Tunable Crystalline Phases in UV-Curable PEG-Grafted Ladder-Structured Silsesquioxane/Polyimide Composites
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Allyl-Terminated PEG (MPEG-Allyl)
2.3. Synthesis of Trimethoxysilane-Terminated PEG (MPEGTMS)
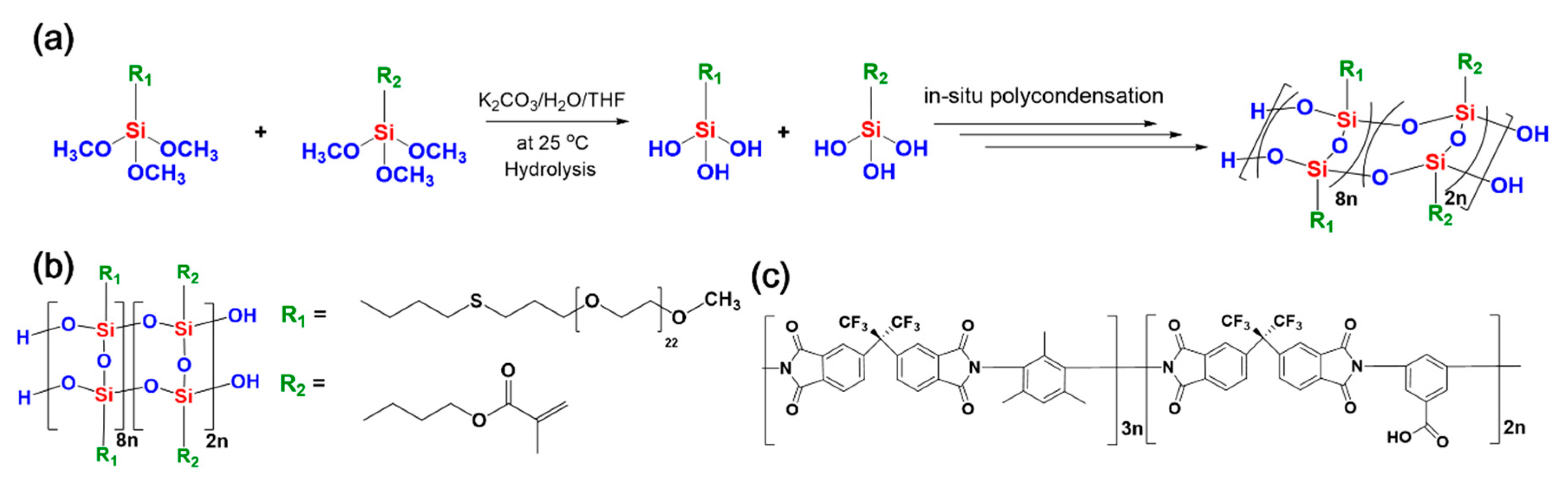
2.4. Synthesis of LPEOMASQ82
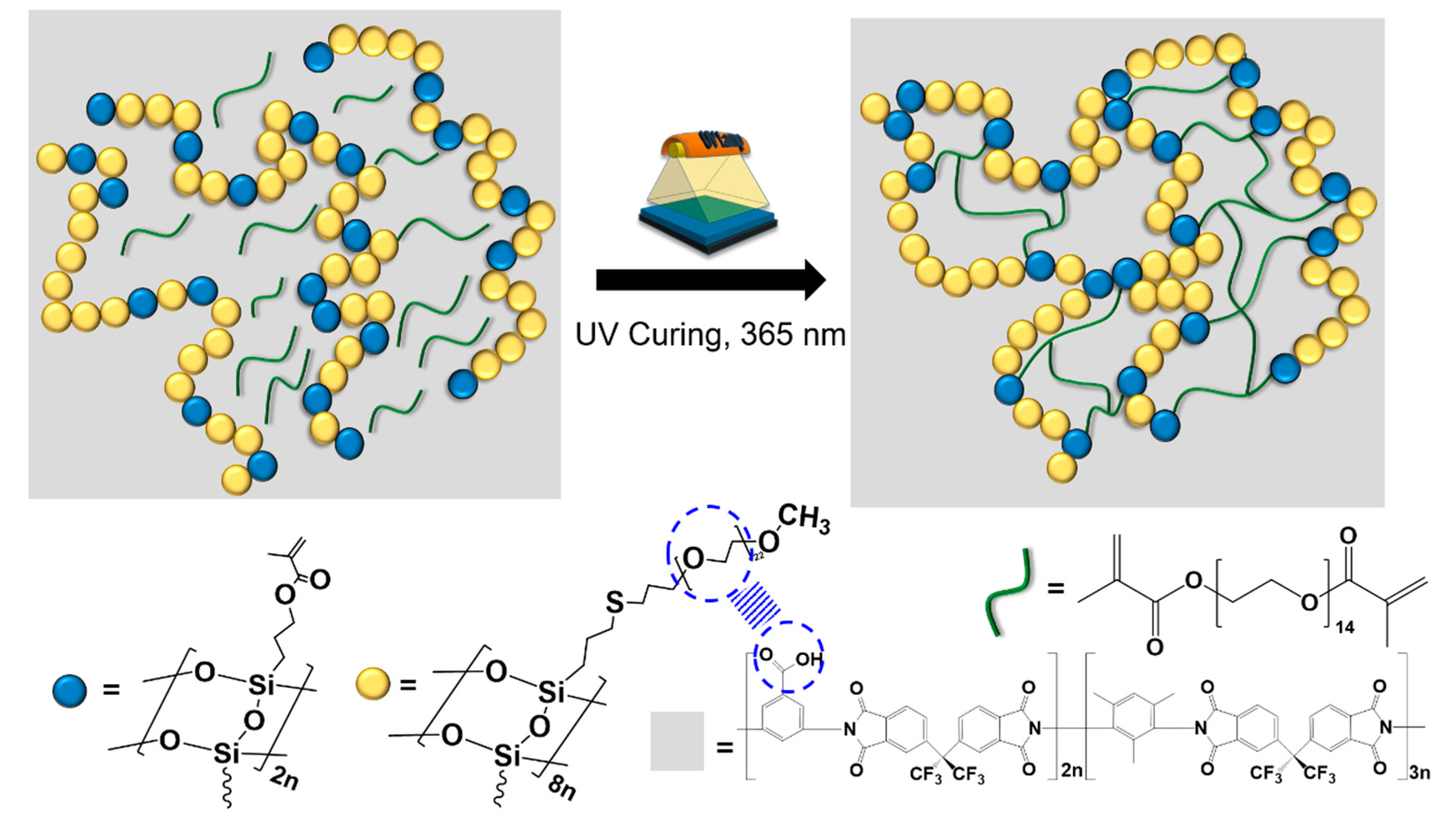
2.5. Fabrication of Composites
2.6. Characterization
3. Results and Discussion
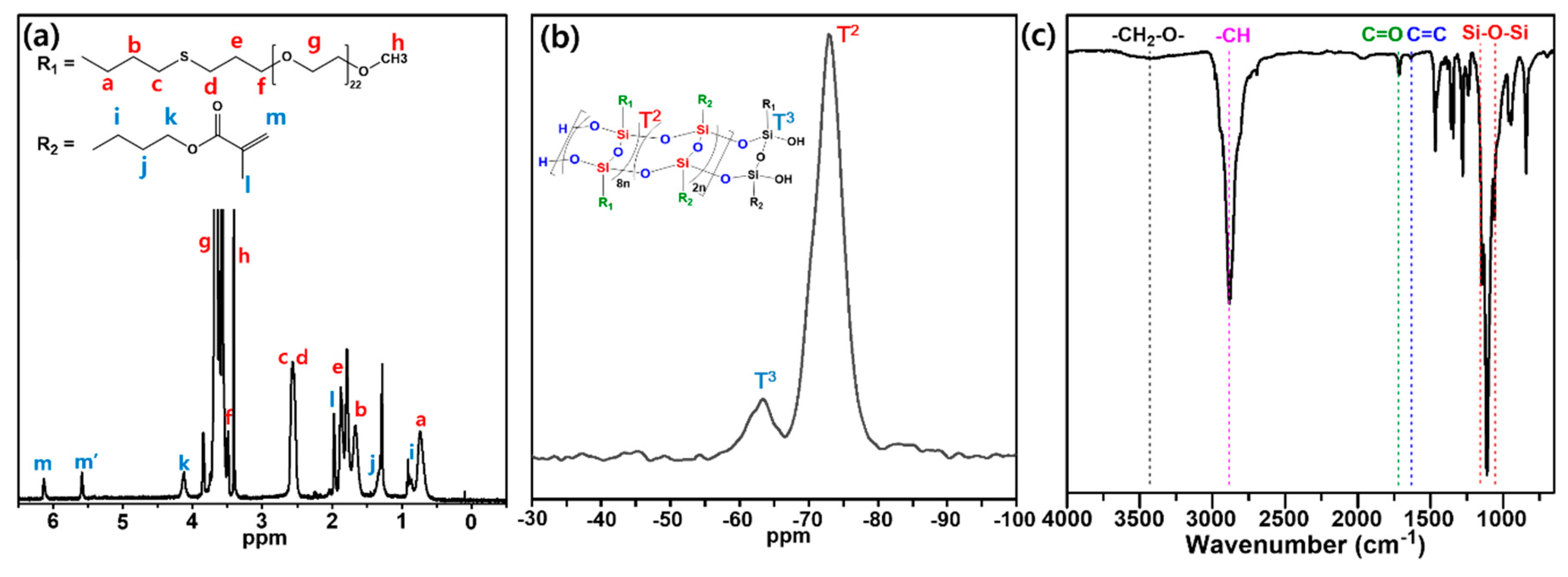
3.1. Synthesis of LPEGMASQ82 and Fabrication of Hybrid Composites
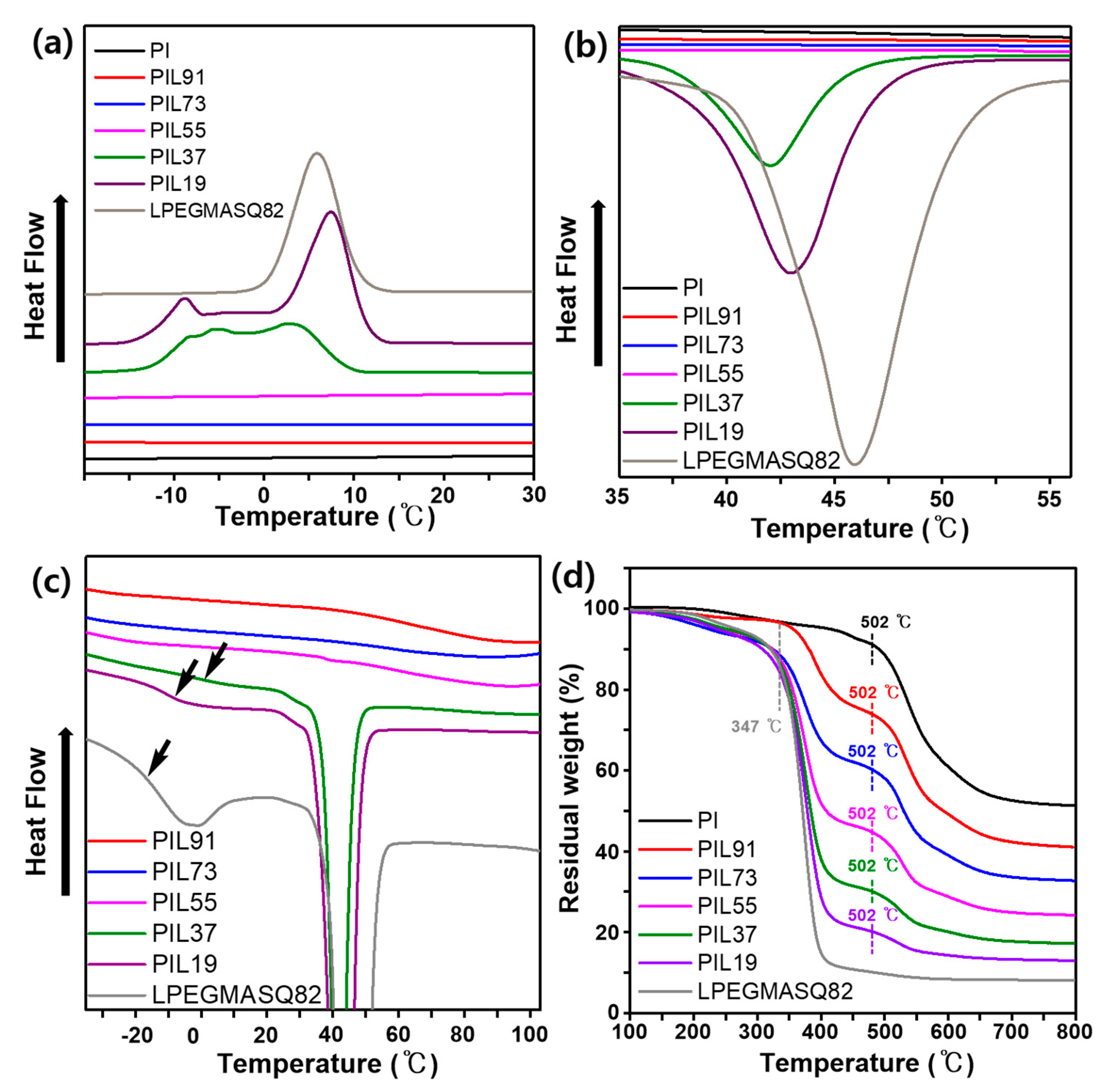
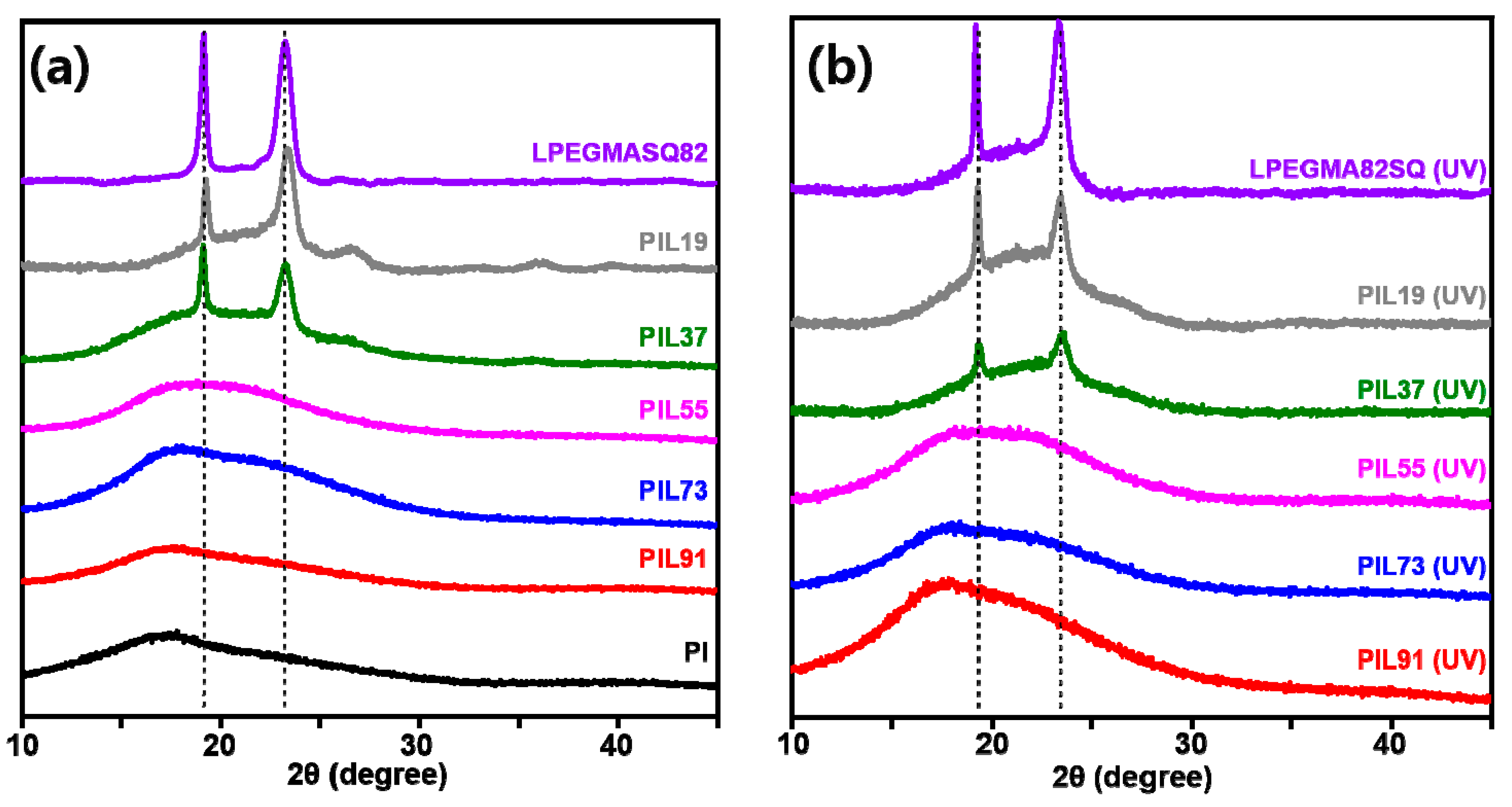
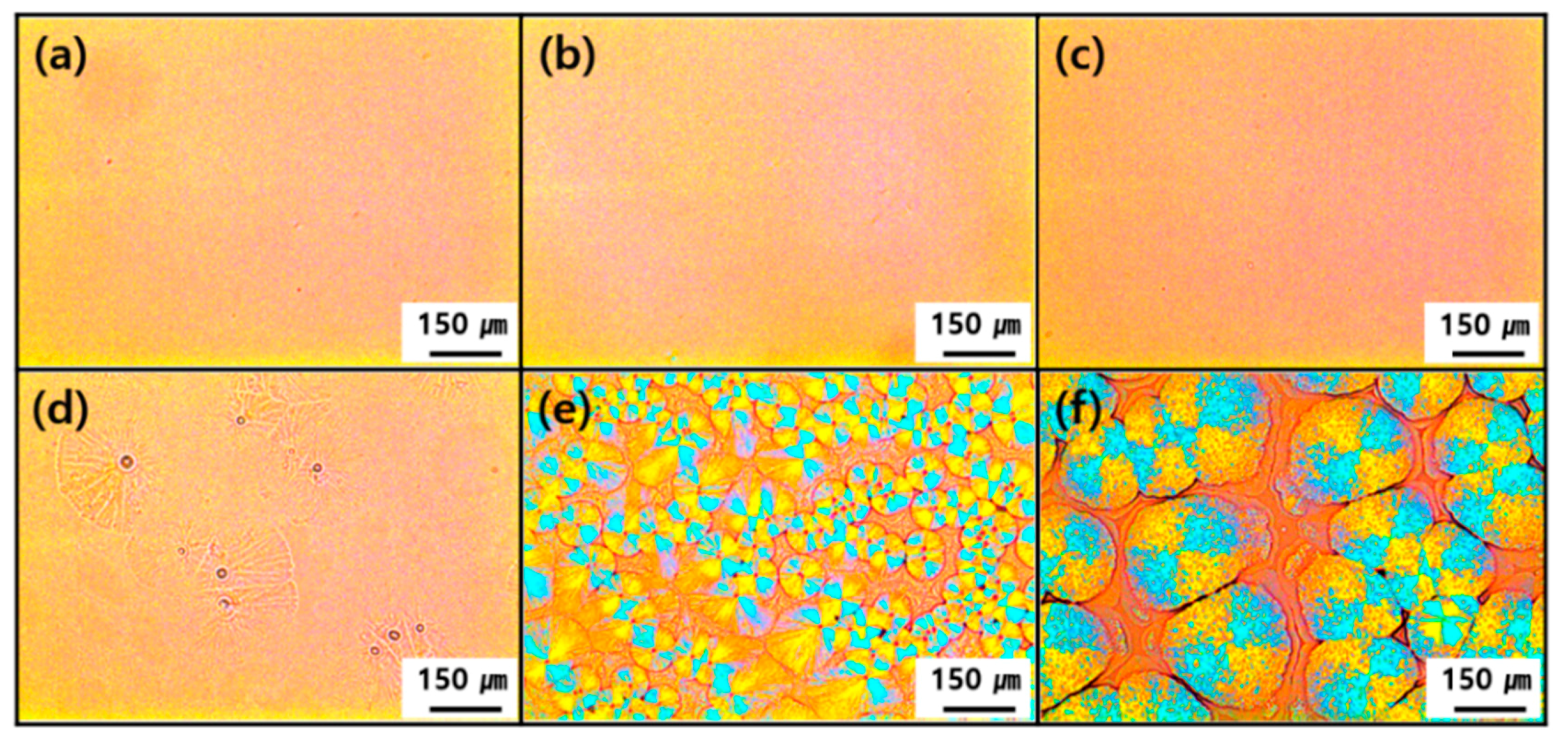
3.2. Thermal and Structural Characterization
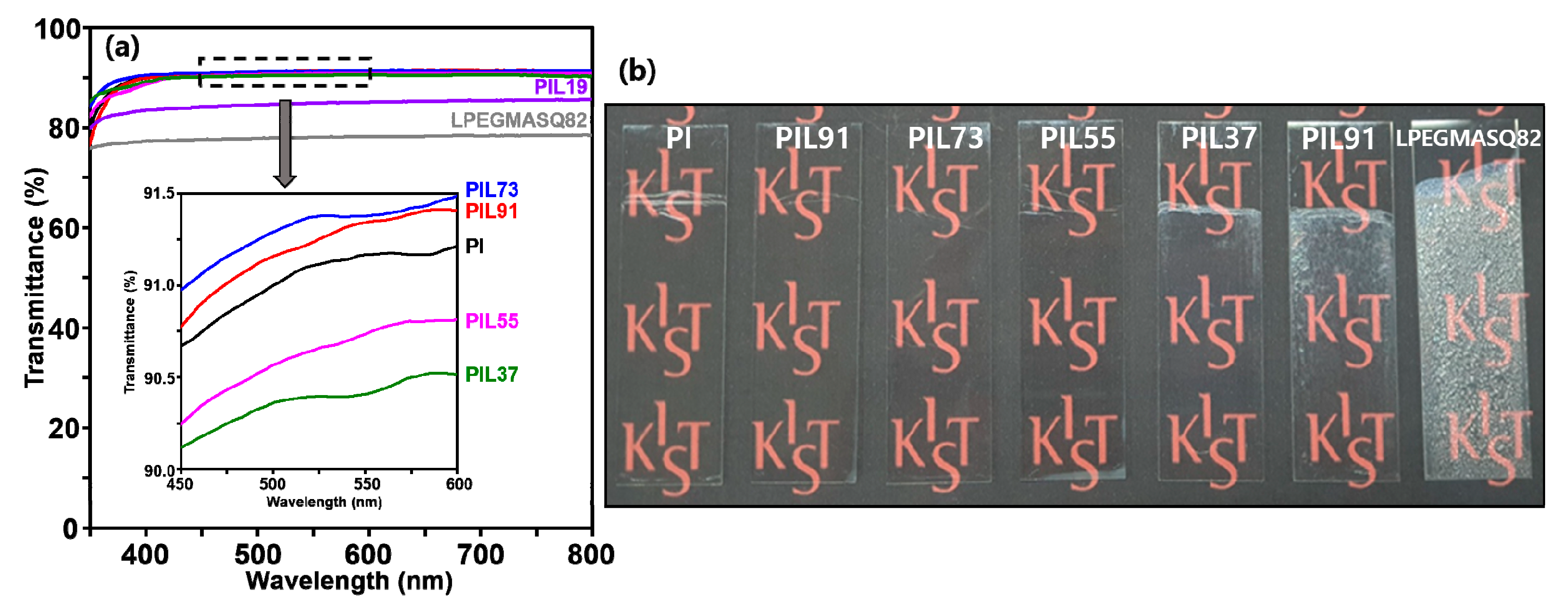
3.3. Optical Properties of Hybrid Composites
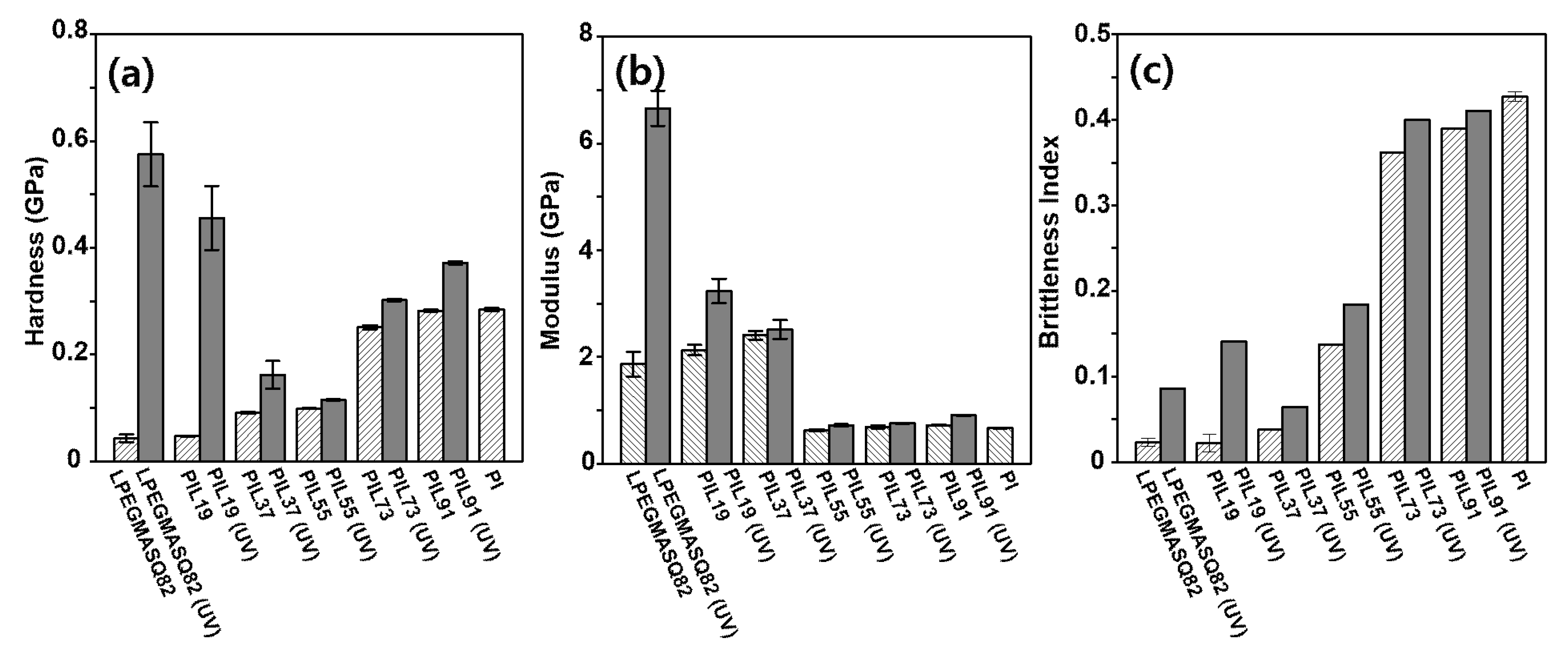
3.4. Surface Mechanical and Wetting Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Gao, S.; You, Q.; Zou, L.; Chen, J.; Liu, J.; Liu, X. Polyimide film with low thermal expansion and high transparency by self-enhancement of polyimide/SiC nanofibers net. RSC Adv. 2018, 8, 19034–19040. [Google Scholar] [CrossRef]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. A review of the latest development of polyimide based membranes for CO2 separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Khanna, D.; Mueller, W. New