Copper Filled Poly(Acrylonitrile-co-Butadiene-co-Styrene) Composites for Laser-Assisted Selective Metallization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- poly(acrylonitrile-co-butadiene-co-styrene) (ABS) Terluran GP-35 Natural with a density of 1.04 g/cm3 and a flow rate of 55 cm3/10 min (10 kg, 220 °C) (Styrolution GmbH, Ludwigshafen, Germany);
- Copper (Cu) in the form of spherical particles (powder) with a purity of 98%, grain size 10–25 μm, density 8,96 g/cm³, melting point 1083.4 °C (Sigma - Aldrich, Poznań, Poland);
- Six components commercial electroless copper bath type M-Copper 85 (MacDermid-Poland, Łysomice, Poland) with formaldehyde 36% (POCH, Gliwice, Poland) as a reducing agent.
2.2. Processing
2.3. Measurements
3. Results and Discussion
3.1. Characterization of the Composites
3.2. Effects of Infrared Lasers Irradiation and Electroless Metallization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coiai, S.; Passaglia, E.; Pucci, A.; Ruggeri, G. Nanocomposites Based on Thermoplastic Polymers and Functional Nanofiller for Sensor Applications. Materials 2015, 8, 3377–3427. [Google Scholar] [CrossRef]
- Amend, P.; Pscherer, C.; Rechtenwald, T.; Frick, T.; Schmidt, M. A fast and flexible method for manufacturing 3D molded interconnect devices by the use of a rapid prototyping technology. Phys. Proc. 2010, 5, 561–572. [Google Scholar] [CrossRef]
