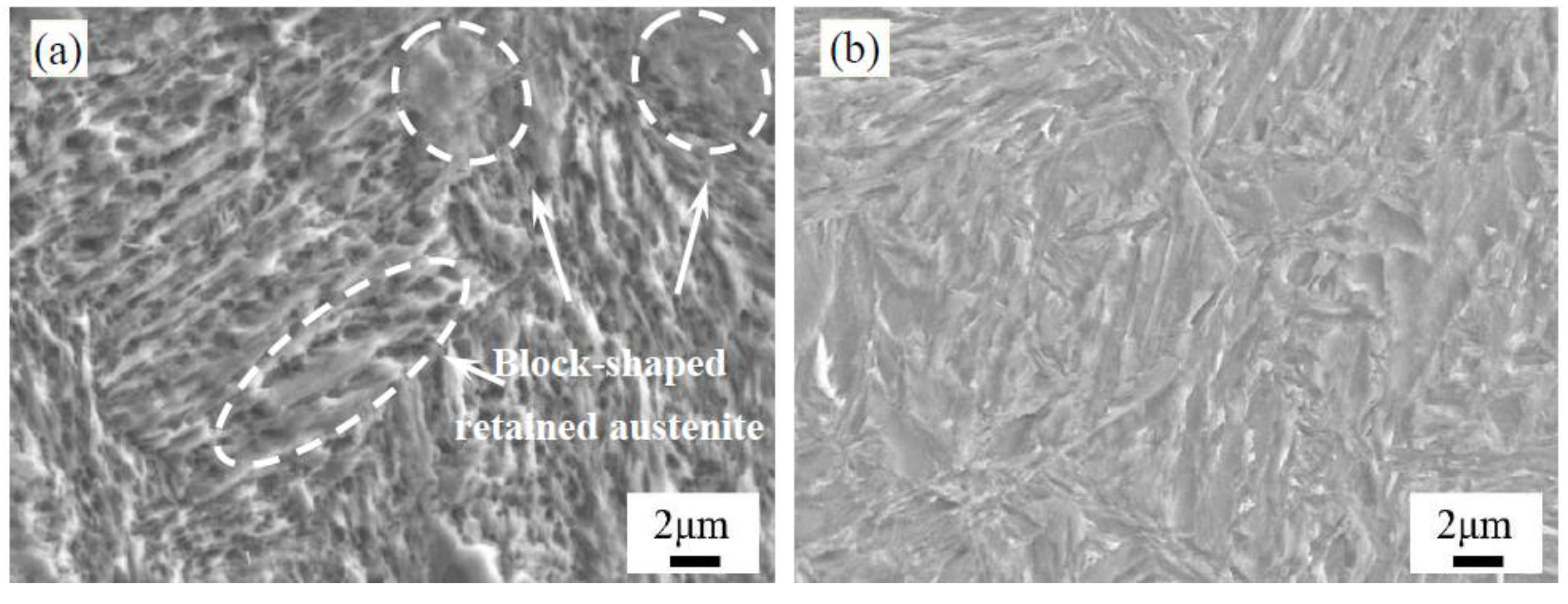
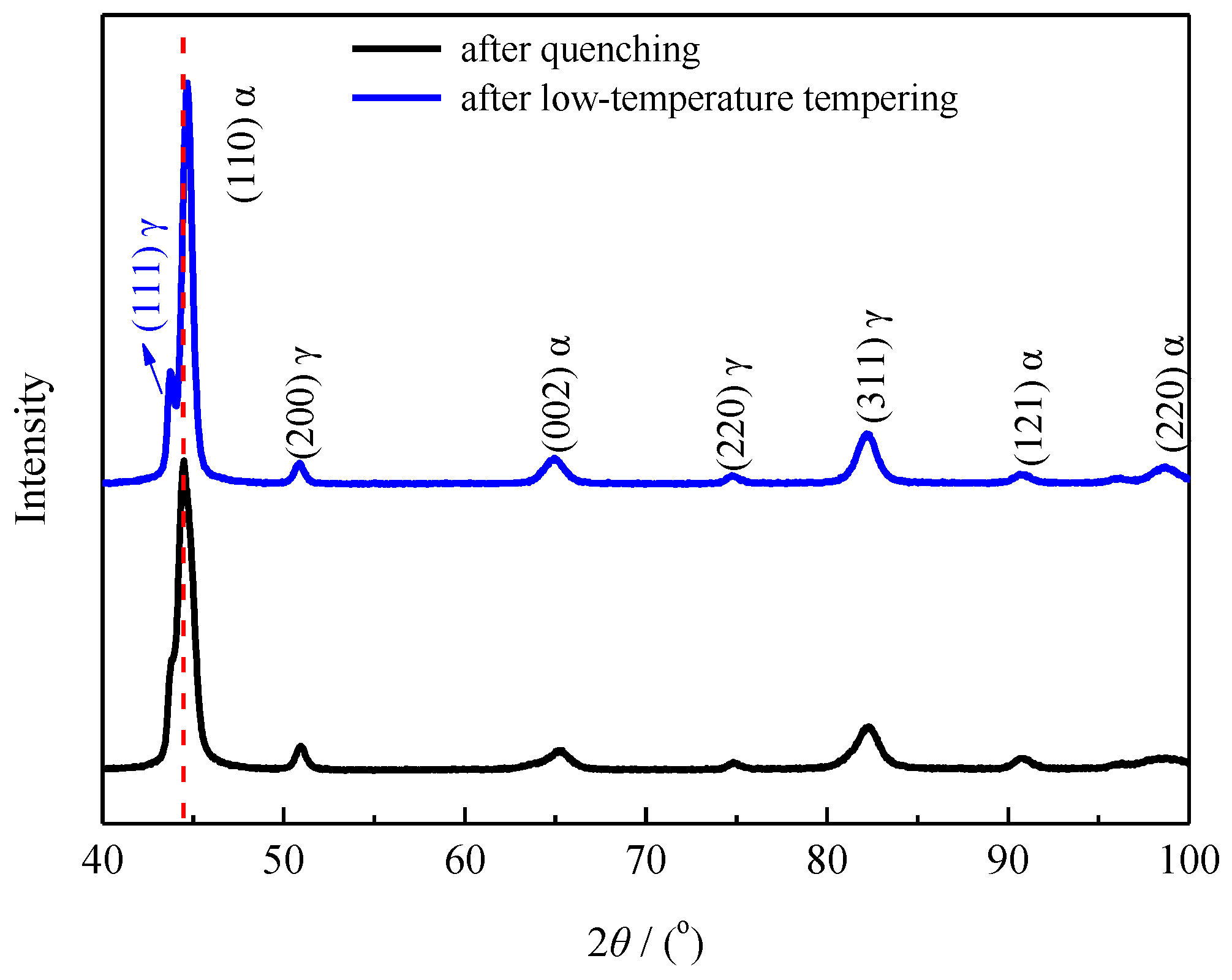
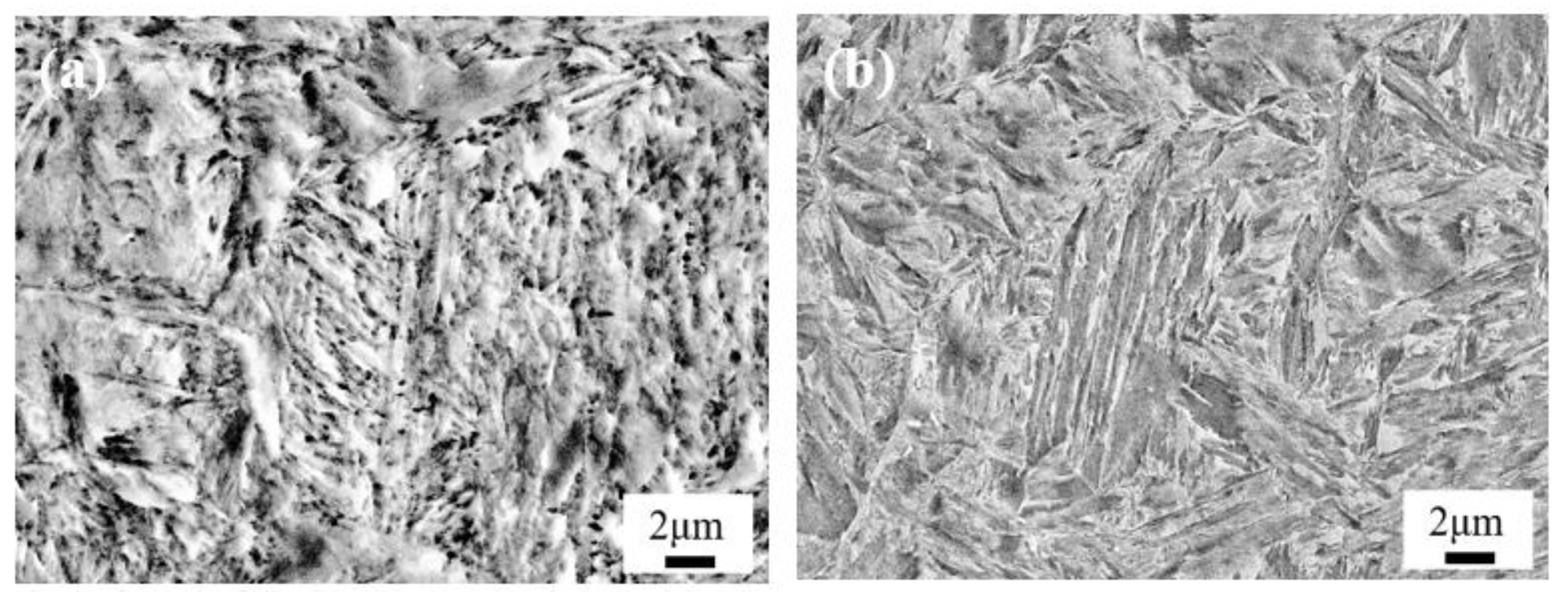
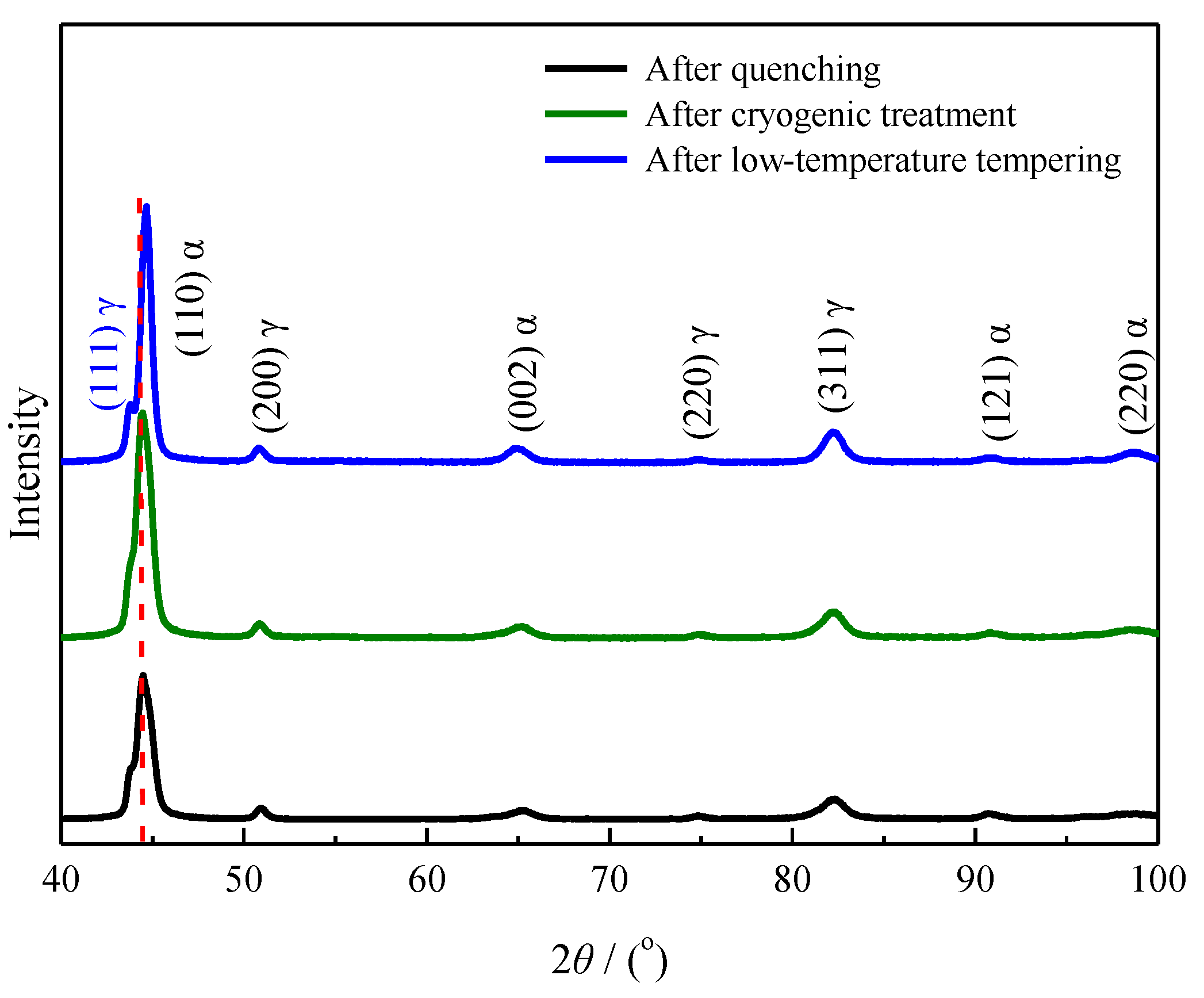
