Titania-Coated Alumina Foam Photocatalyst for Memantine Degradation Derived by Replica Method and Sol-Gel Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation and Characterization of Al2O3 Suspension
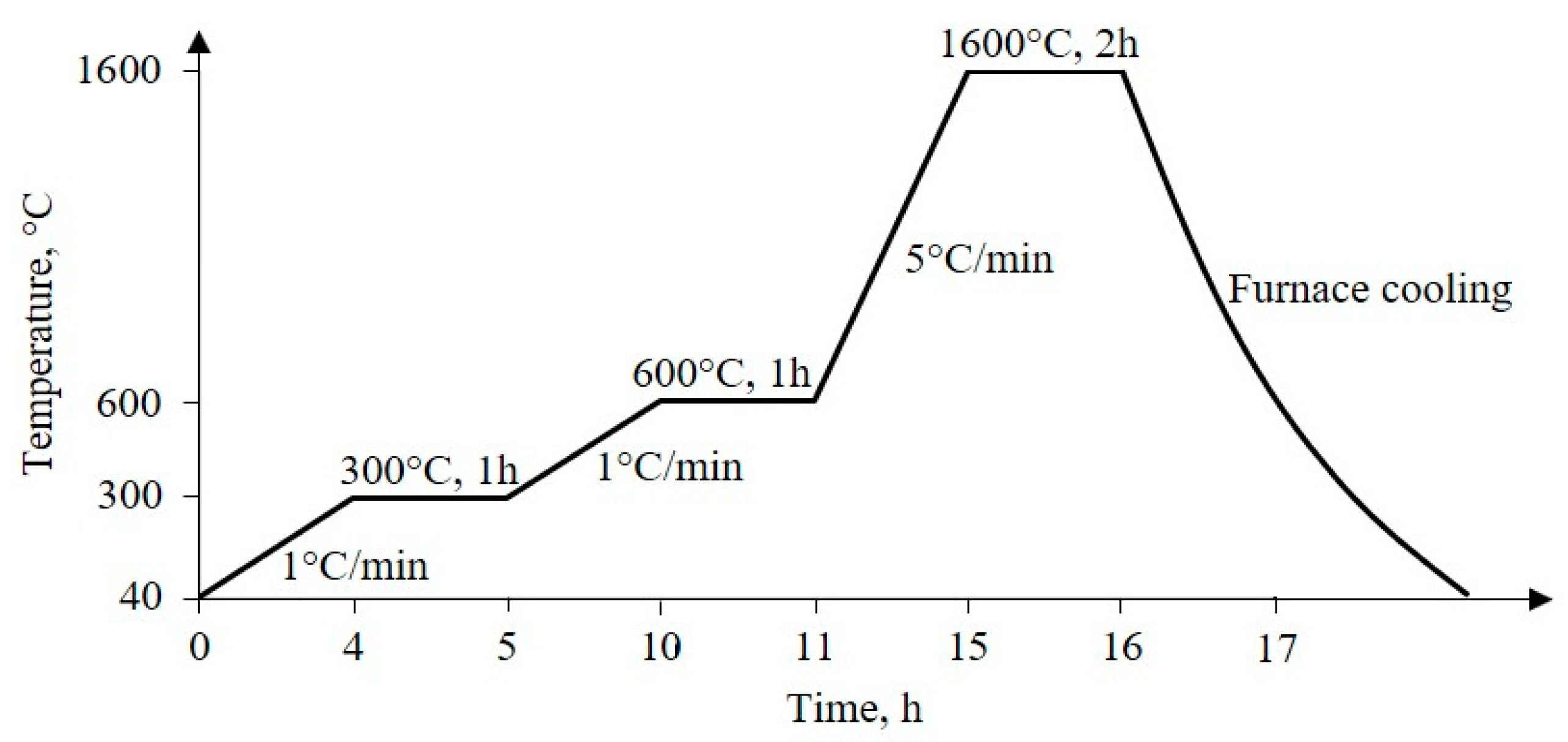
2.3. Preparation of Al2O3 Foam
2.4. Deposition of Nanostructured TiO2 Film on Al2O3 Foam Substrate
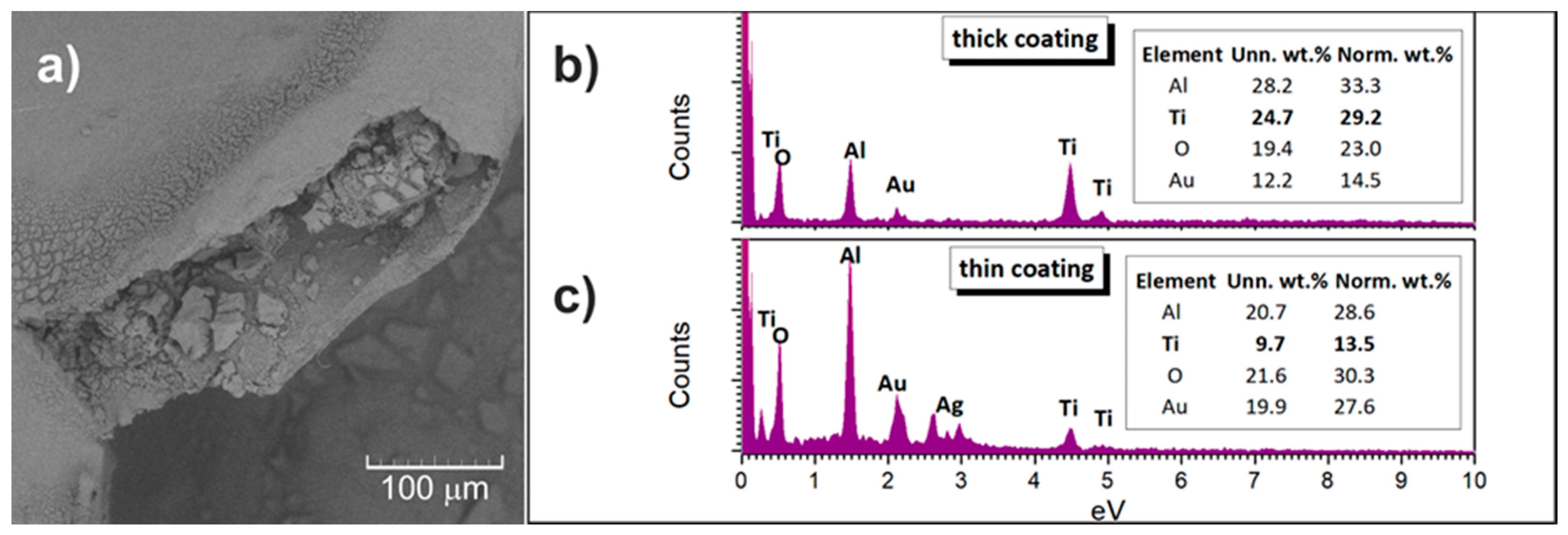
2.5. Characterization of Al2O3 Substrate and TiO2-Coated Al2O3 Foam
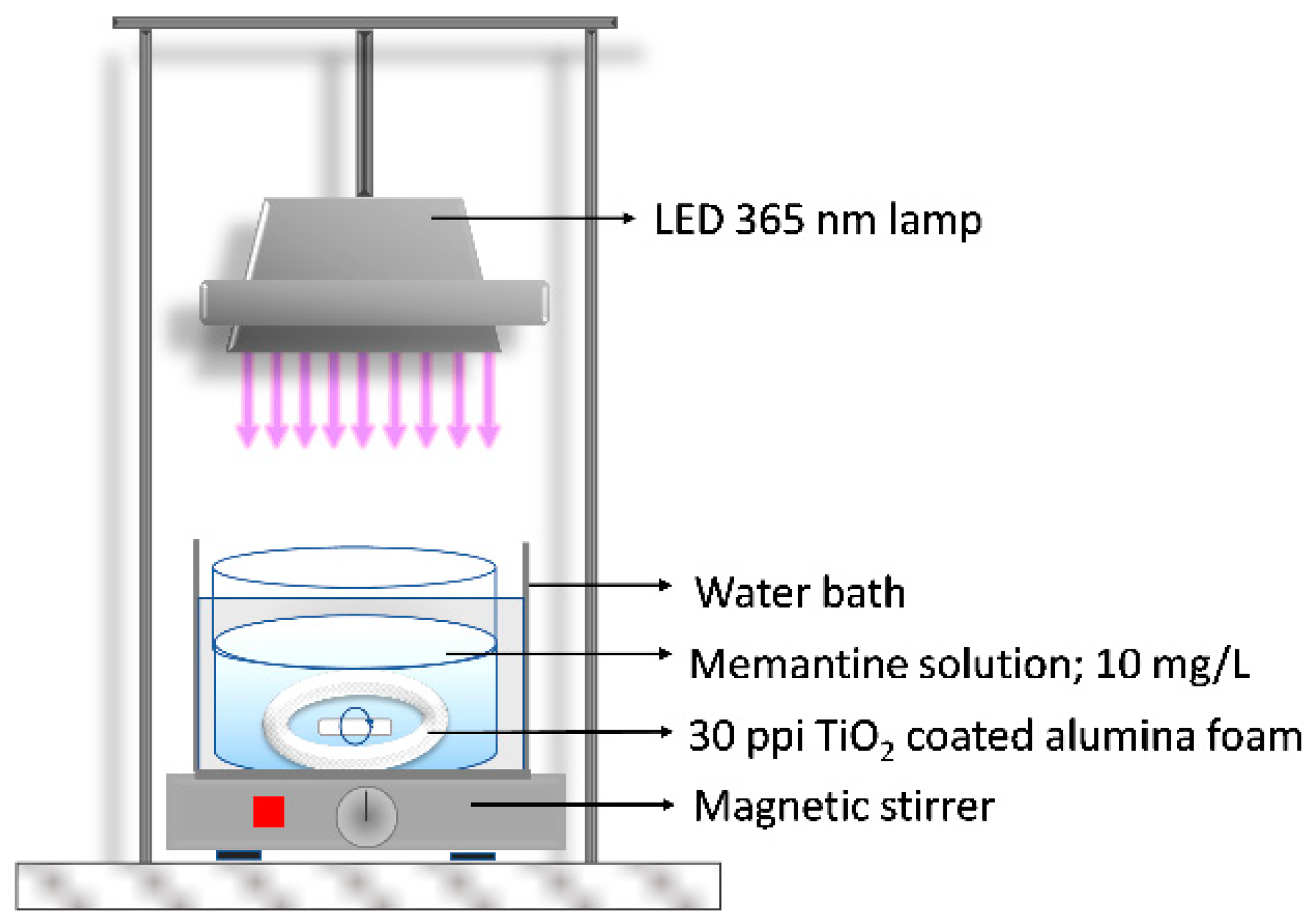
2.6. Photocatalytic Experiment
3. Results and Discussion
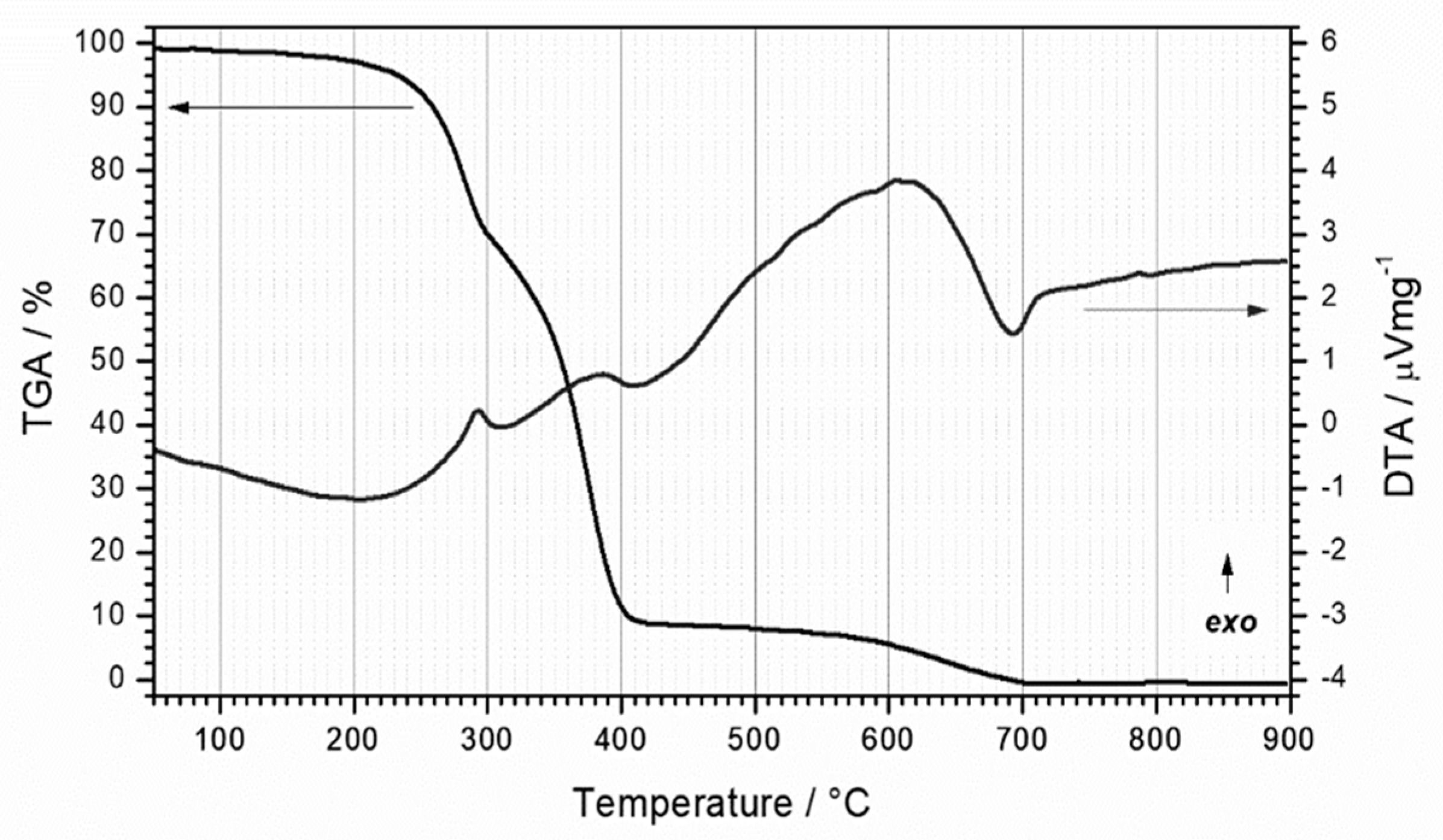
3.1. Thermal Evolution of PU Foam
3.2. Properties of Al2O3 Foam Substrate and TiO2-coated Al2O3 Foam
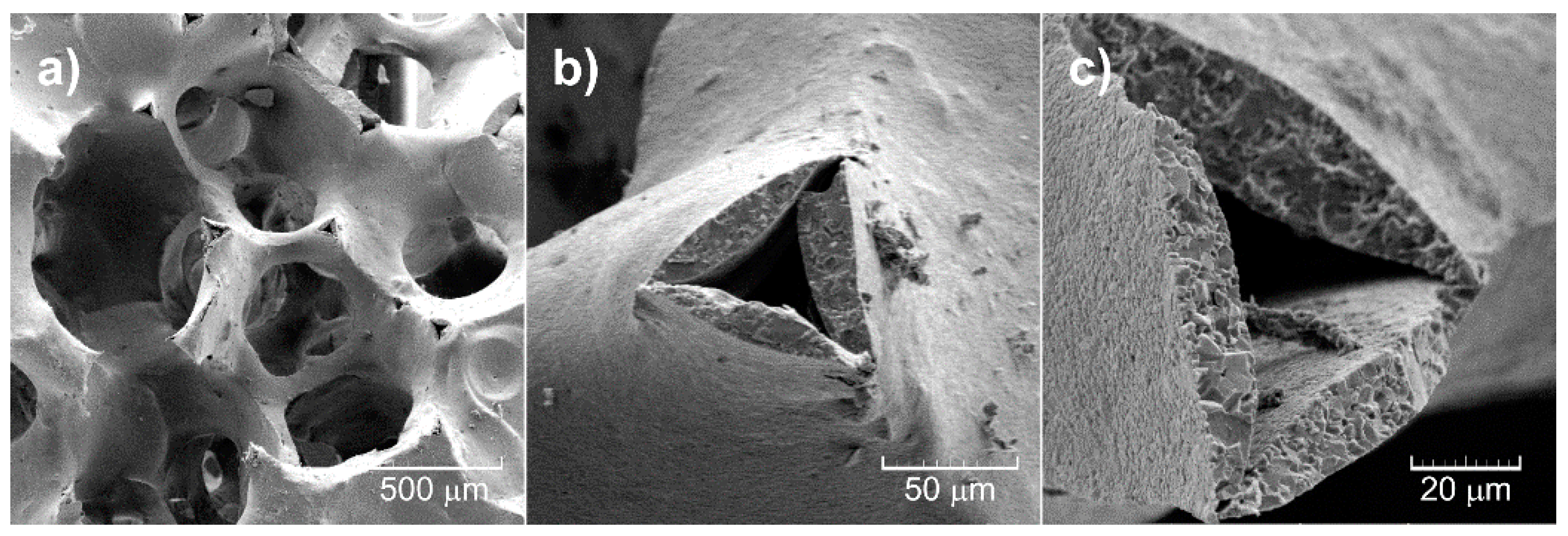
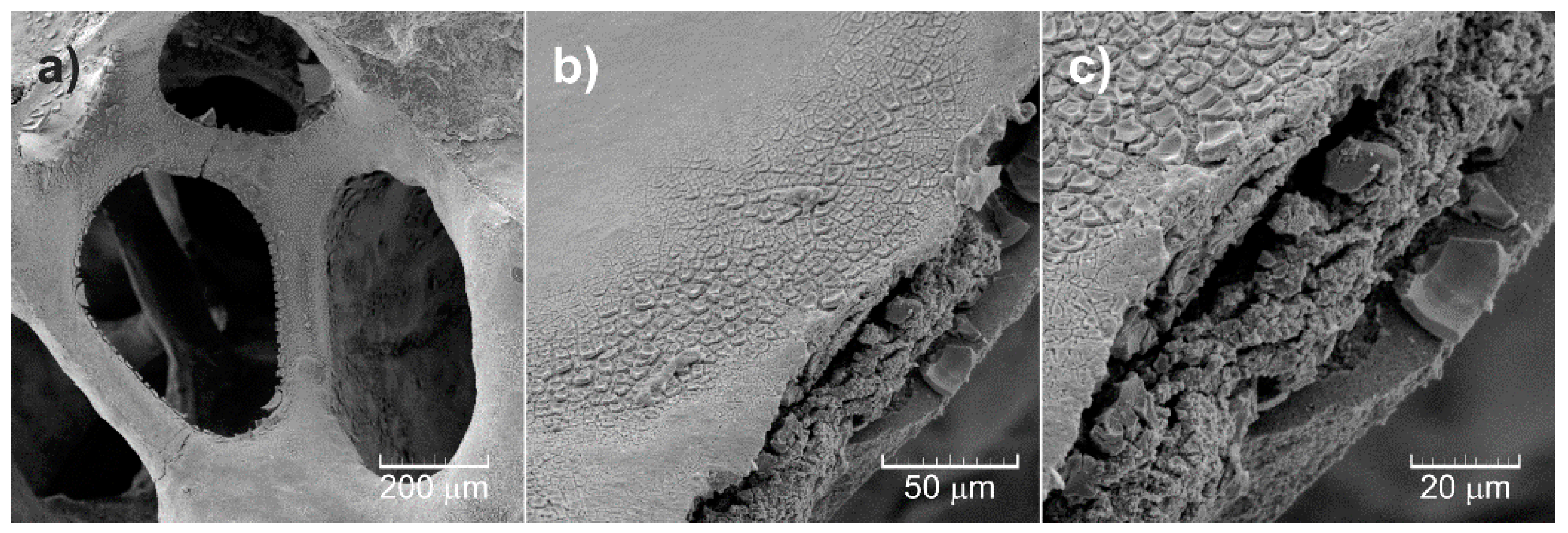
3.3. Morphology of Al2O3 Foam Substrate and TiO2-coated Al2O3 Foam
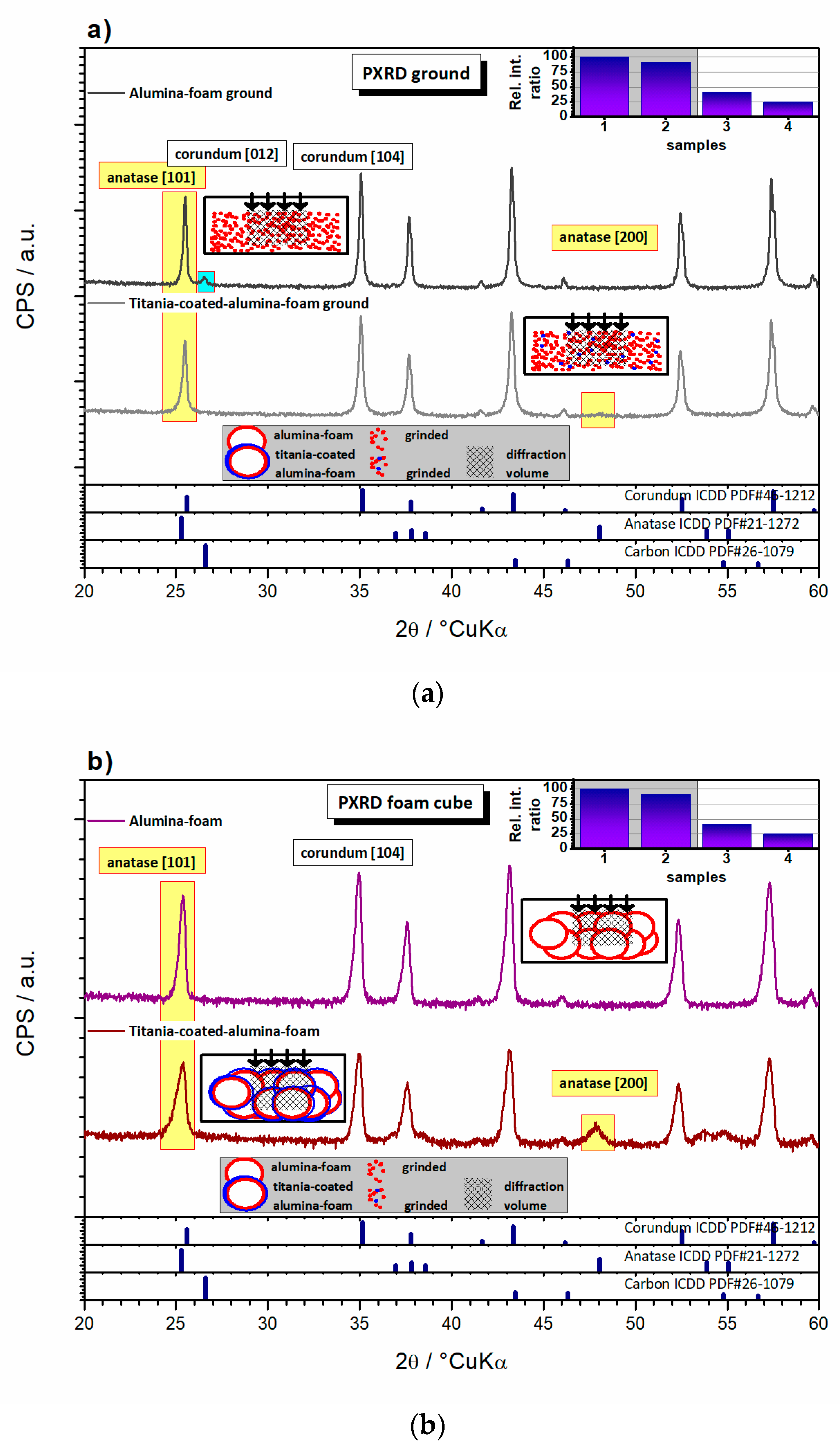
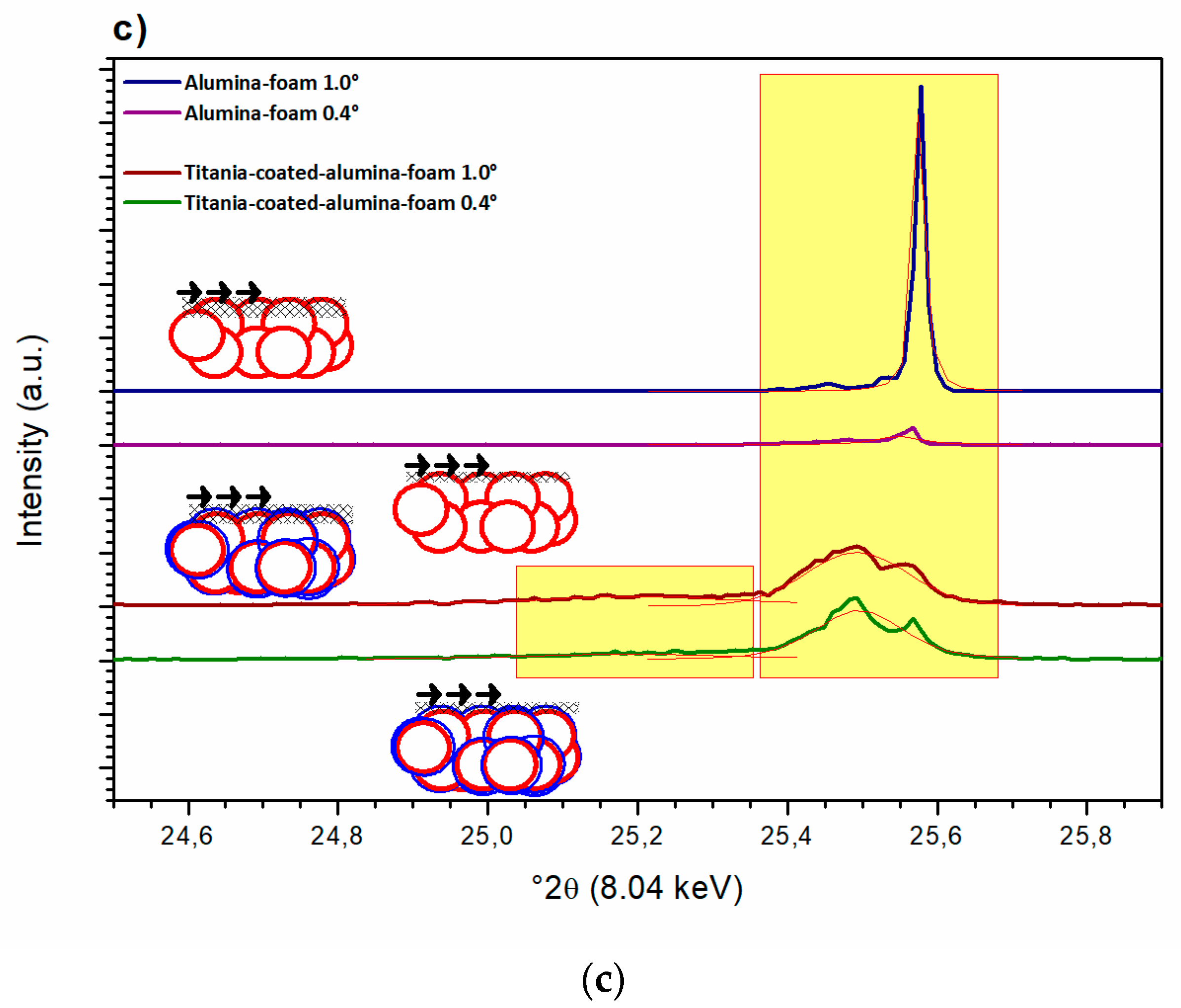
3.4. Structural Properties of the Al2O3 Foam Substrate and TiO2-coated Al2O3 Foam
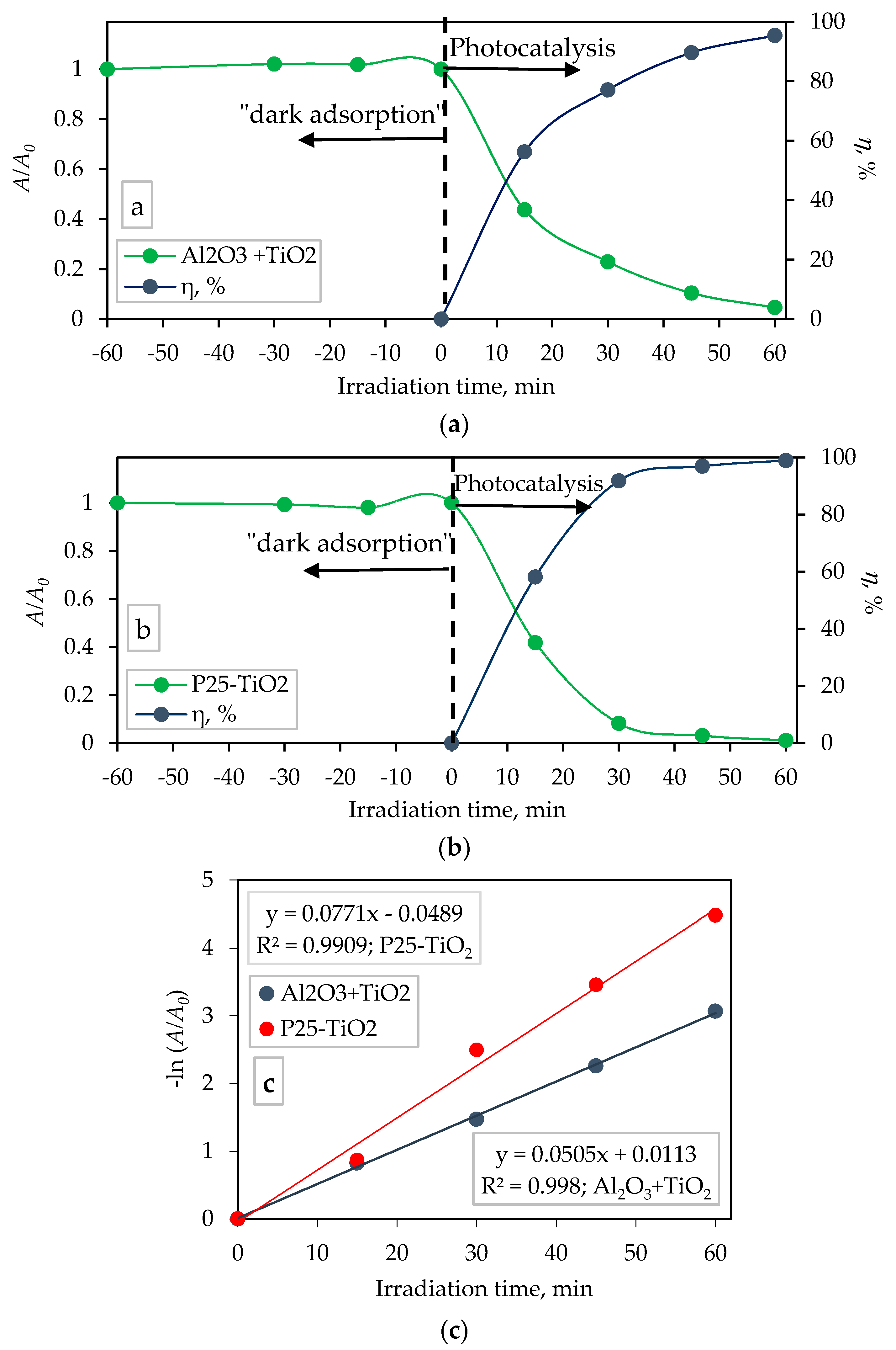
3.5. Photocatalytic Degradation of Memantine on TiO2-coated Al2O3 Foam and TiO2 Nanoparticles P25
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karl, S.; Somers, A.V. Method of Making Porous Ceramic Articles. U.S. Patent 3,090,094, 21 May 1963. [Google Scholar]
- Huo, W.; Zhang, X.; Chen, Y.; Hu, Z.; Wang, D.; Yang, J. Ultralight and high-strength bulk alumina/zirconia composite ceramic foams through direct foaming method. Ceram. Int. 2019, 45, 1464–1467. [Google Scholar] [CrossRef]
