Strength and Microstructure of Class-C Fly Ash and GGBS Blend Geopolymer Activated in NaOH & NaOH + Na2SiO3
Abstract
1. Introduction
2. Experimental Plan
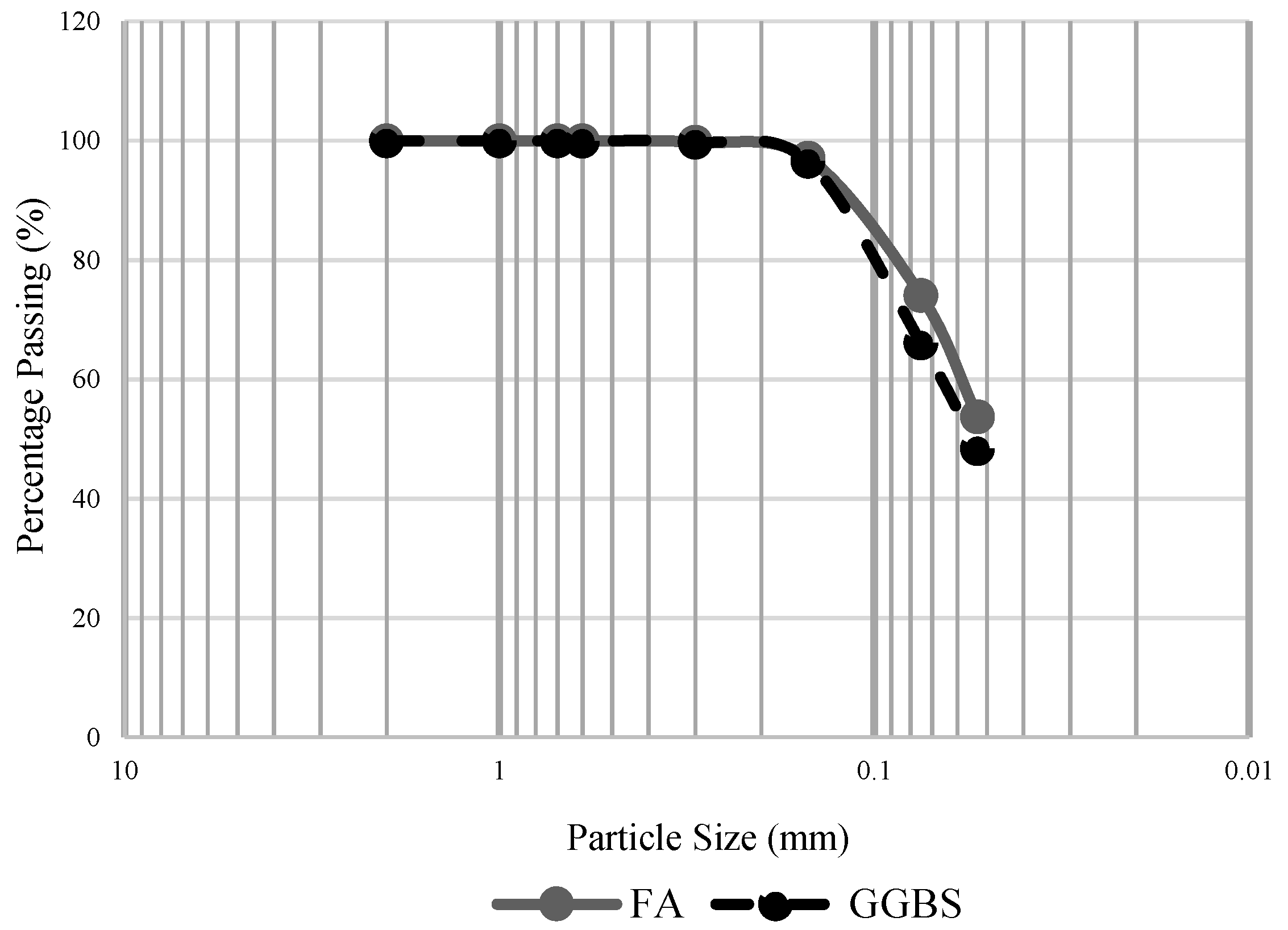
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
3. Results and Discussions
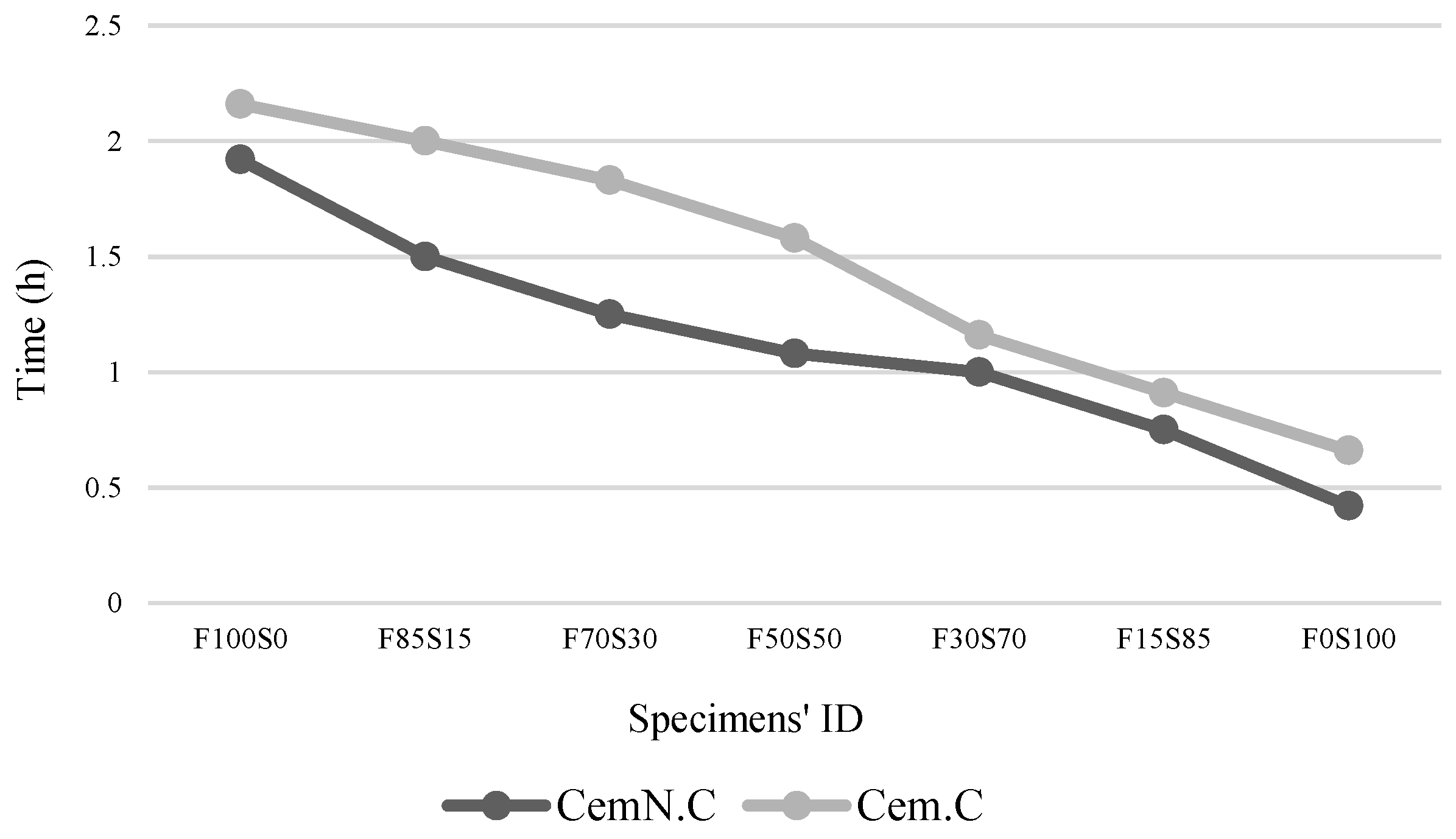
3.1. Final Setting Time