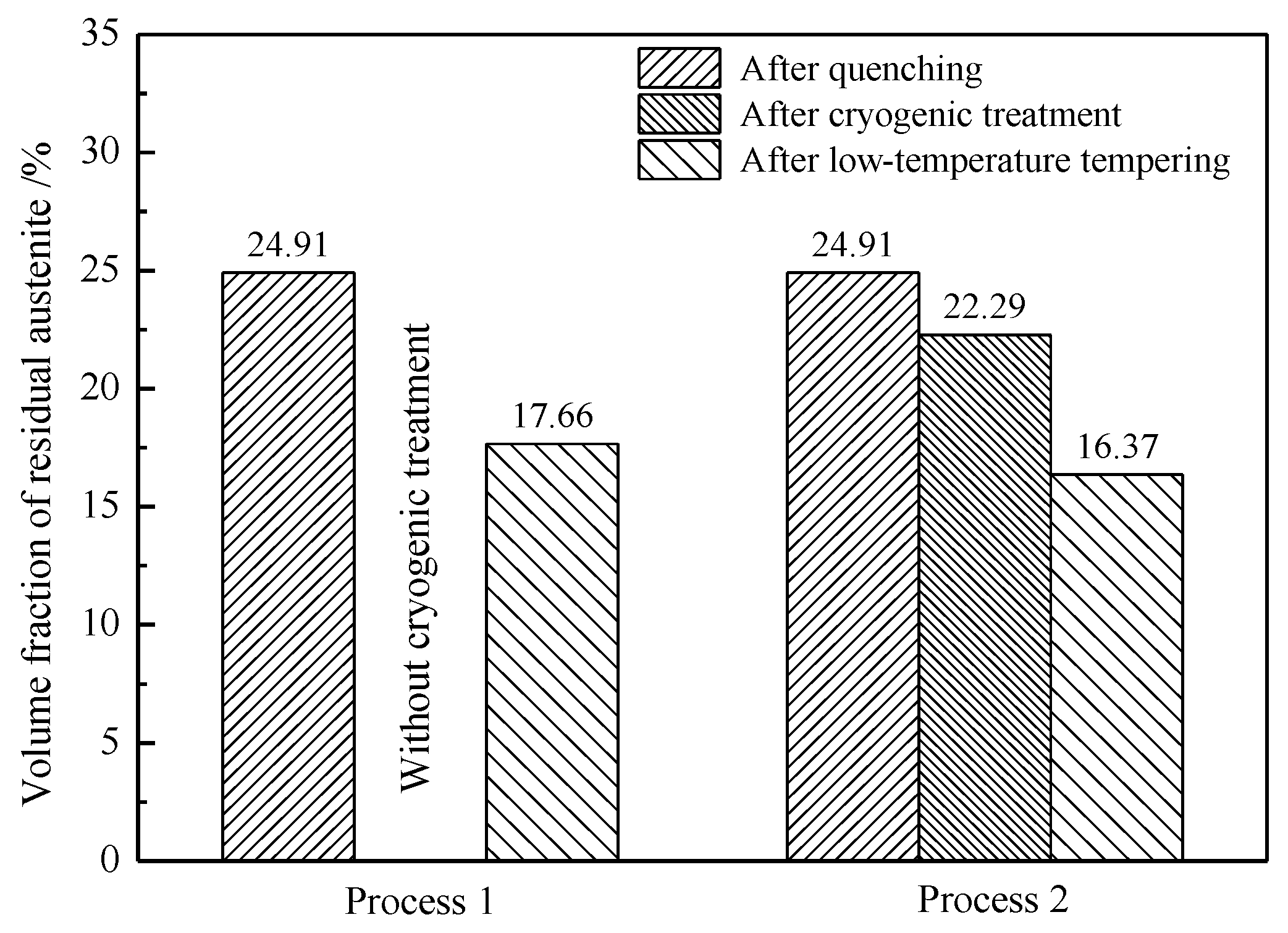
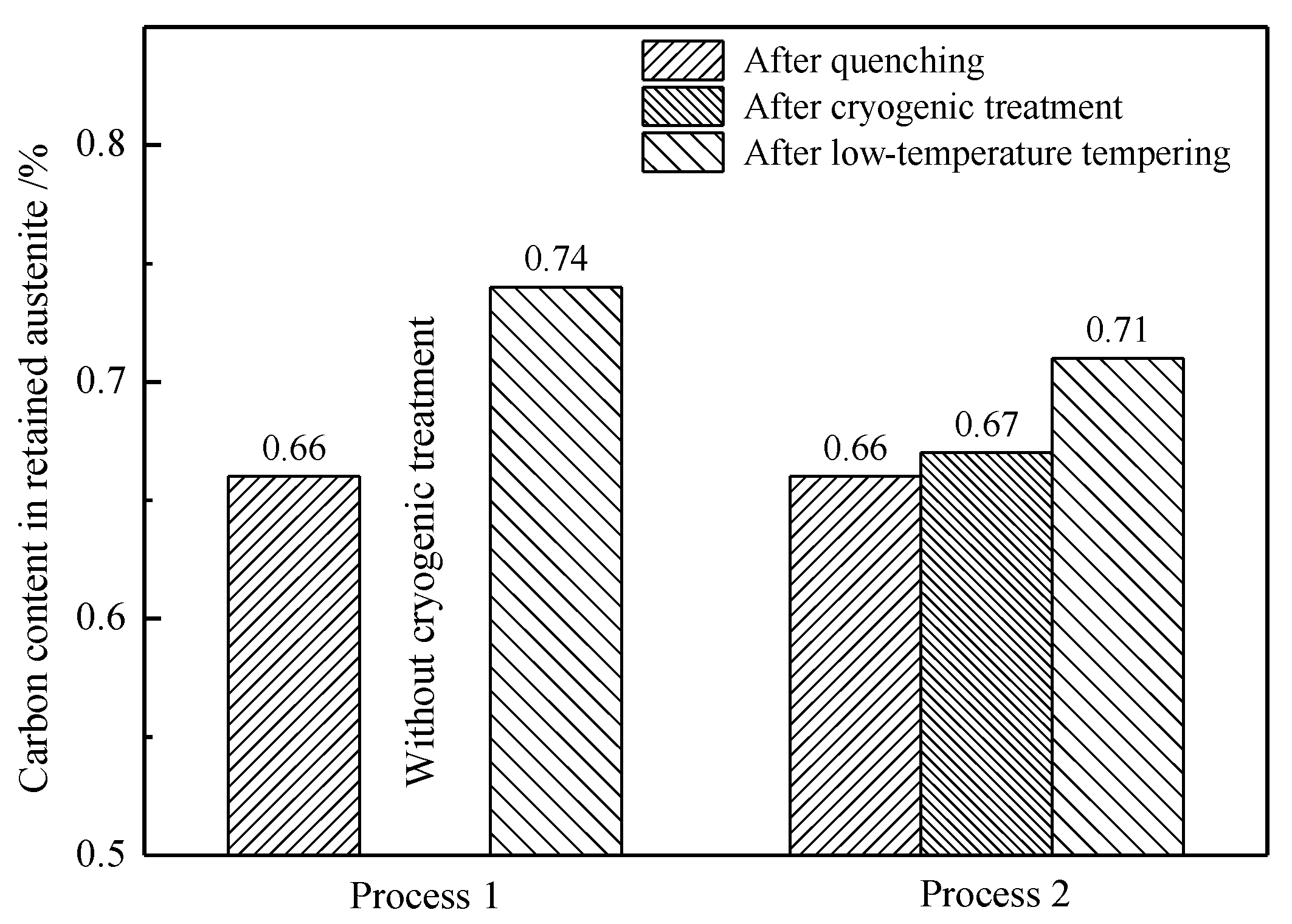
4.1. Effect of Low-Temperature Tempering on the Microstructure and Case Hardness