high temperature stable positive photoresists based on hydroxy polyimides and polyamides containing the hexafluoroisopropylidene (6-f) linking group. Polym. Eng. Sci. 1989, 29, 954–959. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van der Bruggen, B. Polyimides in membrane gas separation: monomer’s molecular design and structural engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films: Heterocyclic structures and nanocomposites. Compos. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, W.C. Synthesis and optical properties of polyimide-silica hybrid thin films. Chem. Mater. 2002, 14, 4242–4248. [Google Scholar] [CrossRef]
- Simpson, J.; Clair, A.K. Fundamental insight on developing low dielectric constant polyimides. Thin Solid Films 1997, 308, 480–485. [Google Scholar] [CrossRef]
- Yi, L.; Huang, W.; Yan, D. Polyimides with side groups: Synthesis and effects of side groups on their properties. J. Polym. Sci. A Polym. Chem. 2017, 55, 533–559. [Google Scholar] [CrossRef]
- Wilson, A. Polyimide insulators for multilevel interconnections. Thin Solid Films 1981, 83, 145–163. [Google Scholar] [CrossRef]
- Ha, C.S.; Park, H.D.; Frank, C.W. Interfacial interaction in polyimide/silica hybrid composites by fluorescence spectroscopy. Chem. Mater. 2000, 12, 839–844. [Google Scholar] [CrossRef]