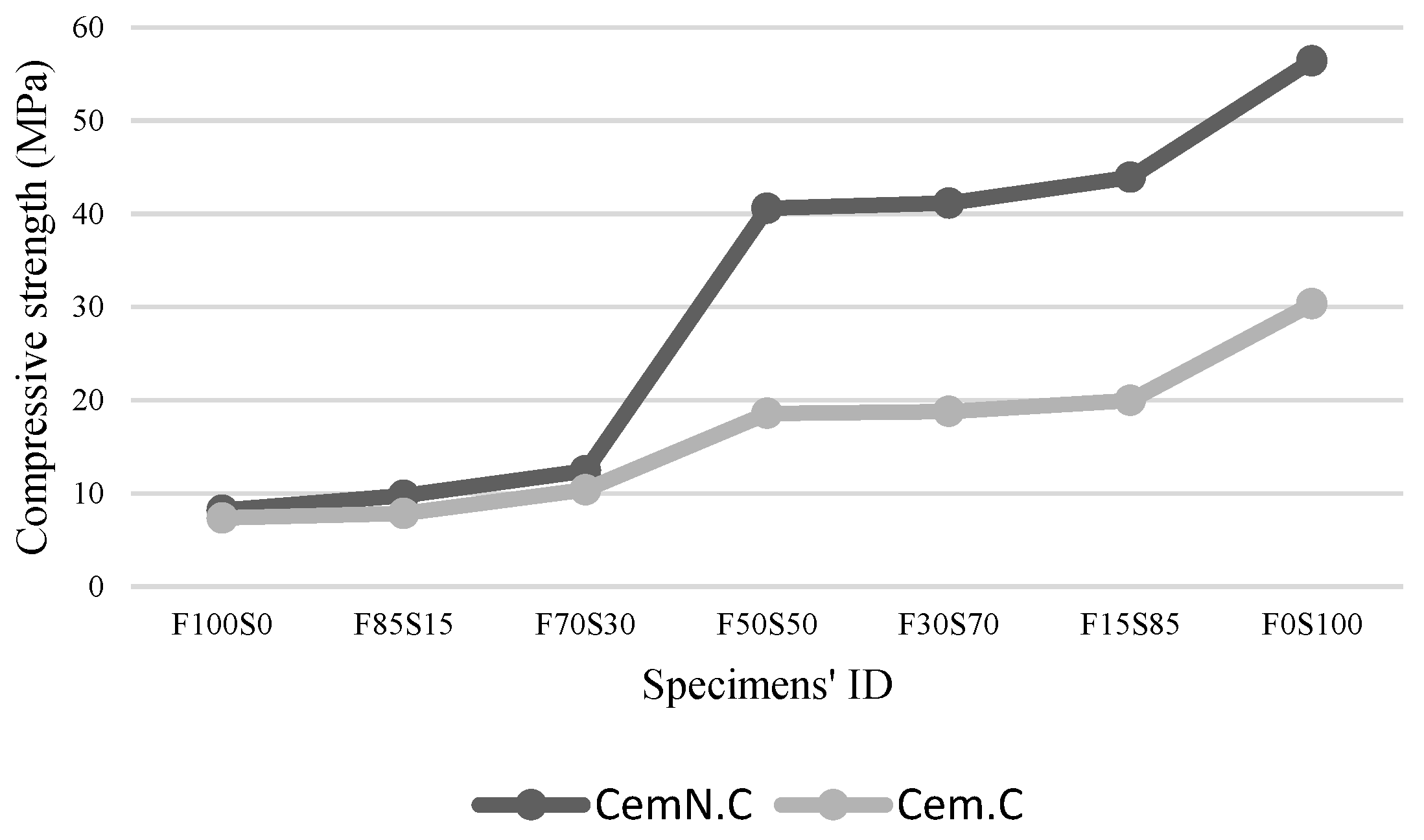
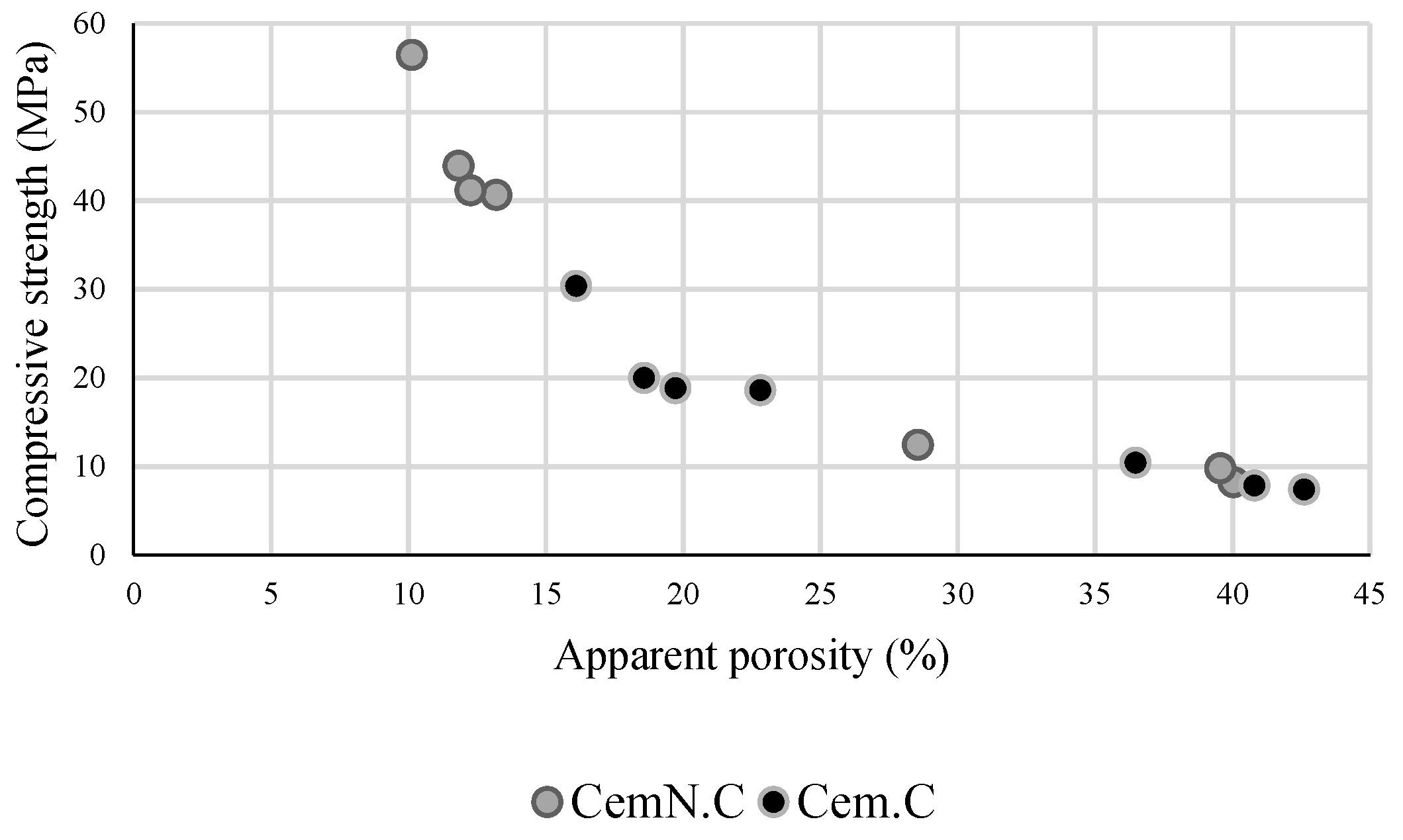
3.2. Compressive Strength
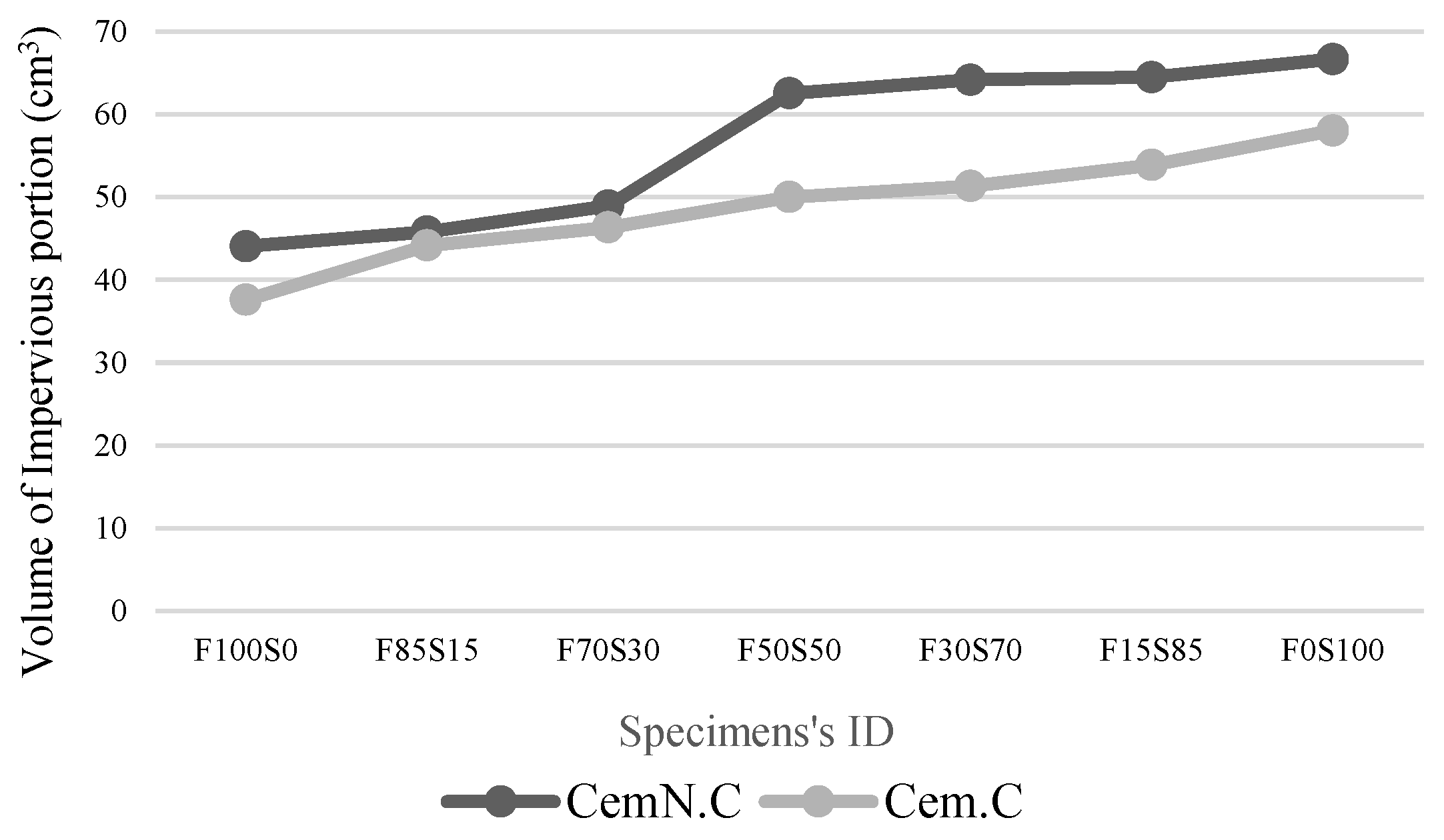
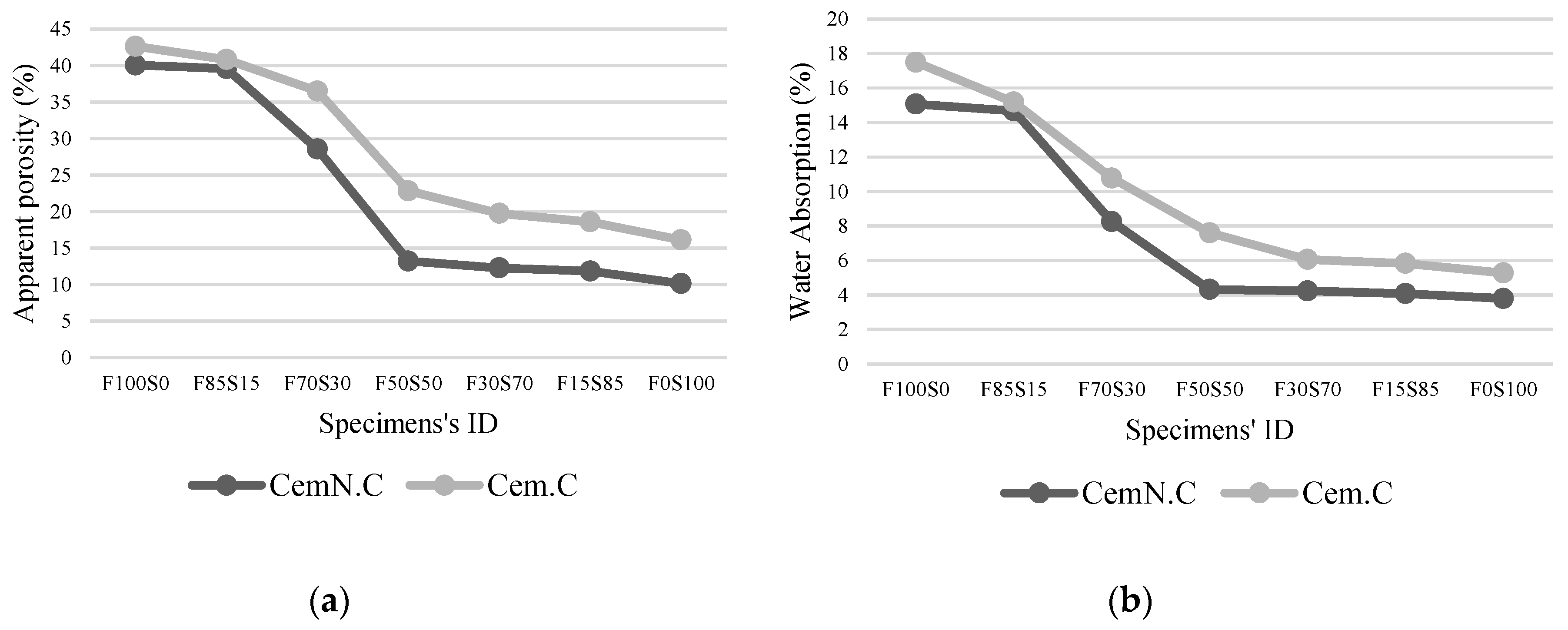
3.3. Volume of Impervious Portion, Apparent Porosity and Water Absorption
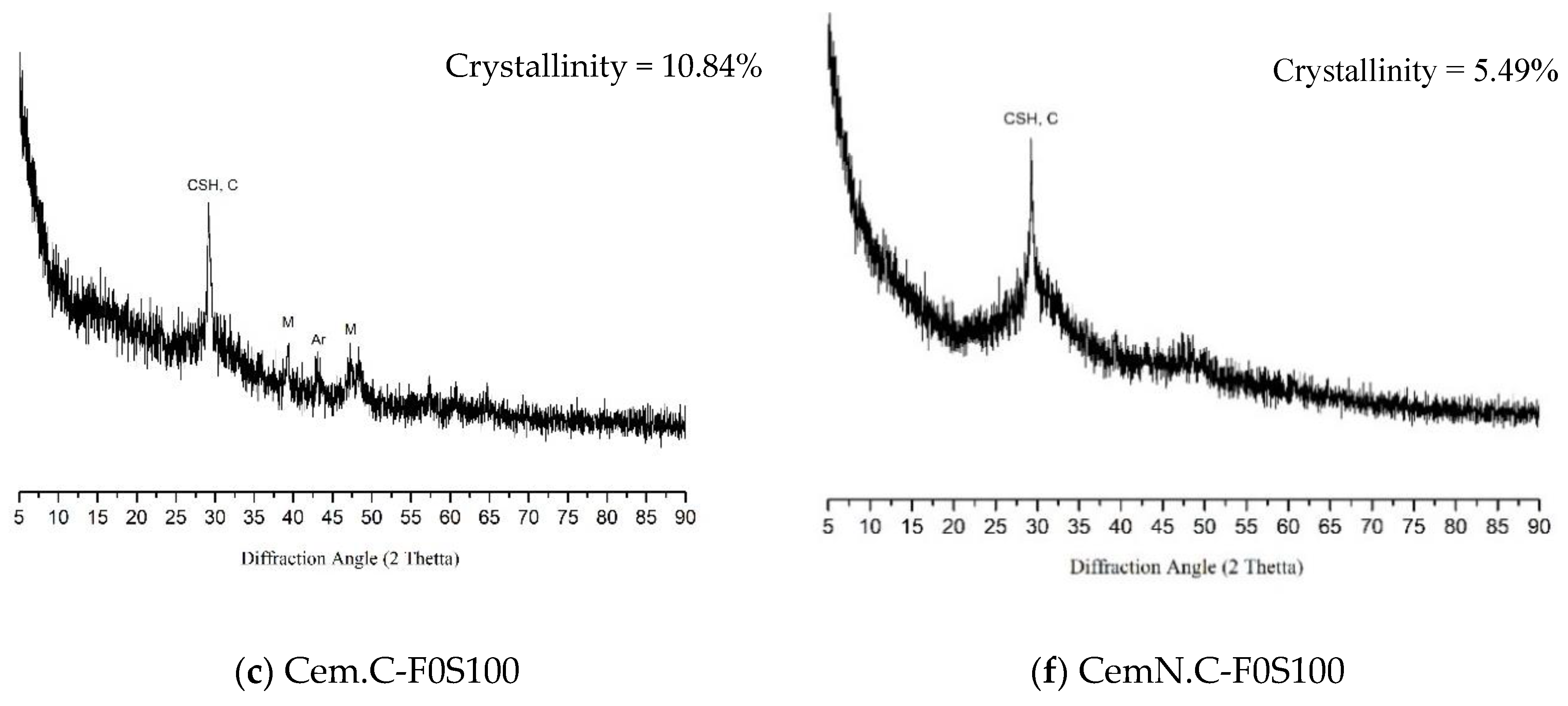
3.4. XRD Analysis
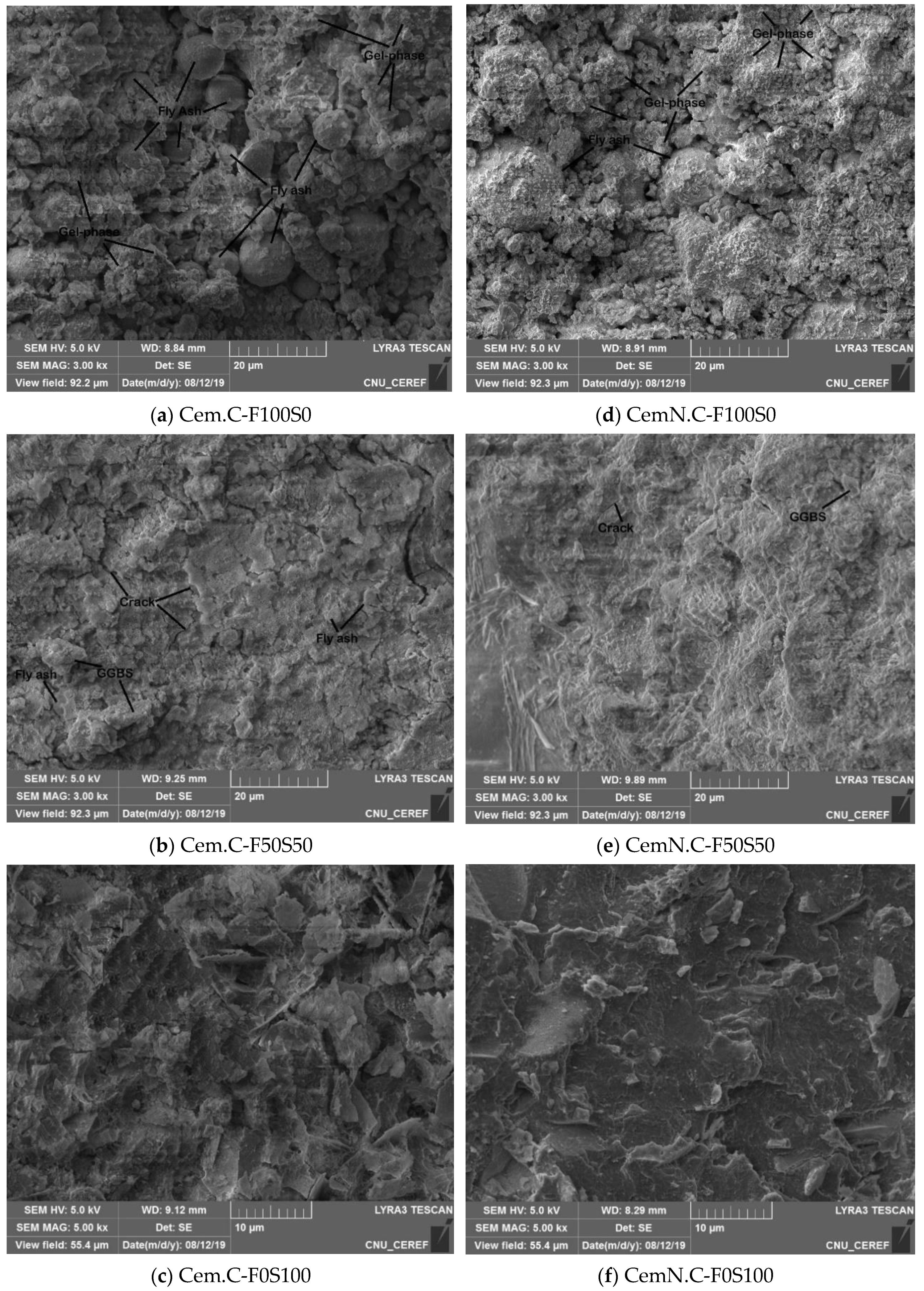
3.5. SEM Micrographs
