Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery
Abstract
1. Introduction
2. Types of Hydrogels
2.1. Physical and Chemical Hydrogels
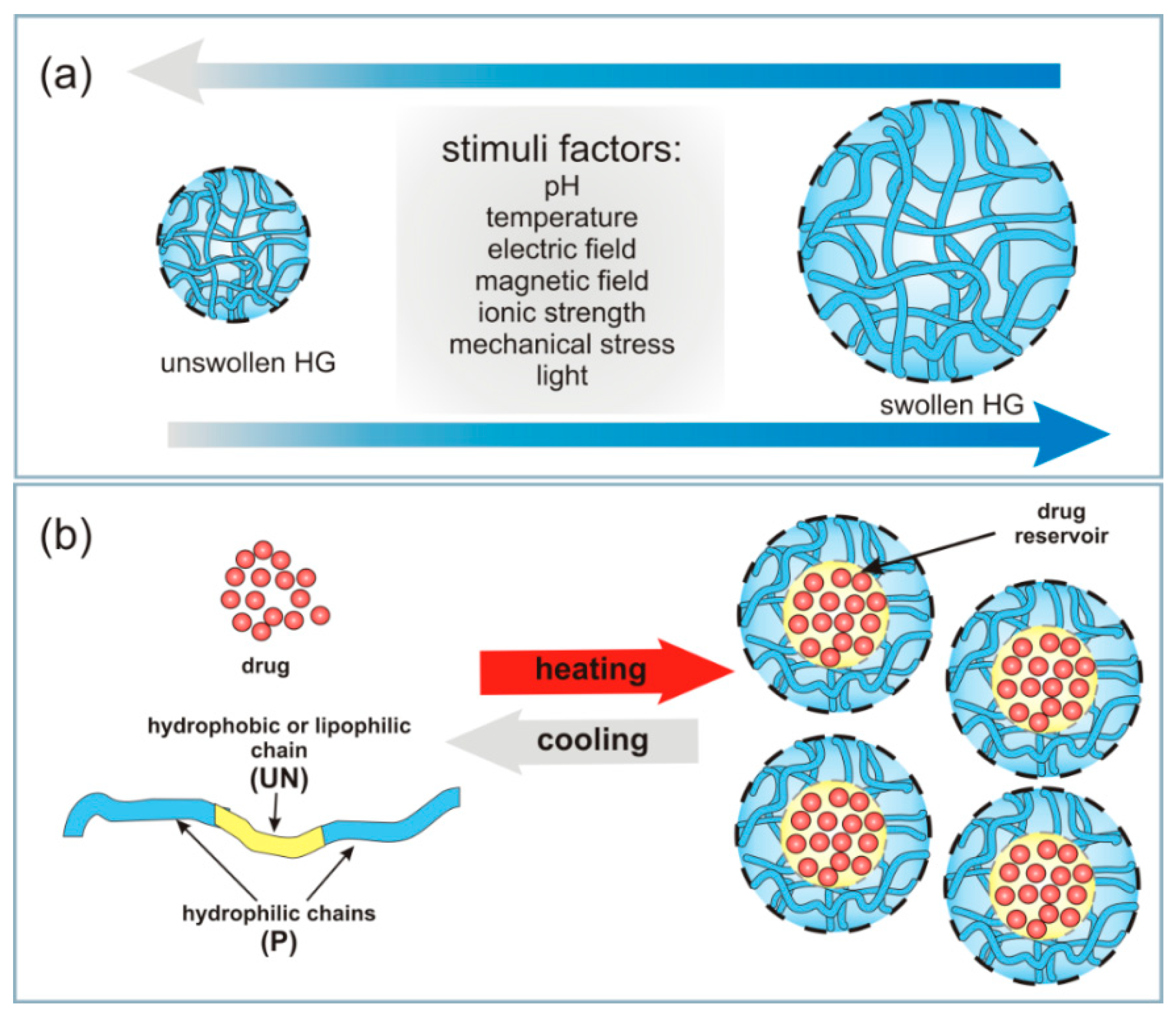
2.2. Conventional and Stimuli-Responsive Hydrogels
2.2.1. Thermosensitive Hydrogels
2.2.2. Photo-Responsive Hydrogels
2.2.3. pH-, Electric- and Magnetic-Responsive Hydrogels
2.3. Other Classifications for Hydrogels
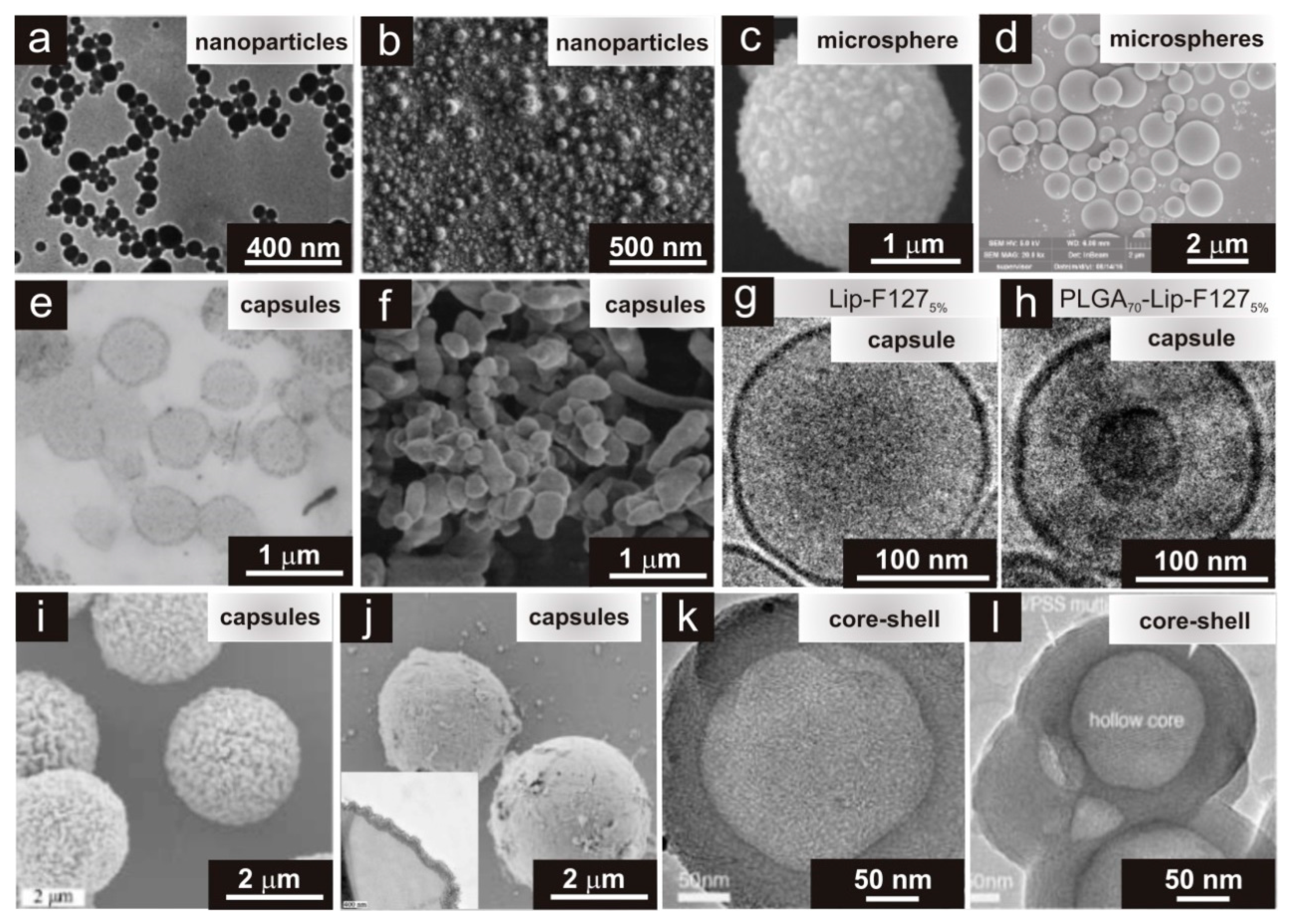
3. Types of Physical Appearance of Hydrogels
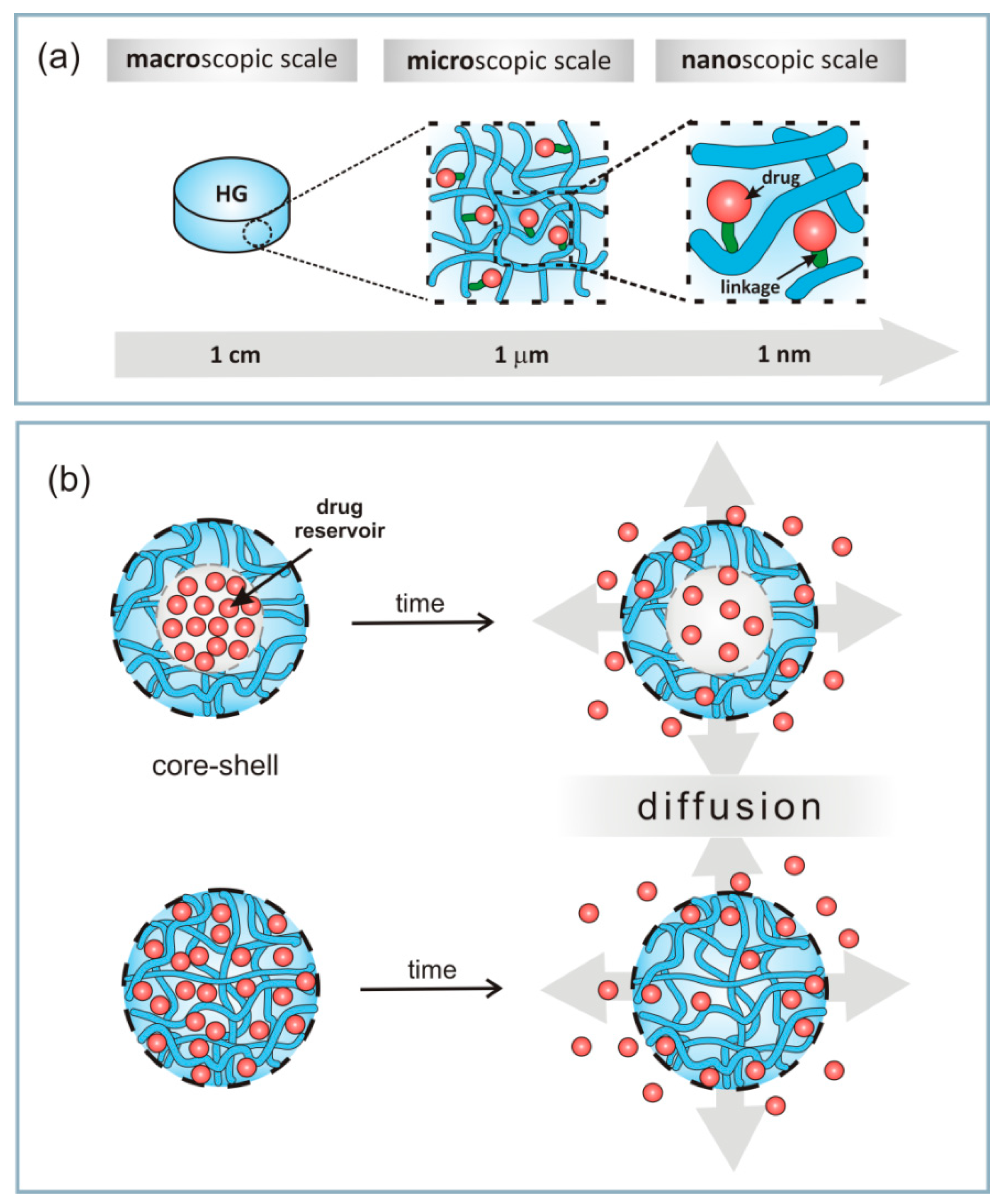
4. Immobilization of Drugs in Hydrogel
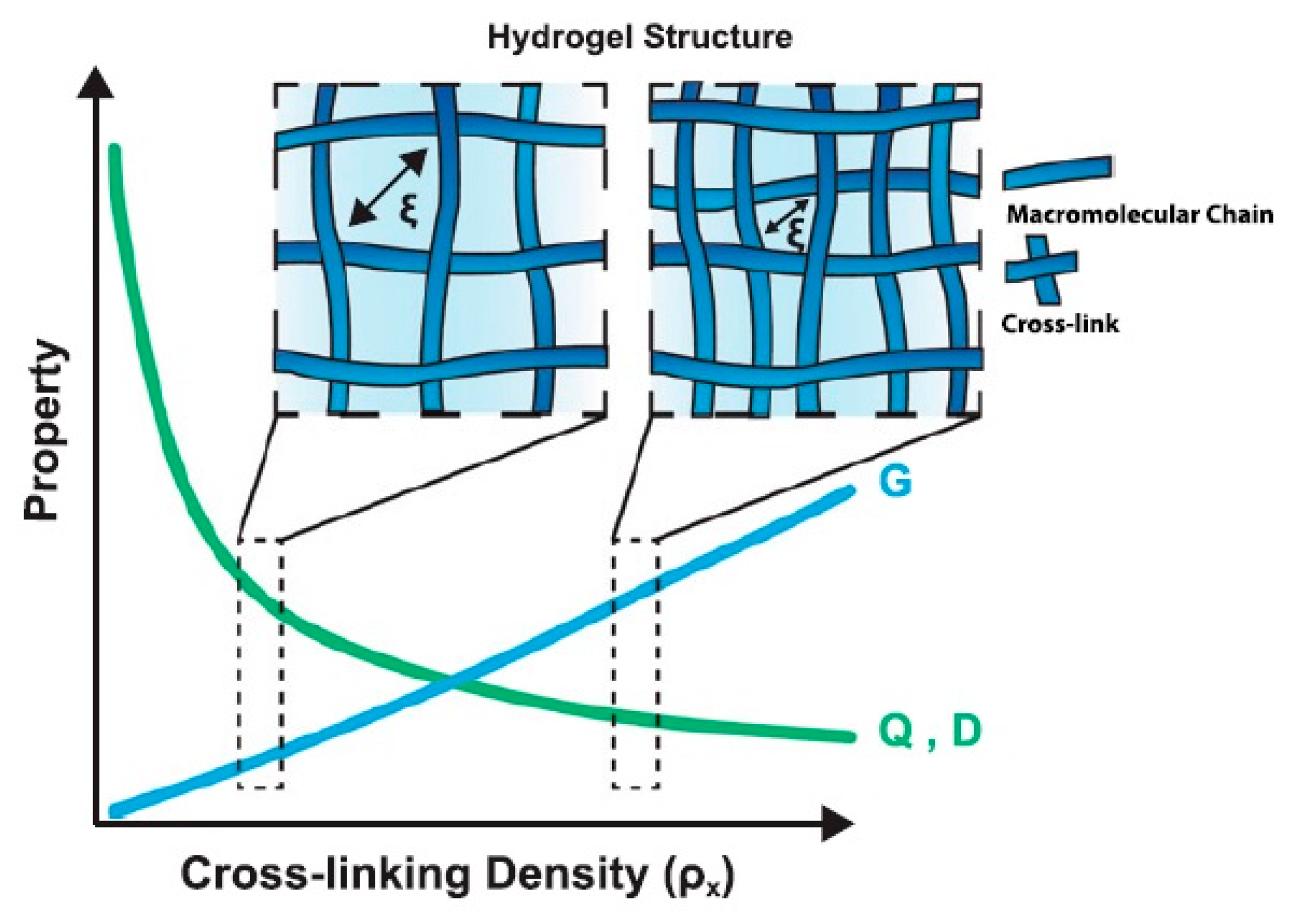
4.1. HG Structure and Physical Properties
4.2. Immobilization of Drugs
Immobilization of Drugs in Hydrogel Composites
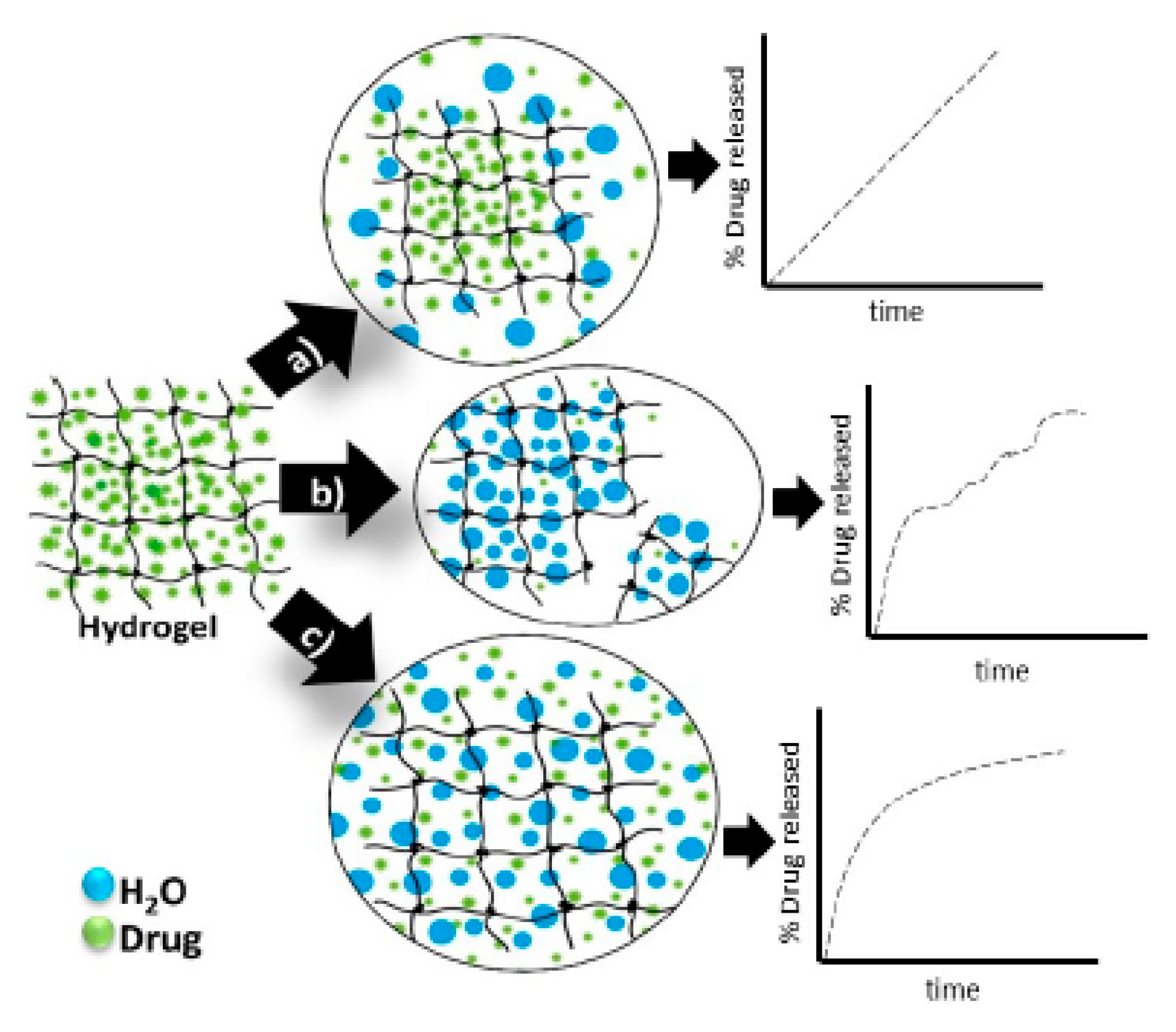
4.3. Release Mechanism of Drug from Hydrogel Matrices
5. The Use of Hydrogels in Modern Pharmacy
5.1. Application of Hydrogels for Oral Administration
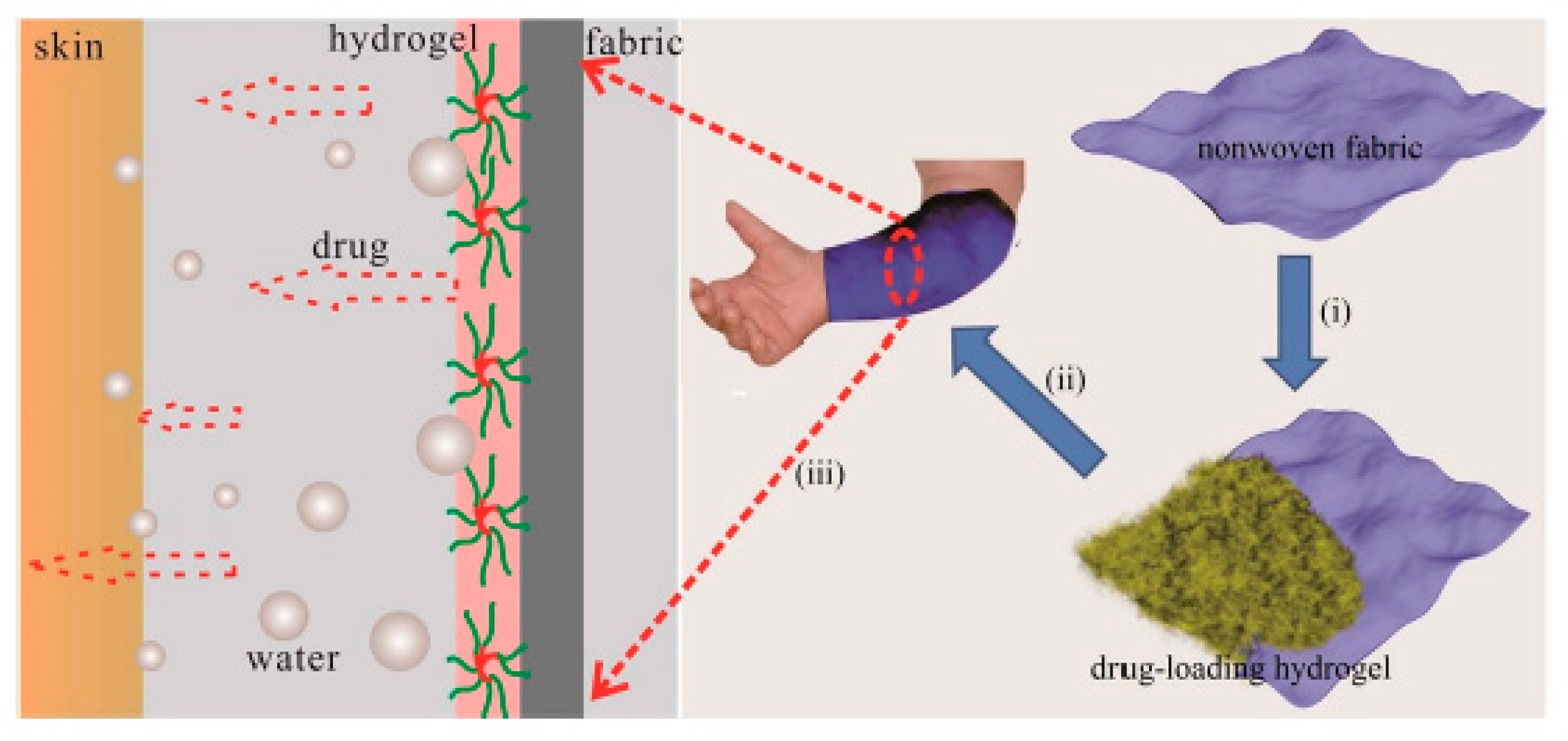
5.2. Hydrogels for Dermal Applications
5.3. Hydrogels for Ocular Applications
5.4. Hydrogels for Vaginal Applications
5.5. Hydrogels for Topical Oral Applications
5.6. Injection of Hydrogels
6. Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Korde, J.M.; Kandasubramanian, B. Naturally biomimicked smart shape memory hydrogels for biomedical functions. Chem. Eng. J. 2020, 379, 122430. [Google Scholar] [CrossRef]
- Tian, W.; Han, S.; Huang, X.; Han, M.; Cao, J.; Liang, Y.; Sun, Y. LDH hybrid thermosensitive hydrogel for intravaginal delivery of anti-HIV drugs. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Fujiyabu, T.; Yoshikawa, Y.; Chung, U.; Sakai, T. Structure-property relationship of a model network containing solvent. Sci. Technol. Adv. Mater. 2019, 20, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, C.M.; Anseth, K.S. Hydrogels in healthcare: From static to dynamic material microenvironments. Acta Mater. 2013, 61, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chi-leung Hui, P. Stimuli-responsive hydrogels: An interdisciplinary overview. In Hydrogels—Smart Materials for Biomedical Applications; Popa, L., Violeta Ghica, M., Dinu-Pîrvu, C.-E., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-875-4. [Google Scholar]
- Shafiee, S.; Ahangar, H.A.; Saffar, A. Taguchi method optimization for synthesis of Fe3O4 @chitosan/Tragacanth Gum nanocomposite as a drug delivery system. Carbohydr. Polym. 2019, 222, 114982. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Sohail, M.; Buabeid, M.A.; Murtaza, G.; Ullah, A.; Rashid, H.; Khan, M.A.; Khan, S.A. Pectin-based (LA-co-MAA) semi-IPNS as a potential biomaterial for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 569, 118557. [Google Scholar] [CrossRef]
- Nazlı, A.B.; Açıkel, Y.S. Loading of cancer drug resveratrol to pH-Sensitive, smart, alginate-chitosan hydrogels and investigation of controlled release kinetics. J. Drug Deliv. Sci. Technol. 2019, 53, 101199. [Google Scholar] [CrossRef]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Cocarta, A.-I.; Hobzova, R.; Sirc, J.; Cerna, T.; Hrabeta, J.; Svojgr, K.; Pochop, P.; Kodetova, M.; Jedelska, J.; Bakowsky, U.; et al. Hydrogel implants for transscleral drug delivery for retinoblastoma treatment. Mater. Sci. Eng. C 2019, 103, 109799. [Google Scholar] [CrossRef]
- Yang, C.; Gao, L.; Liu, X.; Yang, T.; Yin, G.; Chen, J.; Guo, H.; Yu, B.; Cong, H. Injectable Schiff base polysaccharide hydrogels for intraocular drug loading and release. J. Biomed. Mater. Res. 2019, 107. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Serro, A.P.; Silva, D.; Concheiro, A.; Alvarez-Lorenzo, C. Hydrogels for diabetic eyes: Naltrexone loading, release profiles and cornea penetration. Mater. Sci. Eng. C 2019, 105, 110092. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr. Polym. 2019, 225, 115210. [Google Scholar] [CrossRef] [PubMed]
- Duwa, R.; Emami, F.; Lee, S.; Jeong, J.-H.; Yook, S. Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J. Ind. Eng. Chem. 2019, 79, 261–273. [Google Scholar] [CrossRef]
- Riley, L.; Schirmer, L.; Segura, T. Granular hydrogels: Emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr. Opin. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 10671. [Google Scholar] [CrossRef]
- Bakaic, E.; Smeets, N.M.B.; Hoare, T. Injectable hydrogels based on poly (ethylene glycol) and derivatives as functional biomaterials. RSC Adv. 2015, 5, 35469–35486. [Google Scholar] [CrossRef]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Dis. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Huynh, D.P.; Park, J.H.; Lee, D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: A review. Eur. Polym. J. 2015, 72, 602–619. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Rusu, A.G.; Bercea, M.; Ghilan, A.; Dumitriu, R.P.; Mititelu-Tartau, L. New self-healing hydrogels based on reversible physical interactions and their potential applications. Eur. Polym. J. 2019, 118, 176–185. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Chen, J.; Deng, M.; Feng, X.; Chen, L. Supramolecular nanoplatforms via cyclodextrin host-guest recognition for synergistic gene-photodynamic therapy. Eur. Polym. J. 2019, 118, 222–230. [Google Scholar] [CrossRef]
