A Windmill-Shaped Molecule with Anthryl Blades to Form Smooth Hole-Transport Layers via a Photoprecursor Approach
Abstract
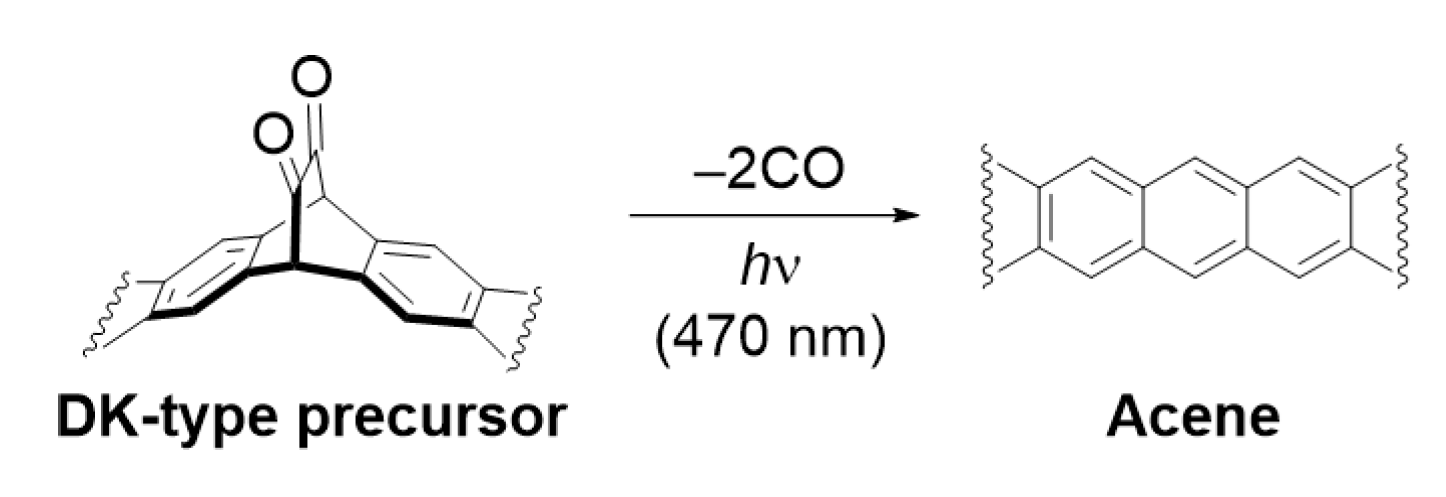
1. Introduction
2. Results and Discussion
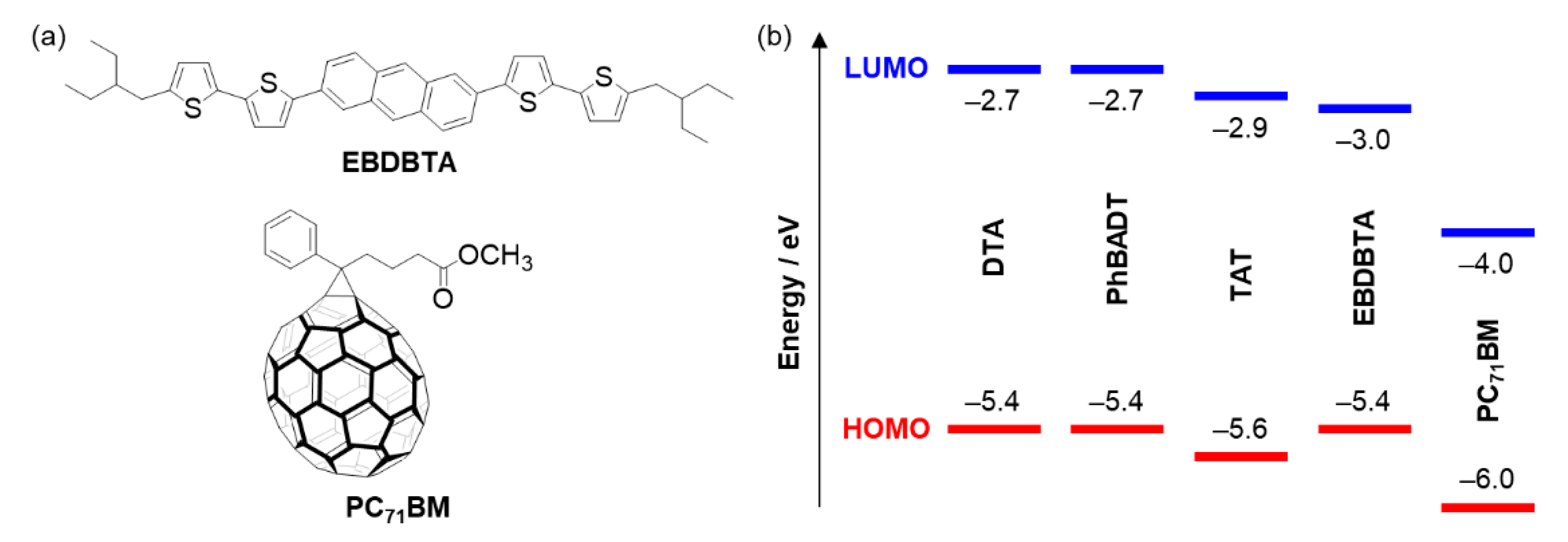
2.1. Molecular Design
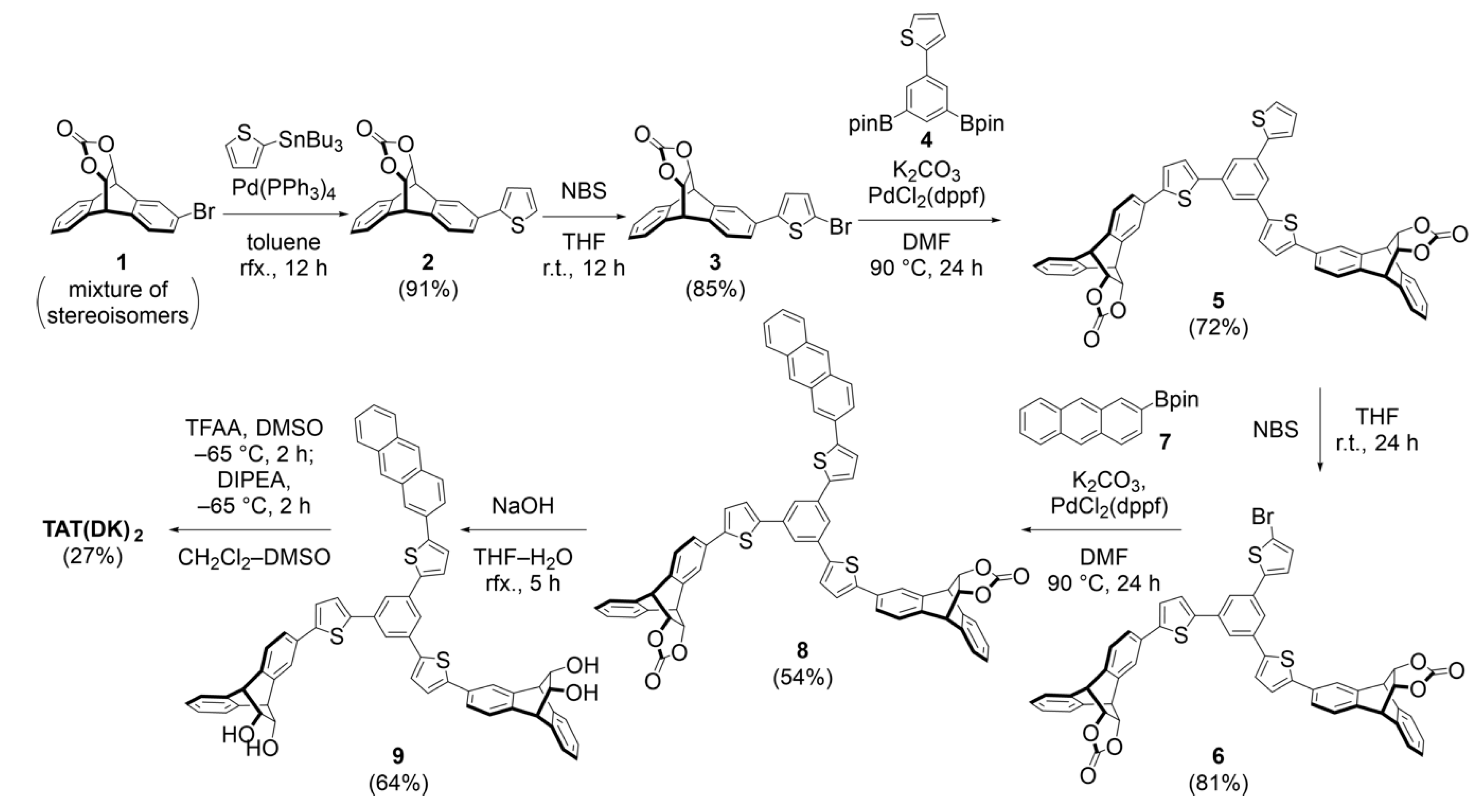
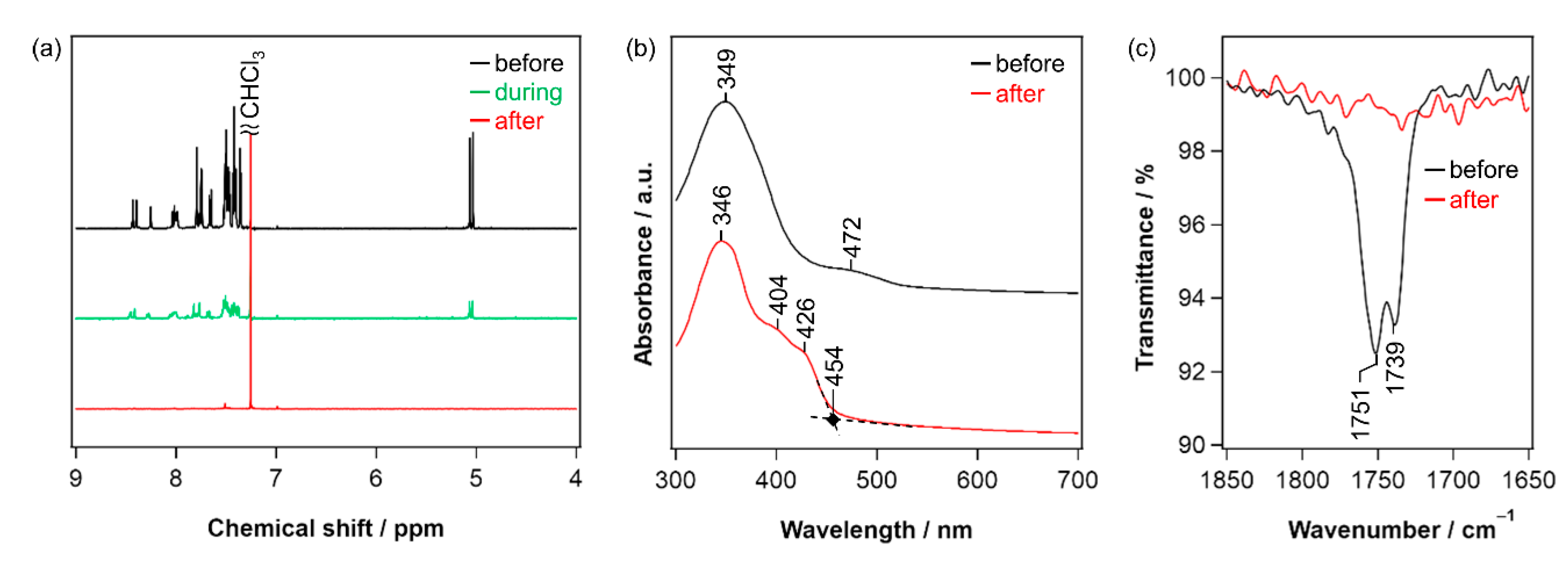
2.2. Synthesis and Photoreaction of the Precursor
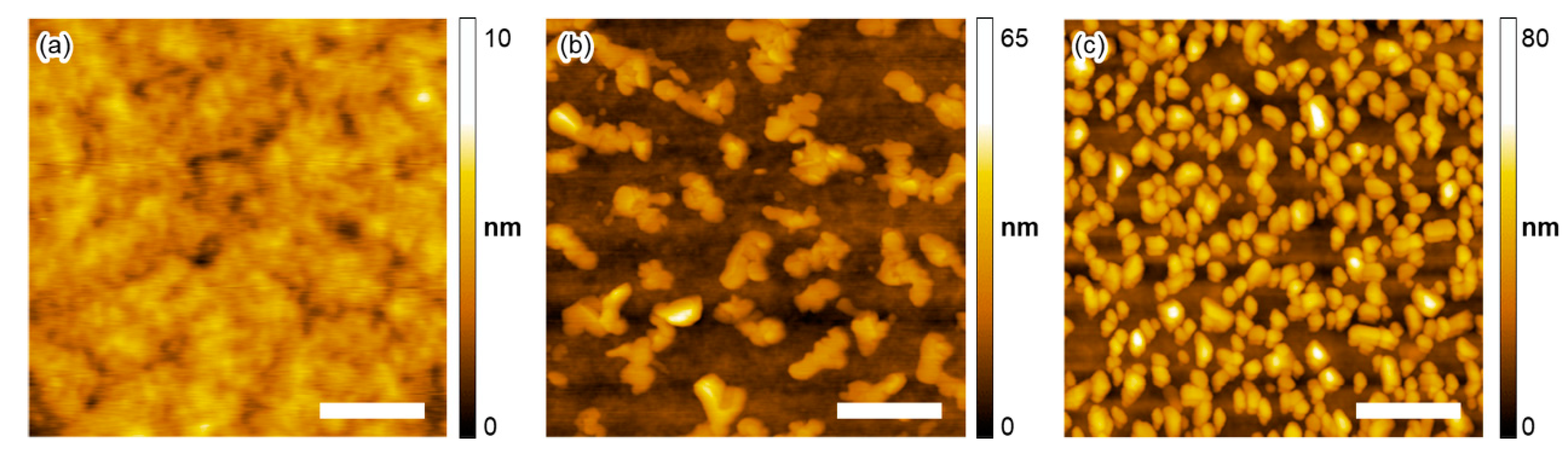
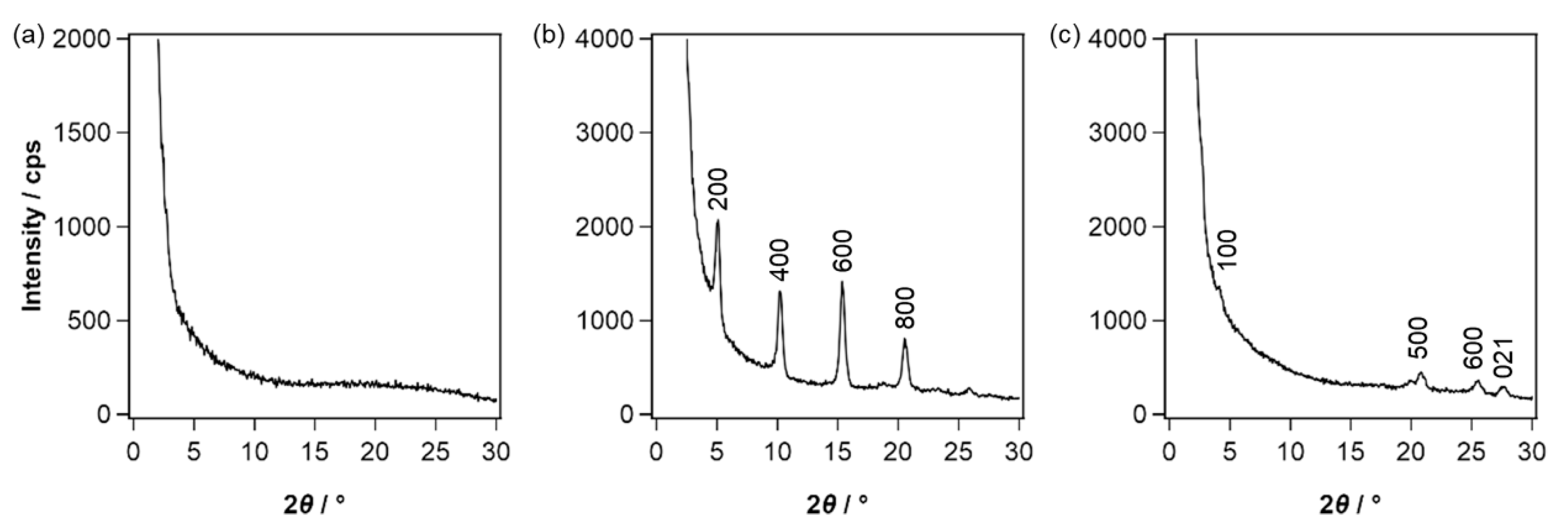
2.3. Thin-Film Characteristics
2.4. Photovoltaic Performance
3. Concluding Remarks
4. Materials and Methods
4.1. General
4.2. Chemical Synthesis and Spectroscopic Data
4.2.1. 2-(Thiophen-2-yl)-9,10-dihydro-9,10-[4,5]epidioxoloanthracen-13-one (2)
4.2.2. 2-(5-Bromothiophen-2-yl)-9,10-dihydro-9,10-[4,5]epidioxoloanthracen-13-one (3)
4.2.3. 2,2′-(5-(thiophen-2-yl)-1,3-phenylene)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) (4)
4.2.4. 2,2′-((5-(thiophen-2-yl)-1,3-phenylene)bis(thiophene-5,2-diyl))bis(9,10-dihydro-9,10-[4,5]epidi- oxoloanthracen-13-one) (5)
4.2.5. 2,2′-((5-(5-bromothiophen-2-yl)-1,3-phenylene)bis(thiophene-5,2-diyl))bis(9,10-dihydro-9,10- [4,5]epidioxoloanthracen-13-one) (6)
4.2.6. 2,2′-((5-(5-(anthracen-2-yl)thiophen-2-yl)-1,3-phenylene)bis(thiophene-5,2-diyl))bis(9,10- dihydro-9,10-[4,5]epidioxoloanthracen-13-one) (8)
4.2.7. 2,2′-((5-(5-(anthracen-2-yl)thiophen-2-yl)-1,3-phenylene)bis(thiophene-5,2-diyl))bis(9,10- dihydro-9,10-ethanoanthracene-11,12-diol) (9)
4.2.8. 2,2′-((5-(5-(anthracen-2-yl)thiophen-2-yl)-1,3-phenylene)bis(thiophene-5,2-diyl))bis(9,10-dihydro-9,10-ethanoanthracene-11,12-dione) (TAT(DK)2)
4.3. Thin-Film Characterization
4.4. Device Fabrication and Characterization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, F.; Wang, C.; Zhan, X. Morphology control in organic solar cells. Adv. Energy Mater. 2018, 8, 1703147. [Google Scholar] [CrossRef]