- Du, Z.; Yao, D.; Xia, Y.; Zuo, K.; Yin, J.; Liang, H.; Zeng, Y.-P. The high porosity silicon nitride foams prepared by the direct foaming method. Ceram. Int. 2019, 45, 2124–2130. [Google Scholar] [CrossRef]
- Pokhrel, A.; Seo, D.N.; Lee, S.T.; Kim, I.J. Processing of Porous Ceramics by Direct Foaming: A Review. J. Korean Ceram. Soc. 2013, 50, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-Y.; Zhou, D.-L.; Chen, Y.; Liang, B.; Zhou, J.-B. Preparation of high open porosity ceramic foams via direct foaming molded and dried at room temperature. J. Eur. Ceram. Soc. 2014, 34, 2443–2452. [Google Scholar] [CrossRef]
- Jang, W.Y.; Seo, D.N.; Basnet, B.; Park, J.G.; Han, I.S.; Kima, I.J. Tailoring the microstructure of Al2O3-SiO2 porous ceramics through starch consolidation by direct foaming. J. Ceram. Process. Res. 2017, 1, 275–279. [Google Scholar]
- Gregorová, E.; Pabst, W.; Uhlírová, T.; Necina, V.; Vesely´, M.; Sedlárová, I. Processing, microstructure and elastic properties of mullite-based ceramic foams prepared by direct foaming with wheat flour. J. Eur. Ceram. Soc. 2016, 36, 109–120. [Google Scholar] [CrossRef]
- Okada, K.; Shimizu, M.; Isobe, T.; Kameshima, Y.; Sakai, M.; Nakajima, A.; Kurata, T. Characteristics of microbubbles generated by porous mullite ceramics prepared by an extrusion method using organic fibers as the pore former. J. Eur. Ceram. Soc. 2010, 30, 1245–1251. [Google Scholar] [CrossRef]
- Song, I.-H.; Kwon, I.-M.; Kim, H.-D.; Kim, Y.-W. Processing of microcellular silicon carbide ceramics with a duplex pore structure. J. Eur. Ceram. Soc. 2010, 30, 2671–2676. [Google Scholar] [CrossRef]
- Mesquita, R.M.; Bressiani, A.H.A. Fabrication of Porous Silicon Nitride by Sacrificing Template Method. Adv. Sci. Technol. 2010, 63, 170–174. [Google Scholar] [CrossRef]
