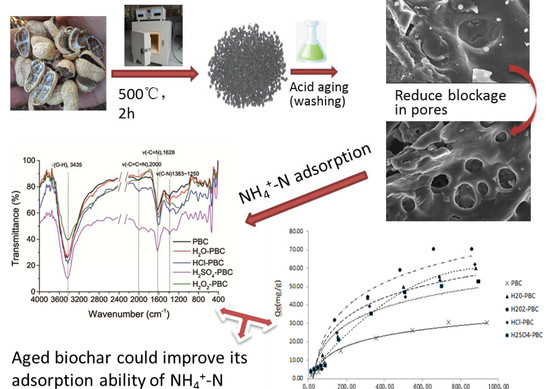
Characterization of Acid-Aged Biochar and Its Ammonium Adsorption in an Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Biochar
2.2. Physical-Chemical Characterization Analysis of the Biochar
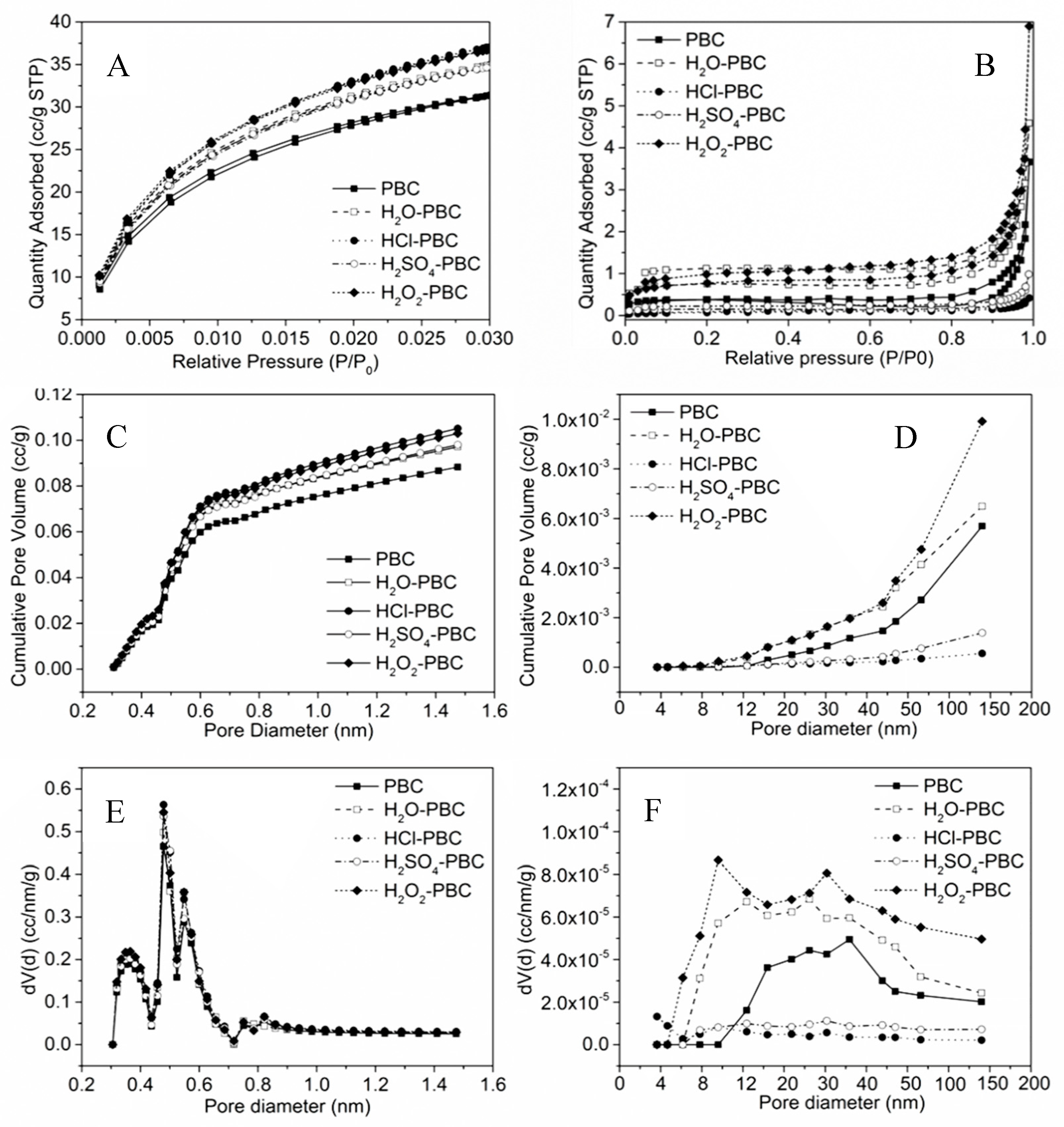
2.2.1. Morphological, Surface, and Pore Structure Analysis
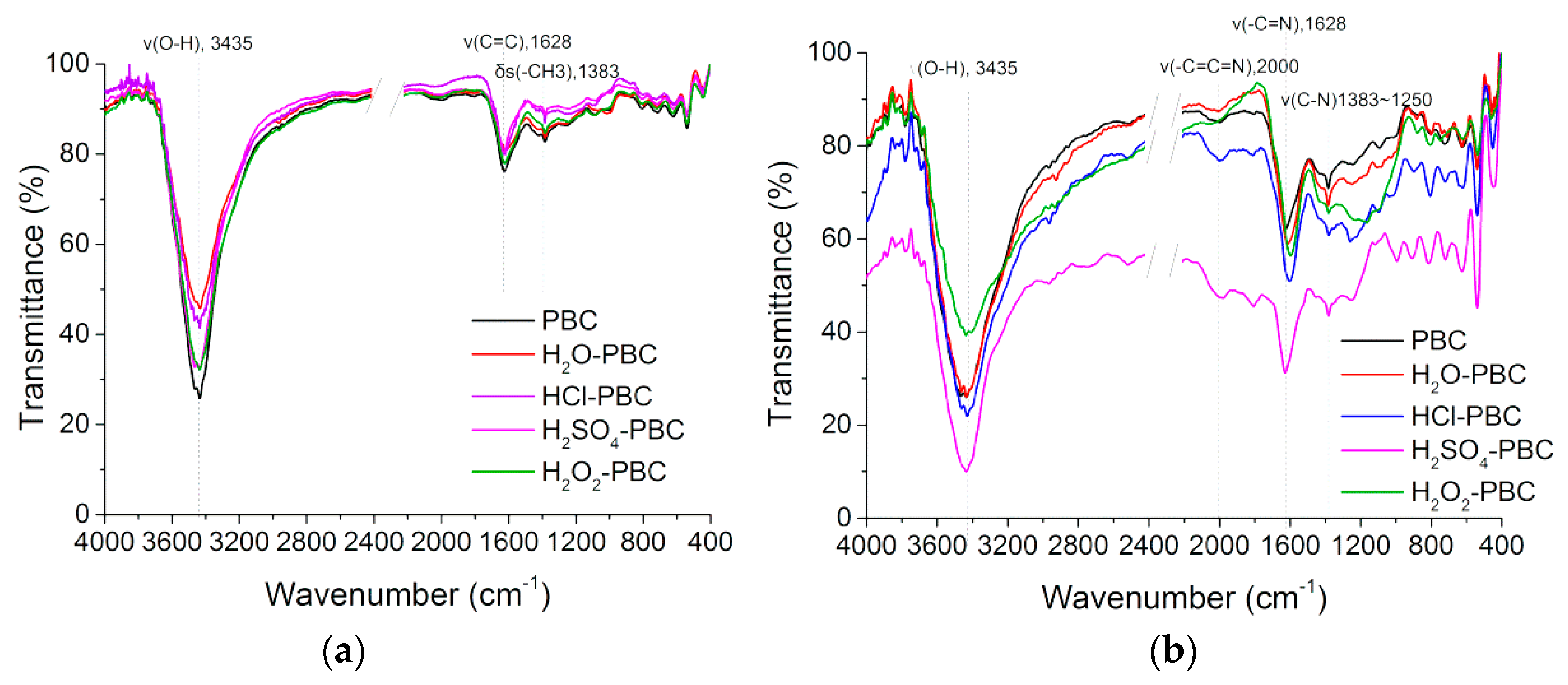
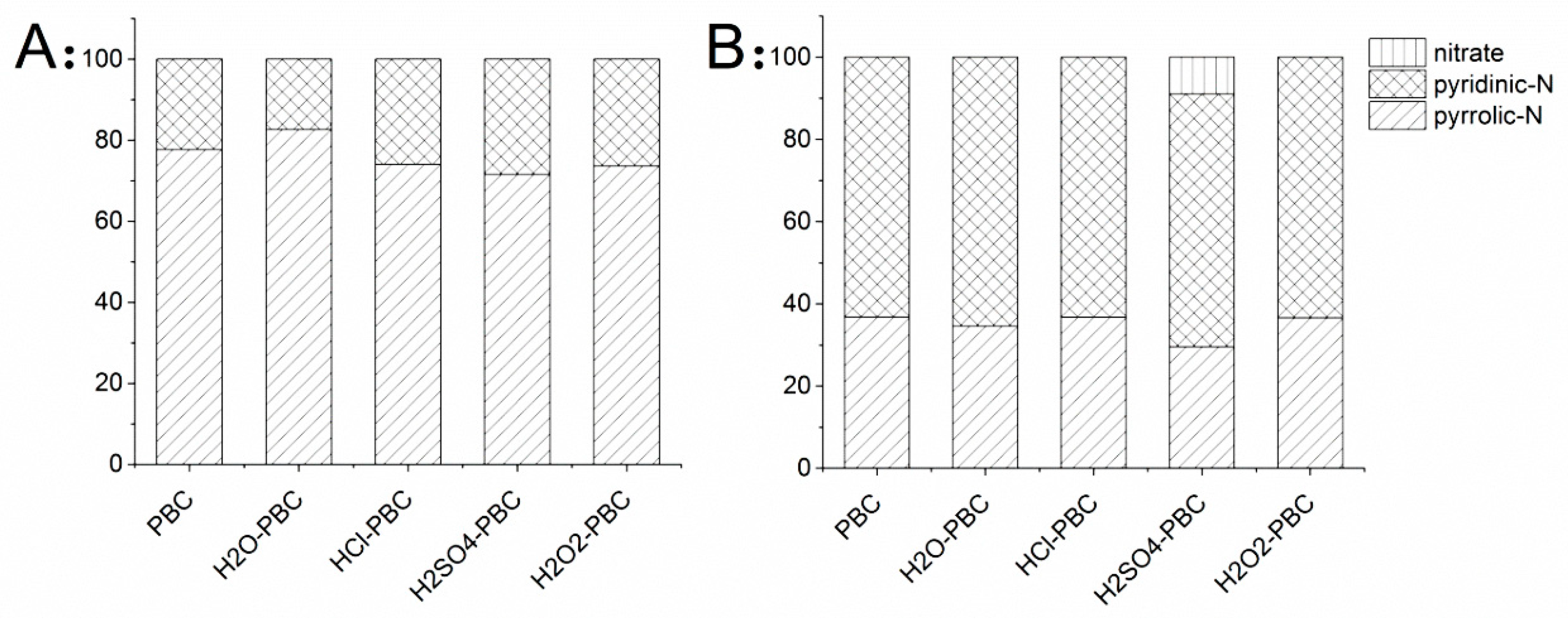
2.2.2. Functional Groups’ Analysis
2.3. Batch Sorption Experiments
2.4. Modeling the Adsorption Data
2.4.1. Adsorption Isotherms
2.4.2. Adsorption Kinetics
3. Results and Discussion
3.1. Characterization of Biochar
3.1.1. Specific Surface Area, Pore Structure, and Elemental Content Analysis
3.1.2. Surface Functional Group Analysis and X-ray Crystal Structure Analysis
3.2. Adsorption Isotherm
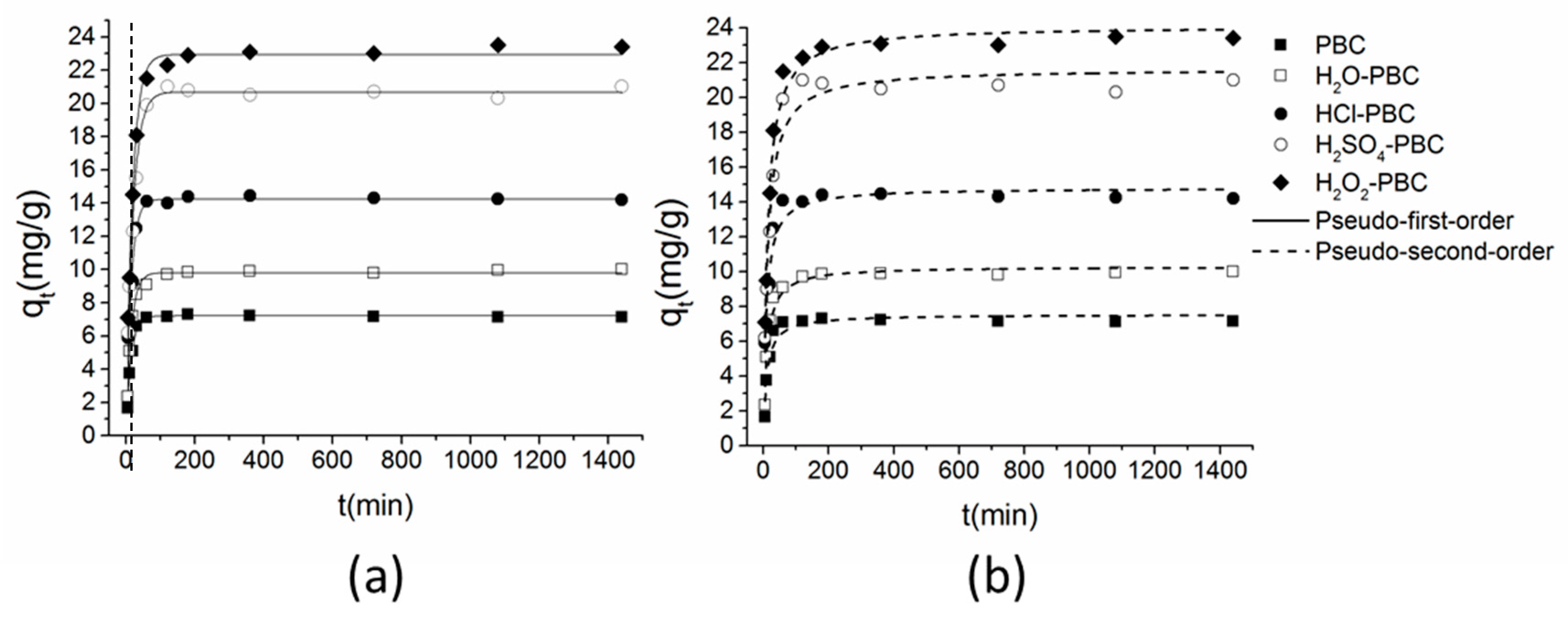
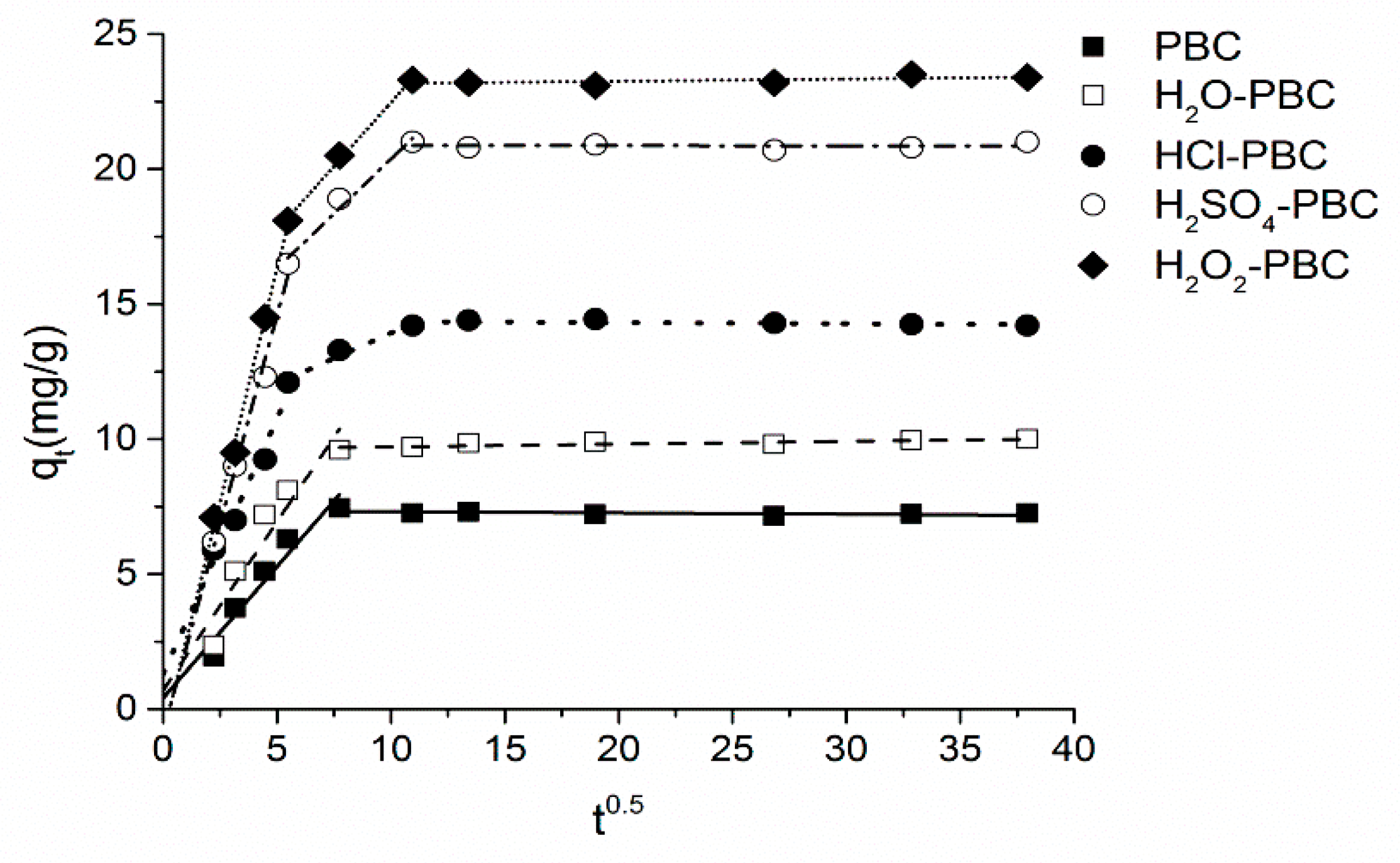
3.3. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ju, X.T.; Wei, Y.P.; Li, B.G.; Zhao, L.L.; Hu, K.L. Simulation of bromide and nitrate leaching under heavy rainfall and high-intensity irrigation rates in North China Plain. Agric. Water Manag. 2010, 97, 1646–1654. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, J.G.; Almoy, T.; Bakken, L.R. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Chang. Biol. 2014, 20, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.B.; Lin, S.; Zhang, X.M.; Jiang, Z.M.; Yang, K.C.; Jian, D.D.; Chen, Y.Z.; Li, J.L.; Chen, Q.; Wang, J.G. Conventional flooding irrigation causes an overuse of nitrogen fertilizer and low nitrogen use efficiency in intensively used solar greenhouse vegetable production. Agric. Water Manag. 2014, 144, 11–19. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Q.; Cao, J.; Zhang, C.; Song, Z.; Zhang, F.; Chen, X. Reducing nitrogen leaching in a subtropical vegetable system. Agric. Ecosyst. Environ. 2017, 241, 133–141. [Google Scholar]
