3.1. Uncoated Samples
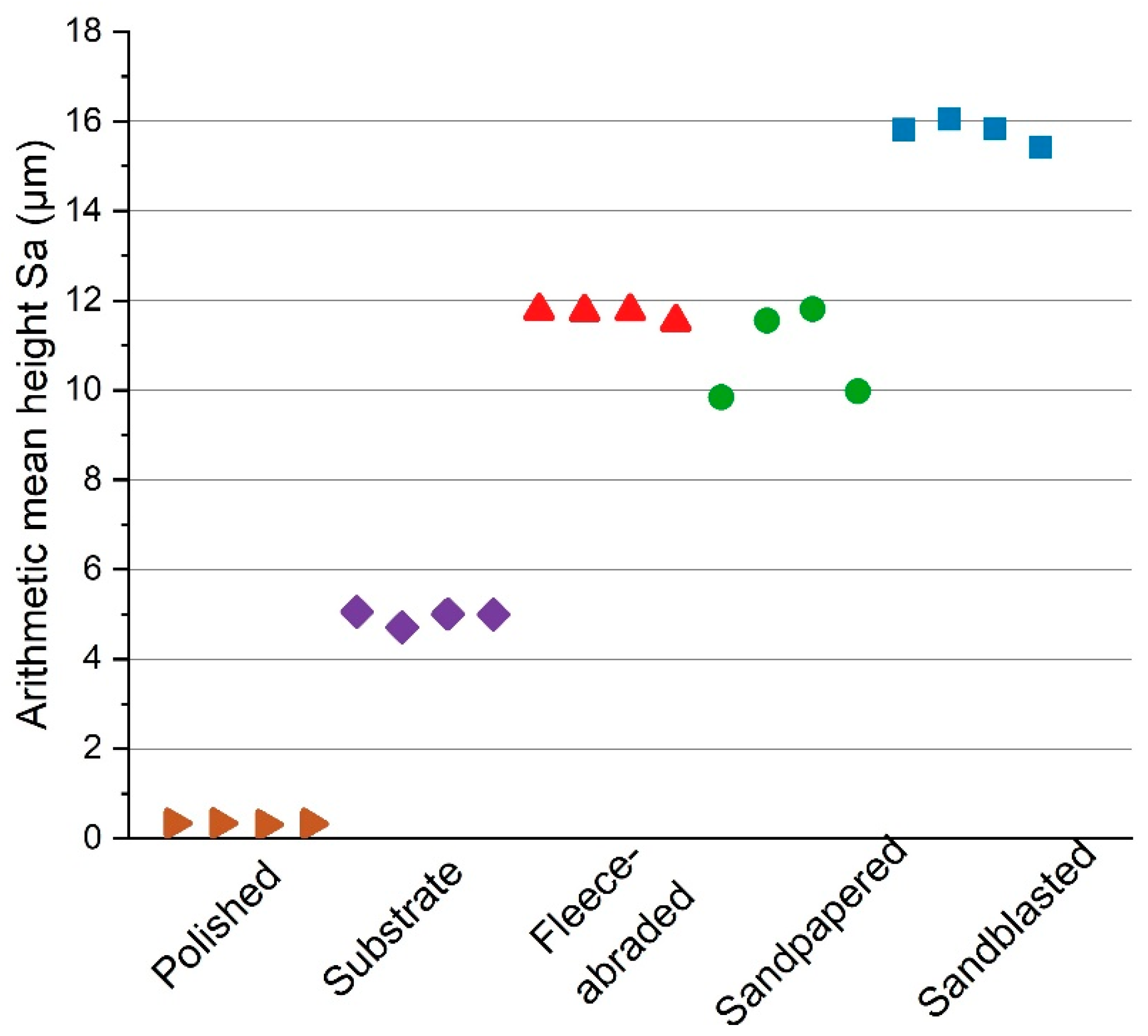
Four sets, each consisting of four samples, were produced by the mechanical finishing methods: polishing, abrading with fleece, sandpapering and sandblasting. The fifth set consisted of four as-received substrates that were untreated and served as reference samples.
All samples prepared with the same method had the similar surface roughness parameter, namely, the arithmetic mean height Sa (
Figure 4). The chosen surface finishing methods resulted in a broad range of values for Sa (between 0 and 16 µm). One sample out of four per each method was used for the further characterisation of the uncoated surfaces.