- Yan, J.; Hong, L.-Y.; Wang, A.; Kim, D.-P. Facile Synthesis of SiCN Ceramic Foam via Self-Sacrificial Template Method. Solid State Phenom. 2007, 124–126, 727–730. [Google Scholar] [CrossRef]
- Kamitani, K.; Hyodo, T.; Shimizu, Y.; Egashira, M. Fabrication of porous alumina ceramics having cell windows with controlled size by PMMA template method. J. Mater. Sci. 2010, 45, 3602–3609. [Google Scholar] [CrossRef] [Green Version]
- Nam, G.; Choi, S.; Byun, H.; Rhym, Y.-M.; Shim, S.E. Preparation of macroporous carbon foams using a polyurethane foam template replica method without curing step. Macromol. Res. 2013, 21, 958–964. [Google Scholar] [CrossRef]
- Betke, U.; Scheunemann, M.; Scheffler, M. Refitting of Zirconia Toughening into Open-Cellular Alumina Foams by Infiltration with Zirconyl Nitrate. Materials 2019, 12, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schelm, K.; Abreu Morales, E.; Scheffler, M. Mechanical and Surface-Chemical Properties of Polymer Derived Ceramic Replica Foams. Materials 2019, 12, 1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappacena, K.E.; Faber, K.T.; Wang, H.; Porter, W.D. Thermal conductivity of porous silicon carbide derived from wood precursors. J. Am. Ceram. Soc. 2007, 90, 2855–2862. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Tian, W.; Wang, Z.; Li, C.-G.; Zhao, Y.; Bian, H.-M. In situ porous alumina/aluminum titanate ceramic composite prepared by spark plasma sintering from nanostructured powders. Scr. Mater. 2009, 60, 578–581. [Google Scholar] [CrossRef]
