Effect of 808 nm Semiconductor Laser on the Stability of Orthodontic Micro-Implants: A Split-Mouth Study
Abstract
1. Introduction
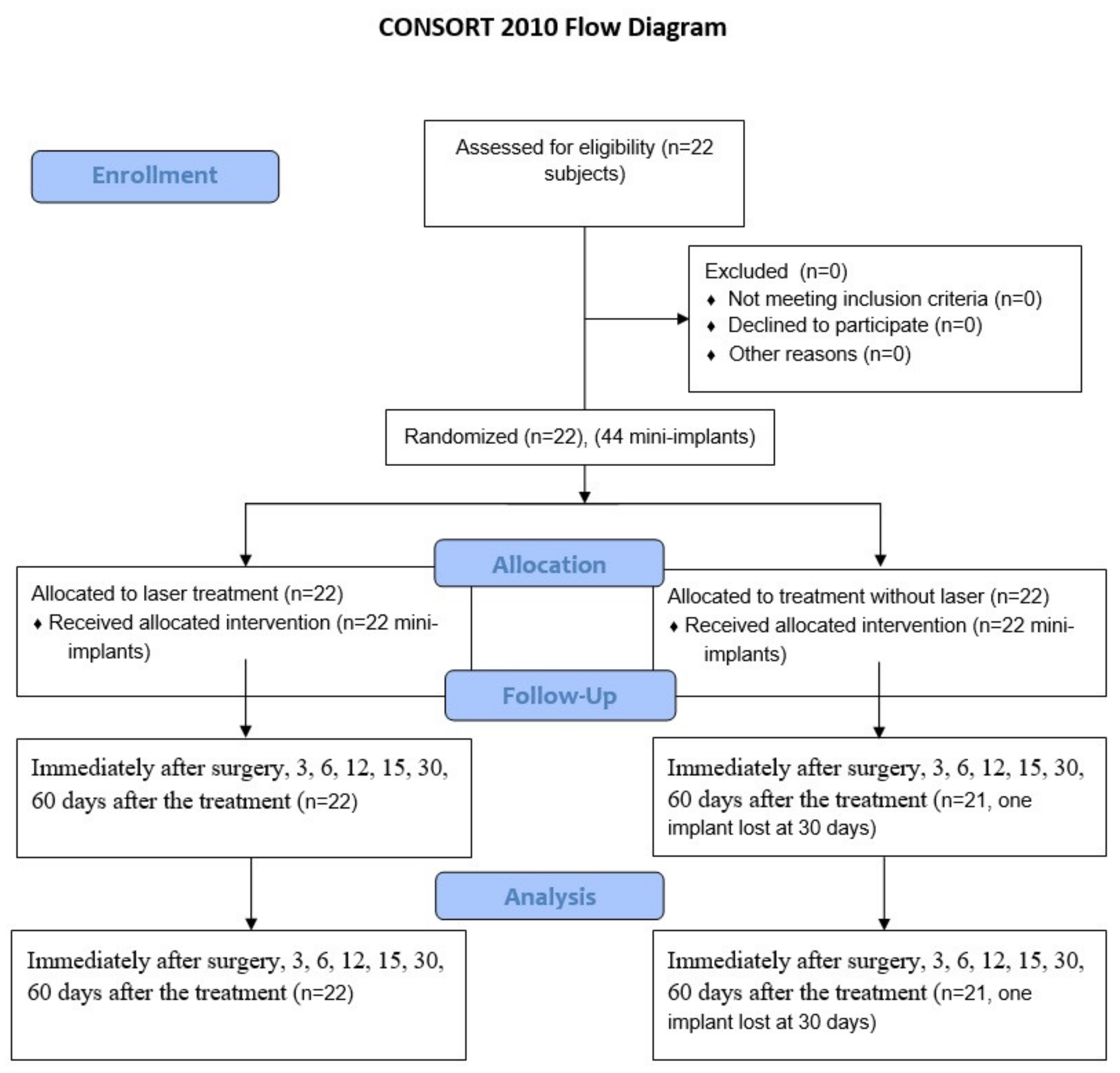
2. Materials and Methods
2.1. Patients
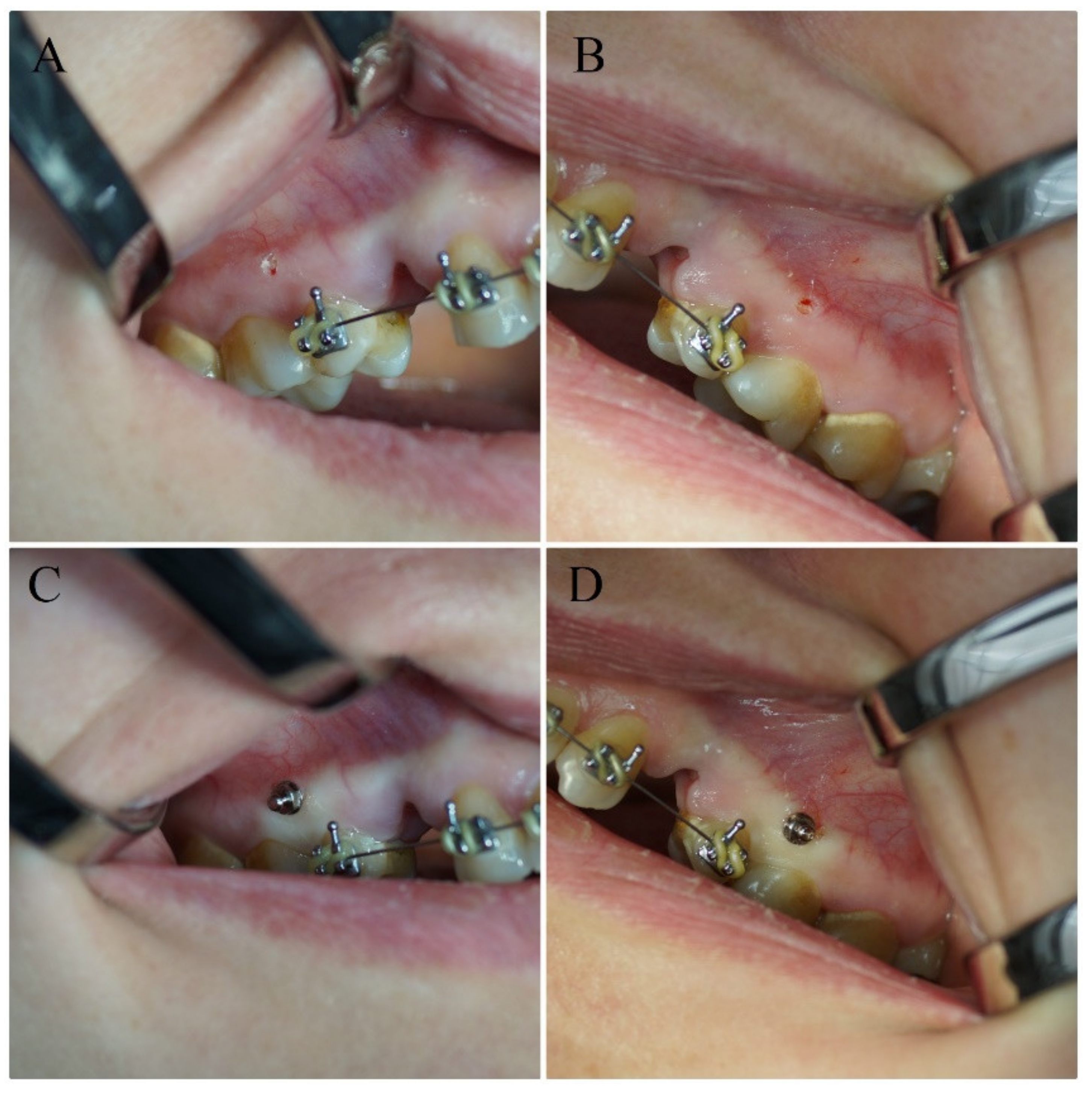
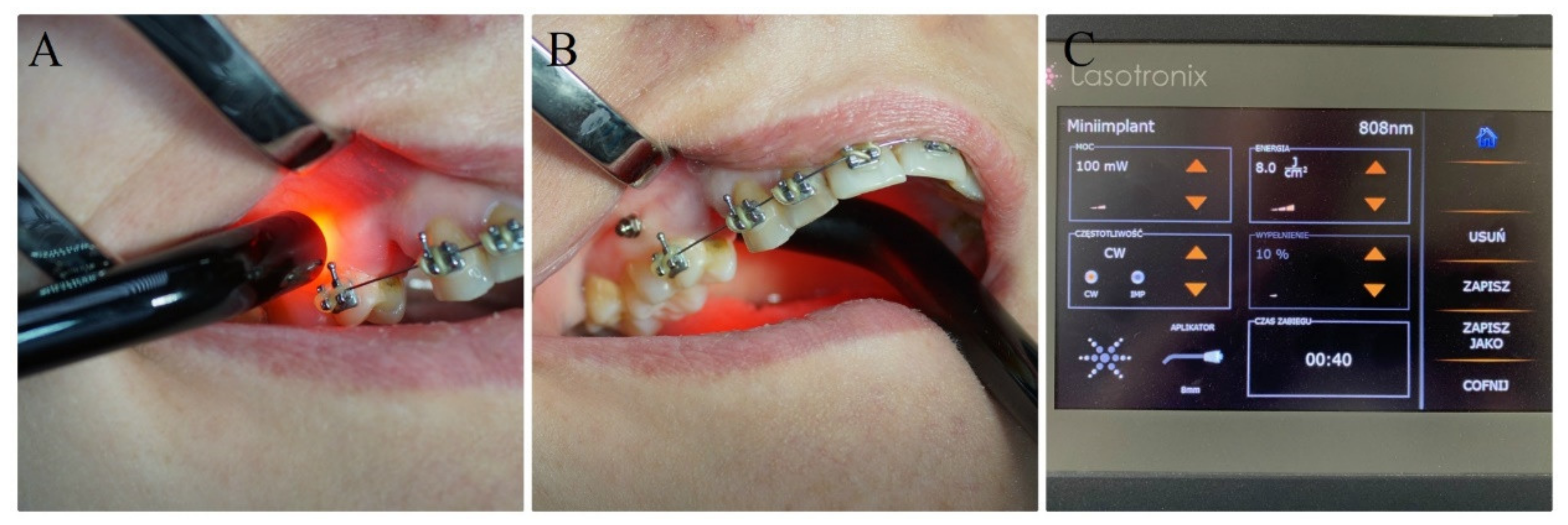
2.2. Orthodontic Treatment, Surgical Procedures and Laser Irradiation
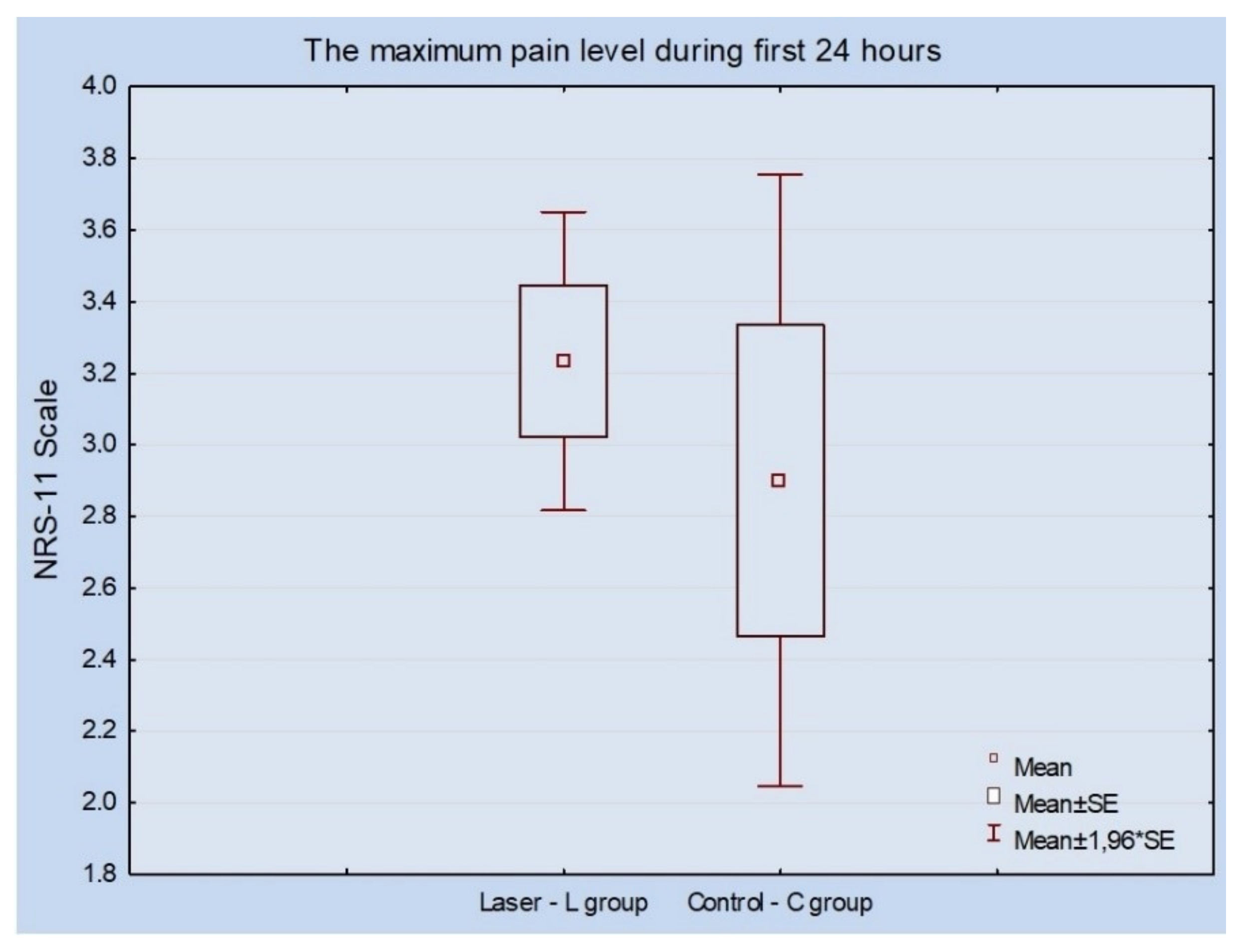
2.3. Measurement of Implants’ Stability, Pain Value and Calculation of Statistical Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, H.S.; Jeong, S.H.; Kwon, O.W. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 18–25. [Google Scholar] [CrossRef]
- Mattos, C.T.; Ruellas, A.C.O.; Elias, C.N. Is it possible to re-use mini-implants for orthodontic anchorage? Results of an In Vitro study. Mater. Res. 2010, 13, 521–525. [Google Scholar] [CrossRef][Green Version]
- Miyawaki, S.; Koyama, I.; Inoue, M.; Mishima, K.; Sugahara, T.; Takano-Yamamoto, T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 373–378. [Google Scholar] [CrossRef]
- Costa, A.; Raffainl, M.; Melsen, B. Miniscrews as orthodontic anchorage: A preliminary report. Int. J. Adult Orthodon. Orthognath. Surg. 1998, 13, 201–209. [Google Scholar]
- Matys, J.; Świder, K.; Flieger, R. Is it possible to re-use mini-implants for orthodontic anchorage? Results of an In Vitro study. Dent. Med. Probl. 2017, 54, 101–106. [Google Scholar] [CrossRef]
- Fritz, U.; Ehmer, A.; Diedrich, P. Klinische eignung von mikrotitanschrauben zur orthodontischen Verankerung-este erfahrungen. J. Orofac. Orthop. 2004, 65, 410–418. [Google Scholar] [PubMed]
- Flieger, R.; Gedrange, T.; Grzech-Leśniak, K.; Dominiak, M.; Matys, J. Low-level laser therapy with a 635 nm diode laser affects orthodontic mini-implants stability: A randomized clinical split-mouth trial. J. Clin. Med. 2019, 9, 112. [Google Scholar] [CrossRef]
- Graham, J.W. Temporary replacement of maxillary lateral incisors with miniscrews and bonded pontics. J. Clin. Orthod. 2007, 41, 321–325. [Google Scholar]
