Prediction of Chloride Distribution for Offshore Concrete Based on Statistical Analysis
Abstract
:1. Introduction
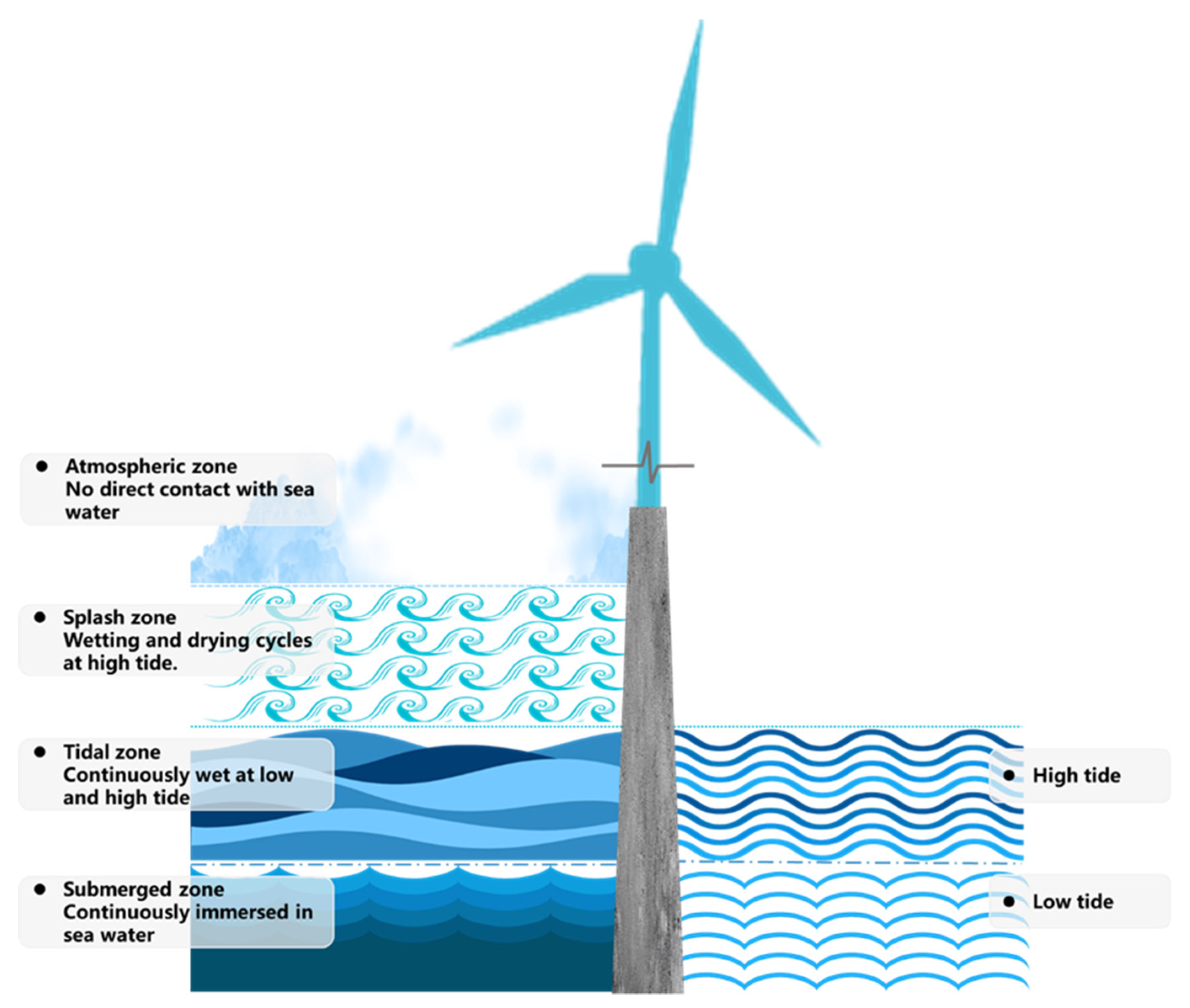
2. Research Background
3. Method Description