- Akhtar, F.; Vasiliev, P.O.; Bergstrom, L. Hierarchically porous ceramics from diatomite powders by pulsed current processing. J. Am. Ceram. Soc. 2009, 92, 338–343. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, W.; Yuan, J.; Shi, J.; He, D.; Shangguan, W. High photocatalytic activity and stability for decomposition of gaseous acetaldehyde on TiO2/Al2O3 composite films coated on foam nickel substrates by sol-gel processes. J. Sol-Gel Sci. Technol. 2008, 45, 1–8. [Google Scholar] [CrossRef]
- Plesch, G.; Gorbár, M.; Vogt, U.F.; Jesenák, K.; Vargová, M. Reticulated macroporous ceramic foam supported TiO2 for photocatalytic applications. Mater. Lett. 2009, 63, 461–463. [Google Scholar] [CrossRef]
- Vargová, M.; Plesch, G.; Vogt, U.F.; Zahoran, M.; Gorbár, M.; Jesenák, K. TiO2 thick films supported on reticulated macroporous Al2O3 foams and their photoactivity in phenol mineralization. Appl. Surf. Sci. 2011, 257, 4678–4684. [Google Scholar] [CrossRef]
- Alijani, S.; Moghaddam, A.Z.; Vaez, M.; Towfighi, J. Characterization of TiO2-coated ceramic foam prepared by modified sol-gel method and optimization of synthesis parameters in photodegradation of Acid Red 73. Korean J. Chem. Eng. 2013, 30, 1855–1866. [Google Scholar] [CrossRef]
- Alijani, S.; Vaez, M.; Moghaddam, A.Z. Photocatalytic degradation of acid red 73 using TiO2 nanoparticles immobilized on alumina foam. Int. J. Environ. Sci. Dev. 2014, 5, 104–107. [Google Scholar]
- Šegota, S.; Ćurković, L.; Ljubas, D.; Svetličić, V.; Fiamengo Houra, I.; Tomašić, N. Synthesis, characterization and photocatalytic properties of sol–gel TiO2 films. Ceram. Int. 2011, 37, 1153–1160. [Google Scholar] [CrossRef]