- Balzereita, S.; Proesb, F.; Altstädta, V.; Emmelmann, C. Properties of copper modified polyamide 12-powders and their potential for the use as laser direct structurable electronic circuit carriers. Addit. Manuf. 2018, 23, 347–354. [Google Scholar] [CrossRef]
- Zhuo, Y.; Du, X.; Zhu, J. Three-dimensional automatic routing for the design of molded interconnect devices. Int. J. Comp. Integr. Manuf. 2011, 24, 302–311. [Google Scholar] [CrossRef]
- Franke, J. Three-Dimensional Molded Interconnect Devices (3D-MID); Carl Hanser: Munich, Germany, 2014. [Google Scholar]
- Jones, A.C.; Hitchman, M.L. Chemical Vapour Deposition Precursors, Processes and Applications; The Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing; Elsevier: Boston, MA, USA, 2010. [Google Scholar]
- Charbonnier, M.; Romand, M.; Goepfert, Y.; Leonard, D.; Bessuueille, F.; Bouadi, M. Palladium (+2) reduction: A key step for the electroless Ni metallization of insulating substrates by a tin-free process. Thin Solid Film. 2006, 515, 1623–1633. [Google Scholar] [CrossRef]
- Bhansali, S.; Sood, D.K. A novel technique for fabrication of metallic structures on polyimide by selective electroless copper plating using ion implantation. Thin Solid Film. 1995, 270, 489–492. [Google Scholar] [CrossRef]
- Yu, Z.J.; Kang, E.T.; Neoh, K.G. Electroless plating of copper on polyimide films modified by surface grafting of tertiary and quaternary amines polymers. Polymer 2002, 43, 4137–4146. [Google Scholar] [CrossRef]
