The Properties of Composites with Recycled Cement Mortar Used as a Supplementary Cementitious Material
Abstract
1. Introduction
2. Technology of Recycled Cement Mortar (RCM) Production
2.1. The Initial Concrete Rubble
2.2. The Recycling Process of Concrete (Thermo-Mechanical Treatment According to PAT.229887)
3. Materials and Methods
3.1. Materials
3.2. The Cement Composites with RCM
3.3. Methods
3.3.1. Physical and Mechanical Properties Test Methods
3.3.2. Analysis of RCM Properties and Microstructure
4. Design of the Experiment
Selection of Variables and Development of the Experimental Plan
5. Test Results and Discussion
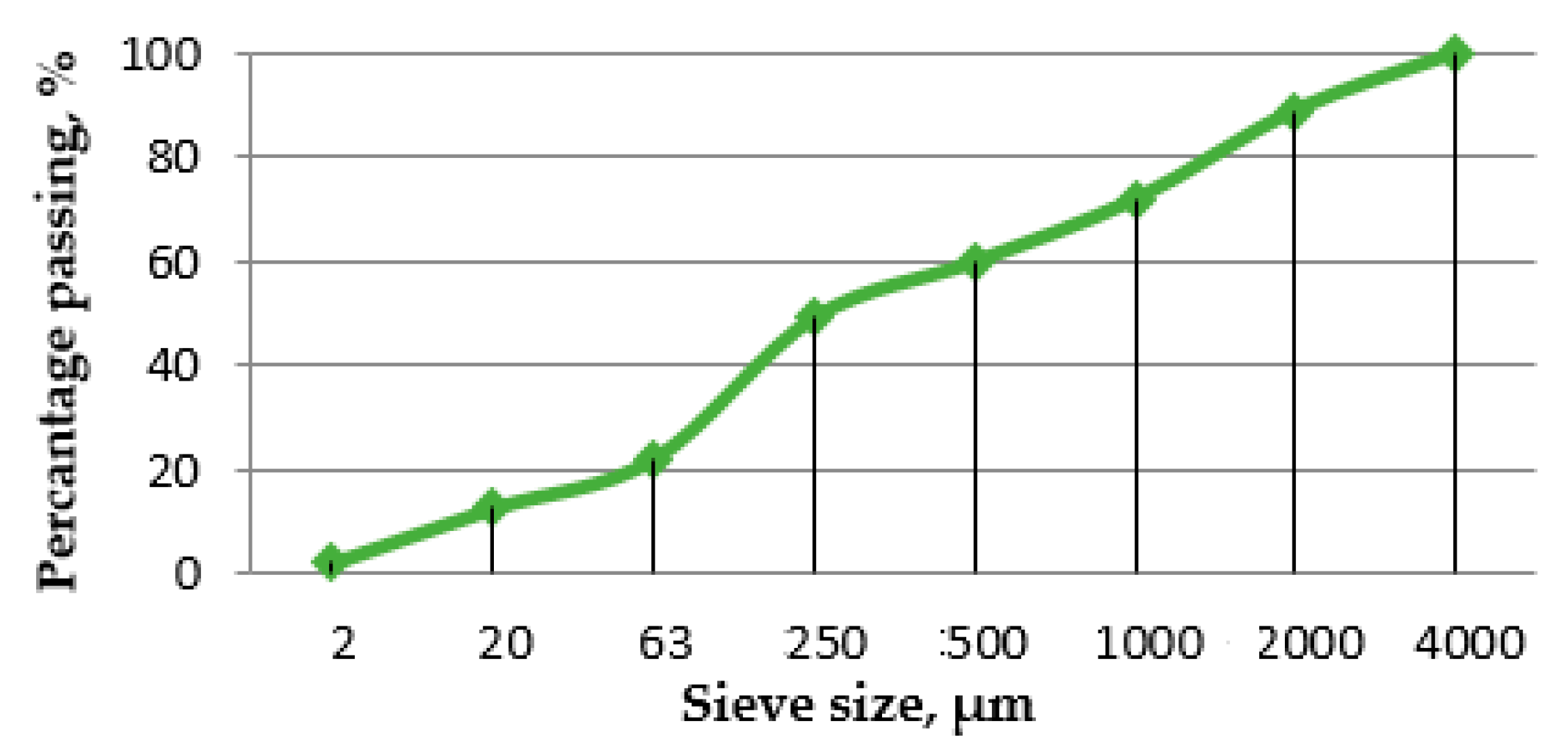
5.1. Characteristics of Recycled Cement Mortar (RCM)
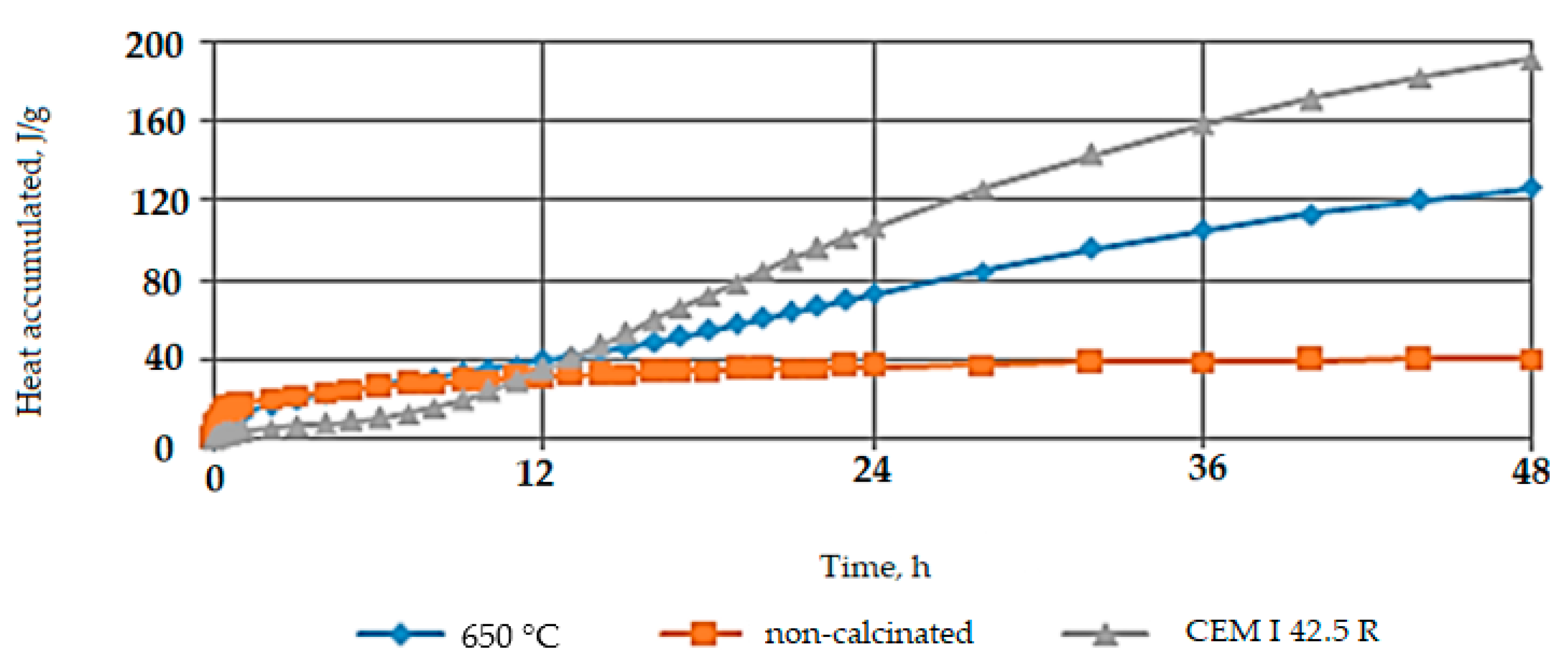
5.1.1. Heat of Hydration
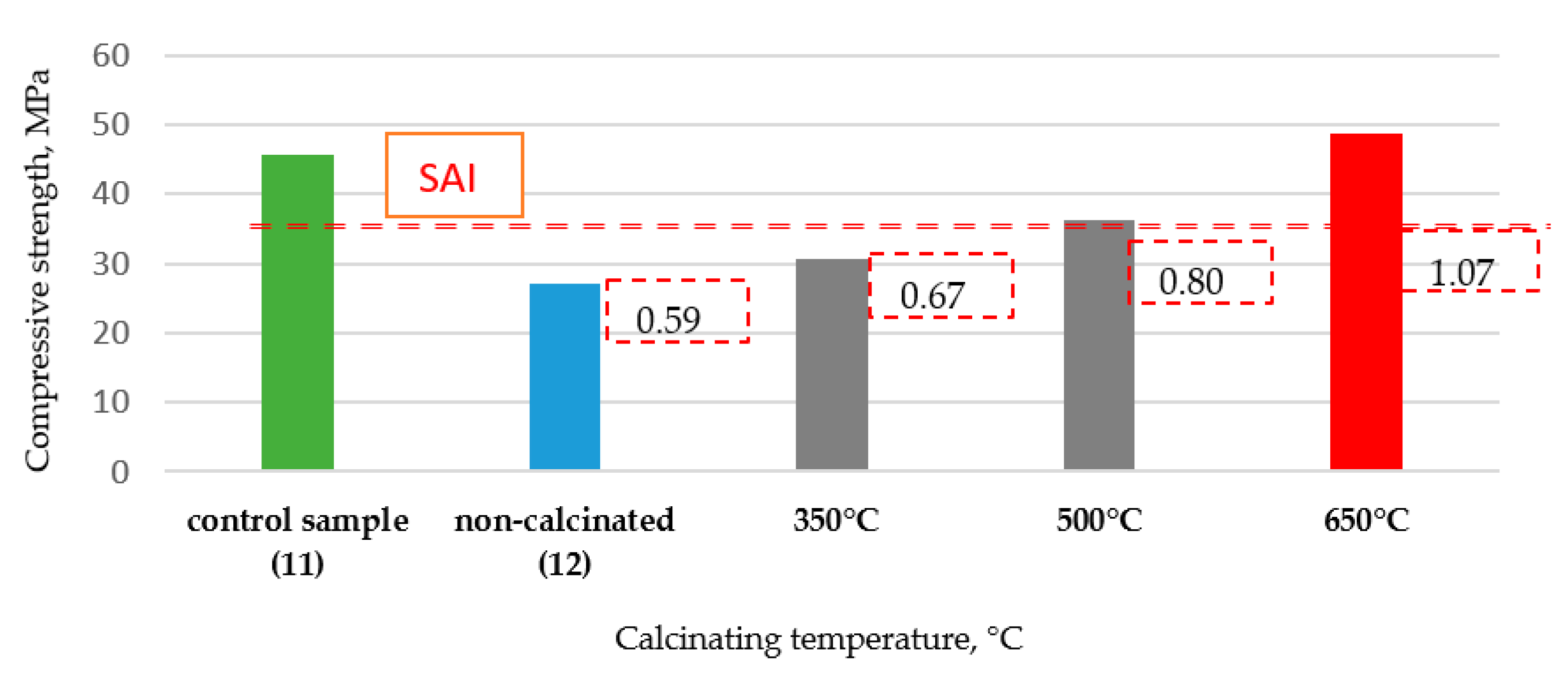
5.1.2. Strength Activity Index (SAI)
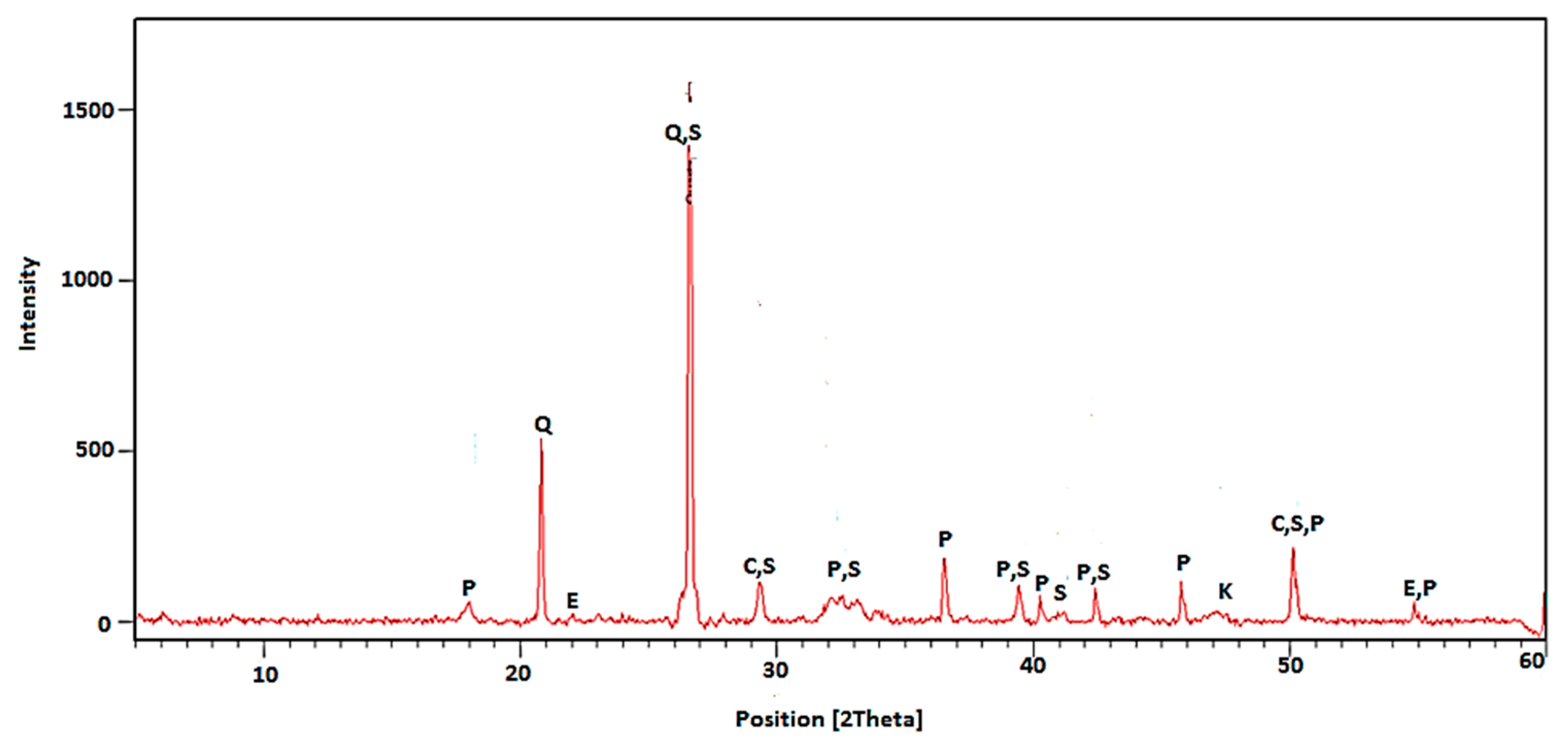
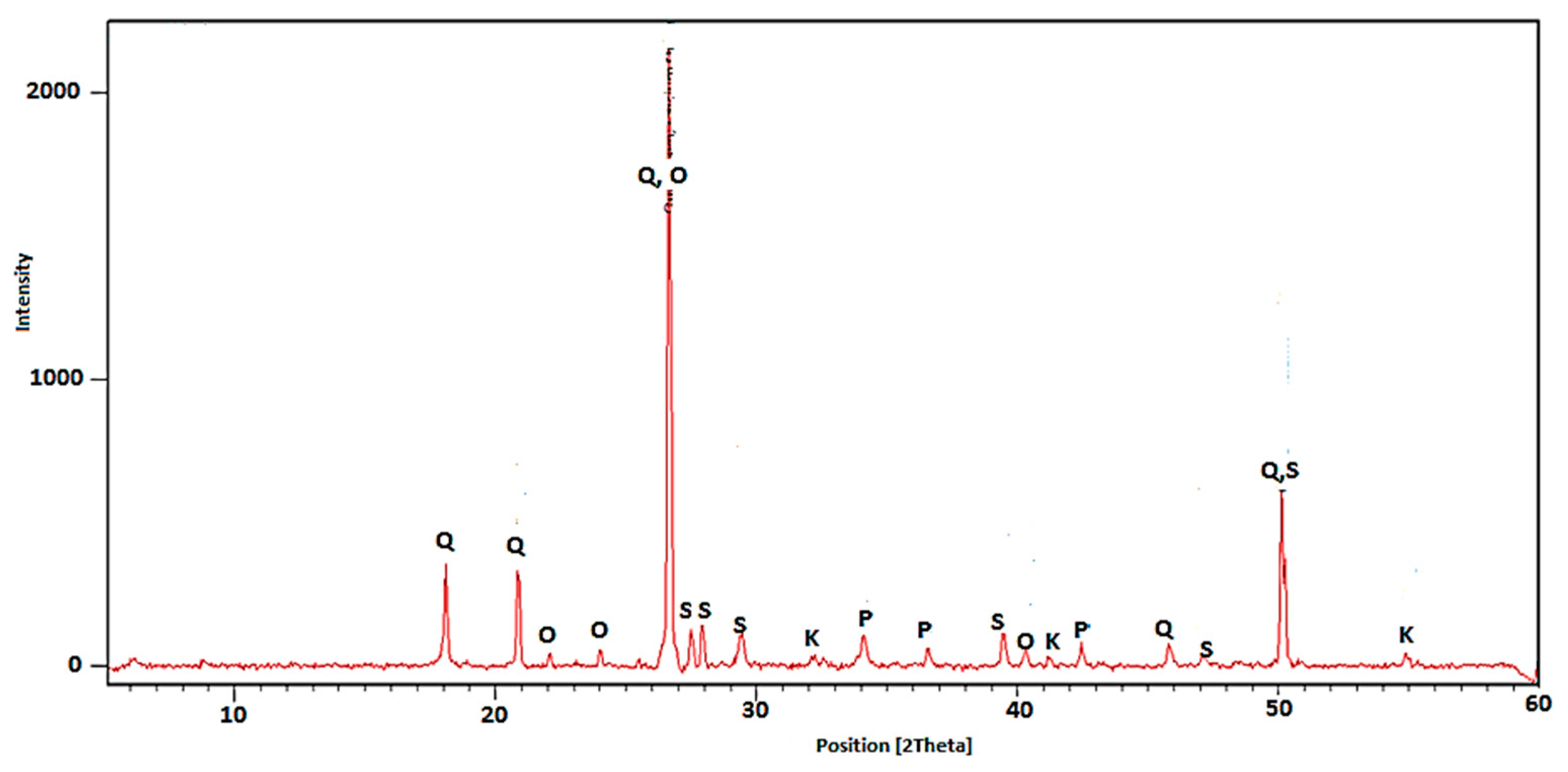
5.1.3. X-ray Diffractometry
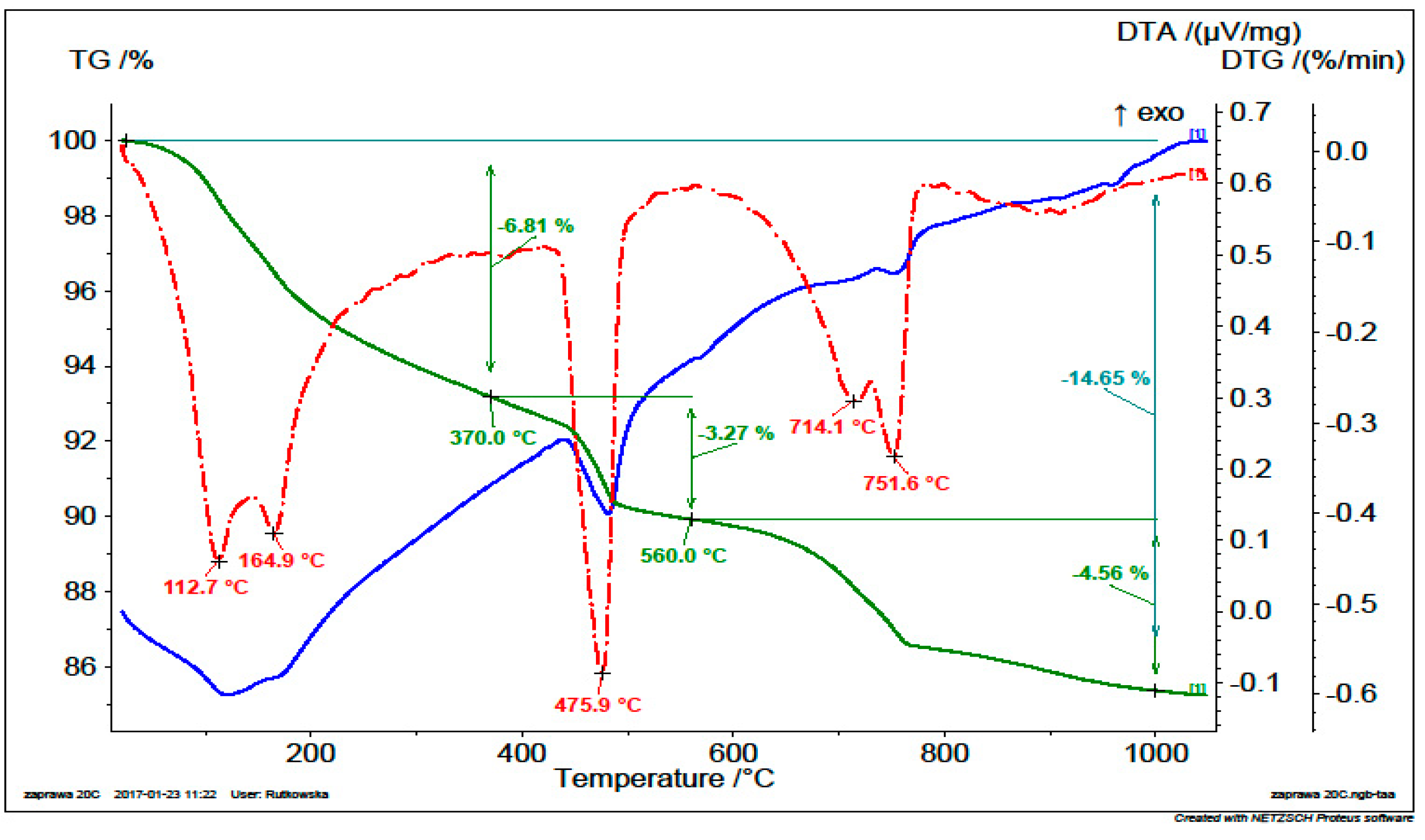
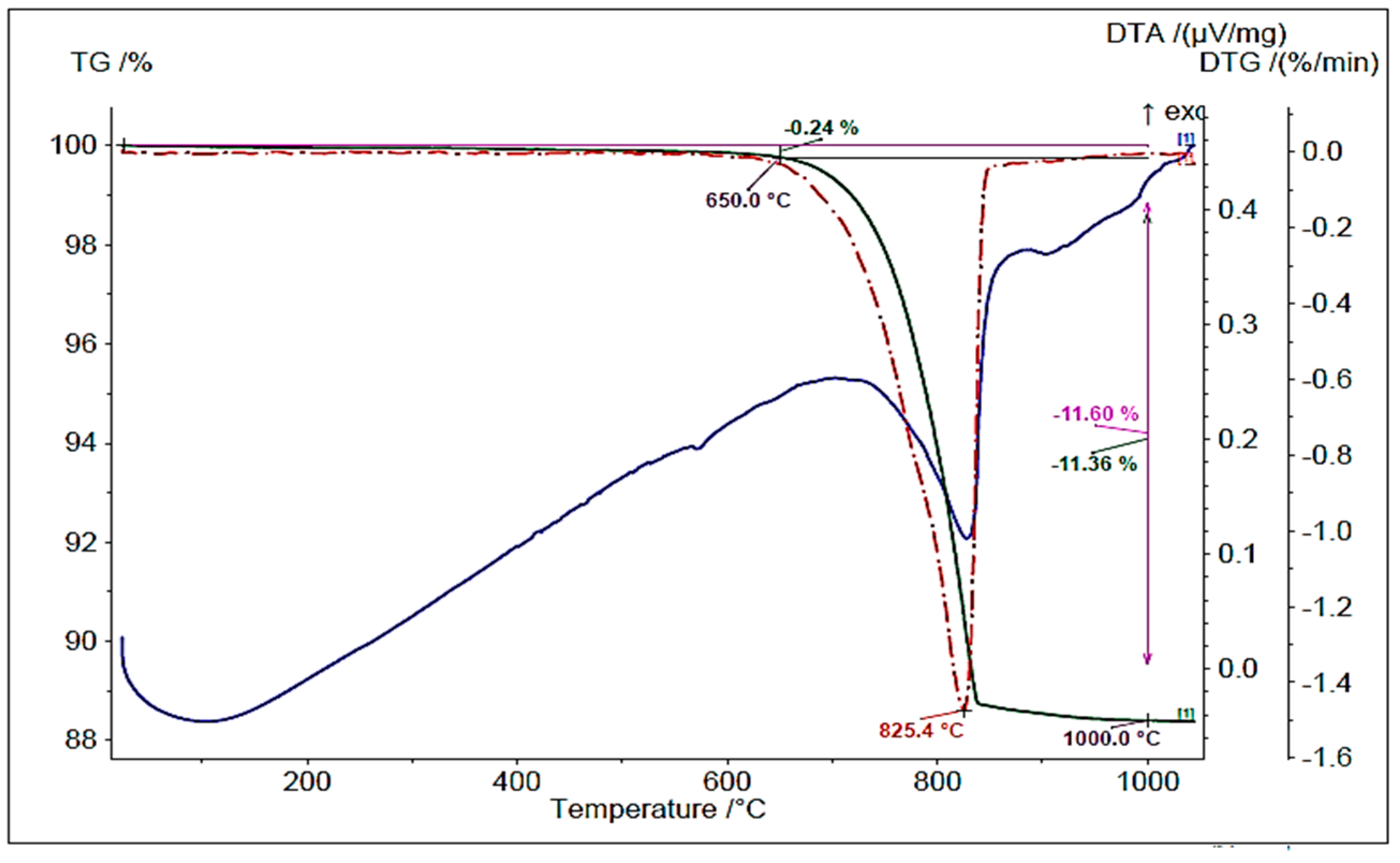
5.1.4. Thermogravimetry (TG) and Differential Thermal Analysis (DTA)
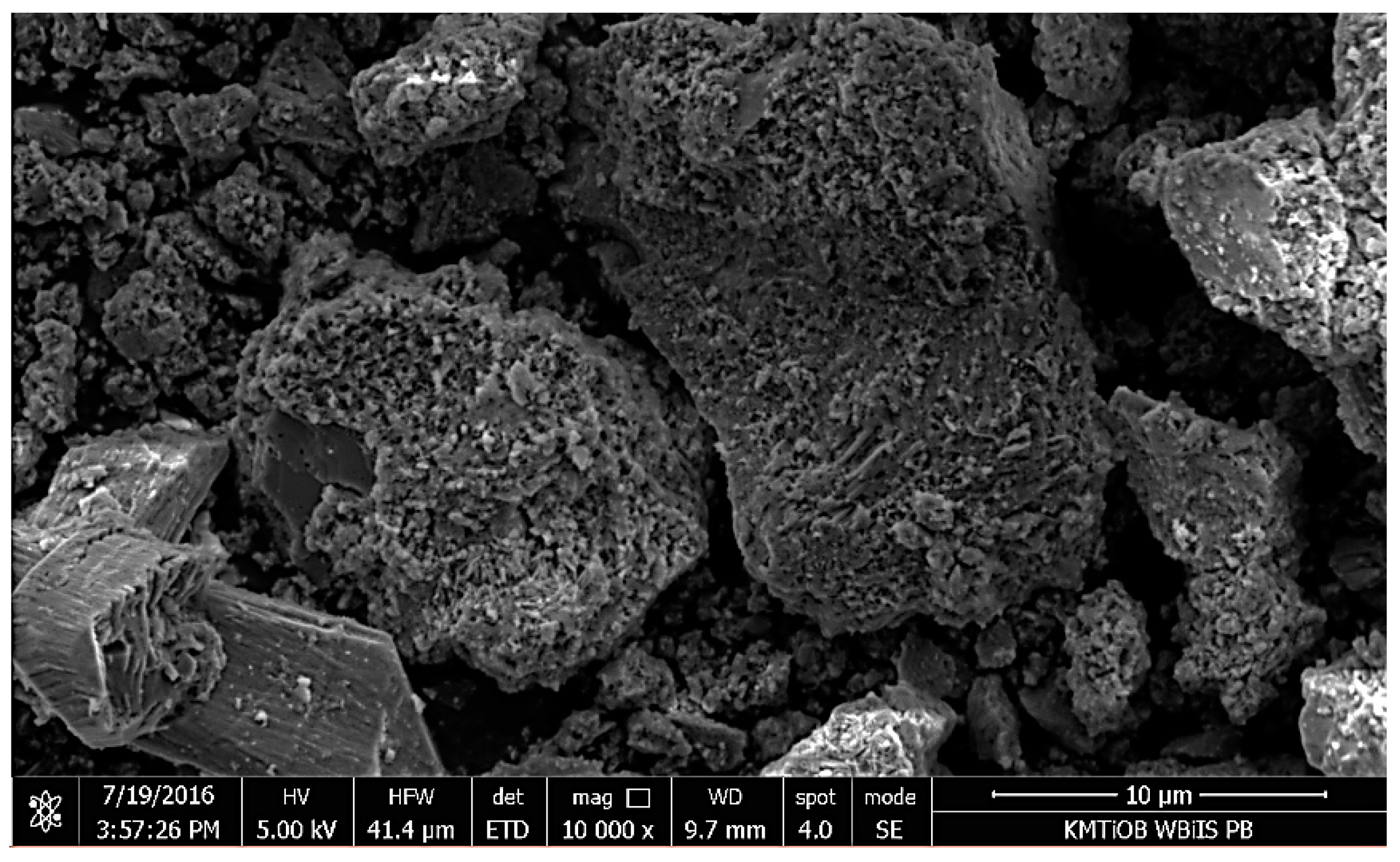
5.1.5. SEM Images Analysis
5.2. Properties of Fresh Mixtures
5.3. Properties of Hardened Cement Composites
5.3.1. Compressive Strength
5.3.2. Flexural Strength
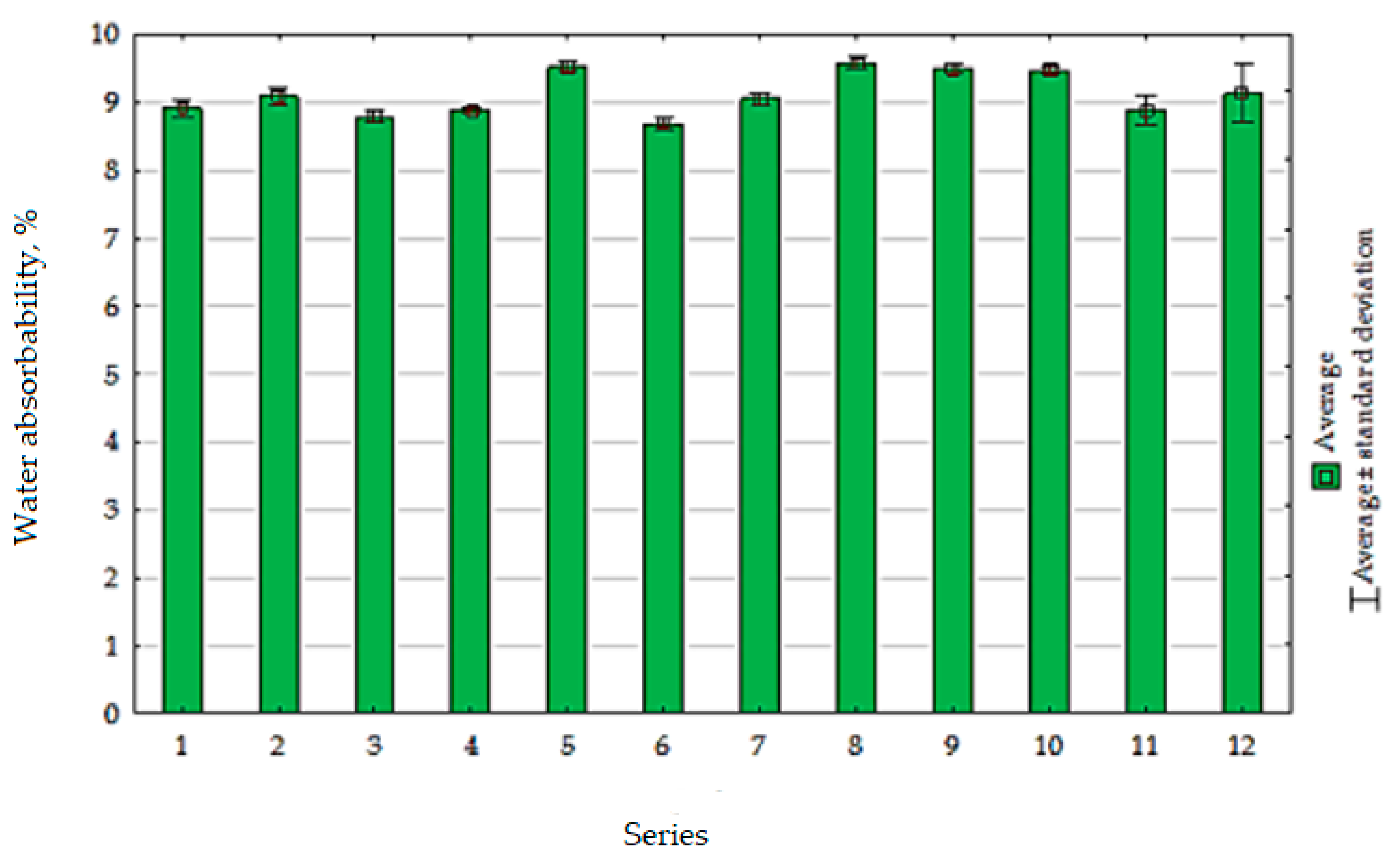
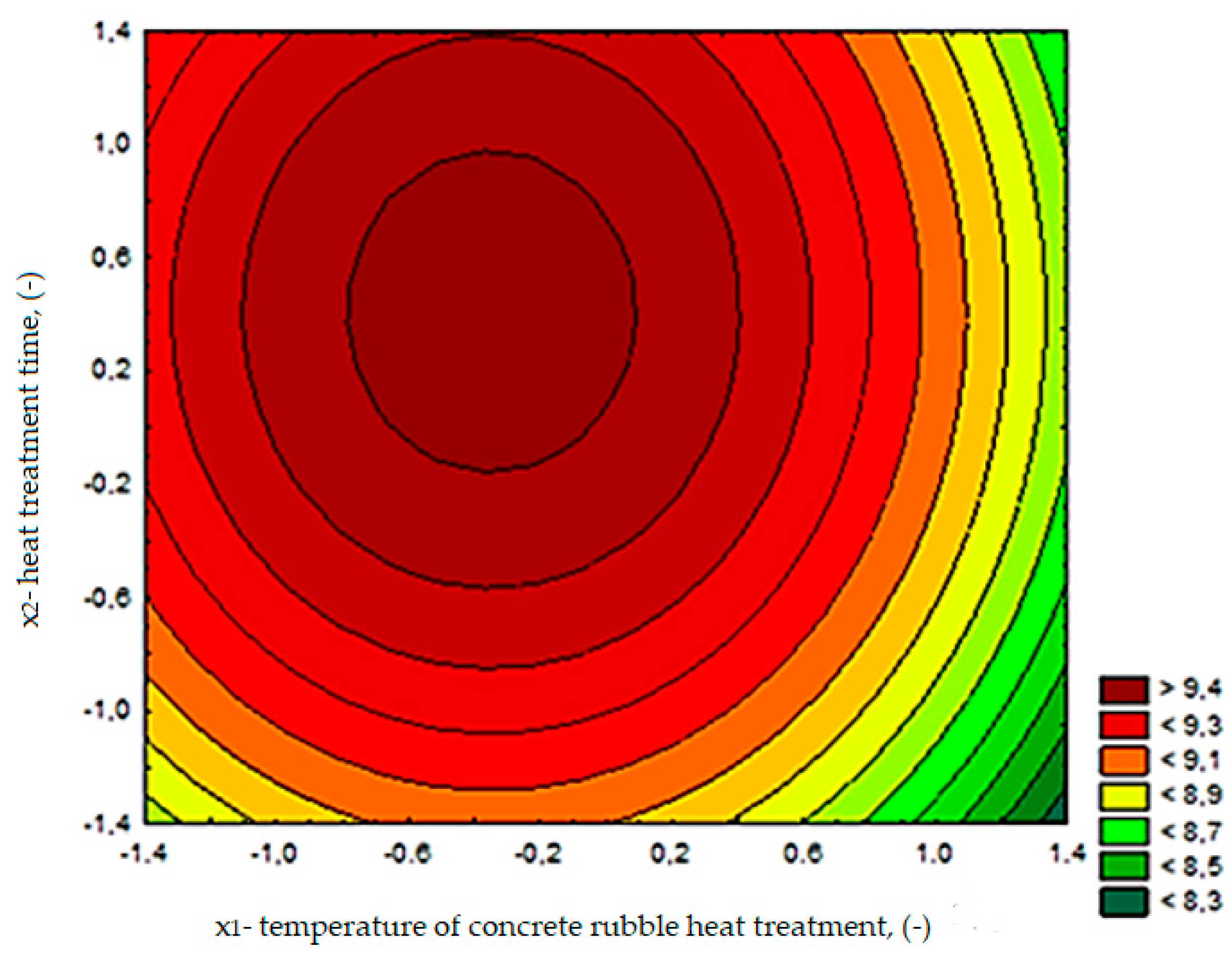
5.3.3. Water Absorbability