- Ruidisch, M.; Bartsch, S.; Kettering, J.; Huwe, B.; Frei, S. The effect of fertilizer best management practices on nitrate leaching in a plastic mulched ridge cultivation system. Agric. Ecosyst. Environ. 2013, 169, 21–32. [Google Scholar] [CrossRef]
- Molnar, M.; Vaszita, E.; Farkas, E.; Ujaczki, E.; Fekete-Kertesz, I.; Kirchkeszner, C.; Gruiz, K.; Uzinger, N.; Feigl, V. Acidic sandy soil improvement with biochar—A microcosm study. Sci. Total Environ. 2016, 563, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Jassal, R.S.; Johnson, M.S.; Molodovskaya, M.; Black, T.A.; Jollymore, A.; Sveinson, K. Nitrogen enrichment potential of biochar in relation to pyrolysis temperature and feedstock quality. J. Environ. Manag. 2015, 152, 140–144. [Google Scholar] [CrossRef]
- Sadegh-Zadeh, F.; Parichehreh, M.; Jalili, B.; Bahmanyar, M.A. Rehabilitation of calcareous saline-sodic soil by means of biochars and acidified biochars. Land Degrad. Dev. 2018, 29, 3262–3271. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Yakout, S.M.; Daifullah, A.M.; El-Reefy, S.A. Pore Structure Characterization of Chemically Modified Biochar Derived from Rice Straw. Environ. Eng. Manag. J. 2015, 14, 473–480. [Google Scholar] [CrossRef]
- Fu, H.C.; Ma, S.L.; Zhao, P.; Xu, S.J.; Zhan, S.H. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: Structure dependence and mechanism. Chem. Eng. J. 2019, 360, 157–170. [Google Scholar] [CrossRef]
- Pereira, R.C.; Arbestain, M.C.; Sueiro, M.V.; Maciá-Agulló, J.A. Assessment of the surface chemistry of wood-derived biochars using wet chemistry, Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy. Soil Res. 2015, 53, 753–762. [Google Scholar] [CrossRef]