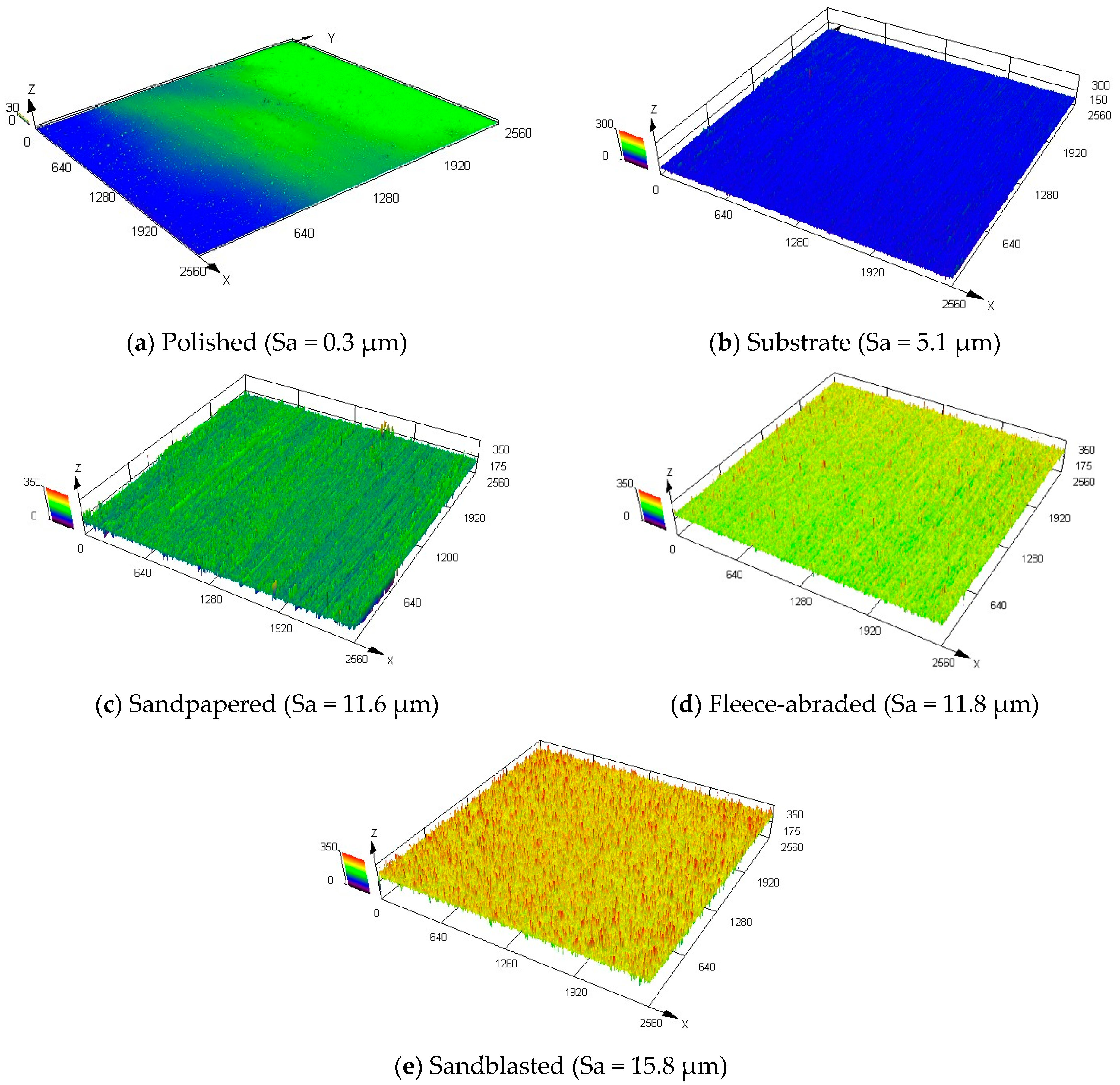
A qualitative overview of the morphology of the examined surfaces, done via confocal laser scanning microscope, for the middle measurement points of the samples, is shown in
Figure 5. The samples were arranged according to the growing average Sa value. The untreated substrate and the polished sample (
Figure 5a,b) had a uniform profile with relatively low roughness, whereas the fleece-abraded and sandblasted samples (
Figure 5d,e) were much rougher. The sandpapered sample had a rough surface that was also strongly directional-oriented (
Figure 5c).
The selected roughness parameters, measured for each uncoated sample, are summarized in
Table 2. As mentioned previously, the initial state of the surface before the treatment corresponds to the substrate. Remarkable for the substrate was that the maximum peak height (Sp) was about four times higher than the maximum valley depth (Sv). These, together with the high mean width of the profile elements (RSm = 100 μm), were produced due to the mechanical rolling of the substrate metal sheet. In general, the surface structure of the substrate was quite uniform (
Figure 5b).
Polishing the surface resulted in the lowest areal roughness parameters among the considered samples, while the arithmetic mean height approached almost zero (Sa = 0.3 μm). As expected, the surface of the polished sample was smooth, with the lowest maximum height (Sz = 75.3 μm) and the broadest profile elements (RSm = 178.4 μm).
As the substrates were roughened, the arithmetic mean height of the samples increased. Thus, using the sandpaper led to an Sa value of 11.6 μm. The sandpapered sample had large standard deviations of Sa and Sq, indicating the significant differences in the height profile. These were reinforced by the much higher peaks, since the Sp was three times higher than the Sv, characterising the structure of the sandpapered sample as strongly non-uniform and directionally oriented.
Using the abrasive fleece resulted in the similar arithmetic mean height (Sa = 11.8 μm) as in the previous case with the sandpaper. The major difference was that the directional orientation of the surface treatment method was less pronounced in case of the fleece-abraded sample. The maximum peak height (Sp) was of the same order of magnitude as the maximum valley depth (Sv), which combined with the lowest mean width of the profile elements (RSm = 73.8 μm) spoke for the uniformly roughened surface structure.
The sandblasted sample, with the largest arithmetic mean height (Sa = 15.8 μm) and the largest maximum height (Sz = 479.7 μm), exhibited the roughest surface of all the tested treatment methods. However, sandblasting created a uniformly rough surface.
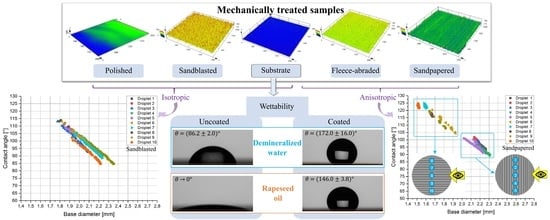
The wettability of the uncoated samples was characterised by measuring the apparent contact angles of water. The measurement results are shown in
Table 3. The polished sample displayed the lowest contact angle of water
= 78° and, together with the substrate and fleece-abraded samples, were classified as hydrophilic surfaces. This reduction of the contact angle by polishing the sample does not correspond to the results published by Torrisi and Scolaro [
24], who reported a slight contact angle increase, from 95° to 99°, by polishing the sample from 0.1 µm to 0.028 µm. However, these authors applied a chemical–mechanical polishing solution, used for polishing optical components in order to evenly remove the oxide layer from the surface. Thus, a very fine surface structure was created. The reduction of the contact angle in the present study could be caused by the newly air-formed oxide layer which distorted the surface profile.