- Tsai, M.H.; Huang, Y.C.; Tseng, I.H.; Yu, H.P.; Lin, Y.K.; Huang, S.L. Thermal and mechanical properties of polyimide/nano-silica hybrid films. Thin Solid Films 2011, 519, 5238–5242. [Google Scholar] [CrossRef]
- Joly, C.; Smaihi, M.; Porcar, L.; Noble, R.D. Polyimide—Silica composite materials: How does silica influence their microstructure and gas permeation properties? Chem. Mater. 1999, 11, 2331–2338. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Rodrigue, D.; Kaliaguine, S. Effect of macrovoids in nano-silica/polyimide mixed matrix membranes for high flux CO2/CH4 gas separation. RSC Adv. 2014, 4, 12235–12244. [Google Scholar] [CrossRef]
- Wang, S.; Ahmad, Z.; Mark, J.E. Polyimide-silica hybrid materials modified by incorporation of an organically substituted alkoxysilane. Chem. Mater. 1994, 6, 943–946. [Google Scholar] [CrossRef]
- Chen, Y.; Iroh, J.O. Synthesis and characterization of polyimide/silica hybrid composites. Chem. Mater. 1999, 11, 1218–1222. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral oligomeric silsesquioxane (POSS)-containing polymer nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef]
- Lee, A.S.; Choi, S.S.; Lee, H.S.; Baek, K.Y.; Hwang, S.S. A new, higher yielding synthetic route towards dodecaphenyl cage silsesquioxanes: Synthesis and mechanistic insights. Dalton Trans. 2012, 41, 10585–10588. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional polyimide/polyhedral oligomeric silsesquioxane nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, H. Pentacyclic laddersiloxane. J. Am. Chem. Soc. 2002, 124, 1574–1575. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, A.S.; Hwang, S.S.; Baek, K.Y. Structural control of fully condensed polysilsesquioxanes: Ladderlike vs cage structured polyphenylsilsesquioxanes. Macromolecules 2015, 48, 6063–6070. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, A.S.; Lee, J.C.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Hybrid ionogel electrolytes for high temperature lithium batteries. J. Mater. Chem. A Mater. 2015, 3, 2226–2233. [Google Scholar] [CrossRef]
- Lee, A.S.; Lee, J.H.; Lee, J.C.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Novel polysilsesquioxane hybrid polymer electrolytes for lithium ion batteries. J. Mater. Chem. A 2014, 2, 1277–1283. [Google Scholar]
- Na, W.J.; Lee, A.S.; Lee, J.H.; Hwang, S.S.; Hong, S.M.; Kim, E.K.; Koo, C.M. Lithium ion capacitors fabricated with polyethylene oxide-functionalized polysilsesquioxane hybrid ionogel electrolytes. Electrochim. Acta 2016, 188, 582–588. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, A.S.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Hybrid ionogels derived from polycationic polysilsesquioxanes for lithium ion batteries. Polymer 2017, 117, 160–166. [Google Scholar] [CrossRef]