4. Results and Discussion
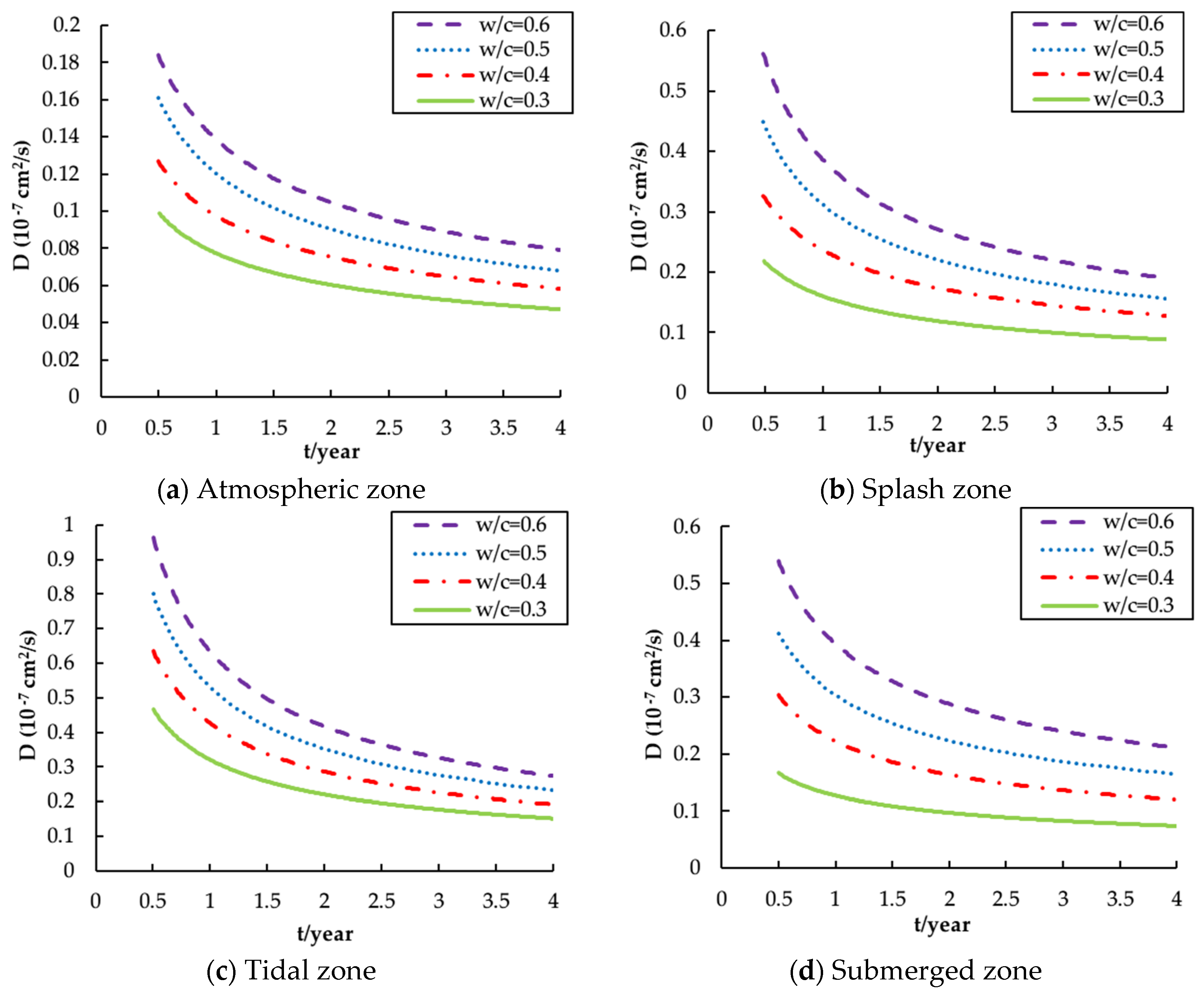
4.1. Diffusion Coefficient of Chloride Ions
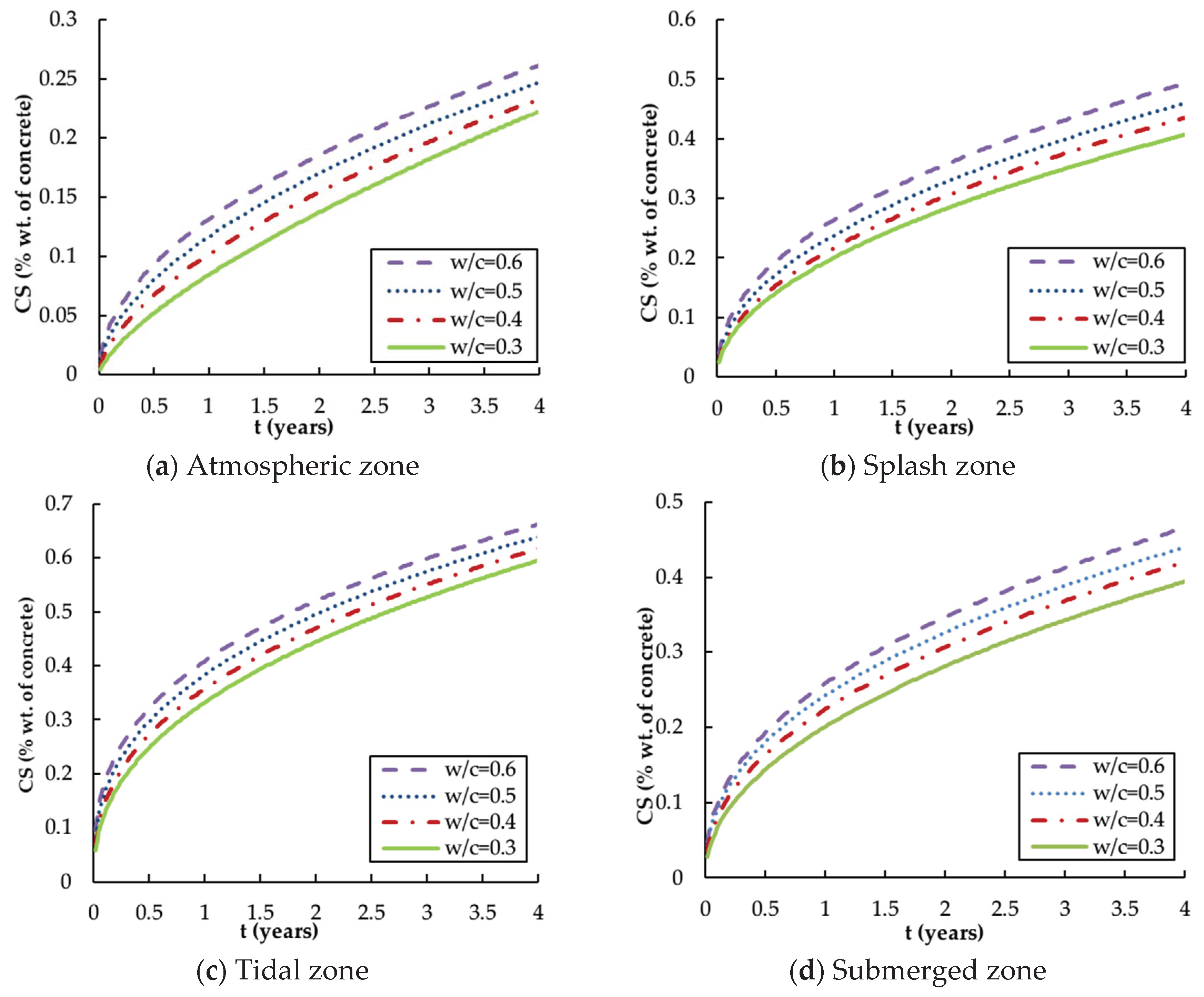
4.2. Surface Chloride Concentration
