Aluminum/Stainless Steel Clad Materials Fabricated via Spark Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
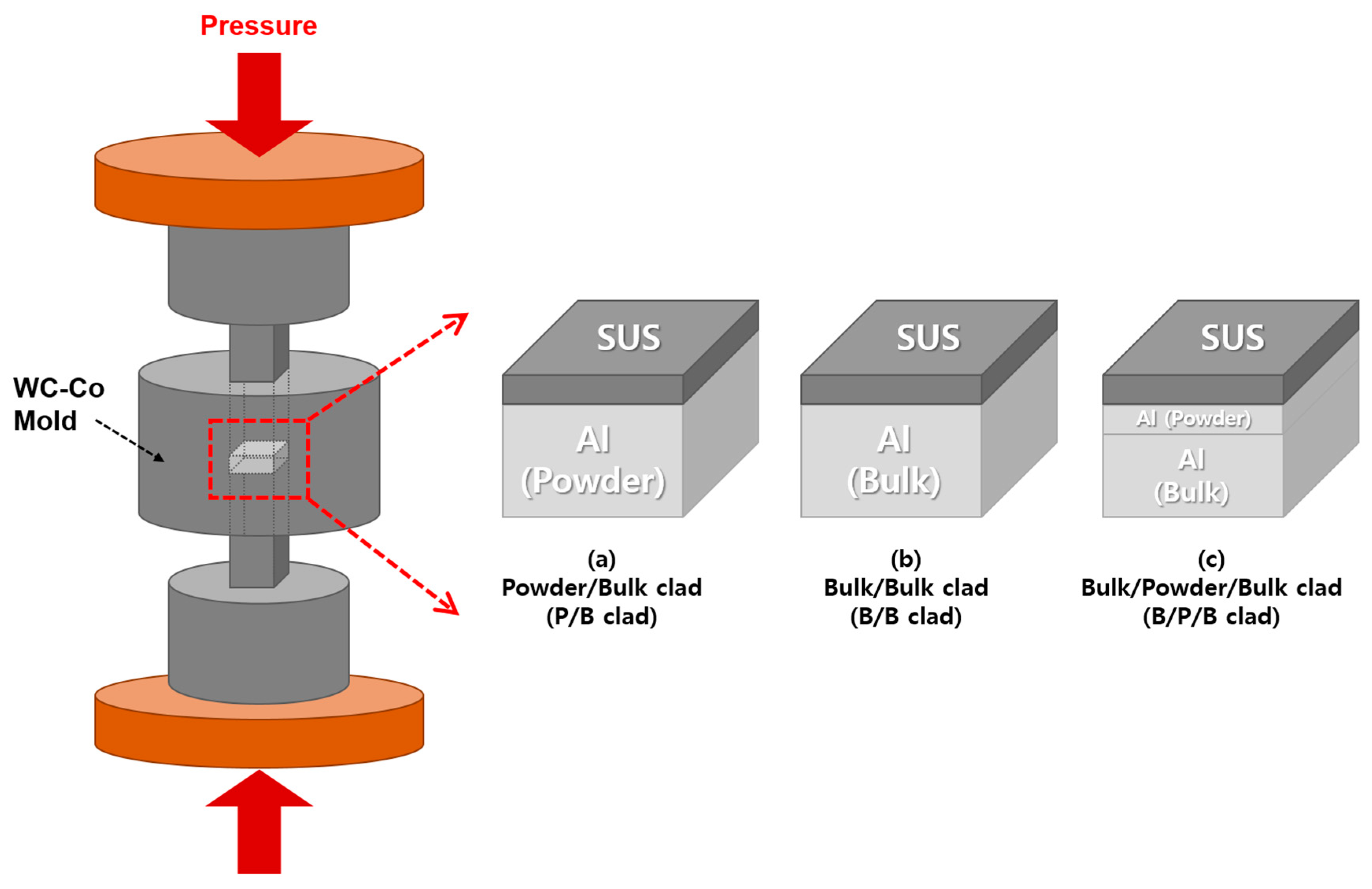
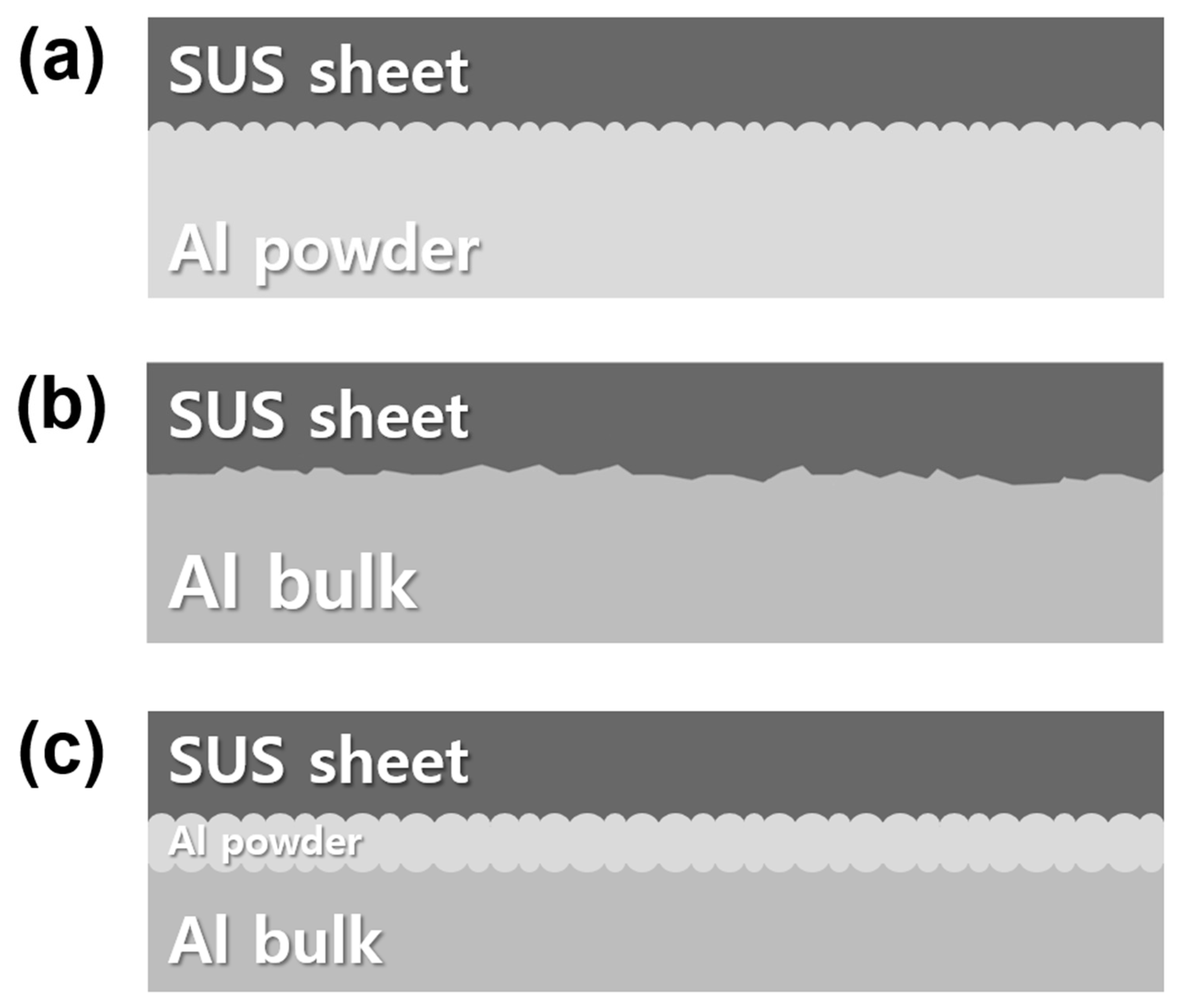
2.1. Al/SUS Clad Material Fabrication
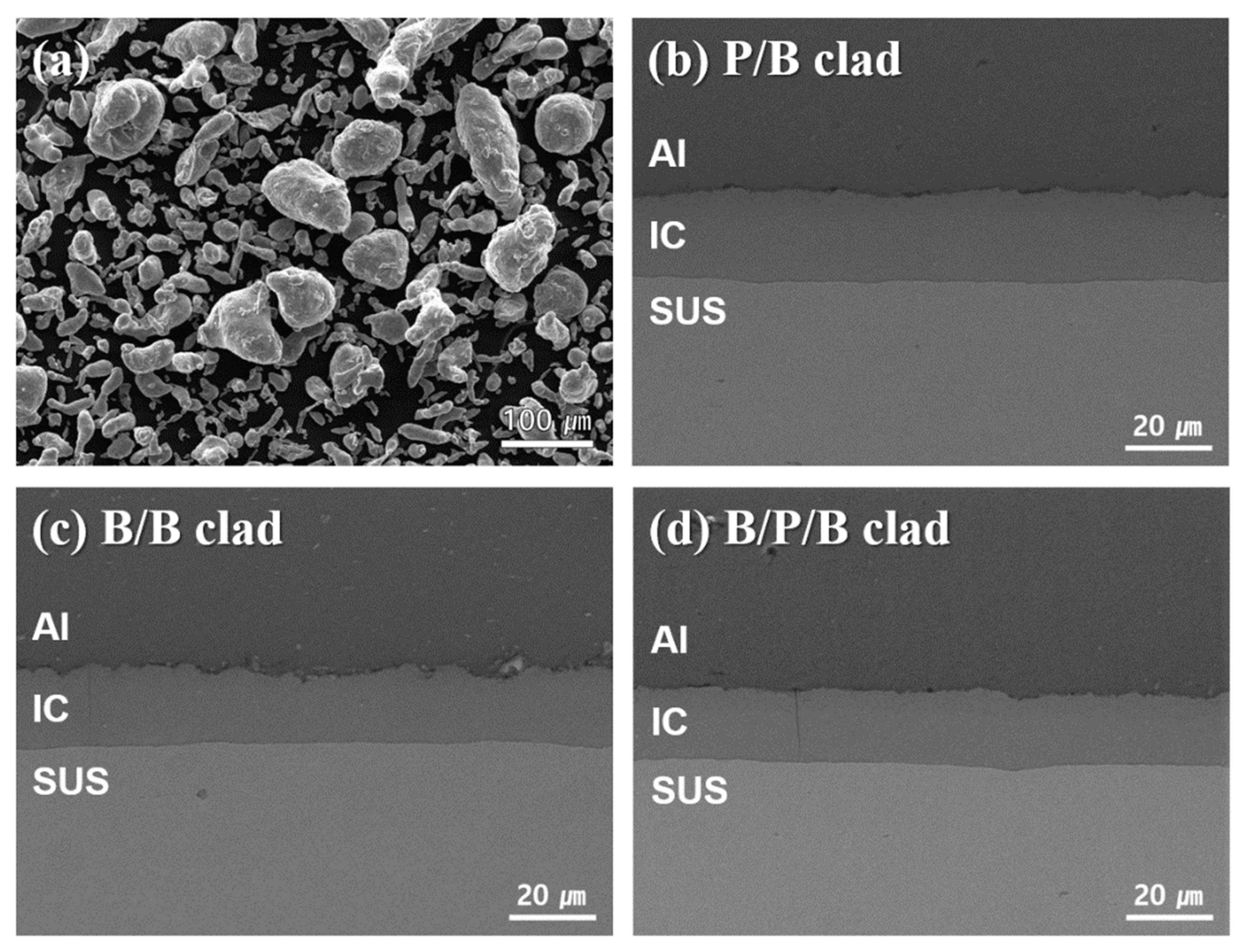
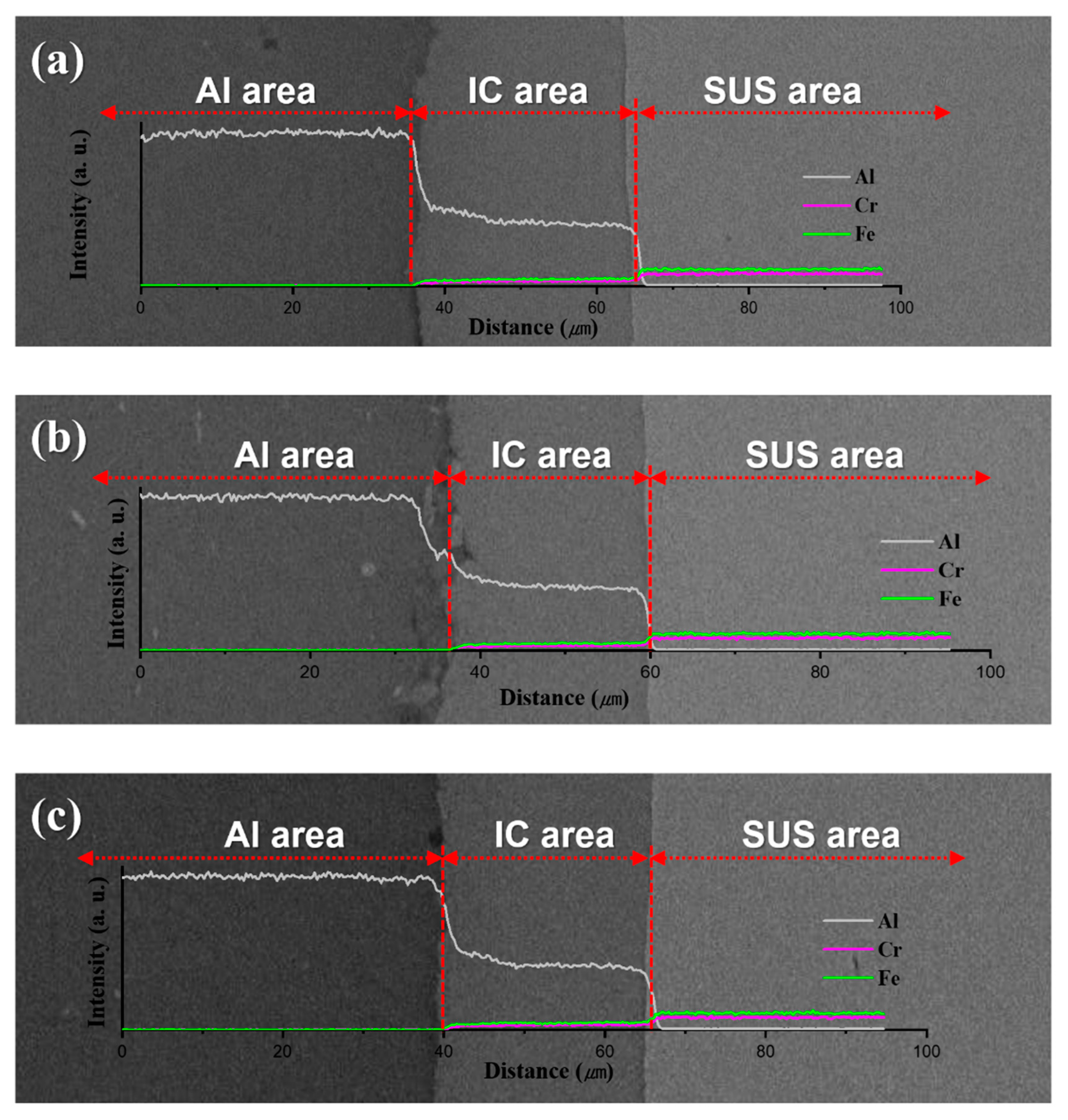
2.2. Al/SUS Clad Material Characterization
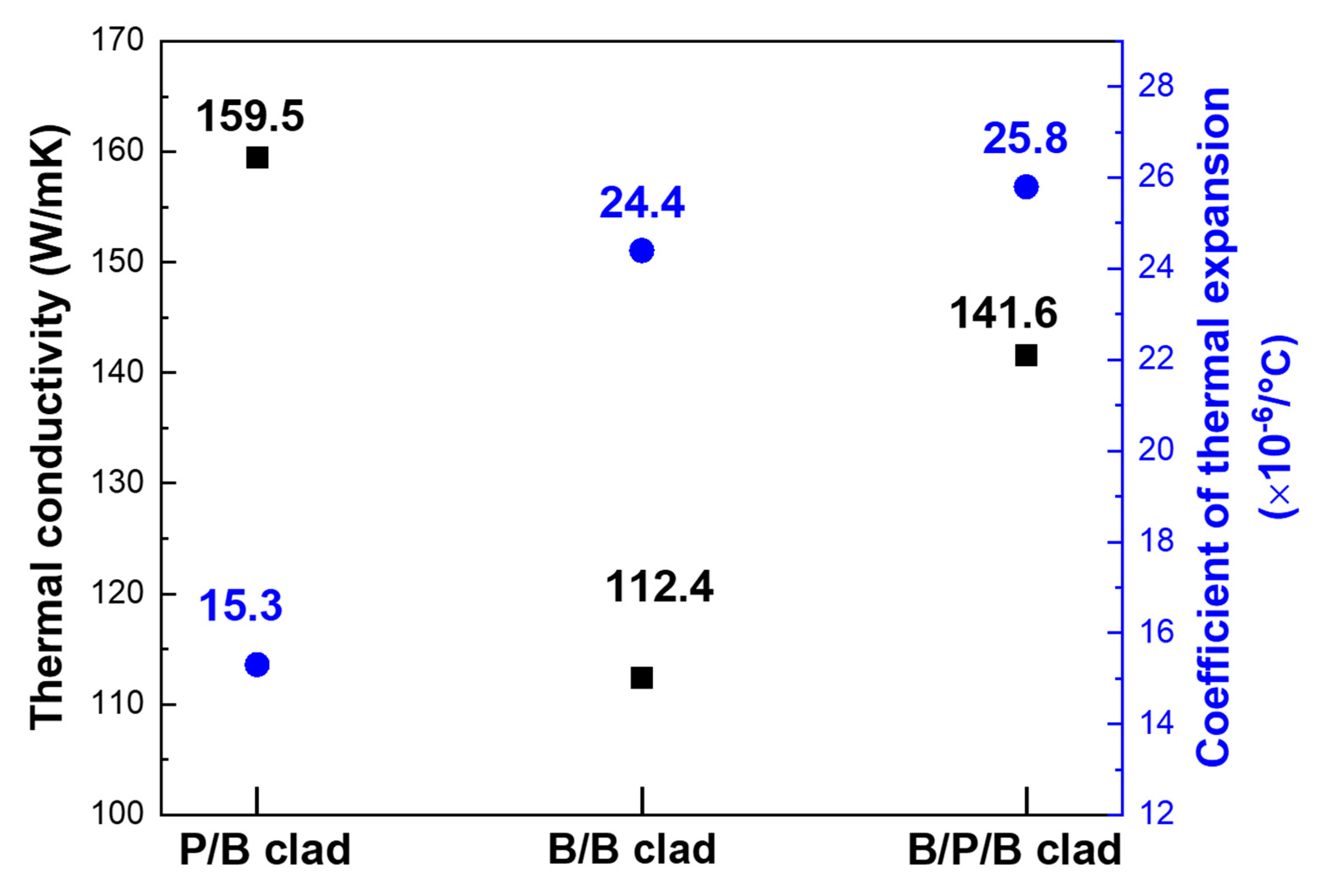
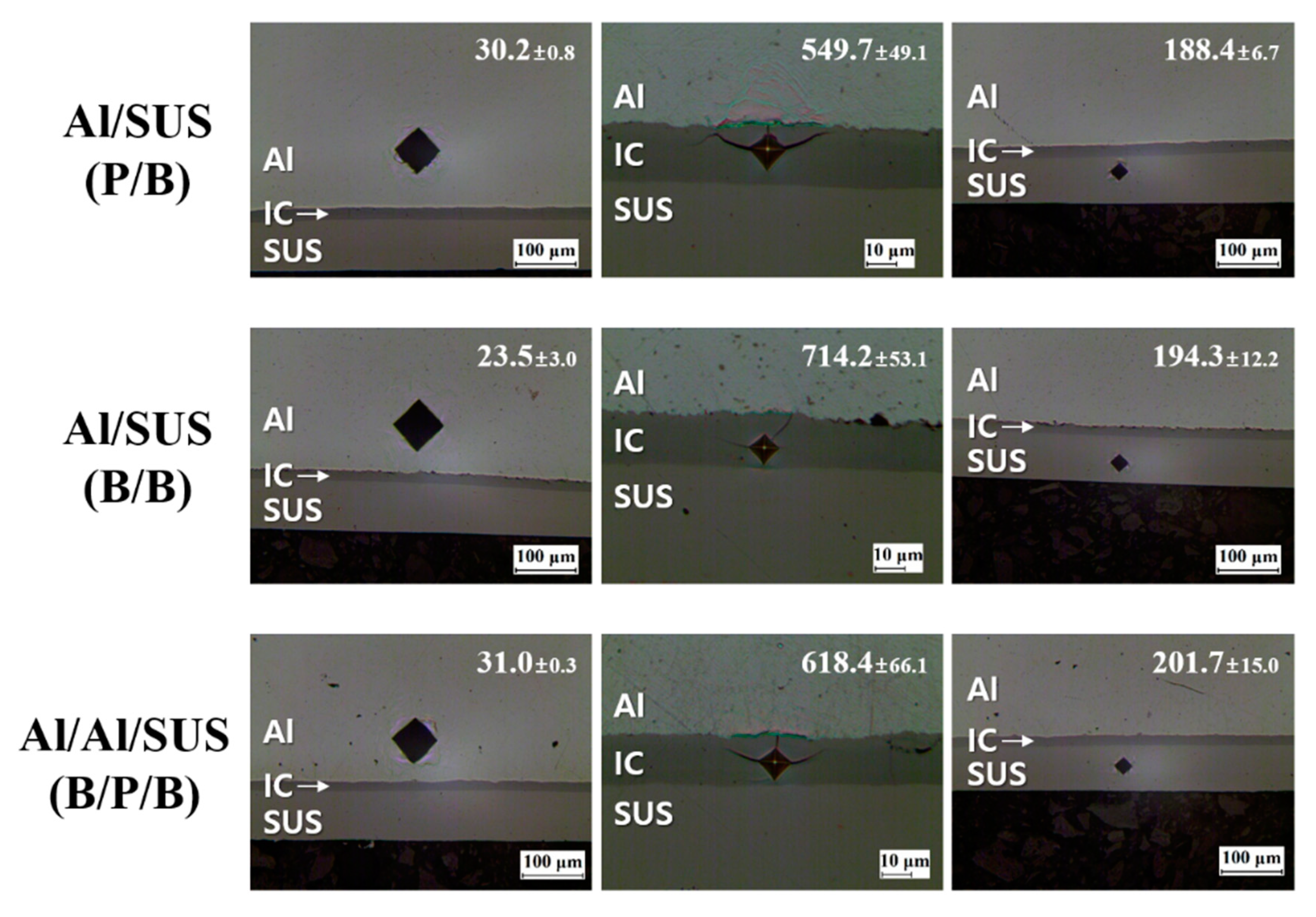
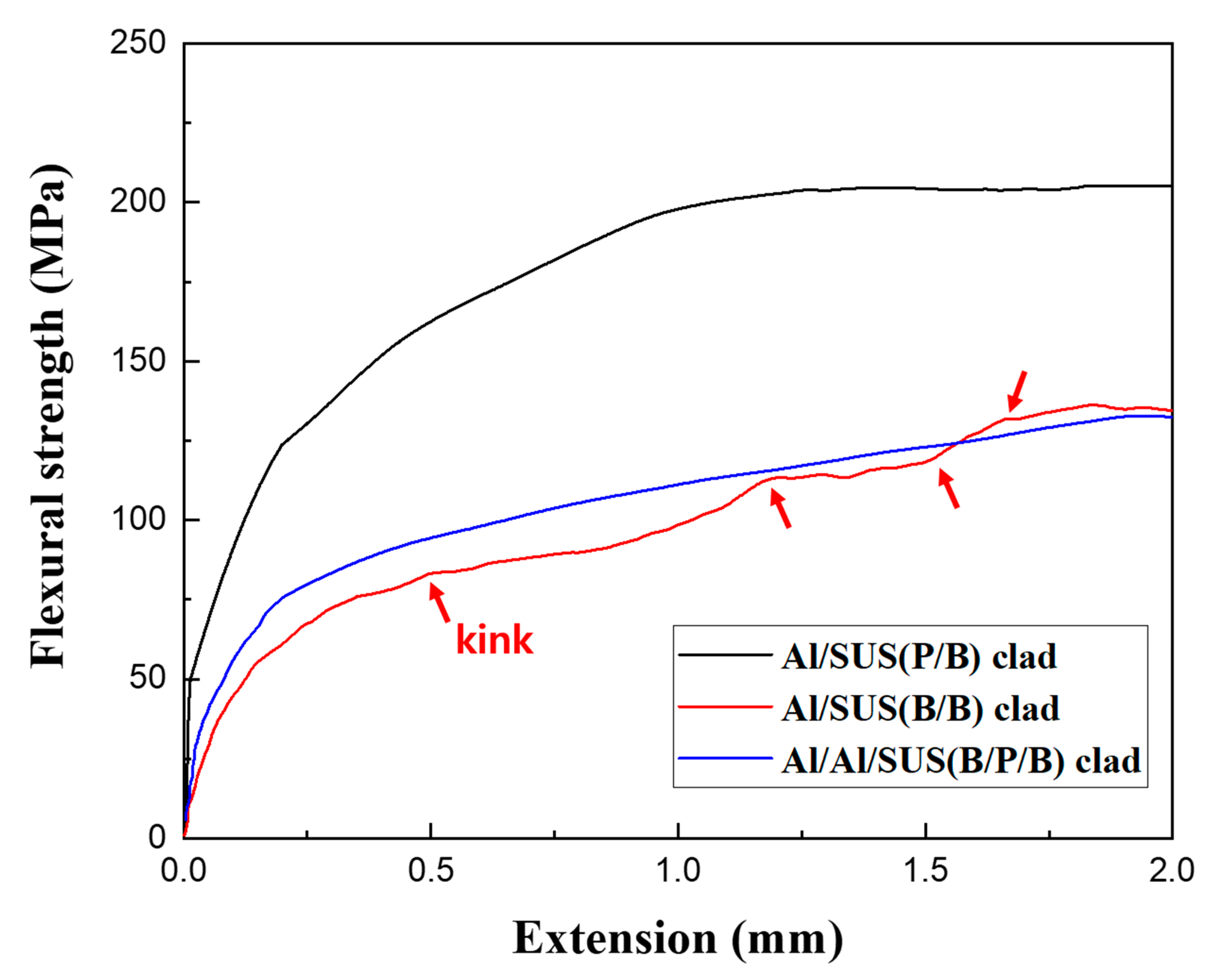
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.H. High-performance stretchable conductive nanocomposites: Materials, process, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef] [PubMed]
- Kravchyk, K.V.; Widmer, R.; Erni, R.; Dubey, R.J.C.; Krumeich, F.; Kovalenko, M.V.; Bodnarchuk, M.I. Copper sulfide nanoparticles as high-performance cathode materials for Mg-ion batteries. Sci. Rep. 2019, 9, 7988. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-G.; Suzuki, A.; Takata, N.; Kobashi, M. Joining of metals and polymers using powder metallurgy with laser irradiation. J. Mater. Process. Technol. 2019, 270, 1–7. [Google Scholar] [CrossRef]
- Liu, R.; Shen, C.; Dong, Y.; Qin, J.; Wang, Q.; Iocozzia, J.; Zhao, S.; Yuan, K.; Han, C.; Li, B.; et al. Sandwich-like CNTs/Si/C nanotubes as high performance anode materials for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14797–14804. [Google Scholar] [CrossRef]
- Kepert, C.J. Advanced functional properties in nanoporous coordination framework materials. Chem. Commun. 2006, 7, 695–700. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional materials for rechargeable batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Shi, G. Functional composite materials based on chemically converted graphene. Adv. Mater. 2011, 23, 1089–1115. [Google Scholar] [CrossRef]
- Ezatpour, H.R.; Sajjadi, S.A.; Sabzevar, M.H.; Huang, Y. Investigation of microstructure and mechanical properties of Al6061-nanocomposite fabricated by stir casting. Mater. Des. 2014, 55, 921–928. [Google Scholar] [CrossRef]
- Carroll, B.E.; Otis, R.A.; Borgonia, J.P.; Suh, J.O.; Dillon, R.P.; Shapiro, A.A.; Hofmann, D.D.; Liu, Z.K.; Beese, A.M. Functionally graded material of 304L stainless steel and inconel 625 fabricated by directed energy deposition: Characterization and thermodynamic modeling. Acta Mater. 2016, 108, 46–54. [Google Scholar] [CrossRef]
- Moghadam, A.D.; Schultz, B.F.; Ferguson, J.B.; Omrani, E.; Rohatgi, P.K.; Gupta, N. Functional metal matrix composites: Self-lubricating, self-healing, and nanocomposites-an outlook. Jom-J. Min. Met. Mat. Soc. 2014, 66, 872–881. [Google Scholar] [CrossRef]
- Lee, J.S.; Son, H.T.; Oh, I.H.; Kang, C.S.; Yun, C.H.; Lim, S.C.; Kwon, H.C. Fabrication and characterization of Ti–Cu clad materials by indirect extrusion. J. Mater. Process. Technol. 2007, 187, 653–656. [Google Scholar] [CrossRef]