- Doydora, S.A.; Cabrera, M.L.; Das, K.C.; Gaskin, J.W.; Sonon, L.S.; Miller, W.P. Release of Nitrogen and Phosphorus from Poultry Litter Amended with Acidified Biochar. Int. J. Environ. Res. Public Health 2011, 8, 1491–1502. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Laird, D.A.; Johnson, R.L.; Jing, D. Accelerated aging of biochars: Impact on anion exchange capacity. Carbon 2016, 103, 217–227. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.D.; Pullammanappallil, P.; Yang, L.Y. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential. Bioresource Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Wu, F.; Zhang, C.Z.; Yang, L.Y. Engineered Biochar from Biofuel Residue: Characterization and Its Silver Removal Potential. ACS Appl. Mater. Interfaces 2015, 7, 10634–10640. [Google Scholar] [CrossRef]
- Kumar, A.; Joseph, S.; Tsechansky, L.; Privat, K.; Schreiter, I.J.; Schuth, C.; Graber, E.R. Biochar aging in contaminated soil promotes Zn immobilization due to changes in biochar surface structural and chemical properties. Sci. Total Environ. 2018, 626, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lian, B. Non-competitive and competitive adsorption of Cd2+, Ni2+, and Cu2+ by biogenic vaterite. Sci. Total Environ. 2019, 659, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Fu, H.; Zhang, J.; Lv, X.; Tang, J.; Xu, X. Studies on removal of NH4+-N from aqueous solution by using the activated carbons derived from rice husk. Biomass Bioenergy 2012, 43, 18–25. [Google Scholar] [CrossRef]