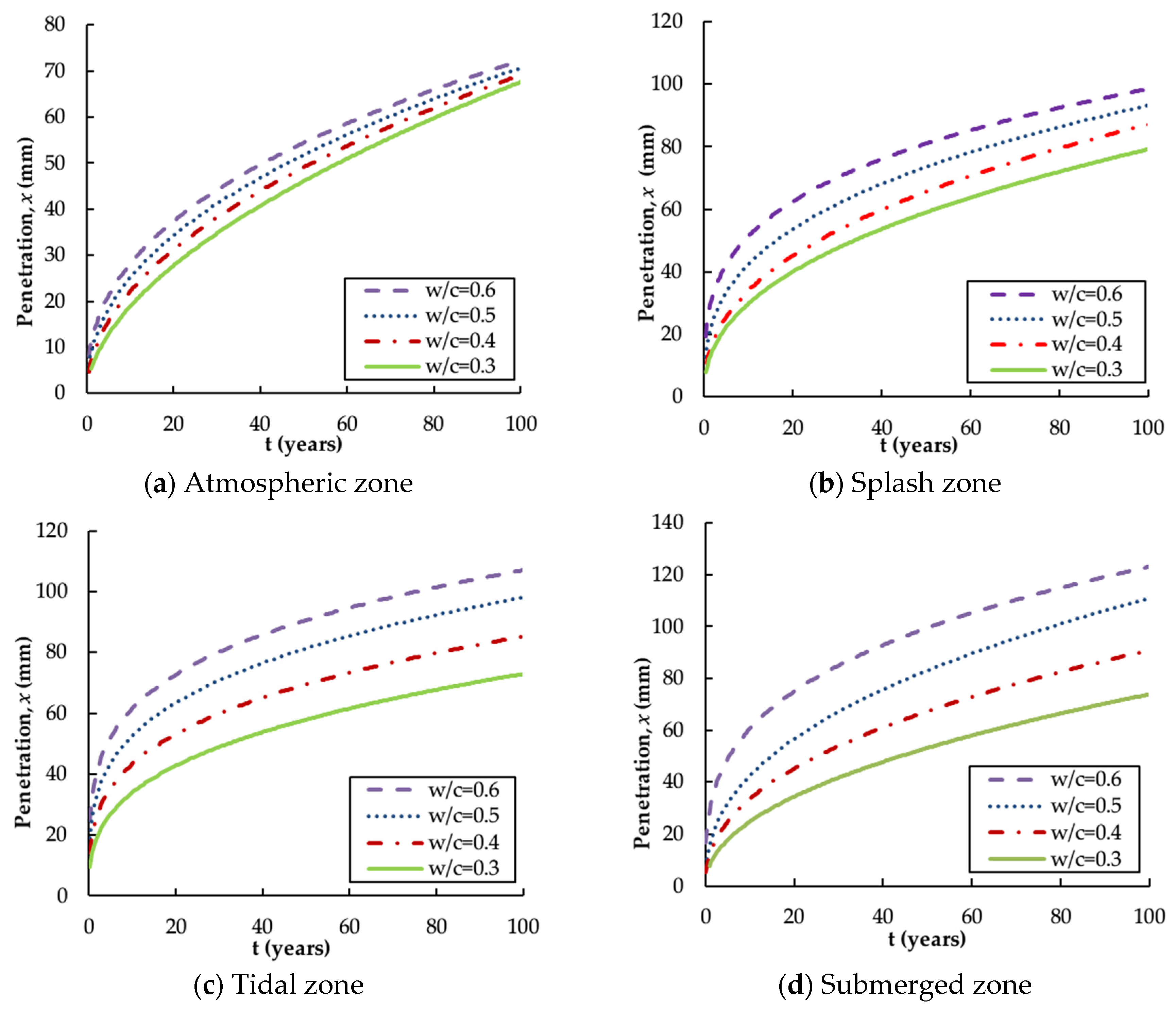
4.3. Penetration Depth
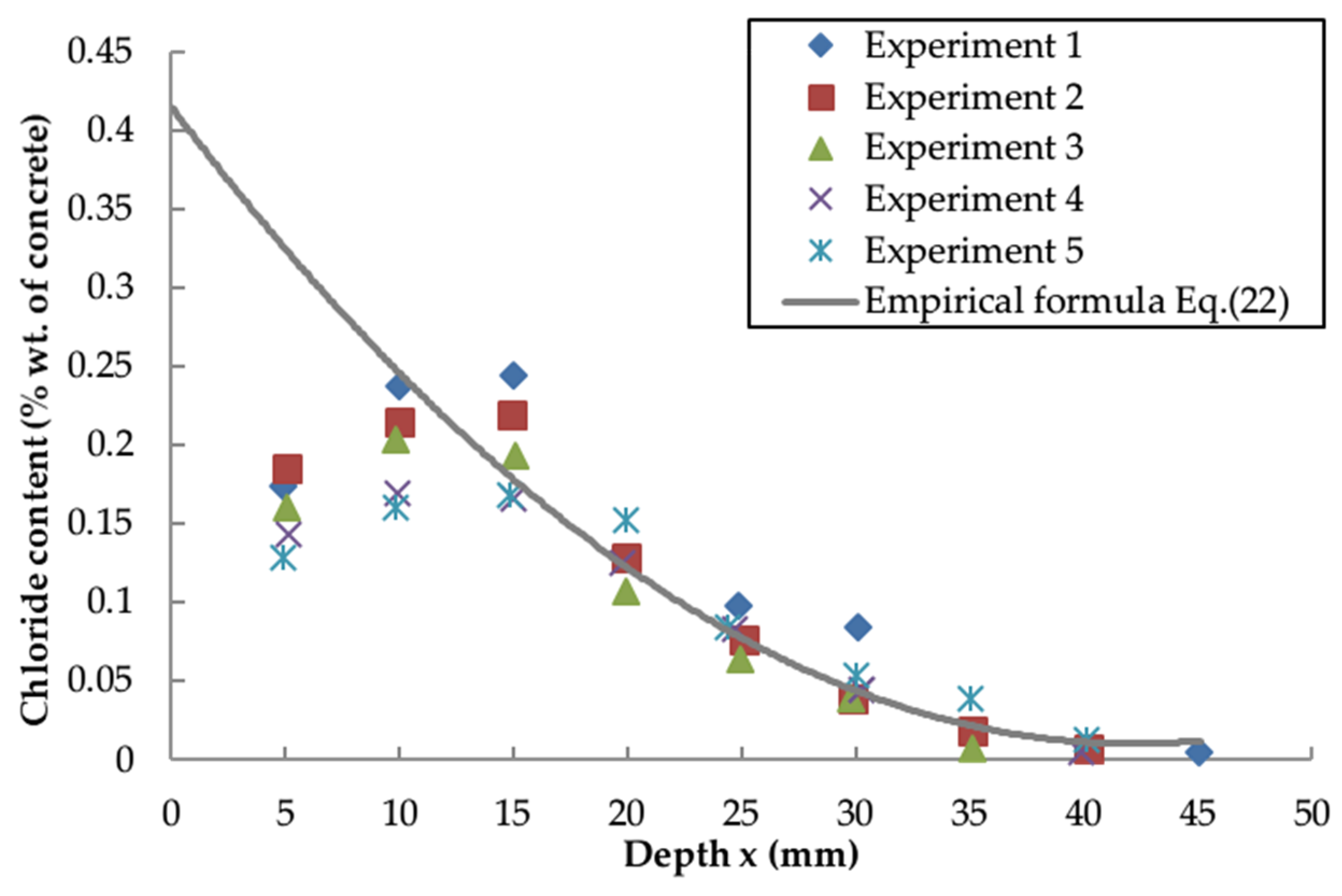
4.4. Empirical Formula
5. Comparison and Validation
5.1. Minimum Cover Thickness