- Zoski, C.G. Handbook of Electrochemistry; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Zhang, Y.; Hansen, H.N.; Grave, A.; Tang, P.T.; Nielsen, J.S. Selective metallization of polymers using laser induced surface activation (LISA)-characterization and optimization of porous surface topography. Int. J. Adv. Manuf. Technol. 2011, 55, 573–580. [Google Scholar] [CrossRef]
- Zhang, Y.; Kontogeorgis, G.M.M.; Hansen, H.N. An explanation of the selective plating of laser machined surfaces using surface tension components. J. Adhes. Sci. Technol. 2011, 25, 2101–2111. [Google Scholar] [CrossRef]
- Ratautas, K.; Andrulevičius, M.; Jagminienė, A.; Stankevičienė, I.; Norkusa, E.; Račiukaitisa, G. Laser-assisted selective copper deposition on commercial PA6 by catalytic electroless plating—Process and activation mechanism. Appl. Surf. Sci. 2019, 470, 405–410. [Google Scholar] [CrossRef]
- Bachya, B.; Franke, J. Experimental investigation and optimization for the effective parameters in the laser direct structuring process. J. Laser Micro Nanoeng. 2015, 10, 202–209. [Google Scholar] [CrossRef][Green Version]
- Wojakowski, B.; Klug, U.; Dusing, J.; Kling, R. Fabrication of Thermoformable Circuits by Laser Patterning of Metallized Thermoplastic Foils. Phys. Procedia 2010, 5, 301–309. [Google Scholar] [CrossRef]
- Charbonnier, M.; Romand, M.; Goepfert, Y. Ni direct electroless metallization of polymers by a new palladium-free process. Surf. Coat. Technol. 2006, 200, 5028–5036. [Google Scholar] [CrossRef]