- Pinto, M.R.; dos Santos, R.L.; Pithon, M.M.; de Souza Araújo, M.T.; Braga, J.P.V.; Nojima, L.I. Influence of low-intensity laser therapy on the stability of orthodontic mini-implants: A study in rabbits. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e26–e30. [Google Scholar] [CrossRef]
- Matys, J.; Świder, K.; Flieger, R.; Dominiak, M. Assessment of the primary stability of root analog zirconia implants designed using cone beam computed tomography software by means of the Periotest device: An ex vivo study. A preliminary report. Adv. Clin. Exp. Med. 2017, 26, 803–809. [Google Scholar] [CrossRef]
- Matys, J.; Flieger, R.; Tenore, G.; Grzech-Leśniak, K.; Romeo, U.; Dominiak, M. Er: YAG laser, piezosurgery, and surgical drill for bone decortication during orthodontic mini-implant insertion: Primary stability analysis—An animal study. Lasers Med. Sci. 2018, 33, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Torkzaban, P.; Kasraei, S.; Torabi, S.; Farhadian, M. Low-level laser therapy with 940 nm diode laser on stability of dental implants: a randomized controlled clinical trial. Lasers Med. Sci. 2018, 33, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Benayahu, D.; Maltz, L.; Oron, U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg. 2005, 23, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Schindl, A.; Schindl, M.; Pernerstorfer-Schön, H.; Schindl, L. Low-intensity laser therapy: A review. J. Investig. Med. 2000, 48, 312–326. [Google Scholar] [PubMed]
- AlGhamdi, K.M.; Kumar, A.; Moussa, N.A. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012, 27, 237–249. [Google Scholar] [CrossRef]
- Barbosa, D.; de Souza, R.A.; Xavier, M.; da Silva, F.F.; Arisawa, E.Â.L.; Villaverde, A.G.J.B. Effects of low-level laser therapy (LLLT) on bone repair in rats: Optical densitometry analysis. Lasers Med. Sci. 2013, 28, 651–656. [Google Scholar] [CrossRef]