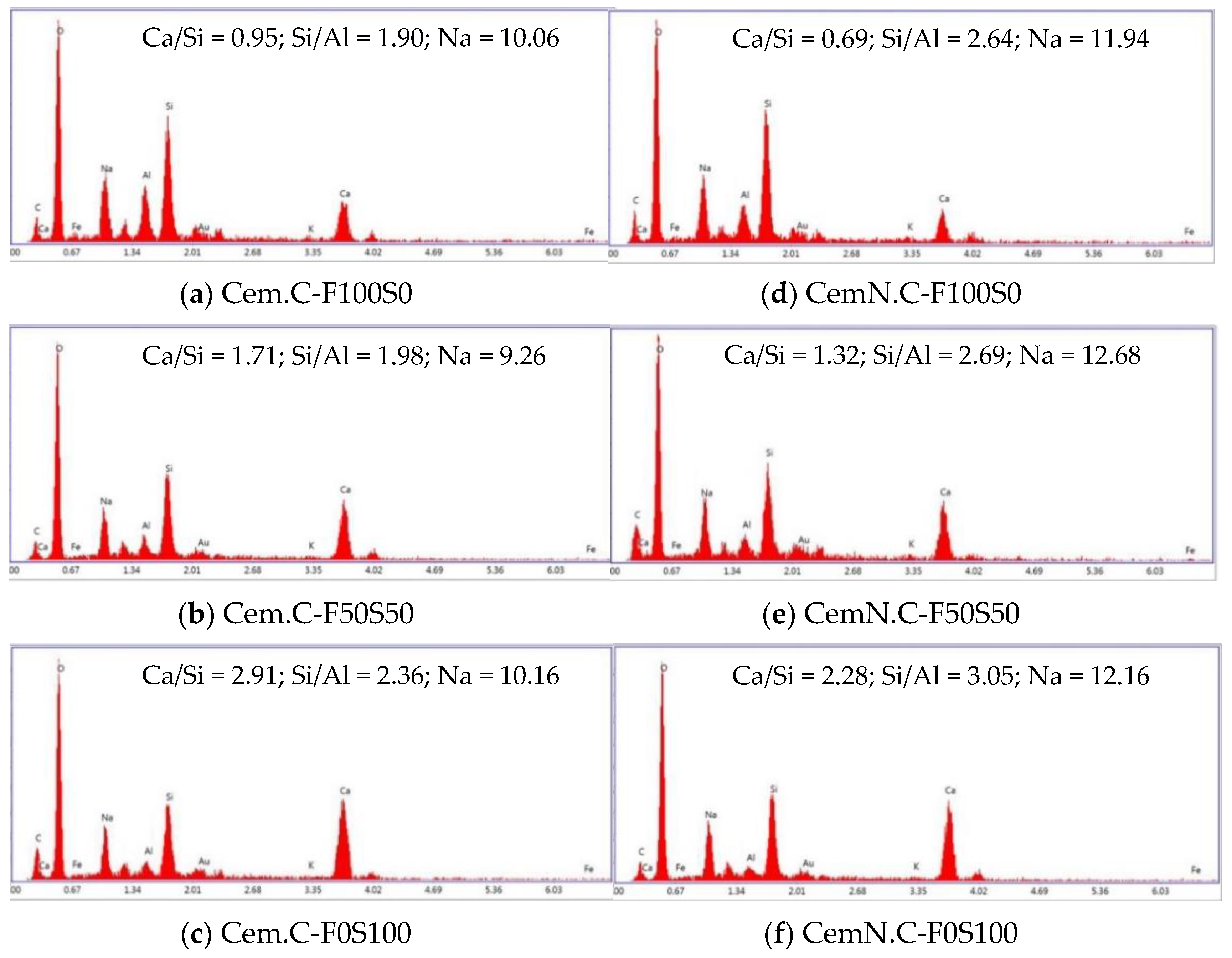
3.6. SEM-EDS Point Analyses
4. Conclusions
- The alkaline activator plays an important role in developing a stronger and denser matrix. As explored, addition of Na2SiO3 was one of the parameters for improving strength and microstructure as it accelerated geopolymerization by supplying the amorphous silica from Na2SiO3 source. This favors the formation of cross-linked structures in the matrix, which is responsible for strength development. The study also examined the improved reactivity of fly ash when activated in NaOH + Na2SiO3, which also contributed to the strength development and improvement in homogeneity of the matrix.
- The other factor effecting the strength and microstructure of FA/GGBS blends is the reactivity of raw materials. Class-C fly ash, when activated, remained unreactive/partially reactive, which limits the leaching of major element such as Si, Al and Ca to the matrix. The limit in the leaching of Ca was initially observed when the setting time increased abruptly with the increasing fly ash content in the sample. Accordingly, XRD analyses reveals the Ca in the fly ash did not contribute in forming C-S-H bonds but the formation of CSH gel was dependent on the reactive Ca from the GGBS source, which resulted in the increased strength, reduced porosity and compact matrix. SEM micrographs further clarified the less reactivity of fly ash in a matrix.