- Marafon, P.; Fachel, F.N.S.; Dal Prá, M.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Development, physico-chemical characterization and in-vitro studies of hydrogels containing rosmarinic acid-loaded nanoemulsion for topical application. J. Pharm. Pharmacol. 2019, 71, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Cruz, M.T.; Vitorino, C.; Cabral, C. Nanostructuring lipid carriers using Ridolfia segetum (L.) Moris essential oil. Mater. Sci. Eng. C 2019, 103, 109804. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Y.; Dang, J.; Zhang, J.; Dong, F.; Wang, K.; Zhang, J. A novel antifungal plasma-activated hydrogel. ACS Appl. Mater. Interfaces 2019, 11, 22941–22949. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Li, L.; Xu, L.; Feng, N.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. Synthesis of a novel anti-freezing, non-drying antibacterial hydrogel dressing by one-pot method. Chem. Eng. J. 2019, 372, 216–225. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Y.; Zhang, Z.; Gao, Y.; Liu, W.; Borjihan, Q.; Qu, H.; Zhang, Y.; Zhang, Y.; Wang, Y.-J.; et al. Engineering a multifunctional N-halamine-based antibacterial hydrogel using a super-convenient strategy for infected skin defect therapy. Chem. Eng. J. 2020, 379, 122238. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Z.; Wu, D.; Gan, W.; Zhu, S.; Li, W.; Tian, J.; Li, L.; Zhou, C.; Lu, L. Antibacterial poly (ethylene glycol) diacrylate/chitosan hydrogels enhance mechanical adhesiveness and promote skin regeneration. Carbohydr. Polym. 2019, 225, 115110. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Coll. Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Solomevich, S.O.; Bychkovsky, P.M.; Yurkshtovich, T.L.; Golub, N.V.; Mirchuk, P.Y.; Revtovich, M.Y.; Shmak, A.I. Biodegradable pH-sensitive prospidine-loaded dextran phosphate based hydrogels for local tumor therapy. Carbohydr. Polym. 2019, 226, 115308. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wei, X.; Chen, H.; Wei, H.; Wang, Y.; Nan, W.; Zhang, Q.; Wen, X. The study of establishment of an in vivo tumor model by three-dimensional cells culture systems methods and evaluation of antitumor effect of biotin-conjugated pullulan acetate nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhang, X.; Akabar, M.D.; Luo, Y.; Wu, H.; Ke, X.; Ci, T. Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 181–191. [Google Scholar] [CrossRef]
- Wu, Q.; He, Z.; Wang, X.; Zhang, Q.; Wei, Q.; Ma, S.; Ma, C.; Li, J.; Wang, Q. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 2019, 10, 240. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, R.; Wu, H.; Yao, H.; Yan, Y.; Liu, J.; Ran, L.; Sun, Z.; Yi, L.; Dang, L.; et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat. Commun. 2019, 10, 1604. [Google Scholar] [CrossRef]
- Uehara, M.; Li, X.; Sheikhi, A.; Zandi, N.; Walker, B.; Saleh, B.; Banouni, N.; Jiang, L.; Ordikhani, F.; Dai, L.; et al. Anti-IL-6 eluting immunomodulatory biomaterials prolong skin allograft survival. Sci. Rep. 2019, 9, 6535. [Google Scholar] [CrossRef]
- García, J.R.; Quirós, M.; Han, W.M.; O’Leary, M.N.; Cox, G.N.; Nusrat, A.; García, A.J. IFN-γ-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials 2019, 220, 119403. [Google Scholar] [CrossRef]
- Hertegård, S.; Nagubothu, S.R.; Malmström, E.; Ström, C.E.; Tolf, A.; Davies, L.C.; Le Blanc, K. Hyaluronan hydrogels for the local delivery of mesenchymal stromal cells to the injured vocal fold. Stem Cells Dev. 2019, 28, 1177–1190. [Google Scholar] [CrossRef]
- Grigoras, A.G. Drug delivery systems using pullulan, a biocompatible polysaccharide produced by fungal fermentation of starch. Environ. Chem. Lett. 2019, 17, 1209–1223. [Google Scholar] [CrossRef]
- Ge, W.; Cao, S.; Shen, F.; Wang, Y.; Ren, J.; Wang, X. Rapid self-healing, stretchable, moldable, antioxidant and antibacterial tannic acid-cellulose nanofibril composite hydrogels. Carbohydr. Polym. 2019, 224, 115147. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.R.; Wagner, A.M.; Shin, S.R.; Hassan, S.; Khademhosseini, A.; Peppas, N.A. Modular fabrication of intelligent material-tissue interfaces for bioinspired and biomimetic devices. Prog. Mater. Sci. 2019, 106, 100589. [Google Scholar] [CrossRef]
- Bezerra, U.T.; Ferreira, H.S.; Barbosa, N.P. Hydrogels: Types, structure, properties, and applications. In Frontiers in Biomaterials; Razavi, M., Ed.; Bentham Science Publishers Ltd.: Sharjah, UAE, 2017; Volume 4, pp. 143–169. ISBN 978-1-68108-536-4. [Google Scholar]
- Chatterjee, S.; Hui, P.; Kan, C. Thermoresponsive hydrogels and their biomedical applications: Special insight into their applications in textile based transdermal therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels … A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Straccia, M.; d’Ayala, G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate hydrogels coated with chitosan for wound dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, Y.; Fang, J.; Wei, X.; Gu, Q.; El-Hamshary, H.; Al-Deyab, S.S.; Morsi, Y.; Mo, X. Modified alginate and gelatin cross-linked hydrogels for soft tissue adhesive. Artif. Cells Nanomed. Biotechnol. 2017, 45, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Pawelska, A. Alginate-based hydrogels in regenerative medicine. In Alginates; IntechOpen: London, UK, 2019. [Google Scholar]
- Hu, Y.; Zheng, M.; Dong, X.; Zhao, D.; Cheng, H.; Xiao, X. Preparation and characterization of alginate-hyaluronic acid-chitosan based composite gel beads. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2015, 30, 1297–1303. [Google Scholar] [CrossRef]
- Parlato, M.; Reichert, S.; Barney, N.; Murphy, W.L. Poly(ethylene glycol) hydrogels with adaptable mechanical and degradation properties for use in biomedical applications. Macromol. Biosci. 2014, 14, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Zustiak, S.P.; Leach, J.B. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef]
- Basu, A.; Kunduru, K.R.; Doppalapudi, S.; Domb, A.J.; Khan, W. Poly(lactic acid) based hydrogels. Adv. Drug Deliv. Rev. 2016, 107, 192–205. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Krylova, M.; Sinitsyna, E.; Ivankova, E.; Averianov, I.; Tennikova, T. Hydrogel layers on the surface of polyester-based materials for improvement of their biointeractions and controlled release of proteins. Polymers 2016, 8, 418. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Stagnaro, P.; Schizzi, I.; Utzeri, R.; Marsano, E.; Castellano, M. Alginate-polymethacrylate hybrid hydrogels for potential osteochondral tissue regeneration. Carbohydr. Polym. 2018, 185, 56–62. [Google Scholar] [CrossRef]
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef]