- Khadra, M.; Kasem, N.; Lyngstadaas, S.P.; Haanaes, H.R.; Mustafa, K. Laser therapy accelerates initial attachment and subsequent behaviour of human oral fibroblasts cultured on titanium implant material: A scanning electron microscopic and histomorphometric analysis. Clin. Oral Implants Res. 2005, 16, 168–175. [Google Scholar] [CrossRef]
- Goymen, M.; Isman, E.; Taner, L.; Kurkcu, M. Histomorphometric evaluation of the effects of various diode lasers and force levels on orthodontic mini screw stability. Photomed. Laser Surg. 2015, 33, 29–34. [Google Scholar] [CrossRef]
- Mohammed, I.F.R.; Al-Mustawfi, N.; Kaka, L.N. Promotion of regenerative processes in injured peripheral nerve induced by low-level laser therapy. Photomed. Laser Surg. 2007, 25, 107–111. [Google Scholar] [CrossRef]
- Omasa, S.; Motoyoshi, M.; Arai, Y.; Ejima, K.I.; Shimizu, N. Low-level laser therapy enhances the stability of orthodontic mini-implants via bone formation related to BMP-2 expression in a rat model. Photomed. Laser Surg. 2012, 30, 255–261. [Google Scholar] [CrossRef]
- Gholami, L.; Asefi, S.; Hooshyarfard, A.; Sculean, A.; Romanos, G.E.; Aoki, A.; Fekrazad, R. Photobiomodulation in periodontology and implant dentistry: Part I. Photobiomodulation Photomed. Laser Surg. 2019, 37, 1–26. [Google Scholar]
- Gholami, L.; Asefi, S.; Hooshyarfard, A.; Sculean, A.; Romanos, G.E.; Aoki, A.; Fekrazad, R. Photobiomodulation in Periodontology and implant dentistry: Part 2. Photobiomodulation Photomed. Laser Surg. 2019, 37, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Caruso-Davis, M.K.; Guillot, T.S.; Podichetty, V.K.; Mashtalir, N.; Dhurandhar, N.V.; Dubuisson, O.; Yu, Y.; Greenway, F.L. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes. Surg. 2011, 21, 722–729. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photobiomodulation for traumatic brain injury and stroke. J. Neurosci. Res. 2018, 96, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Dai, C.; Cao, P.; Sun, D.; Ouyang, R.; Miao, Y. The role of reactive oxygen species in tumor treatment. RSC Adv. 2020, 10, 7740–7750. [Google Scholar] [CrossRef]