Hydrophobic surfaces were achieved by sandblasting and sandpapering, with the latter exhibiting the highest contact angle
= 106°. It is worthwhile noticing that, although the sandpapered and fleece-abraded samples had the similar roughness parameters (Sa = 11.6 μm and Sa = 11.8 μm, respectively), their contact angles were considerably different. This difference is attributed to the strong anisotropy of the surface texture of the sandpapered sample, which is also visible from
Figure 5c. These results emphasize that not only roughness, but also the surface treatment method has a significant impact on the wettability.
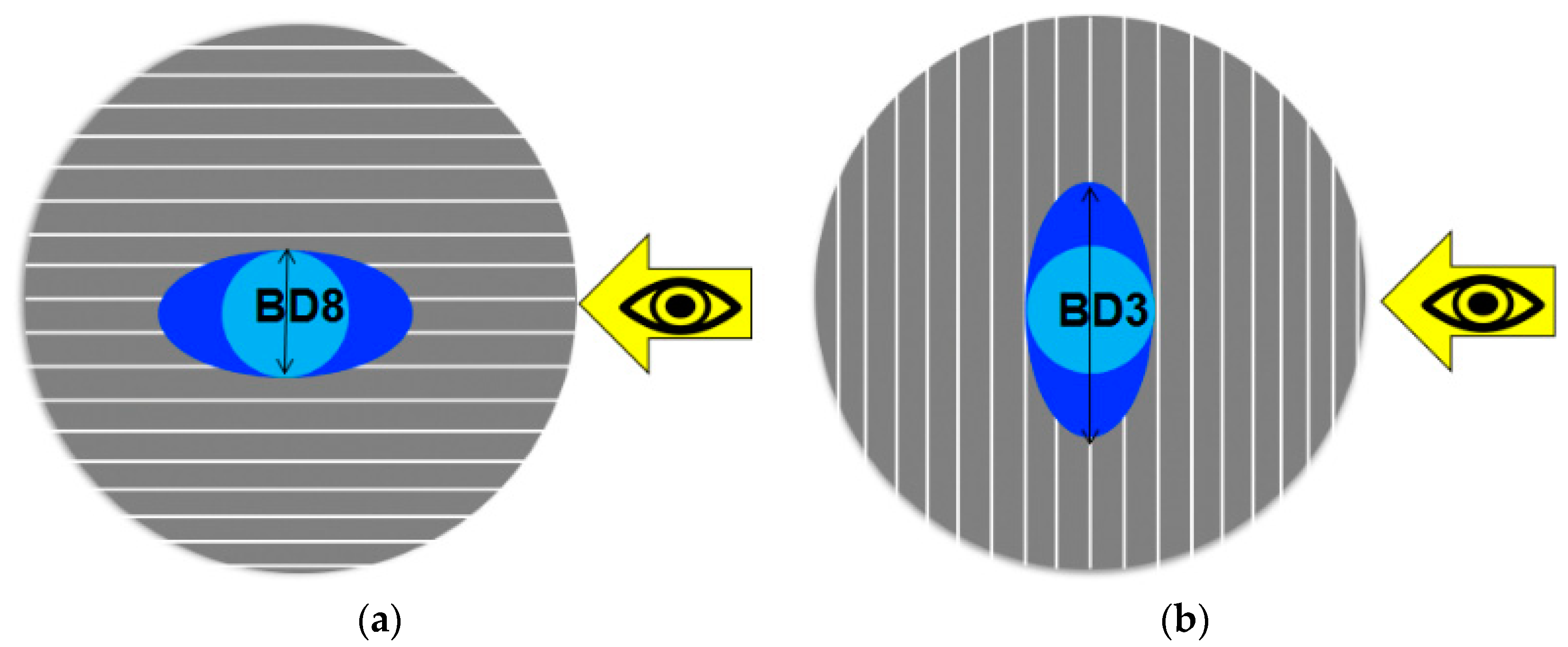
Presented in
Table 3, the ratio between the orthogonally measured base diameters of the third and the eighth droplets (BD3/BD8), both placed in the centres of each sample, helped to estimate whether the sample surface had an isotropic or anisotropic wettability. In the case of isotropic wetting behaviour, the base diameters measured from two orthogonal directions are similar. In its turn, for anisotropic wettability, the droplet base diameter measured in parallel to the surface texture direction (here, BD8) is different from the one measured perpendicular to the surface texture direction (here, BD3) [
32,
33].
When the ratio BD3/BD8 was close to one and was, in our case, in the range of 0.85–1.15, then the wettability was isotropic, as it was in the case with the polished sample and substrate. Due to the directionally oriented production of the metal sheet by mechanical rolling, the surface texture of the substrate was anisotropic. However, the texture anisotropy was not detected in wettability measurements. At a ratio of BD3/BD8 much higher or much lower than one and out of the above-specified range, the wettability was considered anisotropic, as by the sandpapered and fleece-abraded samples. Their surface texture was oriented in one direction (visible in
Figure 5c,d) and was also anisotropic.