- Vieira, V.M.P.; Hay, L.L.; Smith, D.K. Multi-component hybrid hydrogels—Understanding the extent of orthogonal assembly and its impact on controlled release. Chem. Sci. 2017, 8, 6981–6990. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, L.; Tu, L.; Dai, C.; Fan, L.; Zhang, K.; Yao, T.; Chen, J.; Wang, Z.; Xing, J.; et al. Elastomeric conductive hybrid hydrogels with continuous conductive networks. J. Mater. Chem. B 2019, 7, 2389–2397. [Google Scholar] [CrossRef]
- Radvar, E.; Azevedo, H.S. Supramolecular peptide/polymer hybrid hydrogels for biomedical applications. Macromol. Biosci. 2019, 19, 1800221. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Illeperuma, W.R.K.; Suo, Z.; Vlassak, J.J. Hybrid hydrogels with extremely high stiffness and toughness. ACS Macro Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A.; Larsson, A. Swellable hydrogel-based systems for controlled drug delivery. In Smart Drug Delivery System; Sezer, A.D., Ed.; InTech: London, UK, 2016; ISBN 978-953-51-2247-0. [Google Scholar]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Gnavi, S.; Ruini, F.; Gambarotta, G.; Gioffredi, E.; Chiono, V.; Perroteau, I.; Ciardelli, G. Development and characterization of novel agar and gelatin injectable hydrogel as filler for peripheral nerve guidance channels: Novel agar and gelatin injectable hydrogel for peripheral nerve guidance channels. J. Tissue Eng. Regen. Med. 2017, 11, 197–208. [Google Scholar] [CrossRef]
- Wakhet, S.; Singh, V.K.; Sahoo, S.; Sagiri, S.S.; Kulanthaivel, S.; Bhattacharya, M.K.; Kumar, N.; Banerjee, I.; Pal, K. Characterization of gelatin–agar based phase separated hydrogel, emulgel and bigel: A comparative study. J. Mater. Sci. Mater. Med. 2015, 26, 118. [Google Scholar] [CrossRef]
- Sadeghi, M.; Heidari, B. Crosslinked graft copolymer of methacrylic acid and gelatin as a novel hydrogel with pH-responsiveness properties. Materials 2011, 4, 543–552. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Turon, P.; Weis, C.; Alemán, C.; Armelin, E. The mechanism of adhesion and graft polymerization of a PNIPAAm thermoresponsive hydrogel to polypropylene meshes. Soft Matter 2019, 15, 3432–3442. [Google Scholar] [CrossRef]
- Singh, R.; Mahto, V. Synthesis, characterization and evaluation of polyacrylamide graft starch/clay nanocomposite hydrogel system for enhanced oil recovery. Pet. Sci. 2017, 14, 765–779. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Vafaie Sefti, M.; Baghban Salehi, M.; Mousavi Moghadam, A.; Rajaee, S.; Naderi, H. Hydrogel swelling properties: Comparison between conventional and nanocomposite hydrogels for water shutoff treatment: Comparing Conventional hydrogels with Nanocomposite. Asia Pac. J. Chem. Eng. 2015, 10, 743–753. [Google Scholar] [CrossRef]
- Monir, T.S.B.; Afroz, S.; Khan, R.A.; Miah, M.Y.; Takafuji, M.; Alam, M.A. pH-sensitive hydrogel from polyethylene oxide and acrylic acid by gamma radiation. J. Compos. Sci. 2019, 3, 58. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Bauri, K.; Nandi, M.; De, P. Amino acid-derived stimuli-responsive polymers and their applications. Polym. Chem. 2018, 9, 1257–1287. [Google Scholar] [CrossRef]
- Kahn, J.S.; Hu, Y.; Willner, I. Stimuli-responsive dna-based hydrogels: From basic principles to applications. Acc. Chem. Res. 2017, 50, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Poly(ethylene glycol)–poly(lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J. Control. Release 2013, 172, 715–729. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R. Hydrogels for hydrophobic drug delivery. Classification, synthesis and applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer hydrogels: A review. Polym. Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Purushotham, S.; Ramanujan, R.V. Thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy. Acta Biomater. 2010, 6, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogels as drug delivery systems. Curr. Med. Chem. 2012, 20, 79–94. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Cochis, A.; Bonetti, L.; Sorrentino, R.; Contessi Negrini, N.; Grassi, F.; Leigheb, M.; Rimondini, L.; Farè, S. 3D printing of thermo-responsive methylcellulose hydrogels for cell-sheet engineering. Materials 2018, 11, 579. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Bonetti, L.; Contili, L.; Farè, S. 3D printing of methylcellulose-based hydrogels. Bioprinting 2018, 10, e00024. [Google Scholar] [CrossRef]
- Larrañeta, E.; Barturen, L.; Ervine, M.; Donnelly, R.F. Hydrogels based on poly(methyl vinyl ether-co-maleic acid) and Tween 85 for sustained delivery of hydrophobic drugs. Int. J. Pharm. 2018, 538, 147–158. [Google Scholar] [CrossRef]
- Hwang, T.; Frank, Z.; Neubauer, J.; Kim, K.J. High-performance polyvinyl chloride gel artificial muscle actuator with graphene oxide and plasticizer. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Bae, J.W.; Shin, E.-J.; Jeong, J.; Choi, D.-S.; Lee, J.E.; Nam, B.U.; Lin, L.; Kim, S.-Y. High-performance PVC gel for adaptive micro-lenses with variable focal length. Sci. Rep. 2017, 7, 2068. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, B.S.; Park, H.; Lee, J.; Park, W.H. Injectable methylcellulose hydrogel containing calcium phosphate nanoparticles for bone regeneration. Int. J. Biol. Macromol. 2018, 109, 57–64. [Google Scholar] [CrossRef]
- Contessi, N.; Altomare, L.; Filipponi, A.; Farè, S. Thermo-responsive properties of methylcellulose hydrogels for cell sheet engineering. Mater. Lett. 2017, 207, 157–160. [Google Scholar] [CrossRef]
- Safronov, A.P.; Terziyan, T.V. Formation of chemical networks of acrylamide and acrylic acid hydrogels initiated by ammonium persulfate. Polym. Sci. Ser. B 2015, 57, 481–487. [Google Scholar] [CrossRef]
- Jalani, G.; Naccache, R.; Rosenzweig, D.H.; Haglund, L.; Vetrone, F.; Cerruti, M. Photocleavable hydrogel-coated upconverting nanoparticles: A multifunctional theranostic platform for nir imaging and on-demand macromolecular delivery. J. Am. Chem. Soc. 2016, 138, 1078–1083. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H.; Abu-Thabit, N.Y. (Eds.) Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevierz: Amsterdam, The Netherlands, 2018; Volume 1, ISBN 978-0-08-101997-9. [Google Scholar]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef]