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging Energy-Efficiency and CO2 Emission-Reduction Technologies for Cement and Concrete Production: A Technical Review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef]
- Rodríguez-Robles, D.; Van den Heede, P.; De Belie, N. Life Cycle Assessment Applied to Recycled Aggregate Concrete. In New Trends in Eco-Efficient and Recycled Concrete, 1st ed.; de Brito, J., Agrela, F., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 207–256. [Google Scholar] [CrossRef]
- Fernández-Ledesma, E.; Jiménez, J.R.; Ayuso, J.; Corinaldesi, V.; Iglesias-Godino, F.J. A Proposal for the Maximum Use of Recycled Concrete Sand in Masonry Mortar Design. Mater. Constr. 2016, 66, 75. [Google Scholar] [CrossRef]
- Tam, V.W.; Soomro, M.; Evangelista, A.C.J. A Review of Recycled Aggregate in Concrete Applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Evangelista, L.; Guedes, M. Microstructural Studies on Recycled Aggregate Concrete. In New Trends in Eco-Efficient and Recycled Concrete, 1st ed.; de Brito, J., Agrela, F., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 425–451. [Google Scholar] [CrossRef]
- Evangelista, L.; Guedes, M.; de Brito, J.; Ferro, A.C.; Pereira, M.F. Physical, Chemical and Mineralogical Properties of Fine Recycled Aggregates Made from Concrete Waste. Constr. Build. Mater. 2015, 86, 178–188. [Google Scholar] [CrossRef]
- Esmaeeli, H.S.; Shishehbor, M.; Weiss, W.J.; Zavattieri, P.Z. A Two-Step Multiscale Model to Predict Early Age Strength Development of Cementitious Composites Considering Competing Fracture Mechanisms. Constr. Build. Mater. 2019, 208, 577–600. [Google Scholar] [CrossRef]