- Lee, A.S.; Lee, J.H.; Hong, S.M.; Lee, J.C.; Hwang, S.S.; Koo, C.M. Ion conduction behaviour in chemically crosslinked hybrid ionogels: Effect of free-dangling oligoethyleneoxides. RSC Adv. 2015, 5, 94241–94247. [Google Scholar] [CrossRef]
- Kang, W.R.; Lee, A.S.; Park, S.; Park, S.H.; Baek, K.Y.; Lee, K.B.; Lee, S.H.; Lee, J.H.; Hwang, S.S.; Lee, J.S. Free-standing, polysilsesquioxane-based inorganic/organic hybrid membranes for gas separations. J. Memb. Sci. 2015, 475, 384–394. [Google Scholar] [CrossRef]
- Park, S.; Lee, A.S.; Do, Y.S.; Kim, J.F.; Hwang, S.S.; Lee, Y.M.; Lee, J.H.; Lee, J.S. Side-chain engineering of ladder-structured polysilsesquioxane membranes for gas separations. J. Memb. Sci. 2016, 516, 202–214. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, S.S.; Baek, K.Y.; Hong, S.M.; Lee, E.C.; Lee, J.C.; Hwang, S.S. Synthesis and structure characterization of ladder-like polymethylsilsesquioxane (PMSQ) by isolation of stereoisomer. Eur. Polym. J. 2012, 48, 1073–1081. [Google Scholar] [CrossRef]
- Lee, A.S.; Choi, S.S.; Song, S.J.; Baek, K.Y.; Hwang, S.S. Cationically photopolymerizable epoxy-functionalized thermoplastic polysilsesquioxanes: Synthesis and properties. RSC Adv. 2014, 4, 56532–56538. [Google Scholar] [CrossRef]