- Truong, V.X.; Li, F.; Ercole, F.; Forsythe, J.S. Wavelength-selective coupling and decoupling of polymer chains via reversible [2 + 2] photocycloaddition of styrylpyrene for construction of cytocompatible photodynamic hydrogels. ACS Macro Lett. 2018, 7, 464–469. [Google Scholar] [CrossRef]
- Rosales, A.M.; Vega, S.L.; DelRio, F.W.; Burdick, J.A.; Anseth, K.S. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew. Chem. Int. Ed. 2017, 56, 12132–12136. [Google Scholar] [CrossRef]
- Yan, B.; Boyer, J.-C.; Habault, D.; Branda, N.R.; Zhao, Y. near infrared light triggered release of biomacromolecules from hydrogels loaded with upconversion nanoparticles. J. Am. Chem. Soc. 2012, 134, 16558–16561. [Google Scholar] [CrossRef]
- Ariffin, A.; Musa, M.S.; Othman, M.B.H.; Razali, M.A.A.; Yunus, F. Effects of various fillers on anionic polyacrylamide systems for treating kaolin suspensions. Coll. Surf. A Physicochem. Eng. Asp. 2014, 441, 306–311. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Exp. Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, G.; Hu, R.; Chen, S.; Lu, W.; Gao, S.; Xia, H.; Wang, B.; Sun, C.; Nie, X.; et al. A nasal temperature and pH dual-responsive in situ gel delivery system based on microemulsion of huperzine A: Formulation, evaluation, and in vivo pharmacokinetic study. AAPS PharmSciTech 2019, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Kiaee, G.; Mostafalu, P.; Samandari, M.; Sonkusale, S. A pH-mediated electronic wound dressing for controlled drug delivery. Adv. Healthcare Mater. 2018, 7, 1800396. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, A.M. Novel pH switchable gelatin based hydrogel for the controlled delivery of the anti cancer drug 5-fluorouracil. RSC Adv. 2014, 4, 12109. [Google Scholar] [CrossRef]
- Indermun, S.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Luttge, R.; Pillay, V. An interfacially plasticized electro-responsive hydrogel for transdermal electro-activated and modulated (TEAM) drug delivery. Int. J. Pharm. 2014, 462, 52–65. [Google Scholar] [CrossRef]
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Joy, P.A.; Raju, K.M. Magnetic and electric responsive hydrogel-magnetic nanocomposites for drug-delivery application. J. Appl. Polym. Sci. 2011, 122, 1364–1375. [Google Scholar] [CrossRef]
- Siangsanoh, C.; Ummartyotin, S.; Sathirakul, K.; Rojanapanthu, P.; Treesuppharat, W. Fabrication and characterization of triple-responsive composite hydrogel for targeted and controlled drug delivery system. J. Mol. Liq. 2018, 256, 90–99. [Google Scholar] [CrossRef]
- Uva, M.; Pasqui, D.; Mencuccini, L.; Fedi, S.; Barbucci, R. Influence of alternating and static magnetic fields on drug release from hybrid hydrogels containing magnetic nanoparticles. J. Biomater. Nanobiotechnol. 2014, 5, 116–127. [Google Scholar] [CrossRef]
- Crippa, F.; Moore, T.L.; Mortato, M.; Geers, C.; Haeni, L.; Hirt, A.M.; Rothen-Rutishauser, B.; Petri-Fink, A. Dynamic and biocompatible thermo-responsive magnetic hydrogels that respond to an alternating magnetic field. J. Magn. Magn. Mater. 2017, 427, 212–219. [Google Scholar] [CrossRef]
- Jain, E.; Hill, L.; Canning, E.; Sell, S.A.; Zustiak, S.P. Control of gelation, degradation and physical properties of polyethylene glycol hydrogels through the chemical and physical identity of the crosslinker. J. Mater. Chem. B 2017, 5, 2679–2691. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, L. Preparation and characterization of polyethylene glycol (PEG) hydrogel as shape-stabilized phase change material. Appl. Therm. Eng. 2017, 114, 1014–1017. [Google Scholar] [CrossRef]
- Fukasawa, M.; Sakai, T.; Chung, U.; Haraguchi, K. Synthesis and mechanical properties of a nanocomposite gel consisting of a tetra-PEG/clay network. Macromolecules 2010, 43, 4370–4378. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.; Shibayama, M. SANS and SLS studies on tetra-arm PEG gels in as-prepared and swollen states. Macromolecules 2009, 42, 6245–6252. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.; Shibayama, M. Structure characterization of tetra-PEG gel by small-angle neutron scattering. Macromolecules 2009, 42, 1344–1351. [Google Scholar] [CrossRef]
- Fujii, K.; Asai, H.; Ueki, T.; Sakai, T.; Imaizumi, S.; Chung, U.; Watanabe, M.; Shibayama, M. High-performance ion gel with tetra-PEG network. Soft Matter 2012, 8, 1756–1759. [Google Scholar] [CrossRef]
- Asai, H.; Fujii, K.; Ueki, T.; Sakai, T.; Chung, U.; Watanabe, M.; Han, Y.-S.; Kim, T.-H.; Shibayama, M. Structural analysis of high performance ion-gel comprising tetra-PEG network. Macromolecules 2012, 45, 3902–3909. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough hydrophilic polyurethane-based hydrogels with mechanical properties similar to human soft tissues. J. Mater. Chem. B 2019, 7, 3512–3519. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Yan, Q. Building a polysaccharide hydrogel capsule delivery system for control release of ibuprofen. J. Biomater. Sci. Polym. Ed. 2018, 29, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Saha, A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Aceclofenac-loaded chitosan-tamarind seed polysaccharide interpenetrating polymeric network microparticles. Coll. Surf. B Biointerfaces 2013, 105, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-S.; Lin, P.-H.; Yen, H.-J.; Yu, Y.-Y.; Tsai, T.-W.; Chen, W.-C. Highly flexible and optical transparent 6F-PI/TiO2 optical hybrid films with tunable refractive index and excellent thermal stability. J. Mater. Chem. 2010, 20, 531–536. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Sadiku, R.; Raju, K.M. Development of novel biodegradable Au nanocomposite hydrogels based on wheat: For inactivation of bacteria. Carbohydr. Polym. 2013, 92, 2193–2200. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications: Nanocomposite hydrogels. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.-H.; Atluri, P.; Burdick, J.A. Methods to assess shear-thinning hydrogels for application as injectable biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef]