- Pawluczuk, E.; Kalinowska-Wichrowska, K.; Bołtryk, M.; Jiménez, J.R.; Fernández, J.M. The Influence of Heat and Mechanical Treatment of Concrete Rubble on the Properties of Recycled Aggregate Concrete. Materials 2019, 12, 367. [Google Scholar] [CrossRef]
- Ismail, S.; Ramli, M. Engineering Properties of Treated Recycled Concrete Aggregate for Structural Applications. Constr. Build. Mater. 2013, 44, 464–476. [Google Scholar] [CrossRef]
- Shaban, W.M.; Yang, J.; Su, H.; Mo, K.H.; Li, L.; Xie, J. Quality Improvement Techniques for Recycled Concrete Aggregate: A Review. J. Adv. Concr. Technol. 2019, 17, 151–167. [Google Scholar] [CrossRef]
- Dosho, Y. Development of a Sustainable Concrete Waste Recycling System-Application of Recycled Aggregate Concrete Produced by Aggregate Replacing Method. J. Adv. Concr. Technol. 2007, 5, 27–42. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Tam, C.M.; Lea, K.N. Removal of Cement Mortar Remains from Recycled Aggregate Using Pre-Soaking Approaches. Resour. Conserv. Recycl. 2007, 50, 82–101. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Rivera, J.F.; de Gutiérrez, R.M. Alkali-Activated Building Materials Made with Recycled Construction and Demolition Wastes. Constr. Build. Mater. 2017, 149, 130–138. [Google Scholar] [CrossRef]