- Geretovszky, Z.; Boyd, I. Kinetic study of 222 nm excimer lamp induced decomposition of palladium-acetate films. Appl. Surf. Sci. 1999, 138–139, 401–407. [Google Scholar] [CrossRef]
- Charbonnier, M.; Romand, M.; Goepfert, Y.; Leonard, D.; Bouadi, M. Copper metallization of polymers by a palladium-free electroless process. Surf. Coat. Technol. 2006, 200, 5478–5486. [Google Scholar] [CrossRef]
- Hanus, F.; Kolev, K.; Jadin, A.; Laude, L.D. Excimer laser-induced copper nanocluster formation in mixed PMMA/copper acetylacetonate films. Appl. Surf. Sci. 2000, 154–155, 320–323. [Google Scholar] [CrossRef]
- Rytlewski, P.; Żenkiewicz, M.; Tracz, A.; Moraczewski, K.; Mróz, W. Surface morphology studies of laser irradiated and chemically metalized polyamide composites. Surf. Coat. Technol. 2011, 205, 5248–5253. [Google Scholar] [CrossRef]
- Rytlewski, P. Laser induced electroactivity of polyamide composites. Electrochim. Acta 2012, 61, 191–197. [Google Scholar] [CrossRef]
- Rytlewski, P.; Jagodziński, B.; Malinowski, R.; Budner, B.; Moraczewski, K.; Wojciechowska, A.; Augustyn, P. Laser-induced surface activation and electroless metallization of polyurethane coating containing copper (II) L-tyrosine. Appl. Surf. Sci. 2019, 505, 144429. [Google Scholar] [CrossRef]