- The influence of NaOH and NaOH + Na2SiO3 alkali solutions in class-C FA and GGBS is explored in this study where partial reactivity of fly ash in NaOH + Na2SiO3 solution was observed. Based on this study, it is hypothesized that the leaching of Ca from class-C fly ash can be possible if activated in a high concentrated alkali solution. Therefore, the reactivity of class-C fly ash and GGBS in different alkaline solutions with different concentration should be explored in future studies.
Author Contributions
Funding
Conflicts of Interest
References
- Neupane, G.; Donahoe, R.J. Leachability of elements in alkaline and acidic coal fly ash samples during batch and column leaching tests. Fuel 2013, 104, 758–770. [Google Scholar] [CrossRef]
- Nomura, Y.; Fujiwara, K.; Terada, A.; Nakai, S.; Hosomi, M. Prevention of lead leaching from fly ashes by mechanochemical treatment. J. Waste Manag. 2010, 30, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Naik, T.R. Sustainability of concrete construction. Pract. Period. Struct. Des. Constr. 2008, 13, 98–103. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Ravikumar, D.; Neithalath, N. Effects of activator characteristics on the reaction product formation in slag binders activated using alkali silicate powder and NaOH. Constr Build Mater. 2012, 34, 809–818. [Google Scholar] [CrossRef]
- Davidovits, J. Calcium Based Geopolymer: Geopolymer Chemistry and Applications, 3rd ed.; Geopolymer Institute: Saint Quentin, France, 2011; pp. 201–244. [Google Scholar]
- Xu, H.; Van Deventer, J.S. Factors affecting the geopolymerization of alkali-feldspars. Min. Metall. Explor. 2002, 19, 209–214. [Google Scholar] [CrossRef]
- Schmücker, M.; MacKenzie, K.J. Microstructure of sodium polysialate siloxo geopolymer. Ceram Int. 2005, 31, 433–437. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Comp. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Yang, T.; Yao, X.; Zhang, Z.; Wang, H. Mechanical property and structure of alkali-activated fly ash and slag blends. JSCM 2012, 1, 167–178. [Google Scholar] [CrossRef]
- Davidovits, J.; Izquierdo, M.; Querol, X.; Antennuci, D.; Nugteren, H.; Butselaar-Orthlieb, V.; Fernández-Pereira, C.; Luna, Y. The European Research Project GEOASH: Geopolymer Cement Based on European Coal Fly Ashes; Technical Paper; Geopolymer Institute: Aisne, France, 2014. [Google Scholar]
- Wijaya, S.W.; Satria, J.; Sugiarto, A.; Hardjito, D. The use of borax in deterring flash setting of high calcium fly ash based geopolymer. Mater Sci Forum. 2016, 857, 416–420. [Google Scholar] [CrossRef]
- Wijaya, S.W.; Hardjito, D. Factors affecting the setting time of fly ash-based geopolymer. Mater. Sci. Forum 2016, 841, 90–97. [Google Scholar] [CrossRef]
- Wijaya, A.L.; Ekaputri, J.J. Factors Influencing Strength and Setting Time of Fly Ash Based-Geopolymer Paste. In Proceedings of the MATEC Web of Conferences 6th International Conference of Euro Asia Civil Engineering Forum, Seoul, Korea, 22–25 August 2017. [Google Scholar]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Wang, S.-D.; Pu, X.-C.; Scrivener, K.; Pratt, P. Alkali-activated slag cement and concrete: A review of properties and problems. Adv Cem. Res. 1995, 7, 93–102. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. A calorimetric study of early hydration of alkali-slag cements. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Glukhovskij, V.; Zaitsev, Y.; Pakhomov, V. Slag-alkaline cements and concretes-structure, properties, technological and economical aspects of the use. Silic. Ind. 1983, 48, 197–200. [Google Scholar]
- Wang, W.C.; Wang, H.Y.; Lo, M.H. The fresh and engineering properties of alkali activated slag as a function of fly ash replacement and alkali concentration. Constr. Build Mater. 2015, 84, 224–229. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Xing, J.; Sun, X. Fly ash/blast furnace slag-based geopolymer as a potential binder for mine backfilling: Effect of binder type and activator concentration. Adv. Mater. Sci. Eng. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vazquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Chi, M.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar] [CrossRef]
- Wardhono, A.; Law, D.W.; Strano, A. The Strength of Alkali-activated Slag/fly Ash Mortar Blends at Ambient Temperature. Procedia Eng. 2015, 125, 650–656. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Li, W.; Shi, G.; Lu, P. Effect of slag on the mechanical properties and bond strength of fly ash-based engineered geopolymer composites. Compos. Part B Eng. 2019, 164, 747–757. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Microcracking and strength development of alkali activated slag concrete. Cement Concr. Compos. 2001, 23, 345–352. [Google Scholar] [CrossRef]
- Chen, C.C.; Diaz, I.; Menozzi, K.; Murillo, L. An Experimental Study on Slag/Fly ash-based Geopolymer Concrete. In Proceedings of the ISER 11th International Conference, San Francisco, CA, USA, 25–30 September 2015. [Google Scholar]