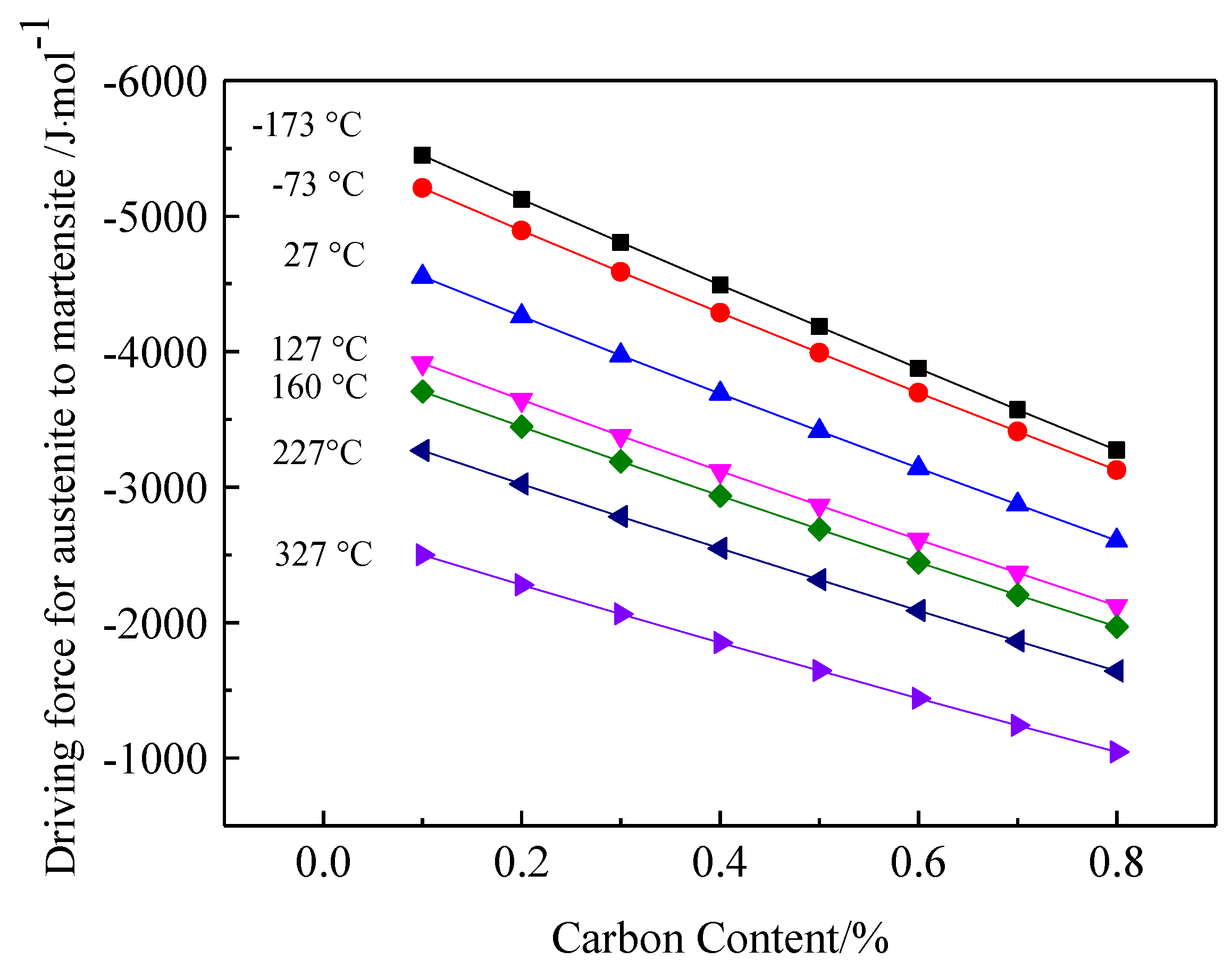
4.2. Driving Force for Phase Transformation from Retained Austenite into Martensite