- Buss, W.; Jansson, S.; Mašek, O. Unexplored potential of novel biochar-ash composites for use as organo-mineral fertilizers. J. Clean. Prod. 2019, 208, 960–967. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.J.; Yang, L.Y. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Hale, S.; Hanley, K.; Lehmann, J.; Zimmerman, A.; Cornelissen, G. Effects of Chemical, Biological, and Physical Aging As Well As Soil Addition on the Sorption of Pyrene to Activated Carbon and Biochar. Environ. Sci. Technol. 2011, 45, 10445–10453. [Google Scholar] [CrossRef]
- Hu, X.; Xue, Y.; Long, L.; Zhang, K. Characteristics and batch experiments of acid- and alkali-modified corncob biomass for nitrate removal from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 19932–19940. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Interactions of Aluminum with Biochars and Oxidized Biochars: Implications for the Biochar Aging Process. J. Agric. Food Chem. 2014, 62, 373–380. [Google Scholar] [CrossRef]
- Zhelezova, A.; Cederlund, H.; Stenström, J. Effect of Biochar Amendment and Ageing on Adsorption and Degradation of Two Herbicides. Water Air Soil Pollut. 2017, 228, 216. [Google Scholar]
- Fan, Q.; Cui, L.; Quan, G.; Wang, S.; Sun, J.; Han, X.; Wang, J.; Yan, J. Effects of Wet Oxidation Process on Biochar Surface in Acid and Alkaline Soil Environments. Materials 2018, 11, 2362. [Google Scholar] [CrossRef]
- Paramashivam, D.; Clough, T.J.; Dickinson, N.M.; Horswell, J.; Lense, O.; Clucas, L.; Robinson, B.H. Effect of Pine Waste and Pine Biochar on Nitrogen Mobility in Biosolids. J. Environ. Qual. 2016, 45, 360–367. [Google Scholar] [CrossRef]
- Manyà, J.J.; González, B.; Azuara, M.; Arner, G. Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity. Chem. Eng. J. 2018, 345, 631–639. [Google Scholar] [CrossRef]