- McDowell, C.; Abdelsamie, M.; Toney, M.F.; Bazan, G.C. Solvent Additives: Key Morphology-Directing Agents for Solution-Processed Organic Solar Cells. Adv. Mater. 2018, 30, 1707114. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, H.; Figueira, F.; Pereira, L.; Mendes, A.; Viana, J.C.; Bernardo, G. Recent developments in the optimization of the bulk heterojunction morphology of polymer: Fullerene solar cells. Materials 2018, 11, 2560. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, C.; Sin, D.H.; Park, J.H.; Cho, K. Recent advances in morphology optimization for organic photovoltaics. Adv. Mater. 2018, 30, 1800453. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, F.; Cao, Y. Layer-by-layer assembly of multilayer thin films for organic optoelectronic devices. Small Methods 2017, 1, 1700264. [Google Scholar] [CrossRef]
- Zuniga, C.A.; Barlow, S.; Marder, S.R. Approaches to solution-processed multilayer organic light-emitting diodes based on cross-linking. Chem. Mater. 2011, 23, 658–681. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Zhang, G.; Zhang, X.; Zhang, D. The effects of side chains on the charge mobilities and functionalities of semiconducting conjugated polymers beyond solubilities. Adv. Mater. 2019, 31, 1903104. [Google Scholar] [CrossRef]
- Huang, W.; Chandrasekaran, N.; Prasad, S.K.K.; Gann, E.; Thomsen, L.; Kabra, D.; Hodgkiss, J.M.; Cheng, Y.-B.; McNeill, C.R. Impact of fullerene mixing behavior on the microstructure, photophysics, and device performance of polymer/fullerene solar cells. ACS Appl. Mater. Interfaces 2016, 8, 29608–29618. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liu, F.; Fu, H. High Performance quinacridone-based polymers in film transistors and photovoltaics: Effects of vinylene linkage on crystallinity and morphology. Polym. Chem. 2015, 6, 3283–3289. [Google Scholar] [CrossRef]