- Rytlewski, P.; Jagodziński, B.; Wojciechowska, A.; Moraczewski, K.; Malinowski, R. TG-FTIR coupled analysis to predetermine effective precursors for laser-activated and electroless metallized materials. J. Therm. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Yang, J.U.; Cho, J.H.; Yoo, M.J. Selective metallization on copper aluminate composite via laser direct structuring technology. Compos. Part B Eng. 2017, 110, 361–367. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, T.; Wen, L. Selective metallization induced by laser activation: Fabricating metallized patterns on polymer via metal oxide composite. ACS Appl. Mater. Interfaces 2017, 9, 8996–9005. [Google Scholar] [CrossRef] [PubMed]
- Rytlewski, P. Laser-assisted metallization of composite coatings containing copper(II) acetylacetonate and copper(II) oxide or copper(II) hydroxide. Surf. Coat. Technol. 2014, 259, 660–666. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, T.; Wen, L.; Zhang, A. Fabricating metallic circuit patterns on polymer substrates through laser and selective metallization. ACS Appl. Mater. Interfaces 2016, 8, 33999–34007. [Google Scholar] [CrossRef]
- ISO 11357-6: Plastics—Differential Scanning Calorimetry (DSC)—Part 6: Determination of Oxidation Induction Time (Isothermal OIT) and Oxidation Induction Temperature (Dynamic OIT); International Organization for Standardization: Geneva, Switzerland, 2018.
- Standard PN-EN ISO 527-2: Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics; International Organization for Standardization: Geneva, Switzerland, 2012.