- Noh, J.S.; Schwarz, J.A. Effect of HNO3 treatment on the surface acidity of activated carbons. Carbon 1990, 28, 675–682. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.B.; Kirui, W.K.; Lei, M.; Lu, Q.M.; Bah, H.; Dong, R.J. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part II.—Liquids. J. Frankl. Inst. 1917, 184, 721. [Google Scholar] [CrossRef]
- Stephen, J.A.; McKay, G.; Khader, K.Y.H. Equilibrium adsorption isotherms for basic dyes onto lignite. J. Chem. Technol. Biotechnol. 1989, 45, 29–32. [Google Scholar]
- Wang, W.; Maimaiti, A.; Shi, H.L.; Wu, R.R.; Wang, R.; Li, Z.L.; Qi, D.L.; Yu, G.; Deng, S.B. Adsorption behavior and mechanism of emerging perfluoro-2-propoxypropanoic acid (GenX) on activated carbons and resins. Chem. Eng. J. 2019, 364, 132–138. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhang, L.; Guo, X.; Jiang, X.H.; Li, T. Adsorption behavior of direct red 80 and congo red onto activated carbon/surfactant: Process optimization, kinetics and equilibrium. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2015, 137, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Zhou, W.; Liang, G.Q.; Song, D.L.; Zhang, X.Y. Characteristics of maize biochar with different pyrolysis temperatures and its effects on organic carbon, nitrogen and enzymatic activities after addition to fluvo-aquic soil. Sci. Total Environ. 2015, 538, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.G.I.D.; Moldrup, P.; Paradelo, M.; de Jonge, L.W. Phenanthrene Sorption on Biochar-Amended Soils: Application Rate, Aging, and Physicochemical Properties of Soil. Water Air Soil Pollut. 2014, 225, 2105. [Google Scholar]
- Yang, M.; Guo, L.; Hu, G.; Hu, X.; Chen, J.; Shen, S.; Dai, W.; Fan, M. Adsorption of CO2 by Petroleum Coke Nitrogen-Doped Porous Carbons Synthesized by Combining Ammoxidation with KOH Activation. Ind. Eng. Chem. Res. 2016, 55, 757–765. [Google Scholar]