- Komarova, S.V.; Ataullakhanov, F.I.; Globus, R.K. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am. J. Physiol. Cell Physiol. 2000, 279, 1220–1229. [Google Scholar] [CrossRef]
- Kim, J.E.; Woo, Y.J.; Sohn, K.M.; Jeong, K.H.; Kang, H. Wnt/β-catenin and ERK pathway activation: A possible mechanism of photobiomodulation therapy with light-emitting diodes that regulate the proliferation of human outer root sheath cells. Lasers Surg. Med. 2017, 49, 940–947. [Google Scholar] [CrossRef]
- Naderi, M.S.; Razzaghi, M.; Djavid, G.E.; Hajebrahimi, Z. A comparative study of 660 nm low-level laser and light emitted diode in proliferative effects of fibroblast cells. J. Lasers Med. Sci. 2017, 8, S46–S50. [Google Scholar] [CrossRef]
- Han, Y.; Kim, Y.M.; Kim, H.S.; Lee, K.Y. Melatonin promotes osteoblast differentiation by regulating Osterix protein stability and expression. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.R.N.; Saberianpour, S.; Geranmayeh, M.H.; Bani, F.; Haghighi, L.; Rahbarghazi, R. Modulatory effect of photobiomodulation on stem cell epigenetic memory: a highlight on differentiation capacity. Lasers Med. Sci. 2020, 35, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Uysal, T.; Ekizer, A.; Akcay, H.; Etoz, O.; Guray, E. Resonance frequency analysis of orthodontic miniscrews subjected to light-emitting diode photobiomodulation therapy. Eur. J. Orthod. 2012, 34, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Suzuki, S.S.; Martinez, E.F.; Iemini, M.G.; Suzuki, H. Effects of low-intensity laser therapy over mini-implants success rate in pigs. Lasers Med. Sci. 2015, 30, 727–732. [Google Scholar] [CrossRef]
- Ekizer, A.; Türker, G.; Uysal, T.; Güray, E.; Taşdemir, Z. Light emitting diode mediated photobiomodulation therapy improves orthodontic tooth movement and miniscrew stability: A randomized controlled clinical trial. Lasers Surg. Med. 2016, 48, 936–943. [Google Scholar] [CrossRef]