- Virkar, A.A.; Mannsfeld, S.; Bao, Z.; Stingelin, N. Organic semiconductor growth and morphology considerations for organic thin-flm transistors. Adv. Mater. 2010, 22, 3857–3875. [Google Scholar] [CrossRef]
- Duan, S.; Gao, X.; Wang, Y.; Yang, F.; Chen, M.; Zhang, X.; Ren, X.; Hu, W. Scalable fabrication of highly crystalline organic semiconductor thin film by channel-restricted screen printing toward the low-cost fabrication of high-performance transistor arrays. Adv. Mater. 2019, 31, 1807975. [Google Scholar] [CrossRef] [PubMed]
- Riera-Galindo, S.; Tamayo, A.; Mas-Torrent, M. Role of polymorphism and thin-film morphology in organic semiconductors processed by solution shearing. ACS Omega 2018, 3, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Shaw, L.; Bao, Z.; Mannsfeld, S.C.B. Morphology control strategies for solution-processed organic semiconductor thin films. Energy Environ. Sci. 2014, 7, 2145–2159. [Google Scholar] [CrossRef]
- Shao, W.; Dong, H.; Jiang, L.; Hu, W. Morphology control for high performance organic thin film transistors. Chem. Sci. 2011, 2, 590–600. [Google Scholar] [CrossRef]
- Kawajiri, K.; Kawanoue, T.; Yamato, M.; Terai, K.; Yamashita, M.; Furukawa, M.; Aratani, N.; Suzuki, M.; Nakayama, K.; Yamada, H. Fullerene-based n-type materials that can be processed by a photoprecursor approach for photovoltaic applications. ECS J. Solid State Sci. Technol. 2017, 6, M3068. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamaguchi, Y.; Takahashi, K.; Takahira, K.; Koganezawa, T.; Masuo, S.; Nakayama, K.; Yamada, H. Photoprecursor approach enables preparation of well-performing bulk-heterojunction layers comprising a highly aggregating molecular semiconductor. ACS Appl. Mater. Interfaces 2016, 8, 8644–8651. [Google Scholar] [CrossRef]