- Choy, K.K.H.; Porter, J.F.; Mckay, G. A Film-Pore-Surface Diffusion Model for the Adsorption of Acid Dyes on Activated Carbon. Adsorption 2001, 7, 305–317. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, C.; Shi, W.; Huang, X.; Ye, Y.; Jiang, W.; Kang, J.; Liu, D.; Ren, Y.; Li, D. Preferable phosphate removal by nano-La(III) hydroxides modified mesoporous rice husk biochars: Role of the host pore structure and point of zero charge. Sci. Total Environ. 2019, 662, 511–520. [Google Scholar] [CrossRef]
- Wu, L.; Li, B.; Liu, M. Influence of aromatic structure and substitution of carboxyl groups of aromatic acids on their sorption to biochars. Chemosphere 2018, 210, 239–246. [Google Scholar] [CrossRef]
- Liu, G.; Wang, P.; Liu, Q.; Han, W. Removal of Cd(II) by Nanometer AlO(OH) Loaded on Fiberglass with Activated Carbon Fiber Felt as Carrier. Chin. J. Chem. Eng. 2008, 16, 805–811. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Fang, J.N.; Zhang, M.; Chen, H.; Zhou, Y.M.; Creamer, A.E.; Sun, Y.N.; Yang, L.Y. Characterization and environmental applications of clay-biochar composites. Chem. Eng. J. 2014, 242, 136–143. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Liu, Y.; Wang, H. Screening of novel low-cost adsorbents from agricultural residues to remove ammonia nitrogen from aqueous solution. J. Hazard. Mater. 2010, 178, 1132–1136. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, Y.; Gao, F.; Cheng, X.; Yang, K. Removal of ammonium from aqueous solutions using alkali-modified biochars. Chem. Speciat. Bioavailab. 2016, 28, 26–32. [Google Scholar]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xue, S.; Zhao, S.C.; Yan, J.C.; Qian, L.B.; Chen, M.F. Biochar Supported Nanoscale Iron Particles for the Efficient Removal of Methyl Orange Dye in Aqueous Solutions. PLoS ONE 2015, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, R.; Deng, S.