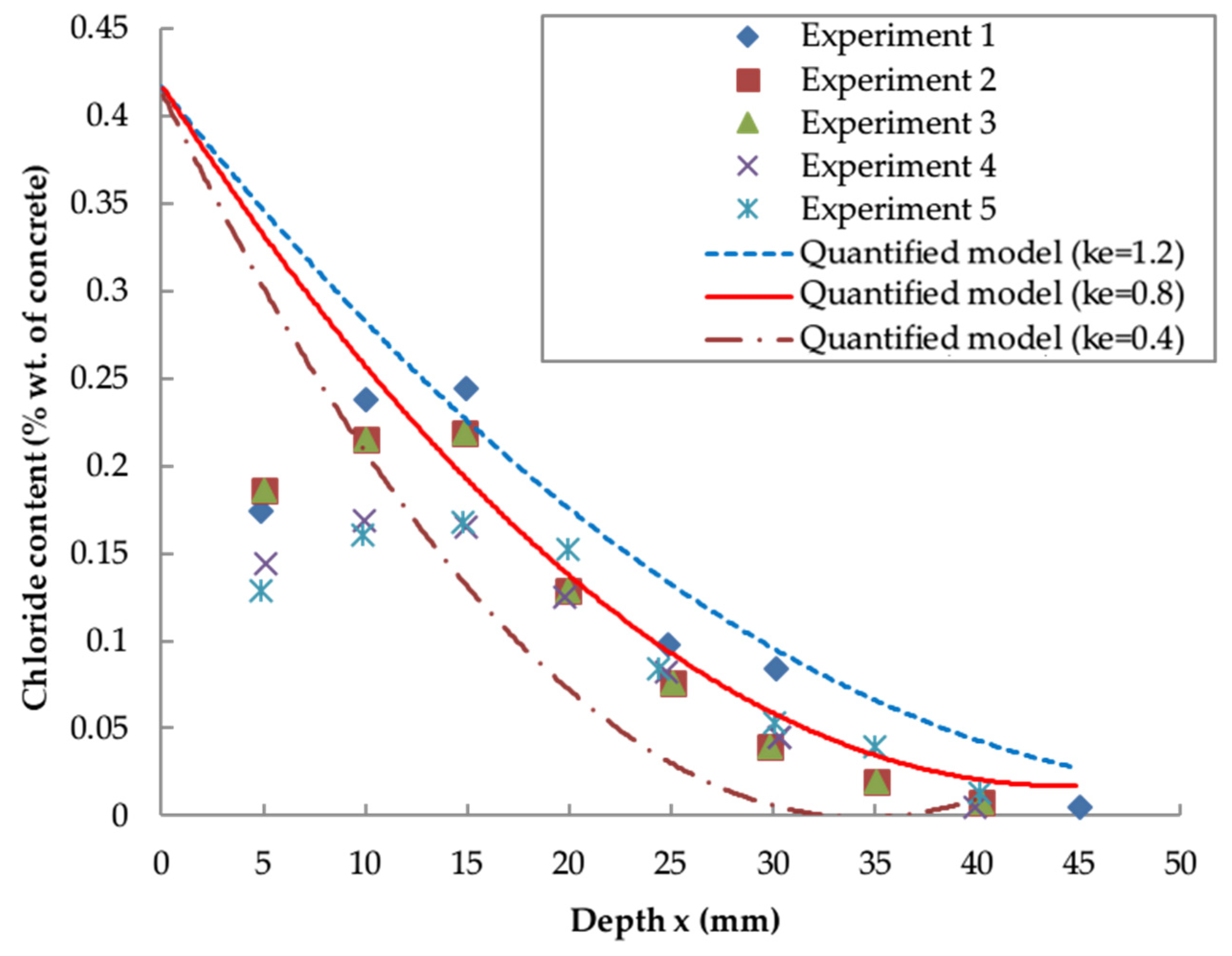
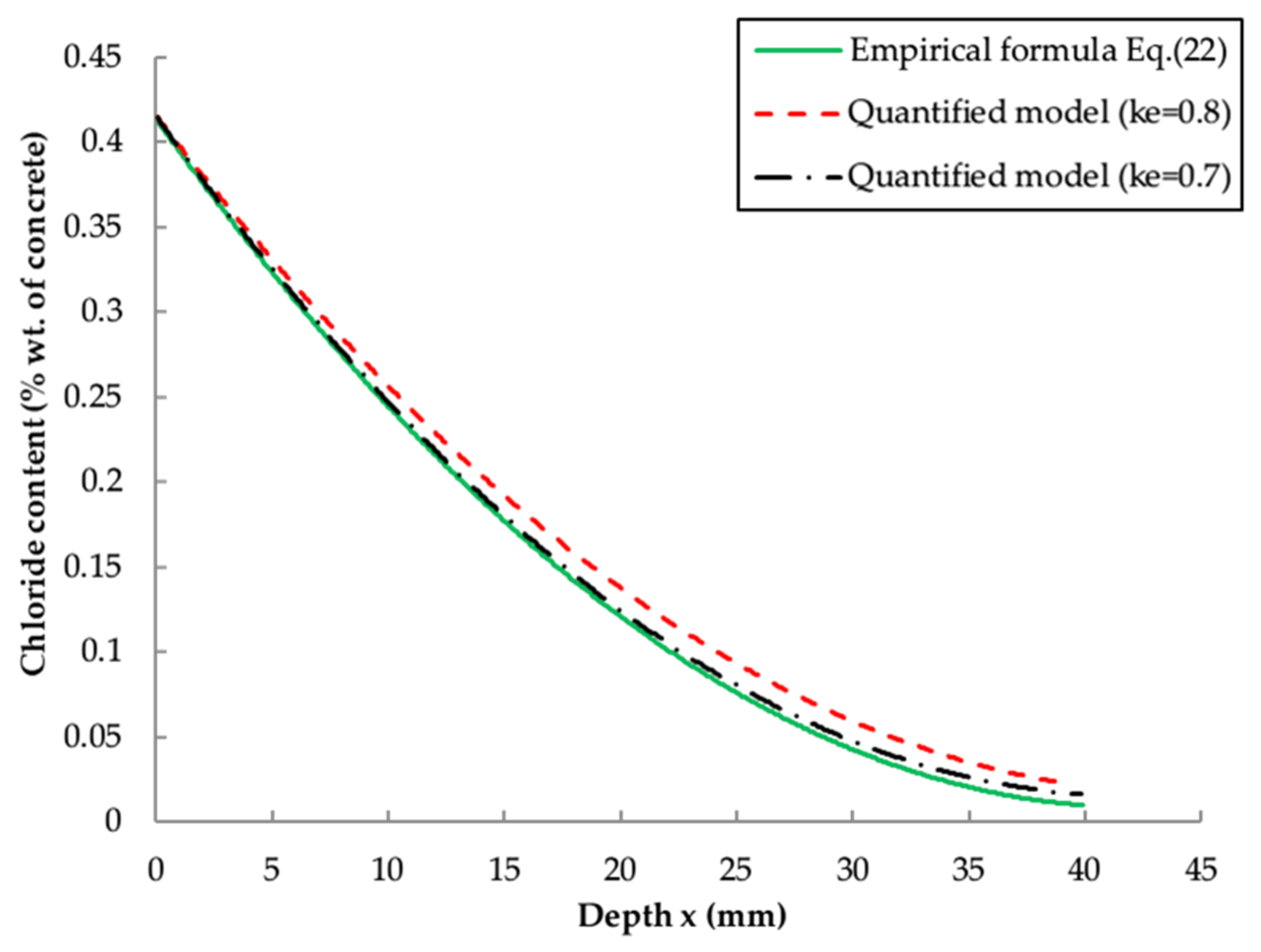
5.2. Comparison and Validation with Existing Models
6. Conclusions
- , and are highly time-dependent parameters and the calibration of these influence parameters is necessary for the reliable prediction of chloride distribution. The revised , and x are functions of exposure zone, ratio and service time.