- Kim, Y.C.; Choi, Y.W. Utilization of Waste Concrete Powder as a Substitution Material for Cement. Constr. Build. Mater. 2012, 30, 500–504. [Google Scholar] [CrossRef]
- Schoon, J.; De Buysser, K.; Van Driessche, I.; De Belie, N. Fines Extracted from Recycled Concrete as Alternative Raw Material for Portland Cement Clinker Production. Cem. Concr. Compos. 2015, 58, 70–80. [Google Scholar] [CrossRef]
- Zhao, Z.; Remond, S.; Damidot, D.; Xu, W. Influence of Fine Recycled Concrete Aggregates on the Properties of Mortars. Constr. Build. Mater. 2015, 81, 179–186. [Google Scholar] [CrossRef]
- Gastaldi, D.; Canonico, F.; Capelli, L.; Buzzi, L.; Boccaleri, L.; Irico, S. An Investigation on the Recycling of Hydrated Cement from Concrete Demolition Waste. Cem. Concr. Compos. 2015, 61, 29–35. [Google Scholar] [CrossRef]
- Bordy, A.; Younsi, A.; Aggoun, S.; Fiorio, B. Cement Substitution by a Recycled Cement Paste Fine: Role of the Residual Anhydrous Clinker. Constr. Build. Mater. 2017, 132, 1–8. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Wan, H.; Cao, B. Rehydration Reactivity of Recycled Mortar from Concrete Waste Experienced to Thermal Treatment. Constr. Build. Mater. 2008, 22, 1723–1729. [Google Scholar] [CrossRef]
- Xuan, D.X.; Shui, Z.H. Rehydration Activity of Hydrated Cement Paste Exposed to High Temperature. Fire Mater. 2011, 35, 481–490. [Google Scholar] [CrossRef]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of Geopolymeric Binder from Blended Waste Concrete Powder and Fly Ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Chai, L.; Monismith, C.L.; Harvey, J. Re-Cementation of Crushed Material in Pavement Bases; UCPRC-TM-2009-04; Pavement Research Center, University of California: Berkeley, CA, USA, 2009. [Google Scholar]