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res. 2009, 43, 1150–1158. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]



| Samples | Ash Content | Volatile Content | Water Content | Fixed Carbon | pHpzc |
|---|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | pH Unit | |
| PBC | 32.54 | 24.36 | 0.74 | 42.36 | 9.20 |
| H2O-PBC | 11.76 | 30.30 | 0.98 | 56.96 | 8.60 |
| HCl-PBC | 8.52 | 27.77 | 0.78 | 62.93 | 5.00 |
| H2O2-PBC | 6.83 | 21.00 | 0.65 | 71.52 | 7.20 |
| H2SO4-PBC | 7.42 | 24.88 | 0.82 | 66.88 | 6.90 |
| Adsorbents | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg·g−1) | b (mg·L−1) | R2 | KF (mg·g−1) | n | R2 | |
| PBC | 24.58 | 5.00 × 10−3 | 0.98 | 0.84 | 2.10 | 0.93 |
| H2O-PBC | 60.50 | 1.78 × 10−3 | 0.99 | 0.49 | 1.56 | 0.99 |
| HCl-PBC | 100.28 | 4.00 × 10−3 | 0.99 | 1.89 | 2.34 | 0.94 |
| H2SO4-PBC | 120.01 | 3.12 × 10−3 | 0.99 | 2.07 | 1.78 | 0.95 |
| H2O2-PBC | 123.23 | 4.40 × 10−3 | 0.99 | 3.03 | 1.90 | 0.95 |
| Adsorbents | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | Qe (mg·g−1) | R2 | v0 (g·mg−1·min−1) | Qe (mg·g−1) | R2 | |
| PBC | 0.15 | 7.20 | 0.99 | 0.79 | 7.53 | 0.92 |
| H2O-PBC | 0.15 | 9.80 | 0.99 | 1.03 | 10.29 | 0.96 |
| HCl-PBC | 0.16 | 14.22 | 0.95 | 1.69 | 14.83 | 0.94 |
| H2SO4-PBC | 0.12 | 20.65 | 0.98 | 1.75 | 21.65 | 0.96 |
| H2O2-PBC | 0.12 | 22.93 | 0.99 | 1.98 | 24.10 | 0.98 |
| Adsorbent | Capacity NH4+-N (mg·g−1) | Concentration Range NH4+-N (mg·L−1) | Contact Time | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|---|
| PBC | 24.58 | 20–1000 | 24 h | ≈7 | 25 | This article |
| H2O-PBC | 60.50 | 20–1000 | 24 h | ≈7 | 25 | This article |