- Inamuddin, R.B.; Sharma, G.; Kumar, A.; Lichtfouse, E.; Asiri, A.M. (Eds.) Nanophotocatalysis and Environmental Applications: Materials and Technology (Environmental Chemistry for a Sustainable World); Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar]
- Liu, Y.; Li, Z.; Green, M.; Just, M.; Li, Y.Y.; Chen, X. Titanium dioxide nanomaterials for photocatalysis. J. Phys. D Appl. Phys. 2017, 50, 193003. [Google Scholar] [CrossRef]
- Wei, S.; Wu, R.; Xu, X.; Jian, J.; Wang, H.; Sun, Y. One-step synthetic approach for core-shelled black anatase titania with high visible light photocatalytic performance. Chem. Eng. J. 2016, 299, 120–125. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Rožman, M.; Ašperger, D.; Ćurković, L.; Babić, S. Photocatalytic degradation of azithromycin by nanostructured TiO2 film: Kinetics, degradation products, and toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef] [Green Version]
- Čizmić, M.; Ljubas, D.; Ćurković, L.; Škorić, I.; Babić, S. Kinetics and degradation pathways of photolytic and photocatalytic oxidation of the anthelmintic drug praziquantel. J. Hazard. Mater. 2017, 323, 500–512. [Google Scholar] [CrossRef]
- Ćurković, L.; Ljubas, D.; Šegota, S.; Bačić, I. Photocatalytic degradation of Lissamine Green B dye by using nanostructured sol–gel TiO2 films. J. Alloys Compd. 2014, 604, 309–316. [Google Scholar] [CrossRef]
- Ćurković, L.; Ljubas, D.; Juretić, H. Photocatalytic decolorization kinetics of diazo dye Congo Red aqueous solution by UV/TiO2 nanoparticles. React. Kinet. Mech. Catal. 2010, 99, 201–208. [Google Scholar] [CrossRef]