- Rodell, C.B.; Lee, M.E.; Wang, H.; Takebayashi, S.; Takayama, T.; Kawamura, T.; Arkles, J.S.; Dusaj, N.N.; Dorsey, S.M.; Witschey, W.R.T.; et al. Injectable shear-thinning hydrogels for minimally invasive delivery to infarcted myocardium to limit left ventricular remodeling. Circ. Cardiovasc. Interv. 2016, 9, e004058. [Google Scholar] [CrossRef]
- Nep, E.I.; Conway, B. Grewia gum 2: Mucoadhesive properties of compacts and gels. Trop. J. Pharm. Res. 2011, 10, 393–401. [Google Scholar] [CrossRef][Green Version]
- Tsintou, M.; Dalamagkas, K.; Seifalian, A. Injectable hydrogel versus plastically compressed collagen scaffold for central nervous system applications. Int. J. Biomater. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Bae, K.H.; Wang, L.-S.; Kurisawa, M. Injectable biodegradable hydrogels: Progress and challenges. J. Mater. Chem. B 2013, 1, 5371. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Chen, G.; Pan, D.; Qi, H.; Li, R.; Tian, J.; Lu, F.; He, M. Highly stretchable and compressible cellulose ionic hydrogels for flexible strain sensors. Biomacromolecules 2019, 20, 2096–2104. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, N.; Wang, L.; Tan, Y.; Liu, C.; Xia, Y.; Nie, Z.; Sui, K. Supramolecular nanofibrillar hydrogels as highly stretchable, elastic and sensitive ionic sensors. Mater. Horiz. 2019, 6, 326–333. [Google Scholar] [CrossRef]
- Liu, S.; Oderinde, O.; Hussain, I.; Yao, F.; Fu, G. Dual ionic cross-linked double network hydrogel with self-healing, conductive, and force sensitive properties. Polymer 2018, 144, 111–120. [Google Scholar] [CrossRef]
- Franzén, H.; Draget, K.; Langebäck, J.; Nilsen-Nygaard, J. Characterization and properties of hydrogels made from neutral soluble chitosans. Polymers 2015, 7, 373–389. [Google Scholar] [CrossRef]
- Shams Es-haghi, S.; Weiss, R.A. Fabrication of tough hydrogels from chemically cross-linked multiple neutral networks. Macromolecules 2016, 49, 8980–8987. [Google Scholar] [CrossRef]
- Weller, C.; Weller, C.; Team, V. Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 105–134. ISBN 978-0-08-102192-7. [Google Scholar]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A. Antimicrobial polymeric gels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 357–371. ISBN 978-0-08-102179-8. [Google Scholar]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.; Cho, H. Hydrogel-based drug delivery systems for poorly water-soluble drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Gu, D.; O’Connor, A.J.; Qiao, G.G.H.; Ladewig, K. Hydrogels with smart systems for delivery of hydrophobic drugs. Exp. Opin. Drug Deliv. 2017, 14, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Barthelat, F.; Yin, Z.; Buehler, M.J. Structure and mechanics of interfaces in biological materials. Nat. Rev. Mater. 2016, 1, 16007. [Google Scholar] [CrossRef]
- Thorne, J.B.; Vine, G.J.; Snowden, M.J. Microgel applications and commercial considerations. Coll. Polym. Sci. 2011, 289, 625–646. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, S.; Pinchuk, A.N.; Xiong, M.P. Active drug encapsulation and release kinetics from hydrogel-in-liposome nanoparticles. J. Coll. Interface Sci. 2013, 406, 247–255. [Google Scholar] [CrossRef][Green Version]
- Nayak, A.K.; Das, B. Introduction to polymeric gels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–27. ISBN 978-0-08-102179-8. [Google Scholar]
- Kawaguchi, H. Micro hydrogels: Preparation, properties, and applications. J. Oleo Sci. 2013, 62, 865–871. [Google Scholar] [CrossRef]
- Vinogradov, S. Colloidal microgels in drug delivery applications. Curr. Pharm. Des. 2006, 12, 4703–4712. [Google Scholar] [CrossRef]
- Panda, J.J.; Mishra, A.; Basu, A.; Chauhan, V.S. Stimuli responsive self-assembled hydrogel of a low molecular weight free dipeptide with potential for tunable drug delivery. Biomacromolecules 2008, 9, 2244–2250. [Google Scholar] [CrossRef]
- Kim, B.; Peppas, N.A. In vitro release behavior and stability of insulin in complexation hydrogels as oral drug delivery carriers. Int. J. Pharm. 2003, 266, 29–37. [Google Scholar] [CrossRef]
- Na, K.; Lee, K.H.; Bae, Y.H. pH-sensitivity and pH-dependent interior structural change of self-assembled hydrogel nanoparticles of pullulan acetate/oligo-sulfonamide conjugate. J. Control. Release 2004, 97, 513–525. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Setia, A.; Ahuja, P. Nanohydrogels. In Organic Materials as Smart Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 293–368. ISBN 978-0-12-813663-8. [Google Scholar]
- Kim, S.H.; Kim, S.-H.; Nair, S.; Moore, E. Reactive electrospinning of cross-linked poly(2-hydroxyethyl methacrylate) nanofibers and elastic properties of individual hydrogel nanofibers in aqueous solutions. Macromolecules 2005, 38, 3719–3723. [Google Scholar] [CrossRef]
- Gil, E.; Hudson, S. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Konwar, R.; Ahmed, A.B. Nanoparticle: An overview of preparation, characterization and application. Int. Res. J. Pharm. 2016, 4, 47–57. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Coll. Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Metters, A.T.; Bowman, C.N.; Anseth, K.S. Verification of scaling laws for degrading PLA-b-PEG-b-PLA hydrogels. AIChE J. 2001, 47, 1432–1437. [Google Scholar] [CrossRef]
- Mason, M.N.; Metters, A.T.; Bowman, C.N.; Anseth, K.S. Predicting controlled-release behavior of degradable PLA-b-PEG-b-PLA hydrogels. Macromolecules 2001, 34, 4630–4635. [Google Scholar] [CrossRef]
- Lustig, S.R.; Peppas, N.A. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J. Appl. Polym. Sci. 1988, 36, 735–747. [Google Scholar] [CrossRef]