- Paige-Green, P. A Preliminary Evaluation of the Reuse of Cementitious Materials. In Proceedings of the 29th Annual Southern African Transport Conference, Pretoria, South Africa, 16–19 August 2010; pp. 520–529. [Google Scholar]
- Kim, J.; Nam, B.H.; Behring, Z.; Al Muhit, B. Evaluation of Recementation Reactivity of Recycled Concrete Aggregate Fines. Transp. Res. Rec. 2014, 2401, 44–51. [Google Scholar] [CrossRef]
- Vegas, I.; Azkarate, I.; Juarrero, A.; Frias, M. Diseño y prestaciones de morteros de albañilería elaborados con áridos reciclados procedentes de escombro de hormigón. Mater. Constr. 2009, 59, 5–18. [Google Scholar] [CrossRef]
- Braga, M.; de Brito, J.; Veiga, R. Incorporation of Fine Concrete Aggregates in Mortars. Constr. Build. Mater. 2012, 36, 960–968. [Google Scholar] [CrossRef]
- Bołtryk, M.; Kalinowska-Wichrowska, K.; Pawluczuk, E. Method for Separation of Set Cement Mortar from Coarse Aggregate and for Crushing that Mortar, and the Device for the Application of This Method, PAT.229887. Available online: http://regserv.uprp.pl/register/application?number=P.417362 (accessed on 1 December 2018).
- EN 197-1:2011 Cement. Composition, Specifications and Conformity Criteria for Common Cements; European Committee for Standardization: Brussels, Belgium, 2011.