- Fernández-Jiménez, A.; Puertas, F. Effect of activator mix on the hydration and strength behaviour of alkali-activated slag cements. Adv. Cem. Res. 2003, 15, 129–136. [Google Scholar] [CrossRef]
- Song, S.; Sohn, D.; Jennings, H.M.; Mason, T.O. Hydration of alkali-activated ground granulated blast furnace slag. J. Matter Sci. 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cem. Concr. Res. 2017, 102, 29–40. [Google Scholar] [CrossRef]
- Tsuji, M.; Komarneni, S.; Malla, P. Substituted tobermorites: 27Al and 29Si MASNMR, cation exchange, and water sorption studies. J. Am. Ceram. Soc. 1991, 74, 274–279. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Srinivasula, R.M.; Dinakar, P.; Rao, B.K.; Satpathy, B.N.; Mohanty, A. Effect of the Na2SiO3/NaOH Ratio and NaOH Molarity on the Synthesis of Fly Ash-Based Geopolymer Mortar. In Proceedings of the Geo-Chicago 2016, Chicago, IL, USA, 14–18 August 2016; pp. 336–344. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Min. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Tan, Z.; Bernal, S.A.; Provis, J.L. Reproducible mini-slump test procedure for measuring the yield stress of cementitious pastes. Mater. Struct. 2017, 50, 235. [Google Scholar] [CrossRef]
- Nedeljković, M.; Li, Z.; Ye, G. Setting, strength, and autogenous shrinkage of alkali-activated fly ash and slag pastes: Effect of slag content. Materials 2018, 11, 2121. [Google Scholar] [CrossRef]
- Siddique, R.; Khan, M.I. Supplementary Cementing Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Bernal, S.A.; San Nicolas, R.; Van Deventer, J.S.; Provis, J.L. Alkali-activated slag cements produced with a blended sodium carbonate/sodium silicate activator. Adv. Cem. Res. 2016, 28, 262–273. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; De-Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F.; Sobrados, I.; Sanz, J. Structure of calcium silicate hydrates formed in alkaline-activated slag: Influence of the type of alkaline activator. J. Am. Ceram. Soc. 2003, 86, 1389–1394. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Ghosh, K.; Ghosh, P. Effect of % Na2O and % SiO2 on apperent porosity and sorptivity of fly ash based geopolymer. IOSR J. Eng. 2012, 2, 96–101. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Ravikumar, D.; Peethamparan, S.; Neithalath, N. Structure and strength of NaOH activated concretes containing fly ash or GGBFS as the sole binder. Cem. Concr. Compos. 2010, 32, 399–410. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Saludung, A.; Ogawa, Y.; Kawai, K. Microstructure and Mechanical Properties of FA/GGBS-Based Geopolymer. In Proceedings of the MATEC Web Conferences the 4th International Conference on Rehabilitation and Maintenance in Civil Engineering (ICRMCE 2018), Solo, Central Java, Indonesia, 11–12 July 2018. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Timakul, P.; Rattanaprasit, W.; Aungkavattana, P. Improving compressive strength of fly ash-based geopolymer composites by basalt fibers addition. Ceram. Int. 2016, 42, 6288–6295. [Google Scholar] [CrossRef]

| Elements (Wt. %) | O | Fe | Na | AL | Si | Au | S | Cl | K | Ca |
| FA | 43.44 | 2.01 | 0.65 | 10.33 | 18.16 | 2.38 | 1.50 | 0.82 | 1.53 | 18.72 |
| GGBS | 35.43 | 0.51 | 0.47 | 7.37 | 14.57 | 1.38 | - | - | 0.35 | 39.92 |
| Oxide (Wt. %) | SiO2 | Al2O3 | CaO | Fe2O3 | Na2O | K2O | ||||
| FA | 38.84 | 19.52 | 26.19 | 2.87 | 0.87 | 1.84 | ||||
| GGBS | 31.17 | 13.92 | 55.85 | 0.729 | 0.63 | 0.421 | ||||
| Specimen ID | FA (wt.%) | GGBS (wt.%) | Alkali Activator | Al/S | W/Mix |
|---|---|---|---|---|---|
| NaOH activated pastes (Cem.C) | |||||
| F100S0 | 100 | 0 | NAOH | 0.4 | 0.075 |
| F85S15 | 85 | 15 | 0.077 | ||
| F70S30 | 70 | 30 | 0.079 | ||
| F50S50 | 50 | 50 | 0.087 | ||
| F30S70 | 30 | 70 | 0.091 | ||
| F15S85 | 15 | 85 | 0.098 | ||
| F0S100 | 0 | 100 | 0.1 | ||
| NaOH + Na2SiO3 activated pastes (CemN.C) | |||||
| F100S0 | 100 | 0 | NaOH+ Na2SiO3 | 0.4 | 0.104 |
| F85S15 | 85 | 15 | 0.107 | ||
| F70S30 | 70 | 30 | 0.112 | ||
| F50S50 | 50 | 50 | 0.123 | ||
| F30S70 | 30 | 70 | 0.129 | ||
| F15S85 | 15 | 85 | 0.132 | ||
| F0S100 | 0 | 100 | 0.138 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasui, S.; Kim, G.; Nam, J.; Koyama, T.; Chansomsak, S. Strength and Microstructure of Class-C Fly Ash and GGBS Blend Geopolymer Activated in NaOH & NaOH + Na2SiO3. Materials 2020, 13, 59. https://doi.org/10.3390/ma13010059
Sasui S, Kim G, Nam J, Koyama T, Chansomsak S. Strength and Microstructure of Class-C Fly Ash and GGBS Blend Geopolymer Activated in NaOH & NaOH + Na2SiO3. Materials. 2020; 13(1):59. https://doi.org/10.3390/ma13010059
Chicago/Turabian StyleSasui, Sasui, Gyuyong Kim, Jeongsoo Nam, Tomoyuki Koyama, and Sant Chansomsak. 2020. "Strength and Microstructure of Class-C Fly Ash and GGBS Blend Geopolymer Activated in NaOH & NaOH + Na2SiO3" Materials 13, no. 1: 59. https://doi.org/10.3390/ma13010059
APA StyleSasui, S., Kim, G., Nam, J., Koyama, T., & Chansomsak, S. (2020). Strength and Microstructure of Class-C Fly Ash and GGBS Blend Geopolymer Activated in NaOH & NaOH + Na2SiO3. Materials, 13(1), 59. https://doi.org/10.3390/ma13010059