- Standard PN-EN ISO 527-1: Plastics—Determination of Tensile Properties—Part 1: General Principles; International Organization for Standardization: Geneva, Switzerland, 2019.
- Standard PN-EN ISO 1133-1: Plastics—Determination of the Melt Mass-Flow Rate (MFR) and the Melt Volume-Flow Rate (MVR) of Thermoplastics; International Organization for Standardization: Geneva, Switzerland, 2011.
- Blom, H.; Yeh, R.; Wojnarowski, R.; Ling, M. Detection of degradation of ABS materials via DSC. Thermochim. Acta 2006, 442, 64–66. [Google Scholar] [CrossRef]
- Bair, H.E.; Boyle, D.J.; Kelleher, P.G. The Effects of Light and Heat on the Rubber Content and Impact Strength of Acrylonitrile-Butadiene-Styrene. Polym. Eng. Sci. 1980, 20, 995–1001. [Google Scholar] [CrossRef]
- Suzuki, M.; Wilkie, C. The thermal degradation of acrylonitrile-butadiene-styrene terpolymer as studied by TGA/FTIR. Polym. Degrad. Stab. 1995, 47, 217–221. [Google Scholar] [CrossRef]
- Ghaemy, M.; Scott, G. Photo- and thermal oxidation of ABS: Correlation of loss of impact strength with degradation of the rubber component. Polym. Degrad. Stab. 1981, 3, 233–242. [Google Scholar] [CrossRef]
- Wolkowicz, M.D.; Gagga, S.K. Effect of thermal aging on impact strength acrylonitrile-butadiene-styrene (ABS) terpolymer. Polym. Eng. Sci. 1981, 21, 571–575. [Google Scholar] [CrossRef]


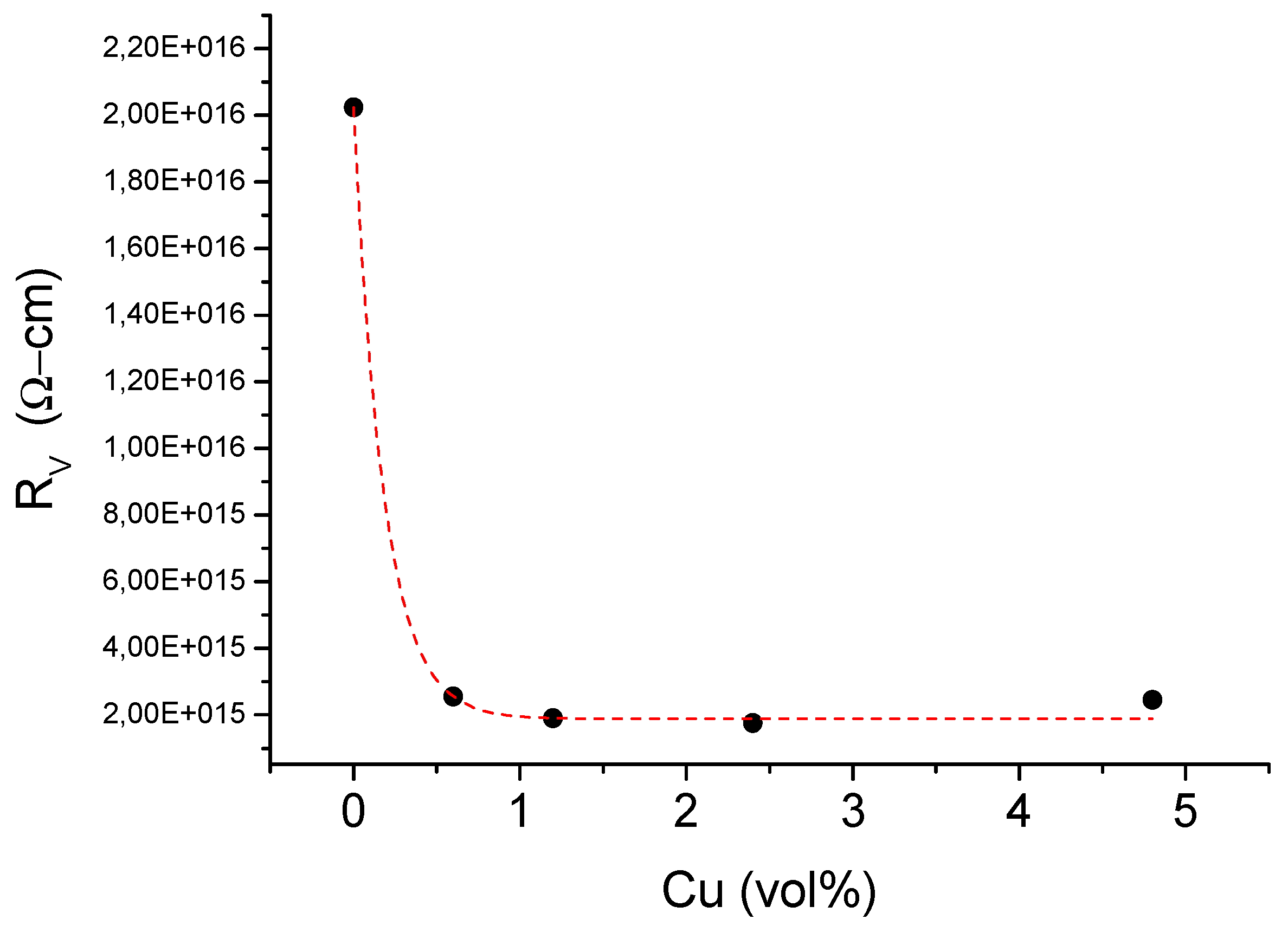
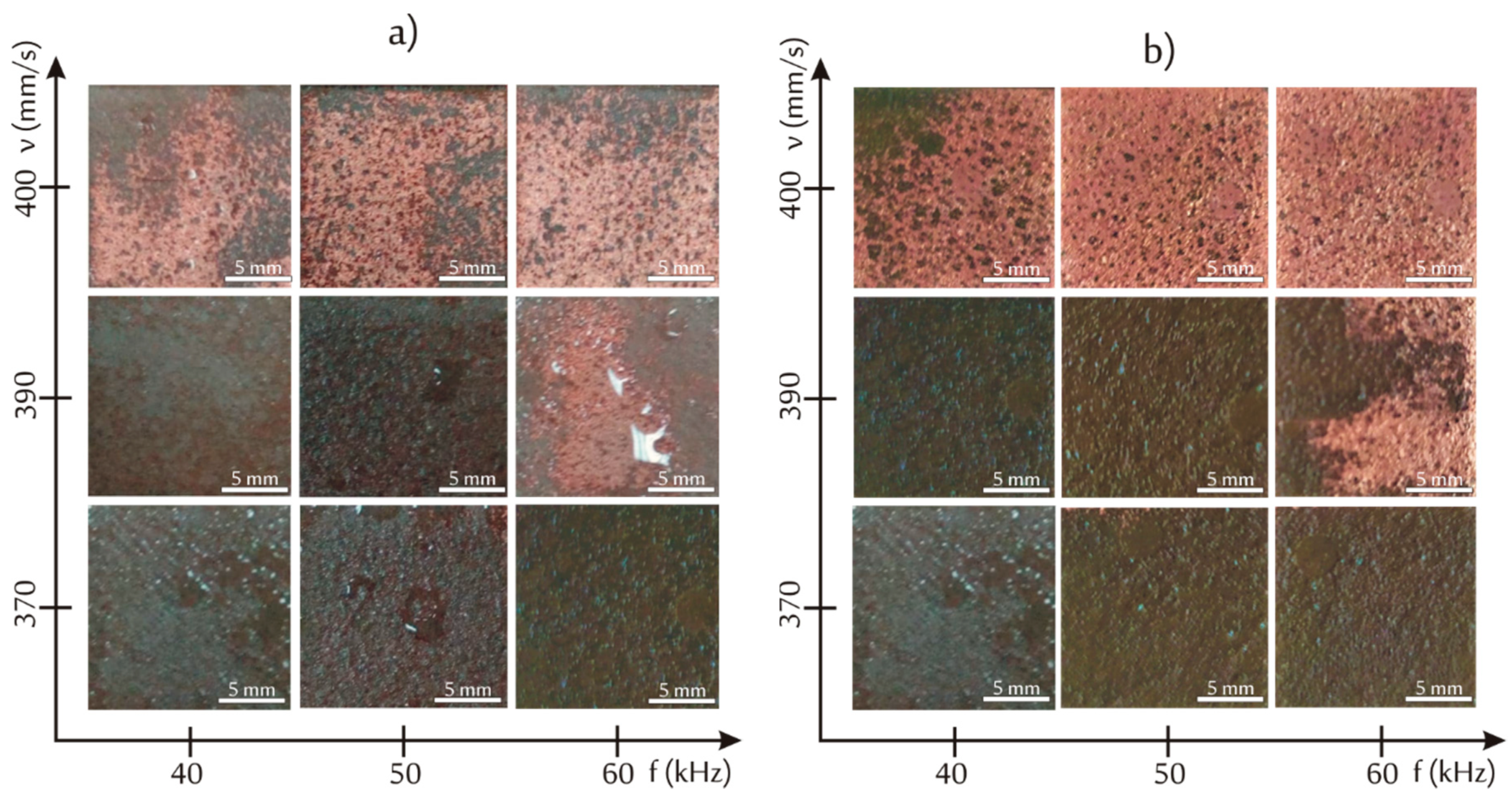
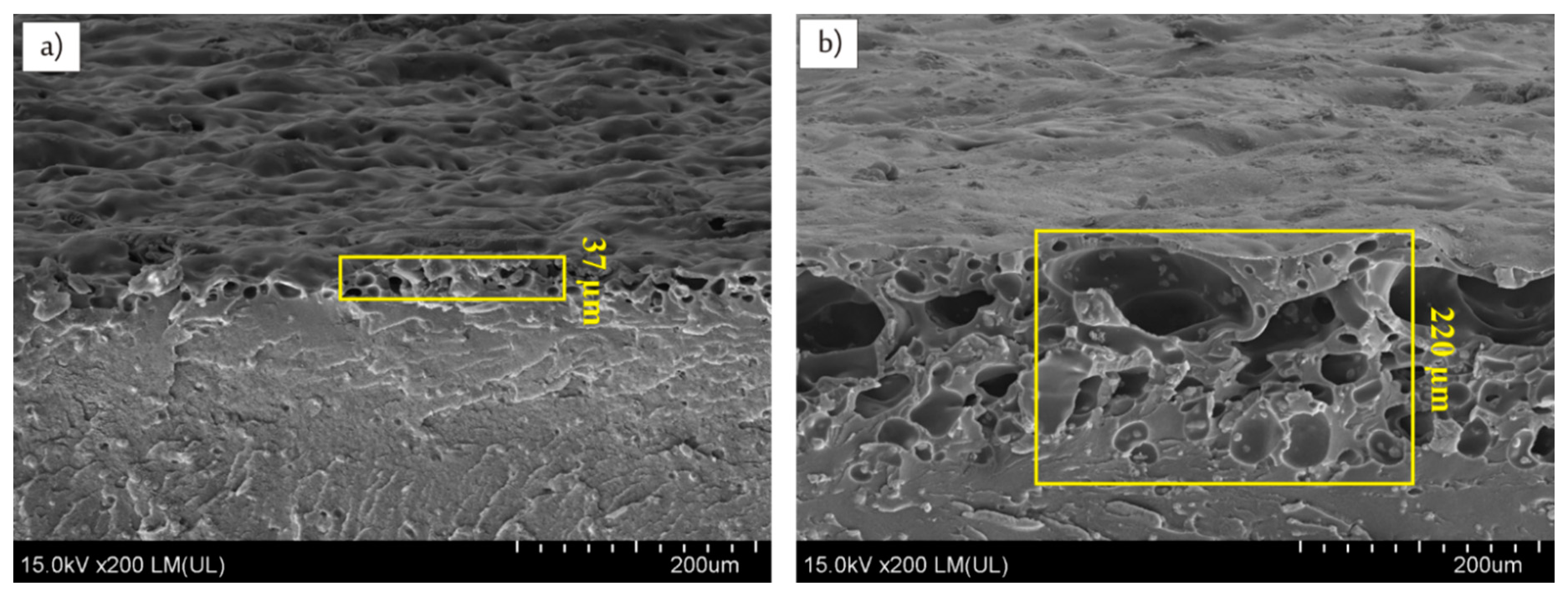