- Plaisier, J.R.; Nodari, L.; Gigli, L.; Miguel, E.P.R.S.; Bertoncello, R.; Lausi, A. The X-ray diffraction beamline MCX at Elettra: A case study of non-destructive analysis on stained glass. ACTA IMEKO 2017, 6, 71–75. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.S.; He, J.J.; Mao, Z.L.; Sun, J.H. Study on the Pyrolytic Behaviors and Kinetics of Rigid Polyurethane Foams. Procedia Eng. 2013, 52, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.-G.; Wang, D.-Y.; Wang, C.; Qu, M.-H.; Wang, J.-S.; Zhao, C.-S.; Jing, X.-K.; Wang, Y.-Z. A novel phosphorus-containing copolyester/montmorillonite nanocomposites with improved flame retardancy. Eur. Polym. J. 2007, 43, 2882–2890. [Google Scholar] [CrossRef]
- Hakim, A.A.A.; Nassar, M.; Emam, A.; Sultan, M. Preparation and characterization of rigid polyurethane foam prepared from sugar-cane bagasse polyol. Mater. Chem. Phys. 2011, 129, 301–307. [Google Scholar] [CrossRef]
- Kramer, R.H.; Zammarano, M.; Linteris, G.T.; Gedde, U.W.; Gilman, J.W. Heat release and structural collapse of flexible polyurethane foam. Polym. Degrad. Stab. 2010, 95, 1115–1122. [Google Scholar] [CrossRef]
- Behravesh, E.; Hupa, L.; Salmi, T.; Murzin, D.Y. Alumina ceramic foams as catalyst supports. Catalysis 2016, 28, 28–50. [Google Scholar]
- Fey, T.; Betke, U.; Rannabauer, S.; Scheffler, M. Reticulated replica ceramic foams: Processing, functionalization, and characterization. Adv. Eng. Mater. 2017, 19, 1700369. [Google Scholar] [CrossRef] [Green Version]
- Bahloul, W.; Bounor-Legare, V.; Seytre, G.; Cassagnau, P. Influence of a non-polar medium (alkane and molten polypropylene) on the titanium n-butoxide hydrolysis-condensation reactions. J. Sol-Gel Sci. Technol. 2011, 57, 86–94. [Google Scholar] [CrossRef]