4.3. Effect of Cryogenic Treatments on the Microstructure and Case Hardness
5. Conclusions
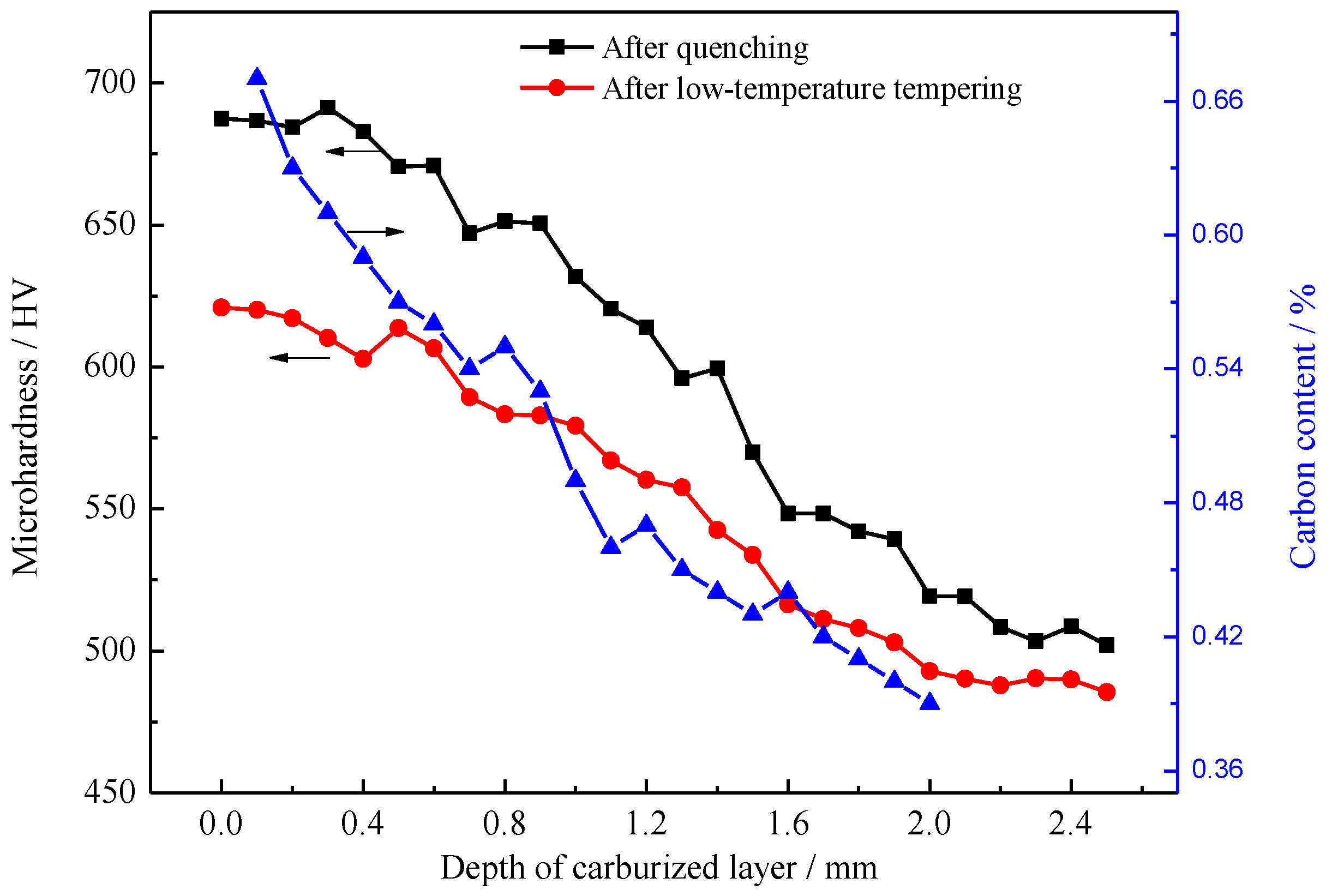
- After carburizing and quenching, the carbon content of the carburized layer decreased gradually and evenly as the depth of the carburized layer increased. The matrix microstructure changed from high-carbon acicular martensite in the surface layer into low-carbon lath martensite in the core and the microhardness gradually decreased.