- Quinton, C.; Suzuki, M.; Kaneshige, Y.; Tatenaka, Y.; Katagiri, C.; Yamaguchi, Y.; Kuzuhara, D.; Aratani, N.; Nakayama, K.; Yamada, H. Evaluation of semiconducting molecular thin films solution-processed via the photoprecursor approach: The case of hexyl-substituted thienoanthracenes. J. Mater. Chem. C 2015, 3, 5995–6005. [Google Scholar] [CrossRef]
- Suzuki, M.; Aotake, T.; Yamaguchi, Y.; Noguchi, N.; Nakano, H.; Nakayama, K.; Yamada, H. Synthesis and photoreactivity of α-diketone-type precursors of acenes and their use in organic-device fabrication. J. Photochem. Photobiol. C 2014, 18, 50–70. [Google Scholar] [CrossRef]
- Suzuki, M.; Terai, K.; Quinton, C.; Hayashi, H.; Aratani, N.; Yamada, H. Open-circuit-voltage shift of over 0.5 V in organic photovoltaic cells induced by a minor structural difference in alkyl substituents. Chem. Sci. 2020, 11, 1825–1831. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamaguchi, Y.; Uchinaga, K.; Takahira, K.; Quinton, C.; Yamamoto, S.; Nagami, N.; Furukawa, M.; Nakayama, K.; Yamada, H. A photochemical layer-by-layer solution process for preparing organic semiconducting thin films having the right material at the right place. Chem. Sci. 2018, 9, 6614–6621. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Suzuki, M.; Motoyama, T.; Sugii, S.; Katagiri, C.; Takahira, K.; Ikeda, S.; Yamada, H.; Nakayama, K. Photoprecursor approach as an effective means for preparing multilayer organic semiconducting thin films by solution processes. Sci. Rep. 2014, 4, 7151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, X. Layer-by-layer processed organic solar cells. Adv. Energy Mater. 2016, 6, 1600414. [Google Scholar] [CrossRef]
- Hiramoto, M.; Kitada, K.; Iketaki, K.; Kaji, T. Near infrared light driven organic p-i-n solar cells incorporating phthalocyanine J.-aggregate. Appl. Phys. Lett. 2011, 98, 023302. [Google Scholar] [CrossRef]