- Osman, A.; Moneim, A.A.; El Harouni, N.; Shokry, M. Long-term evaluation of the effect of low-level laser therapy on orthodontic miniscrew stability and peri-implant gingival condition: A randomized clinical trial. J. World Fed. Orthod. 2017, 6, 109–114. [Google Scholar] [CrossRef]
- Yanaguizawa, M.S.; Suzuki, S.S.; Martinez, E.F.; Suzuki, H.; Pelegrin, M.C.J.; Garcez, A.S. Effects of low-level laser therapy in orthodontic patients on immediate inflammatory response after mini-implants insertion: A preliminary report. Photomed. Laser Surg. 2017, 35, 57–63. [Google Scholar] [CrossRef]
- Pires Oliveira, D.A.A.; de Oliveira, R.F.; Zangaro, R.A.; Soares, C.P. Evaluation of low-level laser therapy of osteoblastic cells. Photomed. Laser Surg. 2008, 26, 401–404. [Google Scholar] [CrossRef]
- Matys, J.; Jaszczak, E.; Flieger, R.; Kostrzewska-Kaminiarz, K.; Grzech-Leśniak, K.; Dominiak, M. Effect of ozone and diode laser (635 nm) in reducing orthodontic pain in the maxillary arch—a randomized clinical controlled trial. Lasers Med. Sci. 2020, 35, 487–496. [Google Scholar] [CrossRef]
- Matys, J.; Świder, K.; Grzech-Leśniak, K.; Dominiak, M.; Romeo, U. Photobiomodulation by a 635 nm diode laser on peri-implant bone: Primary and secondary stability and bone density analysis—a randomized Clinical Trial. Biomed Res. Int. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- AlSayed Hasan, M.M.A.; Sultan, K.; Hamadah, O. Evaluating low-level laser therapy effect on reducing orthodontic pain using two laser energy values: A split-mouth randomized placebo-controlled trial. Eur. J. Orthod. 2018, 40, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Lang, N.P. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin. Oral Implants Res. 2011, 22, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.B.; Pinheiro, A.L.B.; Sathaiah, S.; Silva, N.S.D.; Salgado, M.A.C. Infrared laser photobiomodulation (λ 830 nm) on bone tissue around dental implants: A raman spectroscopy and scanning electronic microscopy study in rabbits. Photomed. Laser Surg. 2007, 25, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Matys, J.; Grzech-Leśniak, K.; Flieger, R.; Dominiak, M. Assessment of an impact of a diode laser mode with wavelength of 980 nm on a temperature rise measured by means of k-02 thermocouple: Preliminary results. Dent. Med. Probl. 2016, 53, 345–351. [Google Scholar] [CrossRef]
- Marquezan, M.; Mattos, C.T.; Sant’Anna, E.F.; de Souza, M.M.G.; Maia, L.C. Does cortical thickness influence the primary stability of miniscrews?: a systematic review and meta-analysis. Angle Orthod. 2014, 84, 1093–1103. [Google Scholar] [CrossRef]
- Casaña-Ruiz, M.D.; Bellot-Arcís, C.; Paredes-Gallardo, V.; García-Sanz, V.; Almerich-Silla, J.M.; Montiel-Company, J.M. Risk factors for orthodontic mini-implants in skeletal anchorage biological stability: A systematic literature review and meta-analysis. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Lang, N.P.; Lindhe, J. Clinical Periodontology and Implant Dentistry, 2 Volume Set, 6th ed.; John Wiley & Sons: Hobeken, NJ, USA, 2015; pp. 256–269. ISBN 978-0-470-67248-8. [Google Scholar]
- Al-Sawai, A.A.; Labib, H. Success of immediate loading implants compared to conventionally-loaded implants: A literature review. J. Investig. Clin. Dent. 2016, 7, 217–224. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, J.H.; Yi, Y.J.; Kwon, M.J.; Yun, P.Y. Prospective comparative study of tapered implants with SLA surfaces in the maxillary posterior area according to 3- and 6-month loading time. Int. J. Periodontics Restor. Dent. 2015, 35, 271–276. [Google Scholar] [CrossRef][Green Version]
- Ramazanzadeh, B.A.; Fatemi, K.; Dehghani, M.; Mohtasham, N.; Jahanbin, A.; Sadeghian, H. Effect of healing time on bone-implant contact of orthodontic micro-implants: A histologic study. ISRN Dent. 2014, 2014. [Google Scholar] [CrossRef][Green Version]