- Calculating the driving force for the phase transformation of austenite showed that low-carbon and low-temperature conditions favored the transformation of retained austenite to martensite. Therefore, some low-carbon retained austenite was more likely to undergo martensitic transformation after low-temperature tempering.
- During low-temperature tempering, the solid-solution carbon content of martensite decreased, the compressive stress on the retained austenite was reduced and the mechanical stability of the retained austenite decreased. Low-temperature tempering, rather than the cryogenic treatment, effectively promoted the transformation of the retained austenite.
Author Contributions
Funding
Conflicts of Interest
References
- Li, L.J.; Sun, L.Y. Experimental and numerical investigations of crack behavior and life prediction of 18Cr2Ni4WA steel subjected to repeated impact loading. Eng. Fail. Anal. 2016, 65, 11–25. [Google Scholar] [CrossRef]
- Xia, C.B.; Tu, M.W.; Pan, Q.J. Performance and property of Ni base coatings by using plasma arc technology for 18Cr2Ni4WA steel repairing. Adv. Mater. Res. 2011, 189–193, 311–315. [Google Scholar]
- Yang, J.J.; Pang, K.J. Elastoplastic behavior of case-carburized 18Cr2Ni4WA steel by indenter testing. J. Aerosp. Eng. 2019, 32, 04019045. [Google Scholar] [CrossRef]
- Xu, Z.J.; Wei, Z.P.; Zhou, P.; Lu, J.C.; Wang, G. Optimization of heat treatment process after vacuum carburizing of 18Cr2Ni4WA steel. Heat Treat. Met. 2014, 39, 32–35. (In Chinese) [Google Scholar]
- Asi, O.; Can, A.C.; Pineault, J.; Belassel, M. The effect of high temperature gas carburizing on bending fatigue strength of SAE 8620 steel. Mater. Des. 2009, 30, 1792–1797. [Google Scholar] [CrossRef]
- Walvekar, A.A.; Sadeghi, F. Rolling contact fatigue of case carburized steels. Int. J. Fatigue 2017, 95, 264–281. [Google Scholar] [CrossRef]
- Woods, J.L.; Daniewicz, S.R.; Nellums, R. Increasing the bending fatigue strength of carburized spur gear teeth by presetting. Int. J. Fatigue 1999, 21, 549–556. [Google Scholar] [CrossRef]
- Loganathan, T.M.; Purbolaksono, J.; Inayat-Hussain, J.I.; Wahab, N. Effects of carburization on expected fatigue life of alloys steel shafts. Mater. Des. 2011, 32, 3544–3547. [Google Scholar] [CrossRef]
- He, S.H.; He, B.B.; Zhu, K.Y.; Ding, R.; Chen, H.; Huang, M.X. Revealing the role of dislocations on the stability of retained austenite in a tempered bainite. Scr. Mater. 2019, 168, 23–27. [Google Scholar] [CrossRef]
- Xiong, X.C.; Chen, B.; Huang, M.X. The effect of morphology on the stability of retained austenite in a quenched and partitioned steel. Scr. Mater 2013, 68, 321–324. [Google Scholar] [CrossRef]
- Shi, Y.F. Effect of high temperature tempering on microstructure of 18Cr2Ni4W steel after carburizing and quenching. Commun. Phys. Chem. Test. (Phys. Vol.) 1978, 4, 1–2. (In Chinese) [Google Scholar]
- Chen, Z.H.; Gu, J.F.; Han, L.Z. Decomposition characteristic of austenite retained in GCr15 bearing steel modified by addition of 1.3 wt % silicon during tempering. J. Mater. Res. Technol. 2019, 8, 157–166. [Google Scholar] [CrossRef]
- Nam, W.J.; Kim, D.S.; Ahn, S.T. Effects of alloying elements on microstructural evolution and mechanical properties of induction quenched-and-tempered steels. J. Mater. Sci. 2003, 38, 3611–3617. [Google Scholar] [CrossRef]
- Wu, Y.X.; Sun, W.W.; Gao, X.; Styles, M.J.; Arlazarov, A.; Hutchinson, C.R. The effect of alloying elements on cementite coarsening during martensite tempering. Acta Mater. 2020, 183, 418–437. [Google Scholar] [CrossRef]
- Feng, F.F.; Wu, H.B.; Yu, X.P. Thermal stability of nanoscale bainite/martensite steel. Chin. J. Mater. Res. 2019, 33, 597–602. (In Chinese) [Google Scholar]
- Chen, W.L.; Wu, W.K.; Li, C.; Meng, X.N. Influence of deep cryogenic treatment and secondary tempering on microstructure and mechanical properties of medium-carbon low-alloy steels. J. Mater. Eng. Perform. 2020, 29, 10–22. [Google Scholar] [CrossRef]
- Steels—Determination and verification of the depth of carburized and hardened cases; GB/T 9450-2005; The General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (AQSIQ): Beijing, China, 21 July 2005.