- Hiramoto, M.; Suemori, K.; Matsumura, Y.; Miyata, T.; Yokoyama, M. P-I-N junction organic solar cells. Mol. Cryst. Liq. Cryst. 2006, 455, 267–275. [Google Scholar] [CrossRef]
- Maennig, B.; Drechsel, J.; Gebeyehu, D.; Simon, P.; Kozlowski, F.; Werner, A.; Li, F.; Grundmann, S.; Sonntag, S.; Koch, M.; et al. Organic p-i-n solar cells. Appl. Phys. A 2004, 79, 1–14. [Google Scholar] [CrossRef]
- Nakamura, T.; Shioya, N.; Hasegawa, T.; Murata, Y.; Murdey, R.; Wakamiya, A. Phthalimide-based transparent electron-transport materials with oriented-amorphous structures: Preparation from solution-processed precursor films. ChemPlusChem 2019, 84, 1396–1404. [Google Scholar] [CrossRef]
- Feng, J.-Y.; Lai, K.-W.; Shiue, Y.-S.; Singh, A.; Kumar, C.P.; Li, C.-T.; Wu, W.-T.; Lin, J.T.; Chu, C.-W.; Chang, C.-C.; et al. Cost-effective dopant-free star-shaped oligo-aryl amines for high performance perovskite solar cells. J. Mater. Chem. A 2019, 7, 14209–14221. [Google Scholar] [CrossRef]
- García-Benito, I.; Zimmermann, I.; Urieta-Mora, J.; Aragó, J.; Calbo, J.; Perles, J.; Serrano, A.; Molina-Ontoria, A.; Ortí, E.; Martín, N.; et al. Heteroatom effect on star-shaped hole-tansporting materials for perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1801734. [Google Scholar] [CrossRef]
- Ge, Z.; Hayakawa, T.; Ando, S.; Ueda, M.; Akiike, T.; Miyamoto, H.; Kajita, T.; Kakimoto, M. Solution-processible bipolar triphenylamine-benzimidazole derivatives for highly efficient single-layer organic light-emitting diodes. Chem. Mater. 2008, 20, 2532–2537. [Google Scholar] [CrossRef]
- Thelakkat, M. Star-shaped, dendrimeric and polymeric triarylamines as photoconductors and hole transport materials for electro-optical applications. Macromol. Mater. Eng. 2002, 287, 442–461. [Google Scholar] [CrossRef]