- The tidal zone is the most severe exposure environment in terms of chloride corrosion, followed by the splash zone, the submerged zone and the atmospheric zone. This affirms that the aggressiveness of the offshore environment varies considerably with the exposure zones and hence the cover thickness should be varied as appropriate for different environments.
- The water–cement ratio is highly relevant to chloride diffusion in that the higher ratio will lead to a greater diffusion coefficient, surface concentration and penetration depth. Thus, it is reasonable to limit the maximum ratio of offshore concrete structures.
- The validity of the proposed empirical formula is confirmed by a successful prediction of a series of independent experiments and the calculated cover thickness is in good agreement with the code UFGS-03. The approach developed in this paper, therefore, provides a simple but reliable means for examining chloride diffusion in offshore structures.
7. Recommendation for Future Work
Author Contributions
Funding
Conflicts of Interest
References
- Harte, R.; Van Zijl, G.P.A.G. Structural stability of concrete wind turbines and solar chimney towers exposed to dynamic wind action. J. Wind Eng. Ind. Aerodyn. 2007, 95, 1079–1096. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Chen, H. The role of fly ash microsphere in the microstructure and macroscopic properties of high-strength concrete. Cem. Concr. Compos. 2017, 83, 125–137. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Chen, H. Long-term properties of concrete containing limestone powder. Mater. Struct. 2017, 50, 168. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Wong, H.S.; Abousnina, R.; AlAjarmeh, O.S.; Zhuge, Y.; Schubel, P. Optimal design for epoxy polymer concrete based on mechanical properties and durability aspects. Constr. Build. Mater. 2020, 232, 117229. [Google Scholar] [CrossRef]
- Li, L.Y.; Xia, J.; Lin, S.S. A multi-phase model for predicting the effective diffusion coefficient of chlorides in concrete. Constr. Build. Mater. 2012, 26, 295–301. [Google Scholar] [CrossRef]
- Liu, Q.F.; Xia, J.; Easterbrook, D.; Yang, J.; Li, L.Y. Three-phase modelling of electrochemical chloride removal from corroded steel-reinforced concrete. Constr. Build. Mater. 2014, 70, 410–427. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, G.; Van Breugel, K. Modeling of ionic diffusivity in non-saturated cement-based materials using lattice Boltzmann method. Cem. Concr. Res. 2012, 42, 1524–1533. [Google Scholar] [CrossRef]
- Dehghanpoor Abyaneh, S.; Wong, H.S.; Buenfeld, N.R. Modelling the diffusivity of mortar and concrete using a three-dimensional mesostructure with several aggregate shapes. Comput. Mater. Sci. 2013, 78, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Šavija, B.; Pacheco, J.; Schlangen, E. Lattice modeling of chloride diffusion in sound and cracked concrete. Cem. Concr. Compos. 2013, 42, 30–40. [Google Scholar] [CrossRef]
- Zhang, W.M.; Ba, H.J. Effect of silica fume addition and repeated loading on chloride diffusion coefficient of concrete. Mater. Struct. Constr. 2013, 46, 1183–1191. [Google Scholar] [CrossRef]
- Shekarchi, M.; Rafiee, A.; Layssi, H. Long-term chloride diffusion in silica fume concrete in harsh marine climates. Cem. Concr. Compos. 2009, 31, 769–775. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, X.; Xia, J.; Mao, L.; Yang, J.; Liu, Q. A numerical study on chloride diffusion in freeze-thaw affected concrete. Constr. Build. Mater. 2018, 179, 553–565. [Google Scholar] [CrossRef]
- Liu, Q.F.; Easterbrook, D.; Yang, J.; Li, L.Y. A three-phase, multi-component ionic transport model for simulation of chloride penetration in concrete. Eng. Struct. 2015, 86, 122–133. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, X.; Hong, S.; Wu, Z.; Liu, Q. Binding capacity and diffusivity of concrete subjected to freeze-thaw and chloride attack: A numerical study. Ocean. Eng. 2019, 186, 106093. [Google Scholar] [CrossRef]
- Hou, D.; Lu, Z.; Li, X.; Ma, H.; Li, Z. Reactive molecular dynamics and experimental study of graphene-cement composites: Structure, dynamics and reinforcement mechanisms. Carbon 2017, 115, 188–208. [Google Scholar] [CrossRef]
- Hou, D.; Yu, J.; Wang, P. Molecular dynamics modeling of the structure, dynamics, energetics and mechanical properties of cement-polymer nanocomposite. Compos. Part. B Eng. 2019, 162, 433–444. [Google Scholar] [CrossRef]