- Lee, A.S.; Jo, Y.Y.; Jeon, H.Y.; Choi, S.S.; Baek, K.Y.; Hwang, S.S. Mechanical properties of thiol-ene UV-curable thermoplastic polysilsesquioxanes. Polymer 2015, 68, 140–146. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, A.S.; Lee, H.S.; Jeon, H.Y.; Baek, K.Y.; Choi, D.H.; Hwang, S.S. Synthesis and characterization of UV-curable ladder-like polysilsesquioxane. J. Polym. Sci. A Polym. Chem. 2011, 49, 5012–5018. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, H.J.; Park, J.H.; Lee, A.S.; Hwang, S.S.; Kim, S.J.; Park, S.H.; Cho, K.Y.; Won, W.G.; Lee, J.S. Fluorine-containing polyimide/polysilsesquioxane carbon molecular sieve membranes and techno-economic evaluation thereof for C3H6/C3H8 separation. J. Memb. Sci. 2020, 598, 117660. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, H.J.; An, H.S.; Lee, A.S.; Hwang, S.S.; Lee, S.Y.; Lee, J.S. Rigid double-stranded siloxane-induced high-flux carbon molecular sieve hollow fiber membranes for CO2/CH4 separation. J. Memb. Sci. 2019, 570, 504–512. [Google Scholar] [CrossRef]
- Lee, A.S.; Choi, S.S.; Lee, H.S.; Jeon, H.Y.; Baek, K.Y.; Hwang, S.S. Synthesis and characterization of organic–inorganic hybrid block copolymers containing a fully condensed ladder-like polyphenylsilsesquioxane. J. Polym. Sci. A Polym. Chem. 2012, 50, 4563–4570. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L.; Sun, D. Nanoindentation and nanoscratch profiles of hybrid films based on (γ-methacrylpropyl) trimethoxysilane and tetraethoxysilane. Acta Mater. 2006, 54, 5469–5475. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, A.S.; Do, Y.S.; Hwang, S.S.; Lee, Y.M.; Lee, J.H.; Lee, J.S. Rational molecular design of PEOlated ladder-structured polysilsesquioxane membranes for high performance CO2 removal. Chem. Commun. 2015, 51, 15308–15311. [Google Scholar] [CrossRef]
- Marzantowicz, M.; Dygas, J.R.; Krok, F.; Nowiński, J.L.; Tomaszewska, A.; Florjańczyk, Z.; Zygadło-Monikowska, E. Crystalline phases, morphology and conductivity of PEO: LiTFSI electrolytes in the eutectic region. J. Power Sources 2006, 159, 420–430. [Google Scholar] [CrossRef]
- Li, X.; Cheng, S.; Zheng, Y.; Li, C.Y. Engineering, Morphology control in semicrystalline solid polymer electrolytes for lithium batteries. Mol. Syst. Des. Eng. 2019, 4, 793–803. [Google Scholar] [CrossRef]



| Sample | Mna | Mnb | Mwc | PDId |
|---|---|---|---|---|
| mPEG | 1,037 | 1,036 | 1,100 | 1.06 |
| Allyl-mPEG | 1,077 | 1,071 | 1,100 | 1.06 |
| mPEG-TMS | 1,276 | 1,303 | 1,400 | 1.06 |
| LPEGMASQ82 | - | 11,000 | 12,300 | 1.12 |
| Weight Ratio of Hybrid Composite Blend | |||