- Aotake, T.; Tanimoto, H.; Hotta, H.; Kuzuhara, D.; Okujima, T.; Uno, H.; Yamada, H. In situ preparation of highly fluorescent pyrene-dyes from non-luminous precursors upon photoirradiation. Chem. Commun. 2013, 49, 3661–3663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aotake, T.; Suzuki, M.; Tahara, K.; Kuzuhara, D.; Aratani, N.; Tamai, N.; Yamada, H. An optically and thermally switchable electronic structure based on an anthracene–BODIPY conjugate. Chem. Eur. J. 2015, 21, 4966–4974. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, S.; Saeki, H.; Tanaka, K.; Murai, Y.; Kuzuhara, D.; Misaki, M.; Aratani, N.; Masuo, S.; Ueda, Y.; Yamada, H. Synthesis and optical reactivity of 6,13-α-diketoprecursors of 2,3,9,10-tetraalkylpentacenes in solution, films and crystals. J. Mater. Chem. C 2014, 2, 986–993. [Google Scholar] [CrossRef]
- Meng, H.; Sun, F.; Goldfinger, M.B.; Jaycox, G.D.; Li, Z.; Marshall, W.J.; Blackman, G.S. High-performance, stable organic thin-film field-effect transistors based on bis-5′-alkylthiophen-2′-yl-2,6-anthracene Semiconductors. J. Am. Chem. Soc. 2005, 127, 2406–2407. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A.; Patrick, B.O.; MacLachlan, M.J.; Wolf, M.O. Conjugated thiophene-containing oligoacenes through photocyclization: Bent acenedithiophenes and a thiahelicene. J. Org. Chem. 2009, 74, 4918–4926. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz; Swayamprabha, S.S.; Nagar, M.R.; Yadav, R.A.K.; Gull, S.; Dubey, D.K.; Jou, J.-H. Hole-transporting materials for organic light-emitting diodes: An overview. J. Mater. Chem. C 2019, 7, 7144–7158. [Google Scholar] [CrossRef]
- Jhulki, S.; Moorthy, J.N. Small molecular hole-transporting materials (HTMs) in organic light-emitting diodes (OLEDs): Structural diversity and classification. J. Mater. Chem. C 2018, 6, 8280–8325. [Google Scholar] [CrossRef]