- Liu, Q.F.; Feng, G.L.; Xia, J.; Yang, J.; Li, L. yuan Ionic transport features in concrete composites containing various shaped aggregates: A numerical study. Compos. Struct. 2017, 183, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.X.; Hu, Z.; Xia, J.; Feng, G.L.; Azim, I.; Yang, J.; Liu, Q.F. Multi-phase modelling of electrochemical rehabilitation for ASR and chloride affected concrete composites. Compos. Struct. 2019, 207, 176–189. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, W.; Hou, D.-S.; Hu, Z.; Yang, J.; Liu, Q. Numerical study of carbonation and its effect on chloride binding in concrete. Cem. Concr. Compos. 2019, 104, 103402. [Google Scholar] [CrossRef]
- Shen, X.H.; Liu, Q.F.; Hu, Z.; Jiang, W.Q.; Lin, X.; Hou, D.; Hao, P. Combine ingress of chloride and carbonation in marine-exposed concrete under unsaturated environment: A numerical study. Ocean. Eng. 2019, 189, 106350. [Google Scholar] [CrossRef]
- Yan, D.; Lin, G.; Chen, G. Dynamic properties of plain concrete in triaxial stress state. ACI Mater. J. 2009, 106, 89. [Google Scholar]
- Ye, H.; Tian, Y.; Jin, N.; Jin, X.; Fu, C. Influence of cracking on chloride diffusivity and moisture influential depth in concrete subjected to simulated environmental conditions. Constr. Build. Mater. 2013, 47, 66–79. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Vogel, M.; Müller, H.S.; Zhao, T. Influence of freeze-thaw cycles on capillary absorption and chloride penetration into concrete. Cem. Concr. Res. 2017, 100, 60–67. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zhang, P.; Ma, Y.; Zhao, T.; Wang, H.; Zhang, Z. Water absorption and chloride diffusivity of concrete under the coupling effect of uniaxial compressive load and freeze–thaw cycles. Constr. Build. Mater. 2019, 209, 566–576. [Google Scholar] [CrossRef]
- Dhir, R.K.; Jones, M.R.; Elghaly, A.E. PFA concrete: Exposure temperature effects on chloride diffusion. Cem. Concr. Res. 1993, 23, 1105–1114. [Google Scholar] [CrossRef]
- Eglinton, M. 7–Resistance of Concrete to Destructive Agencies. In Lea’s Chemistry of Cement and Concrete; Available online: https://www.sciencedirect.com/science/article/pii/B9780750662567500199?via%3Dihub (accessed on 28 December 2019).
- Zhang, P.; Li, D.; Qiao, Y.; Zhang, S.; Sun, C.; Zhao, T. Effect of air entrainment on the mechanical properties, chloride migration, and microstructure of ordinary concrete and fly ash concrete. J. Mater. Civ. Eng. 2018, 30, 4018265. [Google Scholar] [CrossRef]
- Alizadeh, R.; Ghods, P.; Chini, M.; Hoseini, M.; Ghalibafian, M.; Shekarchi, M. Effect of Curing Conditions on the Service Life Design of RC Structures in the Persian Gulf Region. J. Mater. Civ. Eng. 2008, 20, 2–8. [Google Scholar] [CrossRef]
- Ghods, P.; Chini, M.; Alizadeh, R.; Hoseini, M. The effect of Different Exposure Conditions on the Chloride Diffusion into Concrete in the Persian Gulf region. In 3th ConMat; CiteSeer: Delft, The Netherlands, 2005. [Google Scholar]
- Superseding, N. USACE/NAVFAC/AFCEC/NASA UFGS-03 31 29 (February 2019). Available online: https://www.wbdg.org/FFC/DOD/UFGS/UFGS%2003%2031%2029.pdf (accessed on 28 December 2019).
- Pack, S.W.; Jung, M.S.; Song, H.W.; Kim, S.H.; Ann, K.Y. Prediction of time dependent chloride transport in concrete structures exposed to a marine environment. Cem. Concr. Res. 2010, 40, 302–312. [Google Scholar] [CrossRef]
- Chatterji, S. On the applicability of Fick’s second law to chloride ion migration through portland cement concrete. Cem. Concr. Res. 1995, 25, 299–303. [Google Scholar] [CrossRef]
- Nilsson, L.-O.; Massat, M.; Tang, L. The effect of non-linear chloride binding on the prediction of chloride penetration into concrete structures. Spec. Publ. 1994, 145, 469–486. [Google Scholar]
- Markeset, G.; Skjølsvold, O. Time dependent chloride diffusion coefficient: Field studies of concrete exposed to marine environment in Norway. Int. Symp. Serv. Life Des. Infrastruct. 2010, 2010. [Google Scholar]
- Jin, W.; Xue, W.; Chen, J. Effecting coefficients for concrete structure durability design index. J. Build. Struct. 2011, 32, 86–97. [Google Scholar]
- Al-Amoudi, O.S.B.; Al-Kutti, W.A.; Ahmad, S.; Maslehuddin, M. Correlation between compressive strength and certain durability indices of plain and blended cement concretes. Cem. Concr. Compos. 2009, 31, 672–676. [Google Scholar] [CrossRef]
- Ann, K.Y.; Ahn, J.H.; Ryou, J.S. The importance of chloride content at the concrete surface in assessing the time to corrosion of steel in concrete structures. Constr. Build. Mater. 2009, 23, 239–245. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Wang, C.K.; Jin, W.L.; Xu, C. Experimental analysis on time-dependent law of surface chloride ion concentration of concrete. J. Civil. Archit. Environ. Eng. 2010, 32, 8–13. [Google Scholar]
- Mehta, P.K. Concrete in the Marine Environment; Taylor & Francis: New York, NY, USA, 2003. [Google Scholar]
- Gjørv, O.E. Durability Design of Concrete Structures in Severe Environments. Key Eng. Mater. 2015, 629–630, 218–222. [Google Scholar]