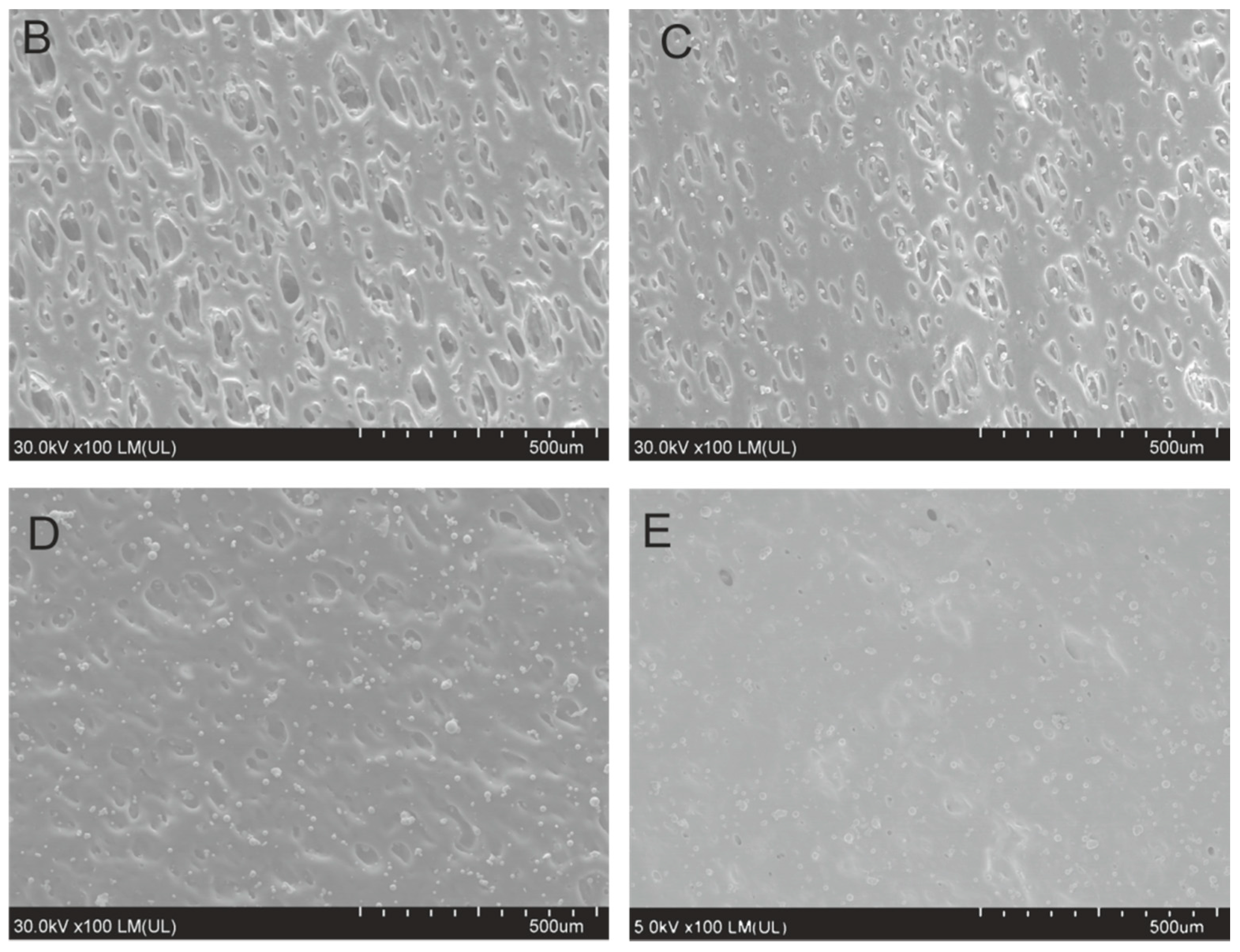
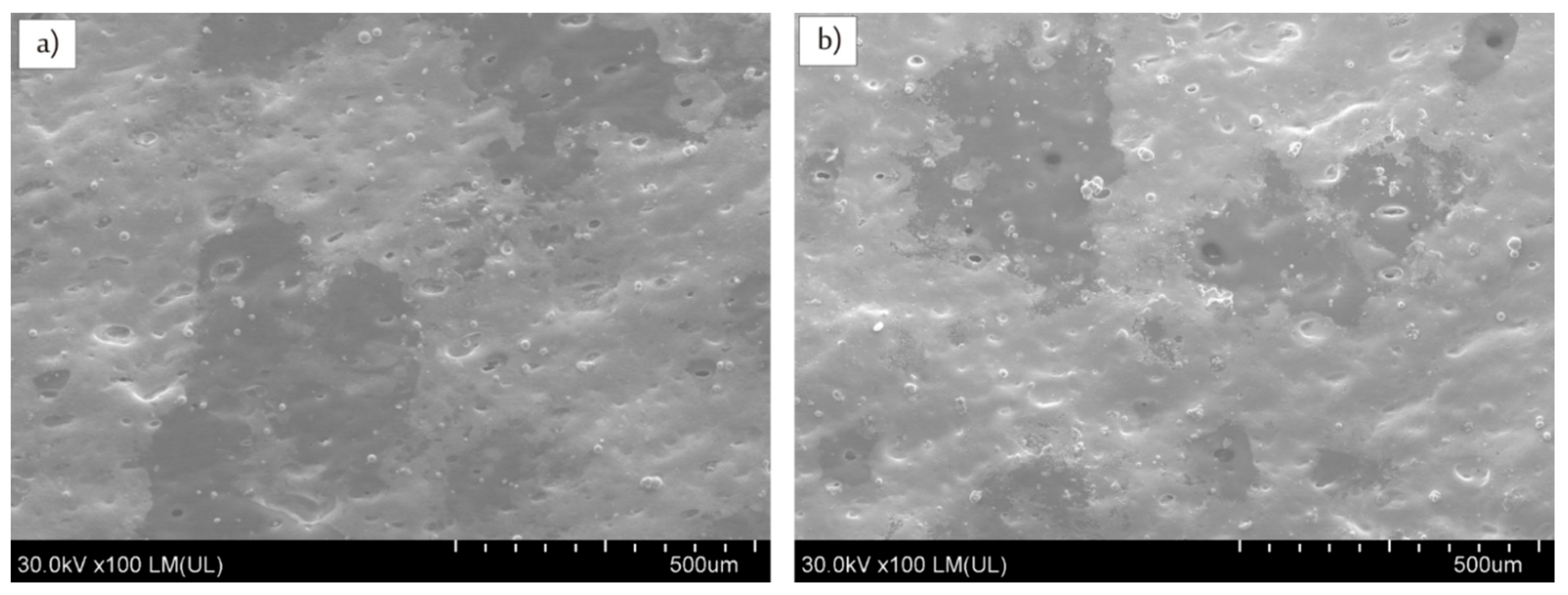
| Samples | Cu (wt %) | Cu (vol %) |
|---|---|---|
| A | 0 | 0 |
| B | 5 | 0.6 |
| C | 10 | 1.2 |
| D | 20 | 2.4 |
| E | 40 | 4.8 |
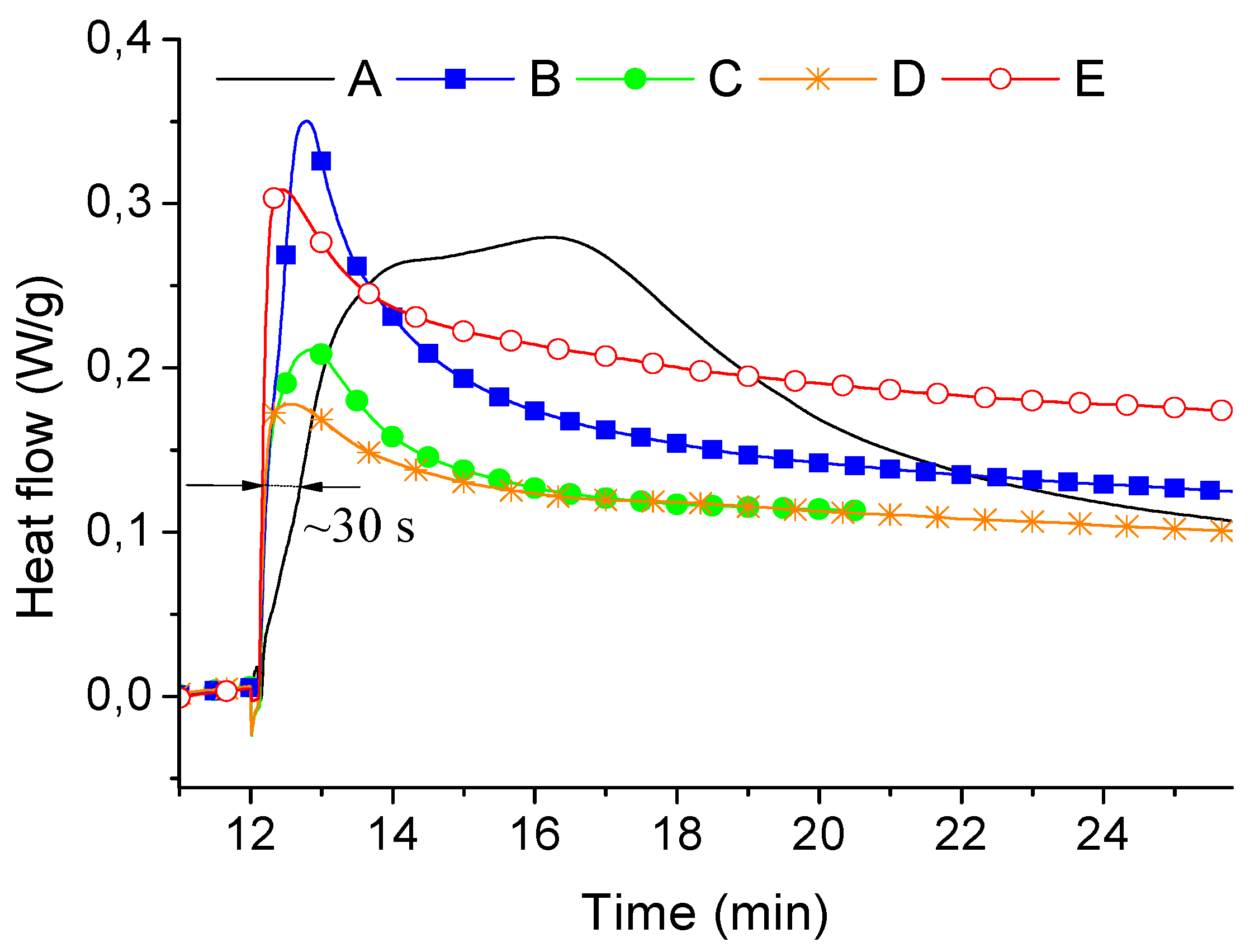
| Samples | Φ | H (J/g) | HABS (J/g) |
|---|---|---|---|
| A | 1 | 8.4 | 8.4 |
| B | 0.95 | 7.5 | 7.9 |
| C | 0.9 | 7.3 | 8.1 |
| D | 0.8 | 6.2 | 7.8 |
| E | 0.6 | 3.53 | 5.9 |
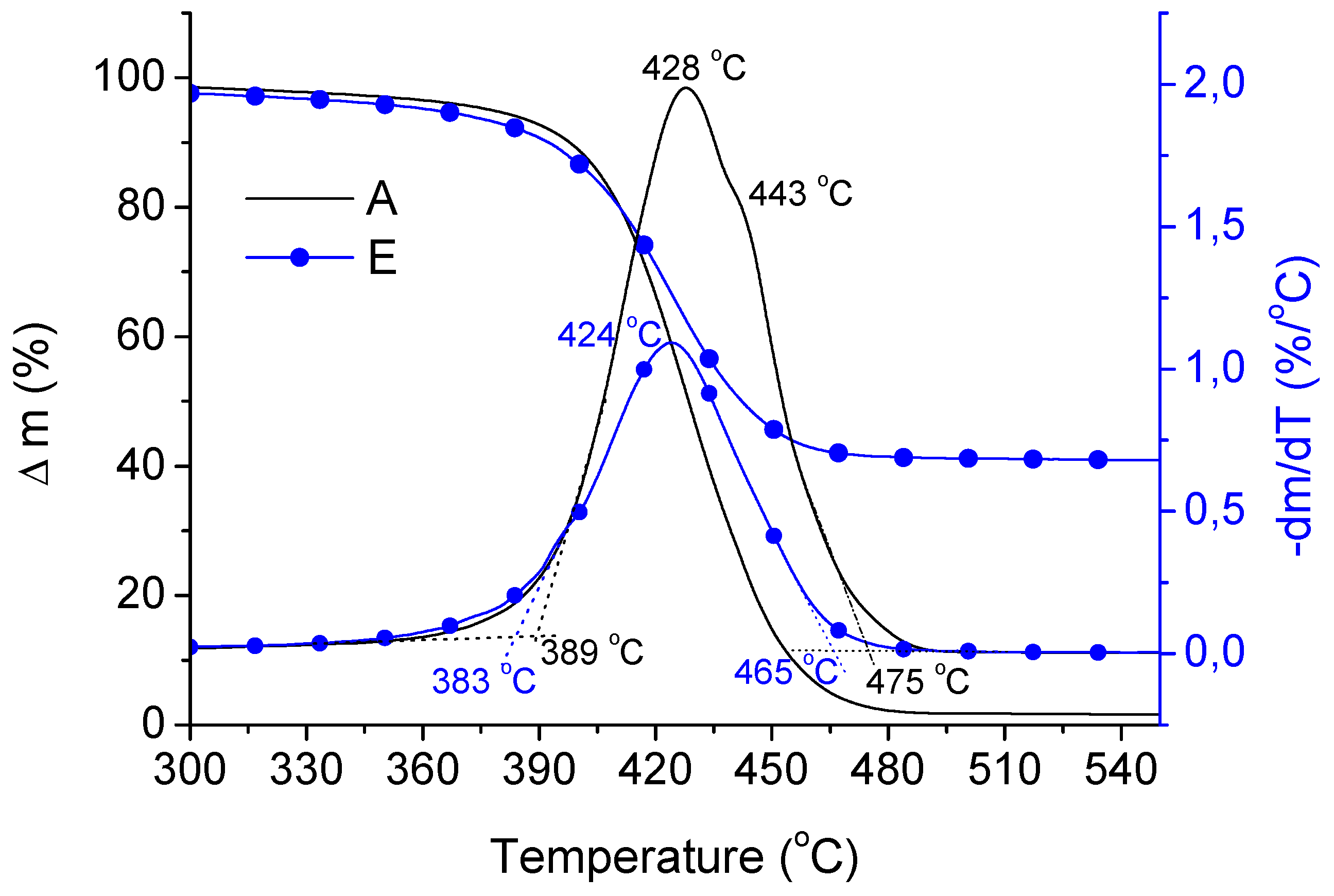
| Samples | TOn (°C) | TMax (°C) | TEnd (°C) |
|---|---|---|---|
| A | 389 | 428 | 475 |
| B | 382 | 428 | 466 |
| C | 385 | 429 | 467 |
| D | 386 | 429 | 466 |
| E | 383 | 424 | 465 |
| Sample | E (MPa) | σM (MPa) | εM (%) | σB (MPa) | εB (%) | EB (kJ/m2) | MFR 220 °C; 5 kg |
|---|---|---|---|---|---|---|---|
| A | 1143 ± 20 | 33.2 ± 1.9 | 4.93 ± 0.38 | 23.7 ± 5.0 | 7.0 ± 1.1 | 16.3 ± 1.6 | 26.5 ± 0.9 |
| B | 1082 ± 38 | 39.3 ± 1.7 | 6.80 ± 0.21 | 38.8 ± 1.6 | 7.0 ± 0.3 | 33.0 ± 2.5 | 21.9 ± 0.6 |
| C | 1103 ± 22 | 36.9 ± 1.8 | 5.42 ± 0.32 | 36.9 ± 1.8 | 5.4 ± 0.4 | 24.6 ± 2.4 | 16.5 ± 0.5 |
| D | 1276 ± 17 | 36.3 ± 1.6 | 4.80 ± 0.31 | 33.8 ± 3.9 | 5.2 ± 0.3 | 21.7 ± 1.5 | 12.0 ± 0.8 |
| E | 1398 ± 24 | 32.5 ±1.6 | 3.92 ± 0.25 | 30.2 ± 3.6 | 4.2 ± 0.3 | 14.9 ± 1.2 | 10.0 ± 0.5 |
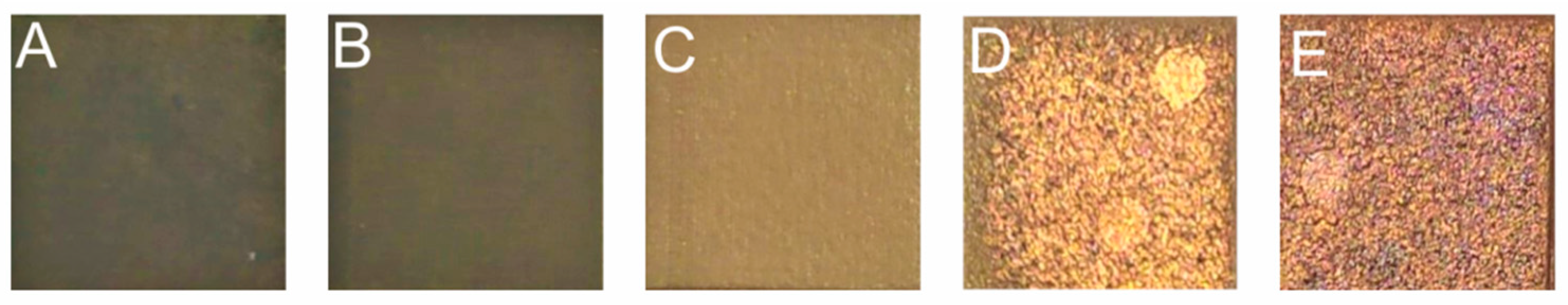
| Composite | Treatment | Element (wt %) | ||
|---|---|---|---|---|
| Cu | C | O | ||
| D | irradiated | 3.7 | 96.3 | 0 |
| D | metalized | 46.3 | 39.3 | 14.2 |