- Metters, A.T.; Anseth, K.S.; Bowman, C.N. A statistical kinetic model for the bulk degradation of PLA-b-PEG-b-PLA hydrogel networks: Incorporating network non-idealities. J. Phys. Chem. B 2001, 105, 8069–8076. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, K.; Wang, C.; Tang, C.; Jiang, X. Synthesis and drug delivery of novel amphiphilic block copolymers containing hydrophobic dehydroabietic moiety. J. Mater. Chem. B 2013, 1, 2324. [Google Scholar] [CrossRef]
- Lengert, E.; Yashchenok, A.M.; Atkin, V.; Lapanje, A.; Gorin, D.A.; Sukhorukov, G.B.; Parakhonskiy, B.V. Hollow silver alginate microspheres for drug delivery and surface enhanced Raman scattering detection. RSC Adv. 2016, 6, 20447–20452. [Google Scholar] [CrossRef]
- Yang, T.-T.; Cheng, Y.-Z.; Qin, M.; Wang, Y.-H.; Yu, H.-L.; Wang, A.-L.; Zhang, W.-F. Thermosensitive chitosan hydrogels containing polymeric microspheres for vaginal drug delivery. BioMed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Nan, J.; Chen, Y.; Li, R.; Wang, J.; Liu, M.; Wang, C.; Chu, F. Polymeric hydrogel nanocapsules: A thermo and pH dual-responsive carrier for sustained drug release. Nano-Micro Lett. 2014, 6, 200–208. [Google Scholar] [CrossRef]
- Yu, M.; Xu, L.; Tian, F.; Su, Q.; Zheng, N.; Yang, Y.; Wang, J.; Wang, A.; Zhu, C.; Guo, S.; et al. Rapid transport of deformation-tuned nanoparticles across biological hydrogels and cellular barriers. Nat. Commun. 2018, 9, 2607. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Möhwald, H. Smart inorganic/organic nanocomposite hollow microcapsules. Angew. Chem. Int. Ed. 2003, 42, 4472–4475. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, J.; Shen, W.; Dong, X.; Feng, J.; Ruan, M.; Li, Y. Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structure. Angew. Chem. Int. Ed. 2005, 44, 5083–5087. [Google Scholar] [CrossRef]
- Bayat, M.; Nasri, S. Injectable microgel–hydrogel composites “plum pudding gels”: New system for prolonged drug delivery. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 343–372. ISBN 978-0-12-816505-8. [Google Scholar]
- Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Hydrogel-based drug delivery nanosystems for the treatment of brain tumors. Gels 2018, 4, 62. [Google Scholar] [CrossRef]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Pertici, V.; Pin-Barre, C.; Rivera, C.; Pellegrino, C.; Laurin, J.; Gigmes, D.; Trimaille, T. Degradable and injectable hydrogel for drug delivery in soft tissues. Biomacromolecules 2019, 20, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K. Polymeric hydrogels as technology platform for drug delivery applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef]
- Song, S.H.; Lee, K.M.; Kang, J.B.; Lee, S.G.; Kang, M.J.; Choi, Y.W. Improved skin delivery of voriconazole with a nanostructured lipid carrier-based hydrogel formulation. Chem. Pharm. Bull. 2014, 62, 793–798. [Google Scholar] [CrossRef]
- Khare, A.; Singh, I.; Pawar, P.; Grover, K. Design and evaluation of voriconazole loaded solid lipid nanoparticles for ophthalmic application. J. Drug Deliv. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- López-Noriega, A.; Hastings, C.L.; Ozbakir, B.; O’Donnell, K.E.; O’Brien, F.J.; Storm, G.; Hennink, W.E.; Duffy, G.P.; Ruiz-Hernández, E. Hyperthermia-induced drug delivery from thermosensitive liposomes encapsulated in an injectable hydrogel for local chemotherapy. Adv. Healthcare Mater. 2014, 3, 854–859. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Park, J.H.; Park, S.H.; Oh, H.J.; Kim, J.H.; Min, B.H.; Park, K.; Kim, M.S. Synergistic anti-tumor activity through combinational intratumoral injection of an in-situ injectable drug depot. Biomaterials 2016, 85, 232–245. [Google Scholar] [CrossRef]
- Sivakumaran, D.; Maitland, D.; Hoare, T. Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery. Biomacromolecules 2011, 12, 4112–4120. [Google Scholar] [CrossRef]
- Town, A.R.; Giardiello, M.; Gurjar, R.; Siccardi, M.; Briggs, M.E.; Akhtar, R.; McDonald, T.O. Dual-stimuli responsive injectable microgel/solid drug nanoparticle nanocomposites for release of poorly soluble drugs. Nanoscale 2017, 9, 6302–6314. [Google Scholar] [CrossRef]
- Hudson, S.P.; Langer, R.; Fink, G.R.; Kohane, D.S. Injectable in situ cross-linking hydrogels for local antifungal therapy. Biomaterials 2010, 31, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, F.C.; Lynch, I.; Rochev, Y.A.; Burke, M.; Dawson, K.A.; Gallagher, W.M.; Keenan, A.K. Novel “plum pudding” gels as potential drug-eluting stent coatings: Controlled release of fluvastatin. J. Biomed. Mater. Res. Part A 2006, 79, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-K.; Park, J.W.; Song, S.-C. Injectable and biodegradable poly(organophosphazene) gel containing silibinin: Its physicochemical properties and anticancer activity. J. Pharm. Sci. 2012, 101, 2382–2391. [Google Scholar] [CrossRef]
- Lei, N.; Gong, C.; Qian, Z.; Luo, F.; Wang, C.; Wang, H.; Wei, Y. Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model. Nanoscale 2012, 4, 5686. [Google Scholar] [CrossRef]
- Chen, M.-C.; Tsai, H.-W.; Liu, C.-T.; Peng, S.-F.; Lai, W.-Y.; Chen, S.-J.; Chang, Y.; Sung, H.-W. A nanoscale drug-entrapment strategy for hydrogel-based systems for the delivery of poorly soluble drugs. Biomaterials 2009, 30, 2102–2111. [Google Scholar] [CrossRef]
- Biondi, M.; Borzacchiello, A.; Mayol, L.; Ambrosio, L. Nanoparticle-integrated hydrogels as multifunctional composite materials for biomedical applications. Gels 2015, 1, 162–178. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Meid, J.; Friedrich, T.; Tieke, B.; Lindner, P.; Richtering, W. Composite hydrogels with temperature sensitive heterogeneities: Influence of gel matrix on the volume phase transition of embedded poly-(N-isopropylacrylamide) microgels. Phys. Chem. Chem. Phys. 2011, 13, 3039–3047. [Google Scholar] [CrossRef]
- Salvati, A.; Söderman, O.; Lynch, I. Plum-pudding gels as a platform for drug delivery: Understanding the effects of the different components on the diffusion behavior of solutes. J. Phys. Chem. B 2007, 111, 7367–7376. [Google Scholar] [CrossRef]