- Teh, C.H.; Daik, R.; Lim, E.L.; Yap, C.C.; Ibrahim, M.A.; Ludin, N.A.; Sopian, K.; Teridi, M.A.M. A review of organic small molecule-based hole-transporting materials for meso-structured organic–inorganic perovskite solar cells. J. Mater. Chem. A 2016, 4, 15788–15822. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.-C.; Hong, Z.; Gao, J.; Yang, Y.; Zhou, H.; Dou, L.; Li, G.; Yang, Y. Solution-processed small-molecule solar cells: Breaking the 10% power conversion efficiency. Sci. Rep. 2013, 3, 3356. [Google Scholar] [CrossRef]
- Li, C.-Z.; Chueh, C.-C.; Yip, H.-L.; O’Malley, K.M.; Chen, W.-C.; Jen, A.K.-Y. Effective interfacial layer to enhance efficiency of polymer solar cells via solution-processed fullerene-surfactants. J. Mater. Chem. 2012, 22, 8574–8578. [Google Scholar] [CrossRef]
- Menke, S.M.; Ran, N.A.; Bazan, G.C.; Friend, R.H. Understanding energy loss in organic solar cells: Toward a new efficiency regime. Joule 2018, 2, 25–35. [Google Scholar] [CrossRef]
- Hirade, M.; Adachi, C. Small molecular organic photovoltaic cells with exciton blocking layer at anode interface for improved device performance. Appl. Phys. Lett. 2011, 99, 153302. [Google Scholar] [CrossRef]
- Zeng, S.; Yin, L.; Ji, C.; Jiang, X.; Li, K.; Li, Y.; Wang, Y. D–π–A–π–D type benzothiadiazole–triphenylamine based small molecules containing cyano on the π-bridge for solution-processed organic solar cells with high open-circuit voltage. Chem. Commun. 2012, 48, 10627–10629. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.R.J.; Huang, T.-H.; Lin, J.T.; Pu, S.-C.; Cheng, Y.-M.; Hsieh, C.-C.; Tai, C.P. Donor–acceptor interactions in red-emitting thienylbenzene-branched dendrimers with benzothiadiazole core. Chem. Eur. J. 2008, 14, 11231–11241. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]