| E | irradiated | 5.2 | 94.7 | 0 |
| E | metalized | 74.7 | 8.9 | 16.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rytlewski, P.; Jagodziński, B.; Karasiewicz, T.; Augustyn, P.; Kaczor, D.; Malinowski, R.; Szabliński, K.; Mazurkiewicz, M.; Moraczewski, K. Copper Filled Poly(Acrylonitrile-co-Butadiene-co-Styrene) Composites for Laser-Assisted Selective Metallization. Materials 2020, 13, 2224. https://doi.org/10.3390/ma13102224
Rytlewski P, Jagodziński B, Karasiewicz T, Augustyn P, Kaczor D, Malinowski R, Szabliński K, Mazurkiewicz M, Moraczewski K. Copper Filled Poly(Acrylonitrile-co-Butadiene-co-Styrene) Composites for Laser-Assisted Selective Metallization. Materials. 2020; 13(10):2224. https://doi.org/10.3390/ma13102224
Chicago/Turabian StyleRytlewski, Piotr, Bartłomiej Jagodziński, Tomasz Karasiewicz, Piotr Augustyn, Daniel Kaczor, Rafał Malinowski, Krzysztof Szabliński, Marcin Mazurkiewicz, and Krzysztof Moraczewski. 2020. "Copper Filled Poly(Acrylonitrile-co-Butadiene-co-Styrene) Composites for Laser-Assisted Selective Metallization" Materials 13, no. 10: 2224. https://doi.org/10.3390/ma13102224
APA StyleRytlewski, P., Jagodziński, B., Karasiewicz, T., Augustyn, P., Kaczor, D., Malinowski, R., Szabliński, K., Mazurkiewicz, M., & Moraczewski, K. (2020). Copper Filled Poly(Acrylonitrile-co-Butadiene-co-Styrene) Composites for Laser-Assisted Selective Metallization. Materials, 13(10), 2224. https://doi.org/10.3390/ma13102224