- EN 933-1:2012 Test for Geometrical Properties of Aggregates. Determination of Particle Size Distribution. Sieving Method; European Committee for Standardization: Brussels, Belgium, 2012.
- EN 206:2013+A1:2016 Concrete. Specification, Performance Production and Conformity; European Committee for Standardization: Brussels, Belgium, 2012.
- EN 196-1:2016 Methods of Testing Cement. Determination of Strength; European Committee for Standardization: Brussels, Belgium, 2016.
- EN 1015-3:1999 Methods of Test for Mortar for Masonry. Determination of Consistence of Fresh Mortar (by Flow Table); European Committee for Standardization: Brussels, Belgium, 1999.
- EN 450-1:2012 Fly Ash for Concrete. Definition, Specifications and Conformity Criteria; European Committee for Standardization: Brussels, Belgium, 2012.
- EN 196-9:2010 Methods of Testing Cement. Heat of Hydration. SEMI-Adiabatic Method; European Committee for Standardization: Brussels, Belgium, 2010.
- Ramachandran, V.S.; Paroli, R.M.; Beaudoin, J.J.; Delgado, A.H. Handbook of Thermal Analysis of Construction Materials; Noyes Publications/William Andrew Publishing: New York, NY, USA, 2003. [Google Scholar]
- Handoo, S.K.; Agarwal, S.; Agarwal, S.K. Physicochemical, Mineralogical, and Morphological Characteristics of Concrete Exposed to Elevated Temperatures. Cem. Concr. Res. 2002, 32, 1009–1018. [Google Scholar] [CrossRef]
- EN ISO 3252:2002 Powder Metallurgy. Vocabulary; European Committee for Standardization: Brussels, Belgium, 2002.