- Li, X.; Rombouts, W.; van der Gucht, J.; de Vries, R.; Dijksman, J.A. Mechanics of composite hydrogels approaching phase separation. PLoS ONE 2019, 14, e0211059. [Google Scholar] [CrossRef]
- Tan, H.-L.; Teow, S.-Y.; Pushpamalar, J. Application of metal nanoparticle–hydrogel composites in tissue regeneration. Bioengineering 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Zhang, Y.; Kuan, H.-C.; Lee, S.-H.; Ma, J. Polymer composite hydrogels containing carbon nanomaterials—Morphology and mechanical and functional performance. Prog. Polym. Sci. 2018, 77, 1–18. [Google Scholar] [CrossRef]
- Rocha-García, D.; Guerra-Contreras, A.; Rosales-Mendoza, S.; Palestino, G. Role of porous silicon/hydrogel composites on drug delivery. Open Mater. Sci. 2016, 3, 93–101. [Google Scholar] [CrossRef]
- Ayub, A.D.; Chiu, H.I.; Mat Yusuf, S.N.A.; Abd Kadir, E.; Ngalim, S.H.; Lim, V. Biocompatible disulphide cross-linked sodium alginate derivative nanoparticles for oral colon-targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Havanur, S.; Batish, I.; Cheruku, S.P.; Gourishetti, K.; JagadeeshBabu, P.E.; Kumar, N. Poly(N,N-diethyl acrylamide)/functionalized graphene quantum dots hydrogels loaded with doxorubicin as a nano-drug carrier for metastatic lung cancer in mice. Mater. Sci. Eng. C 2019, 105, 110094. [Google Scholar] [CrossRef]
- Hou, F.; Xi, B.; Wang, X.; Yang, Y.; Zhao, H.; Li, W.; Qin, J.; He, Y. Self-healing hydrogel with cross-linking induced thermo-response regulated light emission property. Coll. Surf. B Biointerfaces 2019, 183, 110441. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Vatankhah-Varnoosfaderani, M.; Gutiérrez, T.J.; Saeb, M.R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]
- Zhang, Y.; An, Q.; Tong, W.; Li, H.; Ma, Z.; Zhou, Y.; Huang, T.; Zhang, Y. A new way to promote molecular drug release during medical treatment: A polyelectrolyte matrix on a piezoelectric-dielectric energy conversion substrate. Small 2018, 14, 1802136. [Google Scholar] [CrossRef]
- Dadou, S.M.; El-Barghouthi, M.I.; Antonijevic, M.D.; Chowdhry, B.Z.; Badwan, A.A. Elucidation of the controlled-release behavior of metoprolol succinate from directly compressed xanthan gum/chitosan polymers: Computational and experimental studies. ACS Biomater. Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Gu, S.; Yang, L.; Li, S.; Yang, J.; Zhang, B.; Yang, J. Thermo- and glucose-sensitive microgels with improved salt tolerance for controlled insulin release in a physiological environment: Thermo- and glucose-sensitive microgels with improved salt tolerance. Polym. Int. 2018, 67, 1256–1265. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zhang, Q.; Cheng, Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem. Commun. 2016, 52, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.; Dadkhah Tehrani, A.; Adeli, M. pH-responsive hybrid hydrogels as antibacterial and drug delivery systems. Polymers 2018, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, M.; Wu, J.; Zhang, C.; Deng, X.; Dhinakar, A.; Huang, W.; Qian, H.; Ge, L. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017, 51, 294–303. [Google Scholar] [CrossRef]
- Panahi, Y.; Gharekhani, A.; Hamishehkar, H.; Zakeri-Milani, P.; Gharekhani, H. Stomach-specific drug delivery of clarithromycin using asemi interpenetrating polymeric network hydrogel made ofmontmorillonite and chitosan: Synthesis, characterization and in vitro drug release study. Adv. Pharm. Bull. 2019, 9, 159–173. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Salecan-based pH-sensitive hydrogels for insulin delivery. Mol. Pharm. 2017, 14, 431–440. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Huang, Q.; Tong, Z.; Yao, J.; Kong, X. A composite hydrogel system containing glucose-responsive nanocarriers for oral delivery of insulin. Mater. Sci. Eng. C 2016, 69, 37–45. [Google Scholar] [CrossRef]
- Udeni Gunathilake, T.; Ching, Y.; Chuah, C. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Bermejo, M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino, V. Covalently crosslinked organophosphorous derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin. Eur. J. Pharm. Biopharm. 2019, 136, 174–183. [Google Scholar] [CrossRef]
- Tao, G.; Wang, Y.; Cai, R.; Chang, H.; Song, K.; Zuo, H.; Zhao, P.; Xia, Q.; He, H. Design and performance of sericin/poly(vinyl alcohol) hydrogel as a drug delivery carrier for potential wound dressing application. Mater. Sci. Eng. C 2019, 101, 341–351. [Google Scholar] [CrossRef]
- Singh, B.; Varshney, L.; Francis, S.; Rajneesh. Designing tragacanth gum based sterile hydrogel by radiation method for use in drug delivery and wound dressing applications. Int. J. Biol. Macromol. 2016, 88, 586–602. [Google Scholar] [CrossRef]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Peng, X.; Qiao, J.; Jiang, Z.; Han, B.; Yang, C.; Liu, W. Evaluation of a photocrosslinkable hydroxyethyl chitosan hydrogel as a potential drug release system for glaucoma surgery. J. Mater. Sci. Mater. Med. 2017, 28, 149. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Chao, Y.-C.; Hsiao, M.-H.; Chou, H.-S.; Jheng, Y.-H.; Yu, X.-H.; Lee, N.; Yang, C.; Liu, D.-M. Inhibition of periodontitis induction using a stimuli-responsive hydrogel carrying naringin. J. Periodontol. 2017, 88, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for oral local delivery. Mater. Sci. Eng. C 2017, 81, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, M.; Jo, G.; Kim, S.; Chun, H.; Yang, D. Photo-cured glycol chitosan hydrogel for ovarian cancer drug delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Akbari, V.; Amuaghae, E.; Emami, J. Preparation and characterization of an injectable thermosensitive hydrogel for simultaneous delivery of paclitaxel and doxorubicin. Res. Pharm. Sci. 2018, 13, 181. [Google Scholar]
- Songkroh, T.; Xie, H.; Yu, W.; Liu, X.; Sun, G.; Xu, X.; Ma, X. Injectable in situ forming chitosan-based hydrogels for curcumin delivery. Macromol. Res. 2015, 23, 53–59. [Google Scholar] [CrossRef]