Similar results, presented in
Table 4, can be observed from the measured water contact angles perpendicular (
) and parallel (
) to the surface texture direction. These angles were considerably different (
= (10°–23°)) from each other in the case of the fleece-abraded and sandpapered samples, which indicated the strong anisotropy of the samples. Although the sandblasted sample had a ratio of BD3/BD8 much lower than one, the difference in the measured contact angles was minor. Apart from that, there was no grouping of the droplets in the dependence of the measuring direction as shown in
Figure 6a. This let us conclude that the sandblasted sample was rather isotropic.
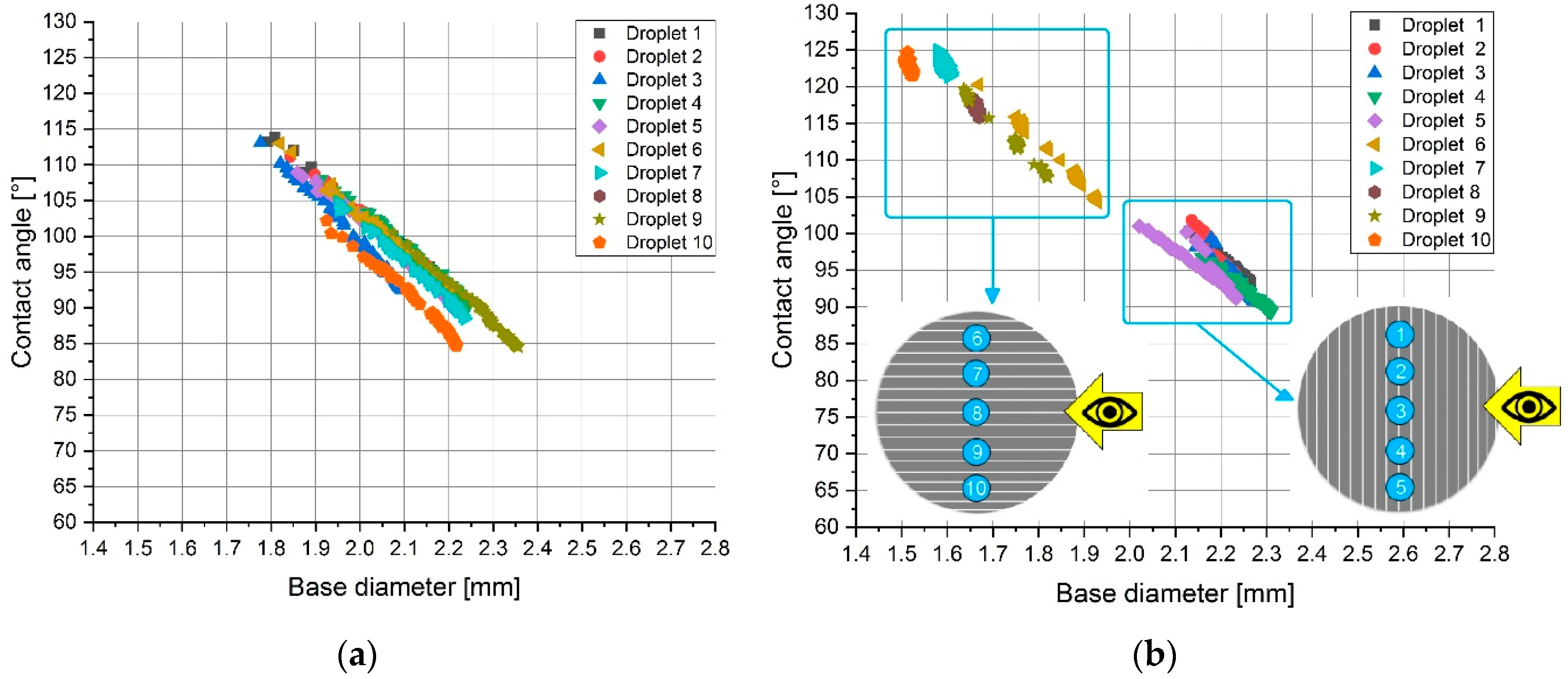
Following the change in the measured contact angles and droplet base diameters (
Figure 6), within the selected timeframe of 45 s at each of the ten different droplet positions for the sandblasted and the sandpapered samples, a clear difference between the isotropic and anisotropic samples could be noticed. In the case of the isotropic sandblasted surface (
Figure 6a), no clear difference between the sets of droplets 1–5 (measured perpendicular to the surface texture direction) and 6–10 (measured parallel to the surface texture direction) was observed. Typical for the anisotropic samples was the clear grouping of the droplets as shown for the sandpapered sample in
Figure 6b. The explanation for this phenomenon is related to the fact that the directionally oriented surface treatment methods formed grooves with large maximum peak heights (e.g., Sp = 334.5 μm for the sandpapered sample).