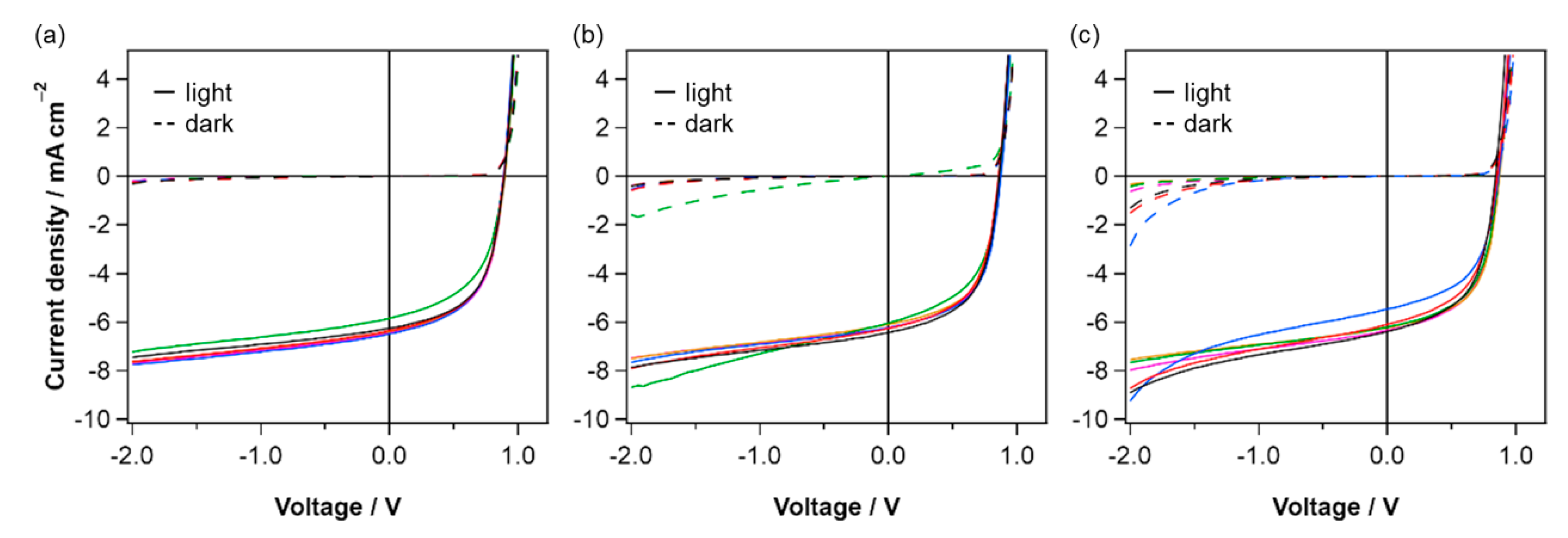
| p-Sublayer Material | JSC/mA cm−2 | VOC/V | FF/% | PCE/% | Jdark (−2 V)/mA cm−2 |
|---|---|---|---|---|---|
| TAT | 6.44 (6.30 ± 0.23) | 0.899 (0.894 ± 0.005) | 55.9 (55.2 ± 1.4) | 3.24 (3.11 ± 0.19) | 0.18 (0.15 ± 0.02) |
| DTA | 6.23 (6.21 ± 0.14) | 0.877 (0.869 ± 0.007) | 57.3 (56.1 ± 1.7) | 3.13 (3.03 ± 0.14) | 0.53 (0.43 ± 0.07) |
| PhBADT | 6.23 (6.12 ± 0.34) | 0.876 (0.863 ± 0.011) | 57.2 (55.3 ± 1.5) | 3.12 (2.93 ± 0.21) | 0.54 (0.46 ± 0.11) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, A.; Nakauchi, A.; Shimizu, Y.; Terai, K.; Sugii, S.; Hayashi, H.; Aratani, N.; Suzuki, M.; Yamada, H. A Windmill-Shaped Molecule with Anthryl Blades to Form Smooth Hole-Transport Layers via a Photoprecursor Approach. Materials 2020, 13, 2316. https://doi.org/10.3390/ma13102316
Maeda A, Nakauchi A, Shimizu Y, Terai K, Sugii S, Hayashi H, Aratani N, Suzuki M, Yamada H. A Windmill-Shaped Molecule with Anthryl Blades to Form Smooth Hole-Transport Layers via a Photoprecursor Approach. Materials. 2020; 13(10):2316. https://doi.org/10.3390/ma13102316
Chicago/Turabian StyleMaeda, Akihiro, Aki Nakauchi, Yusuke Shimizu, Kengo Terai, Shuhei Sugii, Hironobu Hayashi, Naoki Aratani, Mitsuharu Suzuki, and Hiroko Yamada. 2020. "A Windmill-Shaped Molecule with Anthryl Blades to Form Smooth Hole-Transport Layers via a Photoprecursor Approach" Materials 13, no. 10: 2316. https://doi.org/10.3390/ma13102316
APA StyleMaeda, A., Nakauchi, A., Shimizu, Y., Terai, K., Sugii, S., Hayashi, H., Aratani, N., Suzuki, M., & Yamada, H. (2020). A Windmill-Shaped Molecule with Anthryl Blades to Form Smooth Hole-Transport Layers via a Photoprecursor Approach. Materials, 13(10), 2316. https://doi.org/10.3390/ma13102316