|---|---|---|---|
| Sample Name | LPEGMASQ82 | 6FDA-DMA:DABA(3:2) | PEGDMA |
| LPEGMASQ82 | 100 | 0 | 2.5 |
| PIL19 | 90 | 10 | 1.75 |
| PIL37 | 70 | 30 | 1.5 |
| PIL55 | 50 | 50 | 1.25 |
| PIL73 | 30 | 70 | 0.75 |
| PIL91 | 10 | 90 | 0.25 |
| PI | 0 | 100 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, R.I.; Shin, J.H.; Lee, J.S.; Lee, J.-H.; Lee, A.S.; Hwang, S.S. Tunable Crystalline Phases in UV-Curable PEG-Grafted Ladder-Structured Silsesquioxane/Polyimide Composites. Materials 2020, 13, 2295. https://doi.org/10.3390/ma13102295
Kim RI, Shin JH, Lee JS, Lee J-H, Lee AS, Hwang SS. Tunable Crystalline Phases in UV-Curable PEG-Grafted Ladder-Structured Silsesquioxane/Polyimide Composites. Materials. 2020; 13(10):2295. https://doi.org/10.3390/ma13102295
Chicago/Turabian StyleKim, Ryung Il, Ju Ho Shin, Jong Suk Lee, Jung-Hyun Lee, Albert S. Lee, and Seung Sang Hwang. 2020. "Tunable Crystalline Phases in UV-Curable PEG-Grafted Ladder-Structured Silsesquioxane/Polyimide Composites" Materials 13, no. 10: 2295. https://doi.org/10.3390/ma13102295
APA StyleKim, R. I., Shin, J. H., Lee, J. S., Lee, J.-H., Lee, A. S., & Hwang, S. S. (2020). Tunable Crystalline Phases in UV-Curable PEG-Grafted Ladder-Structured Silsesquioxane/Polyimide Composites. Materials, 13(10), 2295. https://doi.org/10.3390/ma13102295