- Yahiro, A.; Masui, T.; Yoshida, T.; Doi, D. Development of nonferrous clad plate and sheet by warm rolling with different temperature of materials. ISIJ Int. 1991, 31, 647–654. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, S.H.; Kim, M.S. Fabrication and brazeability of a three-layer 4343/3003/4343 aluminum clad sheet by rolling. J. Mater. Process. Technol. 2001, 111, 85–89. [Google Scholar] [CrossRef]
- Yilamu, K.; Hino, R.; Hamasaki, H.; Yoshida, F. Air bending and springback of stainless steel clad aluminum sheet. J. Mater. Process. Technol. 2010, 210, 272–278. [Google Scholar] [CrossRef]
- Liu, H.W.; Guo, C.; Cheng, Y.; Liu, X.F.; Shao, G.J. Interfacial strength and structure of stainless steel–semi-solid aluminum alloy clad metal. Mater. Lett. 2006, 60, 180–184. [Google Scholar] [CrossRef]
- Akramifard, H.R.; Mirzadeh, H.; Parsa, M.H. Cladding of aluminum on AISI 304L stainless steel by cold roll bonding: Mechanism, microstructure, and mechanical properties. Mater. Sci. Eng. A 2014, 613, 232–239. [Google Scholar] [CrossRef]
- Guo, X.; Tao, J.; Wang, W.; Li, H.; Wang, C. Effects of the inner mould material on the aluminum-316L stainless steel explosive clad pipe. Mater. Des. 2013, 49, 116–122. [Google Scholar] [CrossRef]
- Park, K.; Kim, D.; Park, J.; Cho, S.; Takamichi, M.; Kwon, H. Intermetallic Compounds in Al-SUS316L Composites. Adv. Eng. Mater. 2018, 20, 1800312. [Google Scholar] [CrossRef]
- Park, K.; Kim, D.; Kim, K.; Cho, S.; Takagi, K.; Kwon, H. Semisolid State Sintering Behavior of Aluminum–Stainless Steel 316L Composite Materials by Powder Metallurgy. Materials 2019, 12, 1473. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Park, K.; Cho, M.; Cho, S.; Kwon, H. Effect of Intermetallic Compounds on the Thermal and Mechanical Properties of Al–Cu Composite Materials Fabricated by Spark Plasma Sintering. Materials 2019, 12, 1546. [Google Scholar] [CrossRef]