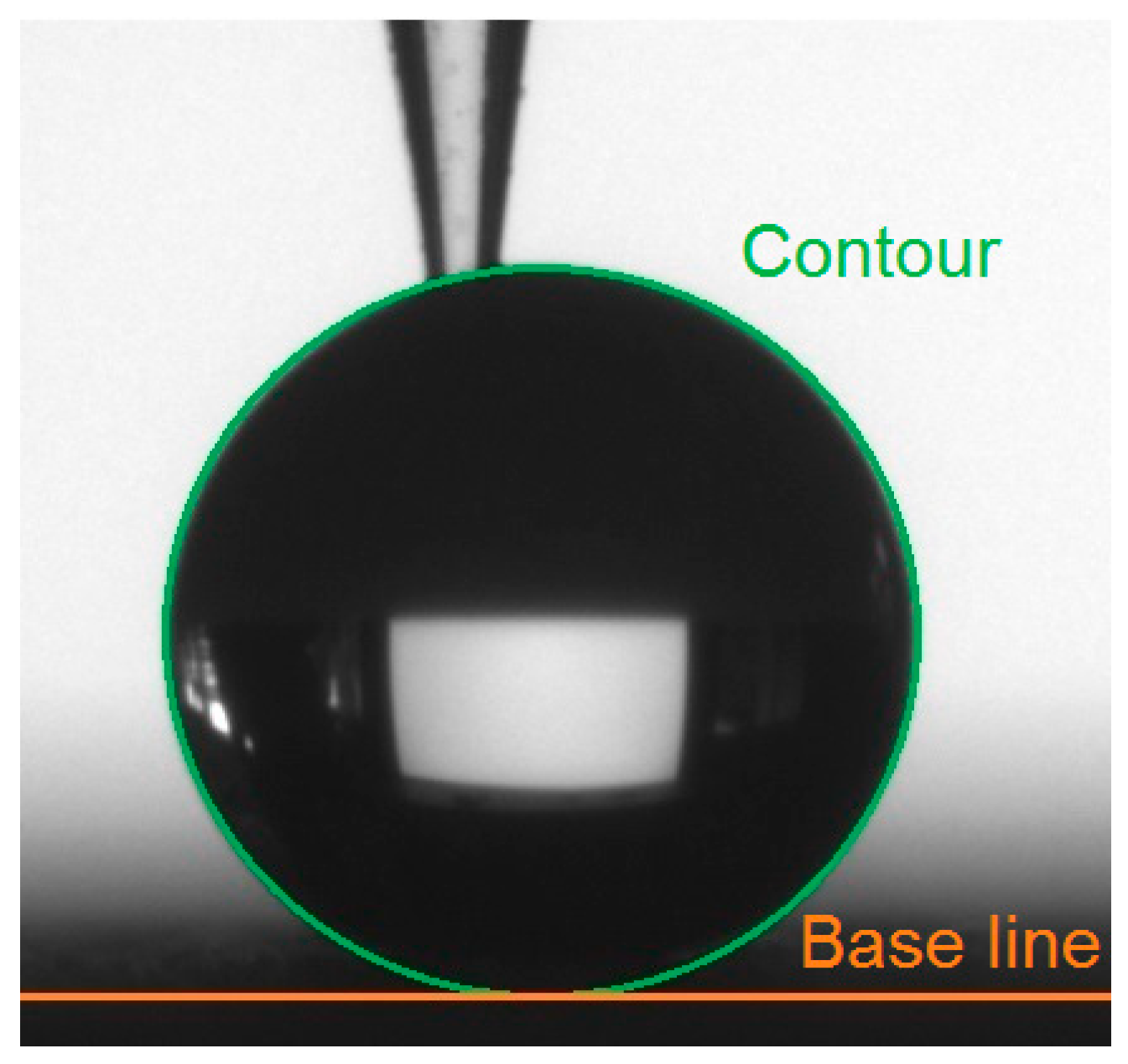
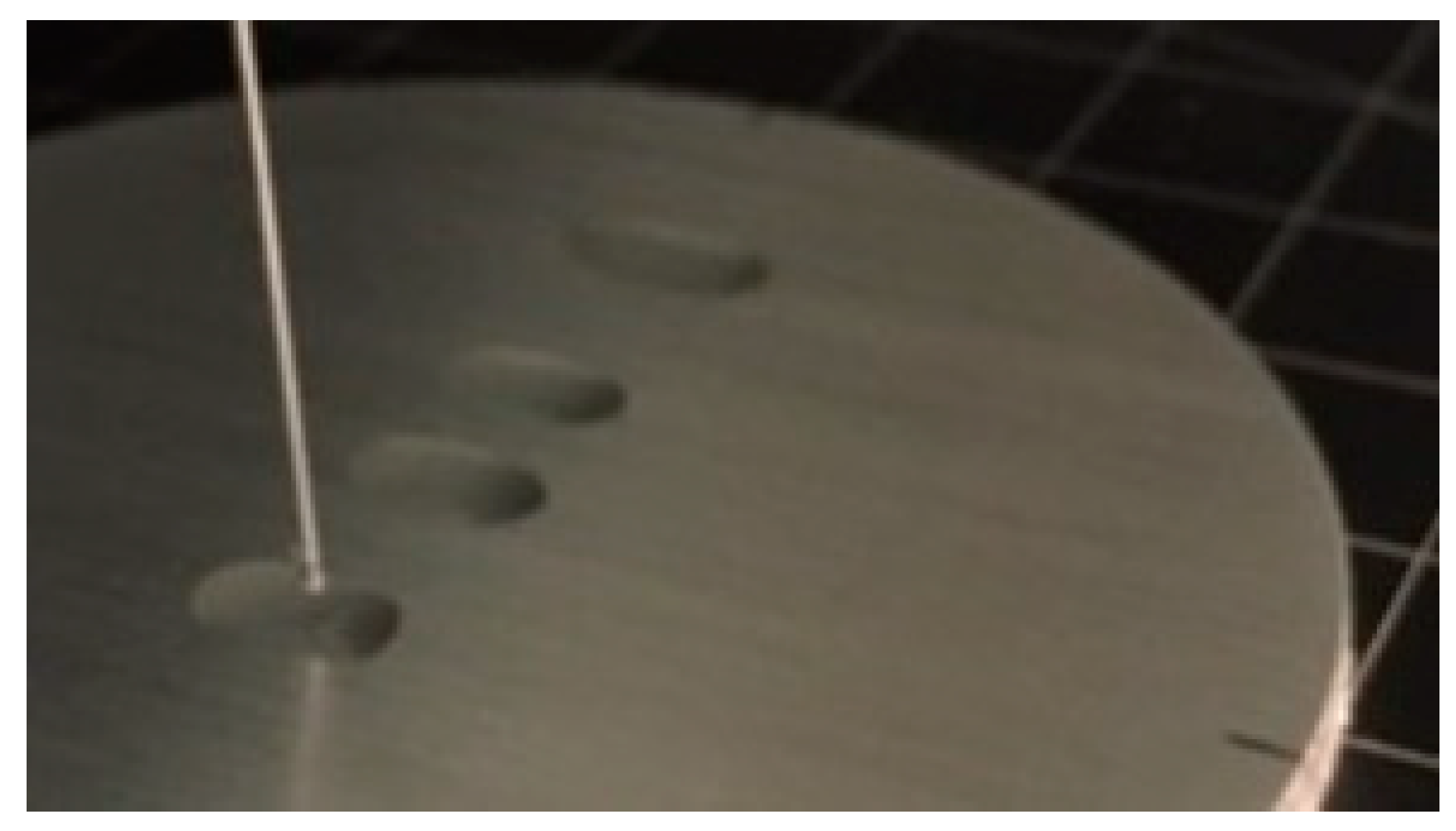
The grooves served as barriers which prevented water droplets from spreading freely across the grooves, as shown schematically in
Figure 7 for the case of droplet 8 (BD8). For droplet 8, the measured base diameter remained quite unchanged, but the contact angle of the drop increased [
33]. While measuring the water contact angle perpendicular to the surface structure (BD3), the contact angle decreased and the base diameter increased due to the capillary force which made the water droplet spread along the groove.