- Brandt, S. Data Analysis. Statistical and Computational Methods for Scientists and Engineers, 3rd ed.; Springer: New York, NY, USA, 1999. [Google Scholar]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7944-0. [Google Scholar]




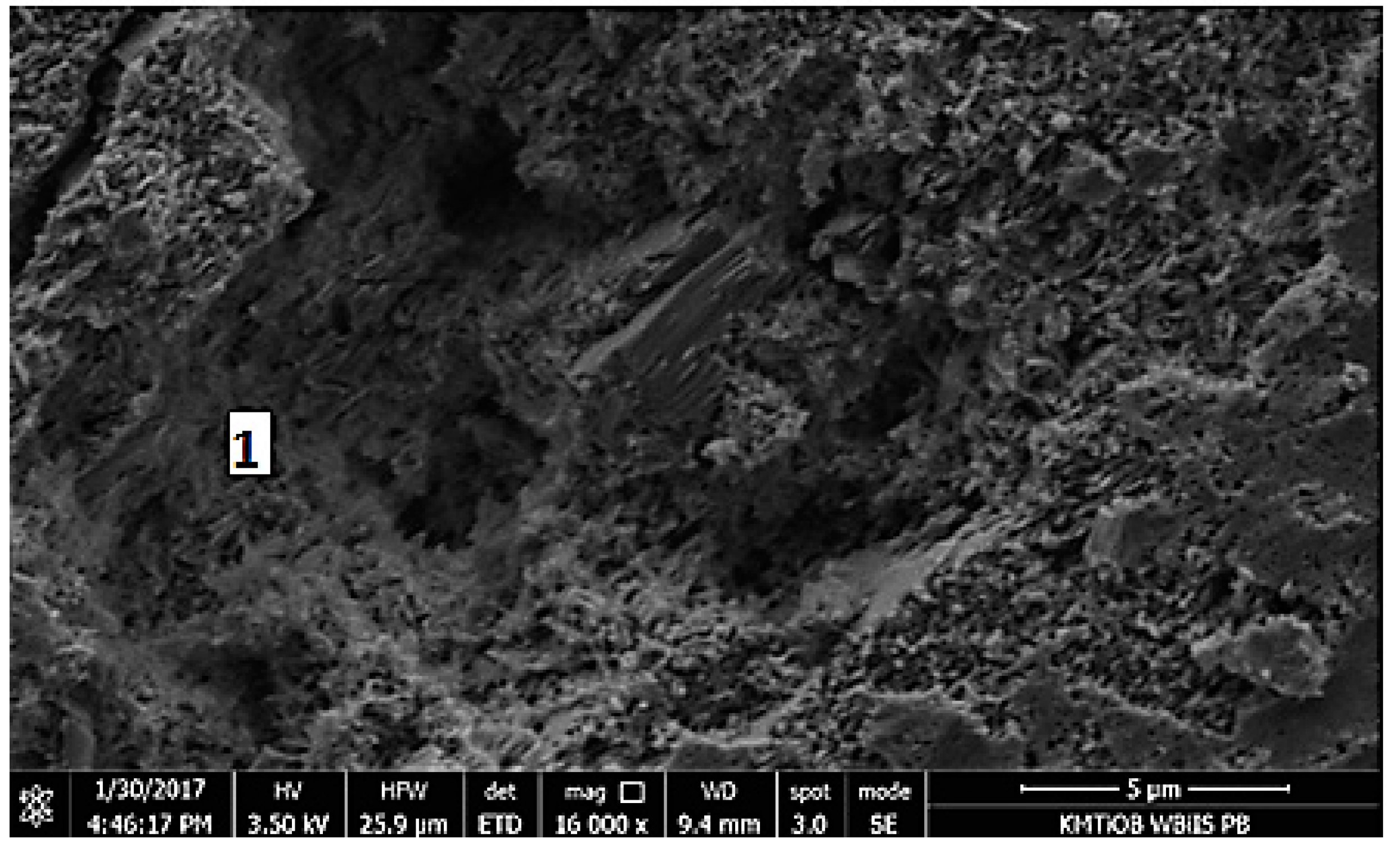
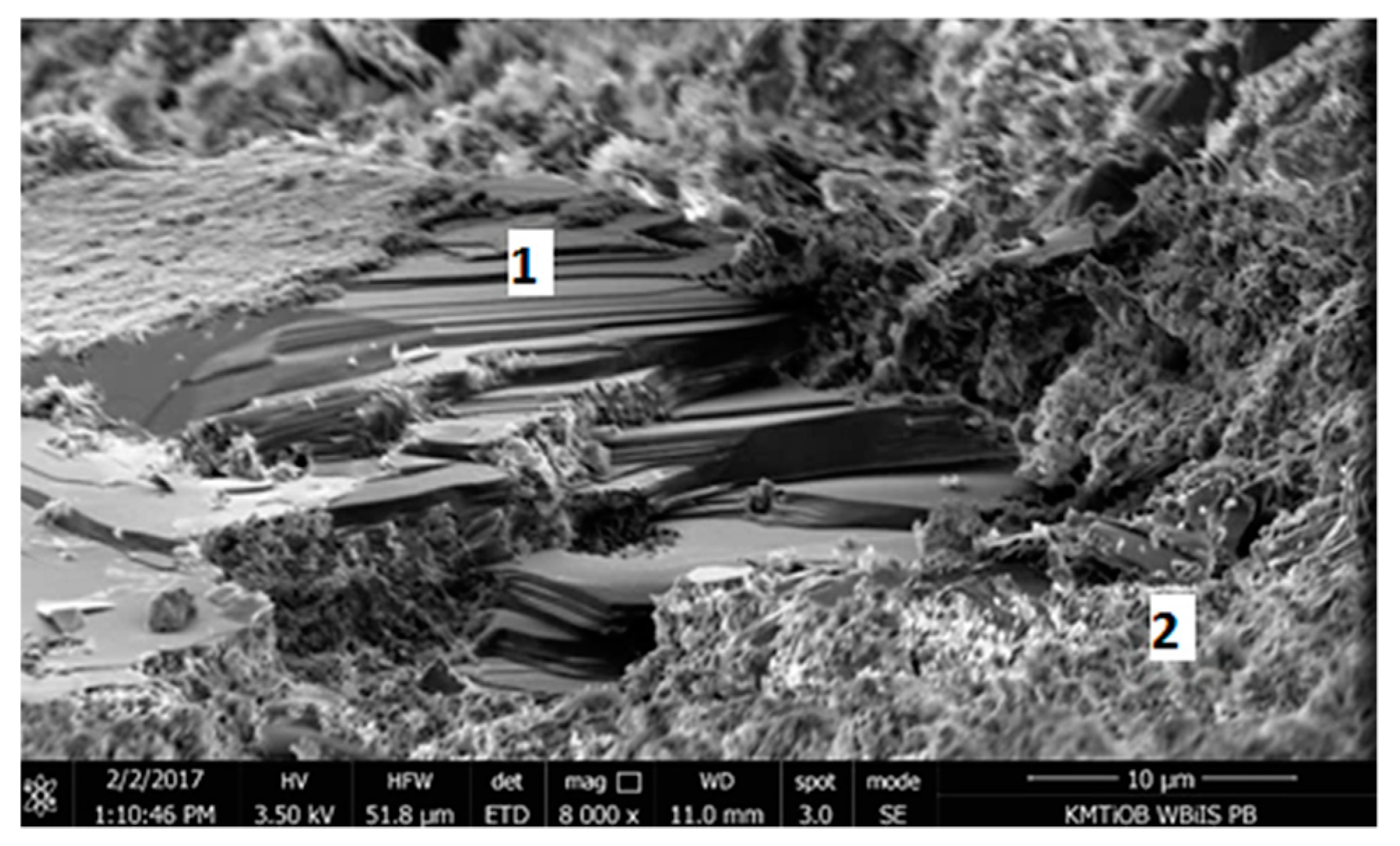
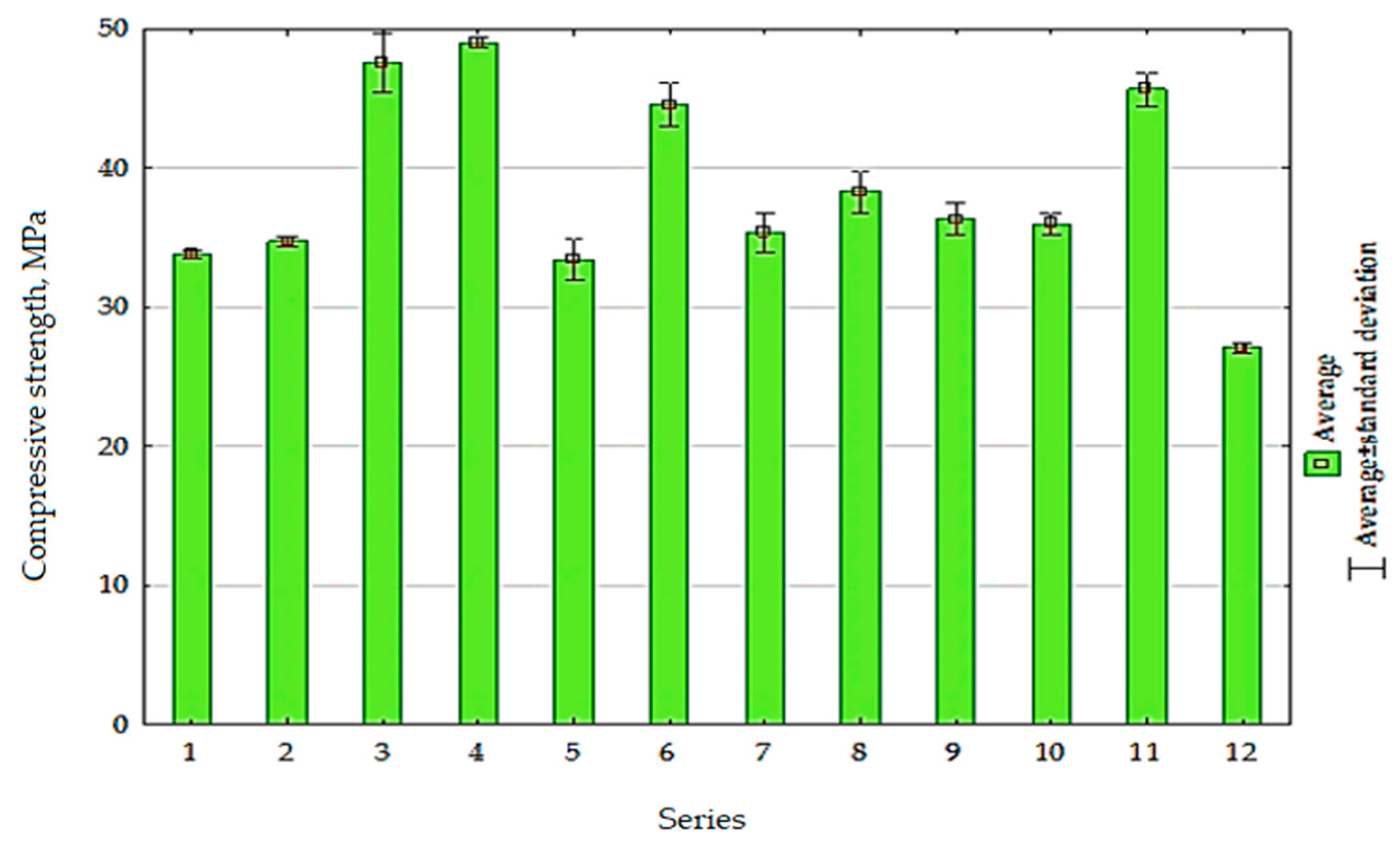


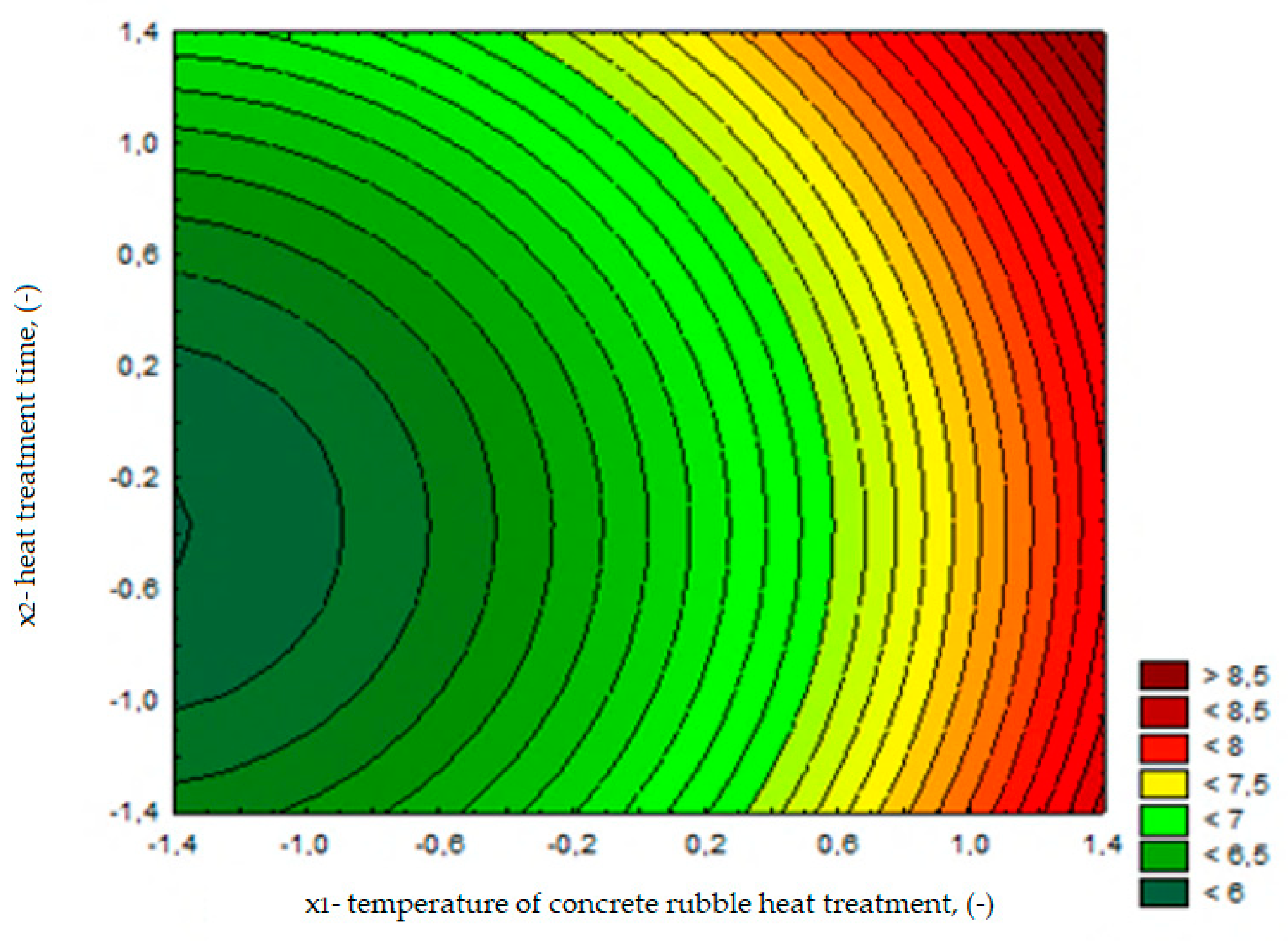
| Component | Content |
|---|---|
| Cement CEM I 42.5 R, kg | 360 |
| Sand 0–2 mm, kg | 641 |
| Gravel 2–16 mm, kg | 1170 |
| Plasticizer, dm3 | 3.2 |
| Water, dm3 | 144 |
| Component | Unit | Cement Composites | |
|---|---|---|---|
| Series 1–10; 12 | Series 11 | ||
| Cement CEM I 42.5 R | g | 337.5 | 450 |
| Water | mL | 225 | 225 |
| Sand 0–2 mm | g | 1350 | 1350 |
| Recycled Cement Mortar | g | 112.5 | - |
| X1 | Temperature of concrete rubble treatment, °C | 288 | 350 | 500 | 650 | 712 |
| X2 | Thermal treatment time, min | 30 | 40 | 60 | 80 | 90 |
| Series | Real Values | Normalized Values | ||
|---|---|---|---|---|
| X1, °C | X2, min | x1 | x2 | |
| 1 | 350 | 40 | −1 | −1 |
| 2 | 350 | 80 | −1 | 1 |
| 3 | 650 | 40 | 1 | −1 |
| 4 | 650 | 80 | 1 | 1 |
| 5 | 288 | 60 | −1.414 | 0 |
| 6 | 712 | 60 | 1.414 | 0 |
| 7 | 500 | 30 | 0 | −1.414 |
| 8 | 500 | 90 | 0 | 1.414 |
| 9 | 500 | 60 | 0 | 0 |
| 10 | 500 | 60 | 0 | 0 |