- Baraton, M.I.; Quintard, P.J. Infrared evidence of order-disorder phase transitions (γ→δα) in Al2O3. Mol. Struct. 1982, 79, 337–340. [Google Scholar] [CrossRef]
- Kurajica, S.; Macan, J.; Mandić, V.; Galjer, M.; Mužina, M.; Plaisier, J.R. Reinforcing blade-cast photocatalytic-titania thin film by titanate nanotubes. Mater. Res. Bull. 2018, 105, 142–148. [Google Scholar] [CrossRef]
- Sacco, O.; Sannino, D.; Matarangolo, M.; Vaiano, V. Room Temperature Synthesis of V-Doped TiO2 and Its Photocatalytic Activity in the Removal of Caffeine under UV Irradiation. Materials 2019, 12, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galata, E.; Georgakopoulou, E.A.; Kassalia, M.E.; Papadopoulou-Fermeli, N.; Pavlato, E.A. Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties. Materials 2019, 12, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, A.; Márquez, A.; Vera, M.L.; Meichtry, J.M.; Litter, M.I. Photocatalytic activity of TiO2 thin films deposited by cathodic arc. Appl. Catal. B: Environ. 2011, 101, 676–681. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Škorić, I.; Rožman, M.; Ašperger, D.; Ćurković, L.; Petrović, M.; Babić, S. Photolytic and photocatalytic degradation of febantel in aqueous media. Desalin. Water Treat. 2018, 104, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Alatrache, A.; Cortyl, A.; Arnoux, P.; Pons, M.N.; Zahraa, O. Sulfamethoxazole removal from polluted water by immobilized photocatalysis. Toxicol. Environ. Chem. 2015, 97, 32–42. [Google Scholar] [CrossRef]
- Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M. Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef] [Green Version]

| ρ, g/cm3 | ϕ, % | Strut Thickness, µm | Pore Size, µm | σ, MPa | |
|---|---|---|---|---|---|
| Al2O3 | 0.46 ± 0.03 | 88.50 ± 0.76 | 52.73 ± 4.79 | 430.14 ± 52.49 | 2.03 ± 0.17 |
| TiO2-Al2O3 | 0.44 ± 0.02 | 88.96 ± 0.70 | 57.46 ± 5.14 | 436.01 ± 57.02 | 2.04 ± 0.19 |
| Photocatalyst | k, min−1 | t1/2, min | R2 |
|---|---|---|---|
| TiO2-coated Al2O3 foam | 0.0505 | 13.73 | 0.9980 |
| TiO2 P25-Degussa | 0.0771 | 8.99 | 0.9909 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švagelj, Z.; Mandić, V.; Ćurković, L.; Biošić, M.; Žmak, I.; Gaborardi, M. Titania-Coated Alumina Foam Photocatalyst for Memantine Degradation Derived by Replica Method and Sol-Gel Reaction. Materials 2020, 13, 227. https://doi.org/10.3390/ma13010227
Švagelj Z, Mandić V, Ćurković L, Biošić M, Žmak I, Gaborardi M. Titania-Coated Alumina Foam Photocatalyst for Memantine Degradation Derived by Replica Method and Sol-Gel Reaction. Materials. 2020; 13(1):227. https://doi.org/10.3390/ma13010227
Chicago/Turabian StyleŠvagelj, Zrinka, Vilko Mandić, Lidija Ćurković, Martina Biošić, Irena Žmak, and Mattia Gaborardi. 2020. "Titania-Coated Alumina Foam Photocatalyst for Memantine Degradation Derived by Replica Method and Sol-Gel Reaction" Materials 13, no. 1: 227. https://doi.org/10.3390/ma13010227
APA StyleŠvagelj, Z., Mandić, V., Ćurković, L., Biošić, M., Žmak, I., & Gaborardi, M. (2020). Titania-Coated Alumina Foam Photocatalyst for Memantine Degradation Derived by Replica Method and Sol-Gel Reaction. Materials, 13(1), 227. https://doi.org/10.3390/ma13010227