Despite the different roughness and structures, the contact angles of oil could not be measured on any of the uncoated samples as exemplified in
Figure 8. Due to the lower surface tension of oil compared to water, the capillary force accelerated the propagation of the oil droplet along the groove. Therefore, the oil penetrated into the surface structures and completely wetted the surface.
3.2. Coated Samples
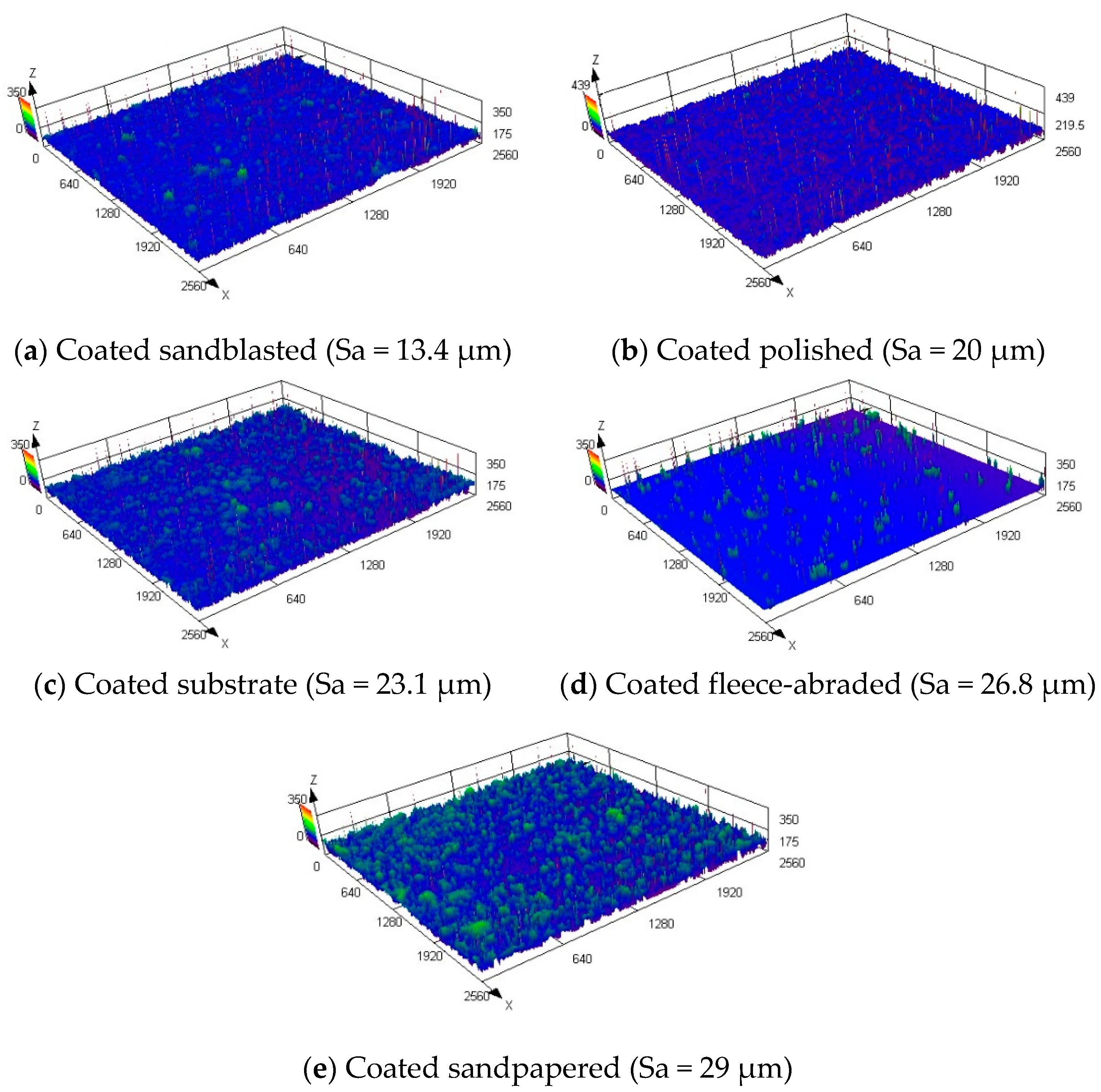
Coating the prepared samples with the amphiphobic layer helped to produce significantly rougher surfaces with the arithmetic mean heights in the range of Sa = 13.4–29 μm. The maximum Sa value achieved by the mechanical treatment of the uncoated sample was 15.8 µm, whereas the coated sample had almost twice as high a value of Sa = 29 µm.