- Naderi, Z.; Azizian, J. Synthesis and characterization of carboxymethyl chitosan/Fe3O4 and MnFe2O4 nanocomposites hydrogels for loading and release of curcumin. J. Photochem. Photobiol. B Biol. 2018, 185, 206–214. [Google Scholar] [CrossRef]
- Li, C.; Ren, S.; Dai, Y.; Tian, F.; Wang, X.; Zhou, S.; Deng, S.; Liu, Q.; Zhao, J.; Chen, X. Efficacy, pharmacokinetics, and biodistribution of thermosensitive chitosan/β-glycerophosphate hydrogel loaded with docetaxel. AAPS PharmSciTech 2014, 15, 417–424. [Google Scholar] [CrossRef]
- Samimi Gharaie, S.; Dabiri, S.; Akbari, M. Smart shear-thinning hydrogels as injectable drug delivery systems. Polymers 2018, 10, 1317. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Zhang, J.; Dong, W. Magnetic field-driven drug release from modified iron oxide-integrated polysaccharide hydrogel. Int. J. Biol. Macromol. 2018, 108, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, Z.; Zhong, Y. Hydrogel loaded with self-assembled dextran sulfate-doxorubicin complexes as a delivery system for chemotherapy. Mater. Sci. Eng. C 2017, 77, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Ferreira, L.M.; Miranda-Gonçalves, V.; Reis, R.M.; Seraphim, T.V.; Borges, J.C.; Baltazar, F.; Gremião, M.P.D. Alginate hydrogel improves anti-angiogenic bevacizumab activity in cancer therapy. Eur. J. Pharm. Biopharm. 2017, 119, 271–282. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Zhang, J.; Wang, Y.; Wang, Y.; Shi, S.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.; et al. Pharmaceutical nanotechnology: A novel composite hydrogel based on chitosan and inorganic phosphate for local drug delivery of camptothecin nanocolloids. J. Pharm. Sci. 2011, 100, 232–241. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Huang, H.; Zhang, H.; Hou, L.; Zhang, Z. Functionalized graphene oxide-based thermosensitive hydrogel for near-infrared chemo-photothermal therapy on tumor. J. Biomater. Appl. 2016, 30, 1230–1241. [Google Scholar] [CrossRef]

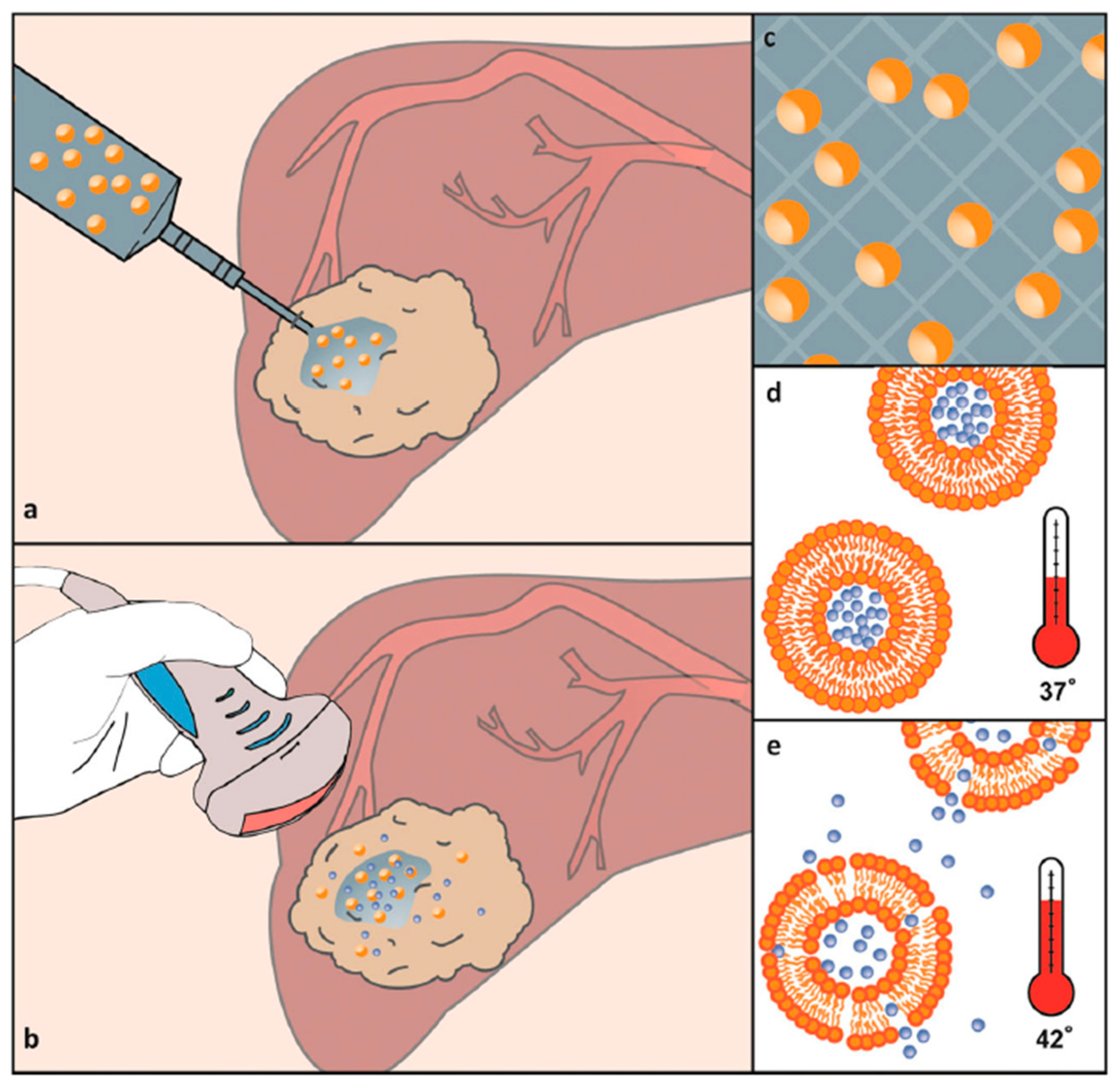


| Polymer Name | Abbreviation | Transition Temperature (in Water) | Refs. |
|---|---|---|---|
| Poly(N-isopropylacrylamide) | PNIPAM | 30–34 °C | [88] |
| Poly(N,N-diethylacrylamide) | PDEAM | 32–34 °C | [88] |
| Poly(methyl vinyl ether) | PMVE | 37 °C | [91] |
| Polyvinyl chloride | PVC | 30–50 °C | [92,93] |
| Gellan gum | - | 50–60 °C | [88] |
| Methylcellulose | - | 40 °C | [94,95] |
| Acrylamide and acrylic acid | AAm and AAc | 15–25 °C | [96] |
| Type of Loading Drugs | Hydrogel Precursors | Drug | Structure | Applications | Refs. |
|---|---|---|---|---|---|
| Post-loading | AA17 –BA18 –DEAP19 | 17-DMAPG 15 | Core-shell 16 | Antitumor activity | [152] |
| Carbopol 940 20 | Vor 11 | Core-shell LPN | Dermal applications | [185] | |
| Carbopol/stearic acid | Vor | Core-shell | Ophthalmic Application | [186] | |
| PNIPAAm-b-PLA-b-PEG-b-PLA7-b-PNIPAAm | Riluzole | - | Neuroprotective drug | [183] | |
| PAA-PNIPAAm | DOX | Core-shell capsule | Antitumor activity | [175] | |
| PEG-b-PDAEMA 8 | PLGM 9 | Nanoparticle | Antitumor activity | [172] | |
| Chitosan/Polysaccharide | Tenofovir | Microsphere | Vaginal drug | [174] | |
| Chitosan/β-GB | DOX-loaded in LTSL 29 | PP33 HG | Antitumor activity | [187] | |
| PLGA 30 MicroC PEG derivatives (HG) | DOX-loaded in MicroC 31 Fu 32-loaded in HG | PP HG | Antitumor activity | [188] | |
| In situ loading | Polysaccharide | Ibuprofen | Core-shell capsule | Oral drug | [126] |
| Carbohydrate-NIPAM 22 | Bupivacaine | HG-microgel composite | Anaesthetic drug | [189] | |
| PNIPAAm-co-AIA 21 | Lopinavir | Microspheres | Antiretroviral drug | [190] | |
| CmetCel 25 -Dextran | AmB 23 | MacroHG | Antifungal therapy | [191] | |
| NiPAAm 26-NtBAAm27 | Fluvastatin | PP HG | HMG-CoA 25 | [192] | |
| PPZ 34 | Silibinin | Microspheres | Anticancer and antiangiogenic activity | [193] | |
| PEG–PCL 35–PEG | PTX micelles 36 | PP HG | Antitumor activity | [194] |
| Hydrogel | Structure/Size | Active Substance | Route of Delivery | Refs. |
|---|---|---|---|---|
| CTS-g-poly(AA-co-AAm)PVP/MMT | Nanogel | Clarithromycin | Oral | [214] |
| Salecan/PAMPS | Microgel | Insulin | Oral | [215] |
| HA | Nanogel | Insulin | Oral | [216] |
| CTS | Nanogel | Curcumin | Oral | [217] |
| CTS | - | Camptothecin | Oral | [218] |
| SS/PVA | Microgel | Gentamycin sulphate | Dermal | [219] |
| TG/SA/PVA | Microgel | Moxifloxacin | Dermal | [220] |
| P407/CMCs | Microgel | Cortex Moutan extract | Dermal | [221] |
| CMCTS/PAD | Macrogel | Voriconazole | Ocular | [12] |
| HECTS | Macrogel | - | Ocular | [222] |
| P407 | Nanogel | Theaflavin/Nifeviroc | Vaginal | [2] |
| CTS | Microgel | Tenofovir | Vaginal | [174] |
| CHC | - | Naringenin | Topical oral | [223] |
| CTS | Nanogel | Thymol | Topical oral | [224] |
| GCTS | - | Paclitaxel | Injection | [225] |
| PF127/HA | Nanogel | Paclitaxel and doxorubicin | Injection | [226] |
| CTS | - | Curcumin | Injection | [227] |
| CMCTS | Nanogel | Curcumin | Injection | [228] |
| CTS/GP | - | Docetaxel | Injection | [229] |
| Gelatine/laponite (+chitosan or PNIPAM-co-AA) | Nanogel | Rhodamine B | Injection | [230] |
| Salecan | - | Doxorubicin | Injection | [231] |
| Agarose | - | Doxorubicin | Injection | [232] |
| SA | Nanogel | Bevacizumab | Injection | [233] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials 2020, 13, 188. https://doi.org/10.3390/ma13010188
Chyzy A, Tomczykowa M, Plonska-Brzezinska ME. Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials. 2020; 13(1):188. https://doi.org/10.3390/ma13010188
Chicago/Turabian StyleChyzy, Adam, Monika Tomczykowa, and Marta E. Plonska-Brzezinska. 2020. "Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery" Materials 13, no. 1: 188. https://doi.org/10.3390/ma13010188
APA StyleChyzy, A., Tomczykowa, M., & Plonska-Brzezinska, M. E. (2020). Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials, 13(1), 188. https://doi.org/10.3390/ma13010188