- Lee, W.B.; Schmuecker, M.; Mercardo, U.A.; Biallas, G.; Jung, S.B. Interfacial reaction in steel–aluminum joints made by friction stir welding. Scr. Mater. 2006, 55, 355–358. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J. Microstructure characteristics and mechanical property of aluminum alloy/stainless steel lap joints fabricated by MIG welding–brazing process. Mat. Sci. Eng. A 2011, 528, 6179–6185. [Google Scholar] [CrossRef]
- Taban, E.; Gould, J.E.; Lippold, J.C. Dissimilar friction welding of 6061-T6 aluminum and AISI 1018 steel: Properties and microstructural characterization. Mater. Des. 2010, 31, 2305–2311. [Google Scholar] [CrossRef]
- Rahman, M.H.; Al Rashed, H.M. Characterization of silicon carbide reinforced aluminum matrix composites. Procedia. Eng. 2014, 90, 103–109. [Google Scholar] [CrossRef]
- Chang, C.N.; Chen, F.S. Wear resistance evaluation of plasma nitrocarburized AISI 316L stainless steel. Mater. Chem. Phys. 2003, 82, 281–287. [Google Scholar] [CrossRef]
- Matysik, P.; Jóźwiak, S.; Czujko, T. Characterization of low-symmetry structures from phase equilibrium of Fe-Al system—Microstructures and mechanical properties. Materials 2015, 8, 914–931. [Google Scholar] [CrossRef]
- Cho, S.; Kikuchi, K.; Miyazaki, T.; Kawasaki, A.; Arami, Y.; Silvan, J.F. Epitaxial growth of chromium carbide nanostructures on multiwalled carbon nanotubes (MWCNTs) in MWCNT-copper composites. Acta Mater. 2013, 61, 708–716. [Google Scholar] [CrossRef]
- Kwon, H.; Estili, M.; Takagi, K.; Miyazaki, T.; Kawasaki, A. Combination of hot extrusion and spark plasma sintering for producing carbon nanotube reinforced aluminum matrix composites. Carbon 2009, 47, 570–577. [Google Scholar] [CrossRef]
| Sample | Density | ||
|---|---|---|---|
| Theoretical Density (g/cm3) | Experimental Density (g/cm3) | Relative Density (%) | |
| Al/SUS (P/B) | 2.964 | 2.869 | 96.8 |
| Al/SUS (B/B) | 3.228 | 3.090 | 95.7 |
| Al/Al/SUS (B/P/B) | 3.101 | 2.994 | 96.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Kim, D.; Kim, K.; Kwon, H. Aluminum/Stainless Steel Clad Materials Fabricated via Spark Plasma Sintering. Materials 2020, 13, 239. https://doi.org/10.3390/ma13010239
Park K, Kim D, Kim K, Kwon H. Aluminum/Stainless Steel Clad Materials Fabricated via Spark Plasma Sintering. Materials. 2020; 13(1):239. https://doi.org/10.3390/ma13010239
Chicago/Turabian StylePark, Kwangjae, Dasom Kim, Kyungju Kim, and Hansang Kwon. 2020. "Aluminum/Stainless Steel Clad Materials Fabricated via Spark Plasma Sintering" Materials 13, no. 1: 239. https://doi.org/10.3390/ma13010239
APA StylePark, K., Kim, D., Kim, K., & Kwon, H. (2020). Aluminum/Stainless Steel Clad Materials Fabricated via Spark Plasma Sintering. Materials, 13(1), 239. https://doi.org/10.3390/ma13010239