The digital reproductions of the morphologies of the coated samples, made via microscope, are shown in
Figure 9. Mainly the random agglomeration of the coating particles contributed to the roughening, since the initial surface under the coating was not observed and the previous order of the uncoated samples, sorted by the increasing Sa value (as in
Figure 5), was no more kept.
In addition to the figures of the samples’ surfaces, the quantitative description by the selected areal roughness parameters are summarized in
Table 5. The determination of the individual profile elements in the horizontal direction was not possible; therefore, the mean width of the profile elements (RSm) was excluded from the analysis.
The coated sample, which had a base that was a sandblasted surface, had the lowest values of the arithmetic mean height (Sa = 13.4 μm), root mean squared height (Sq = 18.6 μm) and maximum peak height (Sp = 222.6 μm). This was remarkable, since the uncoated sandblasted sample exposed the roughest surface. During the coating, the particles within the coating fluid had filled the micro holes that were created arbitrarily by the sandblasting, forming a smooth, uniform surface.
In general, the originally anisotropic samples (fleece-abraded and sandpapered) formed significantly rougher surfaces after being coated than the originally isotropic samples (sandblasted and polished).
The wetting behaviour of the coated samples was again characterised through the measurements of the apparent contact angles of the two tested liquids: demineralized water (
) and rapeseed oil (
), presented in
Table 6.
With the contact angles of water higher than 150° and the contact angles of oil in the range between 139° and 150° for all samples, the coating resulted in the creation of the superhydrophobic and oleophobic surfaces according to the criteria mentioned in the
Section 1. The mechanical surface treatment methods, conducted before the coating, appeared to play a nonessential role, since all the coated samples exhibited similar contact angles of water
= 162°–173° as well as of oil,
= 139°–150°, in a similar range of standard deviations.
Compared to the water contact angles of the uncoated samples, the contact angles almost doubled after the application of the coating. Multiscale roughness was created due to the agglomeration of the coating particles (
Figure 10) which favoured the high contact angles [
34,
35]. Thus, a porous structure with a round, hilly surface was formed as shown in
Figure 9e. The inclusion of air is probable, indicating a Cassie–Baxter wetting state.
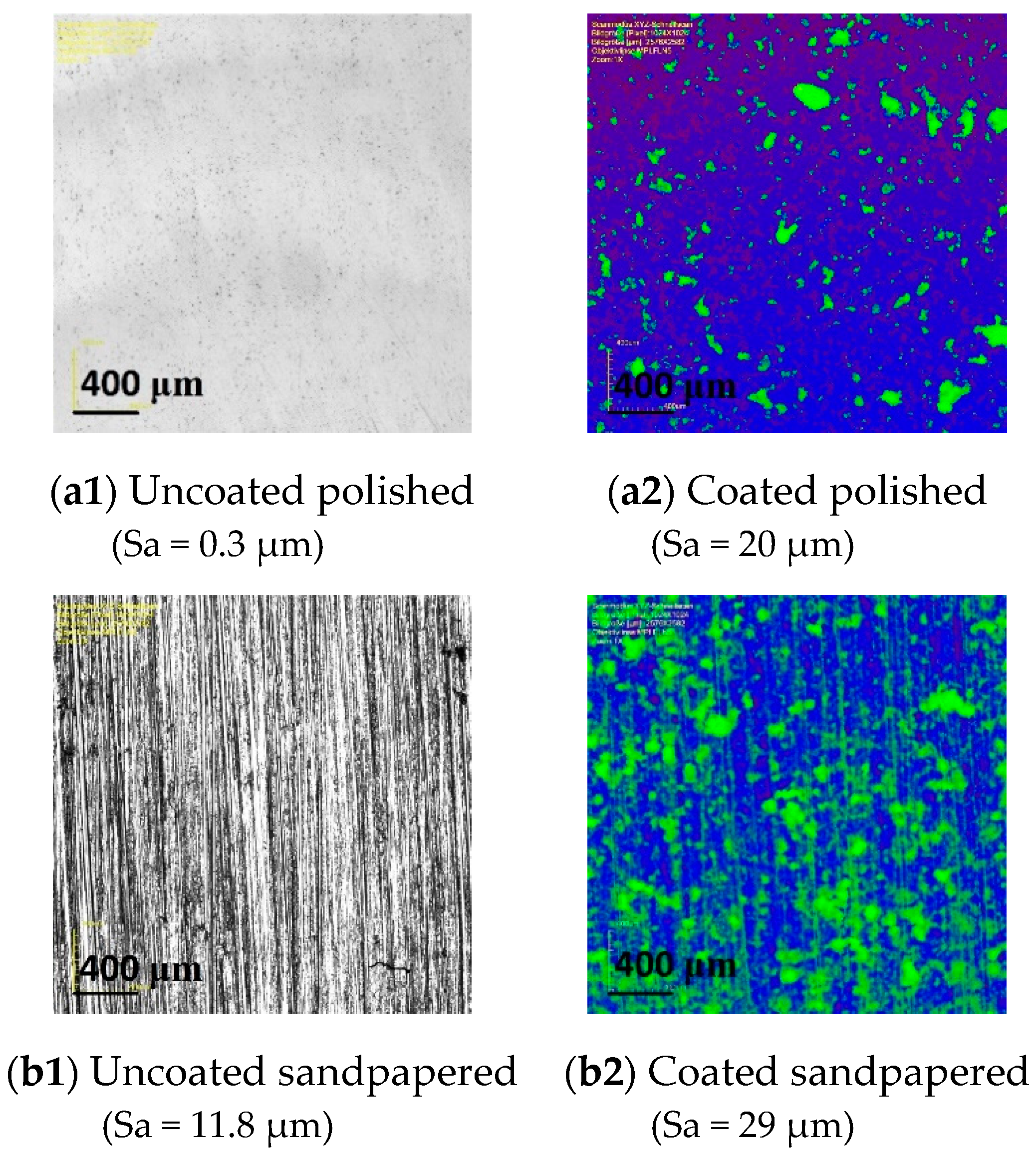
Microscopic images of the polished and sandpapered samples illustrate the coating particles’ arrangement in comparison to the initial uncoated surfaces (
Figure 11). The particles agglomerated on the surface arbitrarily and independently of the initial surface structure. Even in the case of the originally strongly anisotropic sandpapered sample (
Figure 11b1), its base structure was completely cancelled by the coating (
Figure 11b2).