| Type of RCM | Components of the RCM, % of Mass | ||||
|---|---|---|---|---|---|
| Bound Water | Ca(OH)2 | CaCO3 | |||
| HI | HCH | Σ | |||
| Non-calcined | 6.81 | 3.27 | 10.08 | 13.44 | 10.44 |
| Calcined | - | 0.24 | 0.24 | 0 | 25.79 |
| Type of Mixture | Flow Diameter, mm |
|---|---|
| Standard cement mortar (series 11) | 170 |
| Cement composite with RCM (series 4) | 150 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowska-Wichrowska, K.; Kosior-Kazberuk, M.; Pawluczuk, E. The Properties of Composites with Recycled Cement Mortar Used as a Supplementary Cementitious Material. Materials 2020, 13, 64. https://doi.org/10.3390/ma13010064
Kalinowska-Wichrowska K, Kosior-Kazberuk M, Pawluczuk E. The Properties of Composites with Recycled Cement Mortar Used as a Supplementary Cementitious Material. Materials. 2020; 13(1):64. https://doi.org/10.3390/ma13010064
Chicago/Turabian StyleKalinowska-Wichrowska, Katarzyna, Marta Kosior-Kazberuk, and Edyta Pawluczuk. 2020. "The Properties of Composites with Recycled Cement Mortar Used as a Supplementary Cementitious Material" Materials 13, no. 1: 64. https://doi.org/10.3390/ma13010064
APA StyleKalinowska-Wichrowska, K., Kosior-Kazberuk, M., & Pawluczuk, E. (2020). The Properties of Composites with Recycled Cement Mortar Used as a Supplementary Cementitious Material. Materials, 13(1), 64. https://doi.org/10.3390/ma13010064