- Jeong, J.W.; Kim, J.W.; Lee, N.-K.; Kim, Y.-K.; Lee, J.-H.; Kim, T.-W. Analysis of time to failure of orthodontic mini-implants after insertion or loading. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 240–245. [Google Scholar] [CrossRef]
| Period | Laser (L Group) (n = 22) | Std | Control (C Group) (n = 21) | Std | p-Value |
|---|---|---|---|---|---|
| Baseline | −1.25 | 2.65 | −1.08 | 2.34 | 0.824 |
| 3 days | −0.66 | 3.10 | −1.19 | 2.49 | 0.503 |
| 6 days | −0.54 | 2.71 | −0.94 | 2.41 | 0.576 |
| 9 days | −0,79 | 2.37 | −0.83 | 1.43 | 0.949 |
| 12 days | −0.02 | 2.48 | −0.38 | 0.97 | 0.554 |
| 15 days | 1.81 | 3.20 | 1.00 | 0.66 | 0.279 |
| 30 days | 6.32 | 3.62 | 11.34 | 5.76 | 0.004 |
| 60 days | 6.55 | 4.66 | 10.95 | 4.77 | 0.009 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matys, J.; Flieger, R.; Gedrange, T.; Janowicz, K.; Kempisty, B.; Grzech-Leśniak, K.; Dominiak, M. Effect of 808 nm Semiconductor Laser on the Stability of Orthodontic Micro-Implants: A Split-Mouth Study. Materials 2020, 13, 2265. https://doi.org/10.3390/ma13102265
Matys J, Flieger R, Gedrange T, Janowicz K, Kempisty B, Grzech-Leśniak K, Dominiak M. Effect of 808 nm Semiconductor Laser on the Stability of Orthodontic Micro-Implants: A Split-Mouth Study. Materials. 2020; 13(10):2265. https://doi.org/10.3390/ma13102265
Chicago/Turabian StyleMatys, Jacek, Rafał Flieger, Tomasz Gedrange, Krzysztof Janowicz, Bartosz Kempisty, Kinga Grzech-Leśniak, and Marzena Dominiak. 2020. "Effect of 808 nm Semiconductor Laser on the Stability of Orthodontic Micro-Implants: A Split-Mouth Study" Materials 13, no. 10: 2265. https://doi.org/10.3390/ma13102265
APA StyleMatys, J., Flieger, R., Gedrange, T., Janowicz, K., Kempisty, B., Grzech-Leśniak, K., & Dominiak, M. (2020). Effect of 808 nm Semiconductor Laser on the Stability of Orthodontic Micro-Implants: A Split-Mouth Study. Materials, 13(10), 2265. https://doi.org/10.3390/ma13102265