All the coated samples had an isotropic wetting behaviour. The oil contact angles of the coated samples, measured perpendicular and parallel to the surface texture direction (
Table 7), showed slightly differing values between
and
. Nevertheless, these differences are attributed rather to the local surface energy and local surface structure.
The temperature stability of the coating was tested by heating up the coated substrate three times to the temperature of 50 °C for 1 h. After each heating step, the water contact angles were measured, and the results are presented in
Table 8. Under the influence of the increased temperature, the contact angles decreased from 175° to 138°, thus transferring the coated surface from the superhydrophobic to the hydrophobic region.
The main drop in the contact angle (around 30°) was measured after the first heating cycle, while afterwards the contact angle remained almost constant. A similar behaviour was observed with another coated substrate, heated up for only 10 min at 50 °C (
Table 9). The water contact angle decreased by 26° within just ten minutes of heating. On the other side, the elevated temperature did not affect the oil contact angles, which stayed unchanged (about 135°) during the testing. Therefore, heating the coated substrate at 50 °C may remove from the coating the components, that are water-repellent but have no influence on oil/surface interaction.
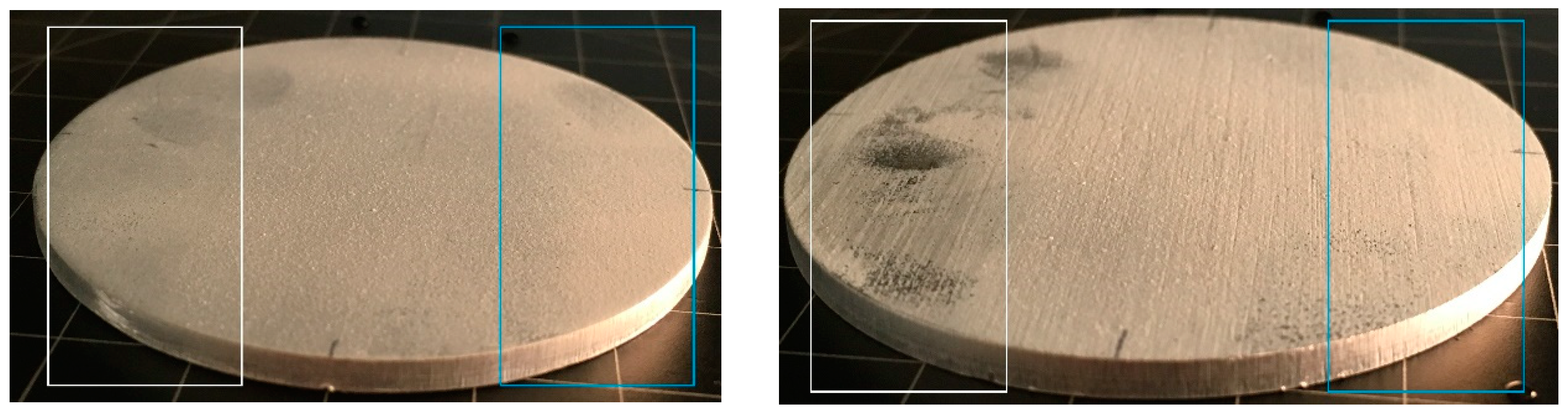
The coating adhesion was initially tested by the tape test using a stronger (to the left side of the coated samples) and weaker (to the right side of the coated samples) adhesive tape. The middle part of the samples was intentionally left unchanged for comparison (
Figure 12).
After using the stronger adhesive tape (white), a significant removal of the coating was observed for all coated samples. Better adhesion of the coating towards the originally rough samples (fleece-abraded and sandblasted), which was expected, could not be observed. A strong adhesion of the tape to the left side of the coated fleece-abraded sample (
Figure 12b) was the most noticeable of all the coated samples. Nevertheless, the measured water contact angles, even in this extreme case, were quite similar (
= 153° and
= 150°) as presented in
Table 10.
The coated as-received substrate showed significant decrease of the contact angle for both tape-tested parts of the surface, and, in addition, high standard deviations were observed for the values. The other tested samples exhibited contact angles of around 150° for the original surface as well as for the tape-tested surface, indicating that the coating layer was sufficiently thick to render the surface superhydrophobic, in spite of the partial removal of the coating. The significantly different behaviour of the coated as-received substrate may indicate that mechanical surface pre-treatments are beneficial for the coating adhesion. This preliminary adhesion test indicated the need for a more detailed investigation of the abrasive wear of the coating, especially in regard to the anisotropic structures, which is a partial outlook of the present study.