| HCl-PBC | 100.28 | 20–1000 | 24 h | ≈7 | 25 | This article |
| H2SO4-PBC | 120.01 | 20–1000 | 24 h | ≈7 | 25 | This article |
| H2O2-PBC | 123.23 | 20–1000 | 24 h | ≈7 | 25 | This article |
| Peanut shell biochar (PS) | 243.30 | 10–500 | 5–10 h | ≈7 | 25–50 | Gao et al., 2015 |
| NaOH modified PS (mPS) | 313.90 | 10–500 | 5–10 h | ≈7 | 25–50 | Liu et al., 2016 |
| Maple wood biochar (MW) | 0.46–0.87 | 0–100 | 16 h | - | - | Wang et al., 2016 |
| H2O2 oxidized MW | 1.35–7.23 | 0–100 | 16 h | - | - | Wang et al., 2016 |
| Rice husk biochar | 39.80 | 250–1400 | 96 h | 5~8 | 25–45 | Kizito et al., 2014 |
| Poulty litter biochar (water washed) | 1.33 | 0–10 | 24 h | ≈7 | - | Tian et al., 2016 |
| Bentonite hydrochar | 23.67 | 200 | 25 h | 6 | 30 | Ismadji et al., 2016 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, J.; Zhang, G.; Zhi, Y.; Yang, D.; Lai, X.; Ren, T. Characterization of Acid-Aged Biochar and Its Ammonium Adsorption in an Aqueous Solution. Materials 2020, 13, 2270. https://doi.org/10.3390/ma13102270
Wang Z, Li J, Zhang G, Zhi Y, Yang D, Lai X, Ren T. Characterization of Acid-Aged Biochar and Its Ammonium Adsorption in an Aqueous Solution. Materials. 2020; 13(10):2270. https://doi.org/10.3390/ma13102270
Chicago/Turabian StyleWang, Zhiwen, Jie Li, Guilong Zhang, Yancai Zhi, Dianlin Yang, Xin Lai, and Tianzhi Ren. 2020. "Characterization of Acid-Aged Biochar and Its Ammonium Adsorption in an Aqueous Solution" Materials 13, no. 10: 2270. https://doi.org/10.3390/ma13102270
APA StyleWang, Z., Li, J., Zhang, G., Zhi, Y., Yang, D., Lai, X., & Ren, T. (2020). Characterization of Acid-Aged Biochar and Its Ammonium Adsorption in an Aqueous Solution. Materials, 13(10), 2270. https://doi.org/10.3390/ma13102270