- Sugimoto, K.; Usui, N.; Kobayashi, M.; Hashimoto, S. Effects of volume fraction and stability of retained austenite on ductility of TRIP-aided dual-phase steels. ISIJ Int. 1992, 32, 1311–1318. [Google Scholar] [CrossRef]
- Tomita, Y.; Morioka, K. Effect of microstructure on transformation-induced plasticity of silicon-containing low-alloy steel. Mater. Charact. 1997, 38, 243–250. [Google Scholar] [CrossRef]
- Tomota, Y.; Tokuda, H.; Adachi, Y.; Wakita, M.; Minakawa, N.; Moriai, A.; Morii, Y. Tensile behavior of TRIP-aided multi-phase steels studied by in-situ neutron diffraction. Acta Mater. 2004, 52, 5737–5745. [Google Scholar] [CrossRef]
- Hidalgo, J.; Findley, K.O.; Santofimia, M.J. Thermal and mechanical stability of retained austenite surrounded by martensite with different degrees of tempering. Mater. Sci. Eng. A 2017, 690, 337–347. [Google Scholar] [CrossRef]
- Nayaka, S.S.; Anumolu, R.; Misra, R.D.K.; Kim, K.H.; Lee, D.L. Microstructure-hardness relationship in quenchedand partitioned medium-carbon and partitioned medium-carbon and high-carbon steels containing silicon. Mater. Sci. Eng. A 2008, 498, 442–456. [Google Scholar] [CrossRef]
- Toji, Y.; Miyamoto, G.; Raabe, D. Carbon partitioning during quenching and partitioning heat treatment accompanied by carbide precipitation. Acta Mater. 2015, 86, 137–147. [Google Scholar] [CrossRef]
- Kaufman, L.; Radcliffe, S.V.; Cohen, M. Decomposition of Austenite by Diffusional Processes; Interscience: New York, NY, USA, 1962. [Google Scholar]
- Kaufman, L.; Clougherty, E.V.; Weiss, R.J. The lattice stability of metals—III. Iron. Acta Metall. 1963, 11, 323–335. [Google Scholar] [CrossRef]
- Mogutnov, B.M.; Tomilin, I.A.; Shartsman, L.A. Thermodynamics of Fe-C Alloys; Metallurgy Press: Moscow, Russia, 1972. [Google Scholar]
- Orr, R.L.; Chipman, J. Thermodynamic functions of iron. Trans. Metall. Soc. AIME 1967, 239, 630–634. [Google Scholar]
- Shiflet, G.J.; Bradley, J.R.; Aaronson, H.I. A re-examination of the thermodynamics of the proeutectoid ferrite transformation in Fe-C alloys. Metall. Trans. A 1978, 9, 999–1008. [Google Scholar] [CrossRef]
- Ishida, K. Calculation of the effect of alloying elements on the MS temperature in steels. J. Alloys Compd. 1995, 220, 126–131. [Google Scholar] [CrossRef]



| Element | C | Si | Mn | Cr | Ni | W |
|---|---|---|---|---|---|---|
| Content | 0.2 | 0.33 | 0.5 | 1.4 | 4.3 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; He, Y.; Liu, Y.; Tian, Y.; You, J.; Wang, Z.; Wang, G. Mechanism of the Microstructural Evolution of 18Cr2Ni4WA Steel during Vacuum Low-Pressure Carburizing Heat Treatment and Its Effect on Case Hardness. Materials 2020, 13, 2352. https://doi.org/10.3390/ma13102352
Wang B, He Y, Liu Y, Tian Y, You J, Wang Z, Wang G. Mechanism of the Microstructural Evolution of 18Cr2Ni4WA Steel during Vacuum Low-Pressure Carburizing Heat Treatment and Its Effect on Case Hardness. Materials. 2020; 13(10):2352. https://doi.org/10.3390/ma13102352
Chicago/Turabian StyleWang, Bin, Yanping He, Ye Liu, Yong Tian, Jinglin You, Zhaodong Wang, and Guodong Wang. 2020. "Mechanism of the Microstructural Evolution of 18Cr2Ni4WA Steel during Vacuum Low-Pressure Carburizing Heat Treatment and Its Effect on Case Hardness" Materials 13, no. 10: 2352. https://doi.org/10.3390/ma13102352
APA StyleWang, B., He, Y., Liu, Y., Tian, Y., You, J., Wang, Z., & Wang, G. (2020). Mechanism of the Microstructural Evolution of 18Cr2Ni4WA Steel during Vacuum Low-Pressure Carburizing Heat Treatment and Its Effect on Case Hardness. Materials, 13(10), 2352. https://doi.org/10.3390/ma13102352