- Page, C.L.; Page, M.M. Durability of Concrete and Cement Composites; CRC Press: Boca Raton, FA, USA, 2007. [Google Scholar]
- Costa, A.; Appleton, J. Chloride penetration into concrete in marine environment—Part I: Main parameters affecting chloride penetration. Mater. Struct. 1999, 32, 252–259. [Google Scholar] [CrossRef]
- Costa, A.; Appleton, J. Chloride penetration into concrete in marine environment-Part II: Prediction of long term chloride penetration. Mater. Struct. 1999, 32, 354–359. [Google Scholar] [CrossRef]
- Mackechnie, J.R. Predictions of Reinforced Concrete Durability in the Marine Environment; University of Cape Town: Cape Town, South Africa, 1995. [Google Scholar]
- Lawrence, C.D. The Constitution and Specification of Portland Cements. In Lea’s Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Pech-Canul, M.A.; Castro, P. Corrosion measurements of steel reinforcement in concrete exposed to a tropical marine atmosphere. Cem. Concr. Res. 2002, 32, 491–498. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Xi, Y. The effect of w/c ratio and aggregate volume fraction on chloride penetration in non-saturated concrete. Constr. Build. Mater. 2018, 191, 260–269. [Google Scholar] [CrossRef]
- Lahalle, H.; Cau Dit Coumes, C.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c ratio on the hydration process of a magnesium phosphate cement and on its retardation by boric acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Amey, S.L.; Johnson, D.A.; Miltenberger, M.A.; Farzam, H. Predicting the service life of concrete marine structures: An environmental methodology. Aci. Struct. J. 1998, 95, 205–214. [Google Scholar]
- Concrete, C.; Australia, A. Chloride Resistance of Concrete. 2009. Available online: https://www.ccaa.com.au/imis_prod/documents/Library%20Documents/CCAA%20Reports/Report%202009%20ChlorideResistance.pdf (accessed on 28 December 2019).
- AASHTO T259-02-UL. Standard Method of Test for Resistance of Concrete to Chloride Ion Penetration; American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2006. [Google Scholar]
- Ferdous, W.; Bai, Y.; Almutairi, A.D.; Satasivam, S.; Jeske, J. Modular assembly of water-retaining walls using GFRP hollow profiles: Components and connection performance. Compos. Struct. 2018, 194, 1–11. [Google Scholar] [CrossRef]
- Ferdous, W.; Almutairi, A.D.; Huang, Y.; Bai, Y. Short-term flexural behaviour of concrete filled pultruded GFRP cellular and tubular sections with pin-eye connections for modular retaining wall construction. Compos. Struct. 2018, 206, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. Investigation on the poor fluidity of electrically conductive cement-graphite paste: Experiment and simulation. Mater. Des. 2019, 169, 107679. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Liu, Q.F.; Azim, I.; Zhu, X.; Yang, J.; Javed, M.F.; Rauf, M. Prediction of mechanical properties of green concrete incorporating waste foundry sand based on gene expression programming. J. Hazard. Mater. 2020, 384, 121322. [Google Scholar] [CrossRef] [PubMed]
- Association, I.; Construction, I.R. C674: The Use of Concrete in Maritime Engineering–a Good Practice Guide; Ciria: London, UK, 2010. [Google Scholar]
- Liu, J.; Xing, F.; Dong, B.; Ding, Z. Diffusion of chloride ions into concrete in salt spray environment. J. Shenzhen Univ. 2010, 27, 192–198. [Google Scholar]
| Exposure Zone | Empirical Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Atmospheric | 0.16 | 0.04 | 0.58 | 0.20 × 10−7 | 0.02 × 10−7 | 0.61 | 0 | 18.94 | −0.43 | 0.48 |
| Splash | 0.21 | 0.13 | 0.48 | 0.76 × 10−7 | −0.01 × 10−7 | 0.53 | 139.50 | −72.71 | 20.38 | 0.36 |
| Tidal | 0.26 | 0.25 | 0.38 | 1.05 × 10−7 | 0.01 × 10−7 | 0.42 | 0 | 64.92 | −3.84 | 0.28 |
| Submerged | 0.19 | 0.14 | 0.45 | 0.88 × 10−7 | −0.13 × 10−7 | 0.57 | 238.78 | −146.21 | 31.18 | 0.41 |
| Zone | Cover Thickness (mm) |
|---|---|
| Atmospheric zone not subject to salt spray | 65 |
| Tidal, splash and atmospheric zone subject to salt spray | 75 |
| Submerged zone | 75 |
| Exposure Zone | Critical Depth(mm) |
|---|---|
| Atmospheric | 50 |
| Splash | 64 |
| Tidal | 68 |
| Submerged | 67 |
| Exposure Zone | Critical Depth(mm) |
|---|---|
| Atmospheric | 56 |
| Splash | 66 |
| Tidal | 75 |
| Submerged | 64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.-f.; Hu, Z.; Lu, X.-y.; Yang, J.; Azim, I.; Sun, W. Prediction of Chloride Distribution for Offshore Concrete Based on Statistical Analysis. Materials 2020, 13, 174. https://doi.org/10.3390/ma13010174
Liu Q-f, Hu Z, Lu X-y, Yang J, Azim I, Sun W. Prediction of Chloride Distribution for Offshore Concrete Based on Statistical Analysis. Materials. 2020; 13(1):174. https://doi.org/10.3390/ma13010174
Chicago/Turabian StyleLiu, Qing-feng, Zhi Hu, Xian-yang Lu, Jian Yang, Iftikhar Azim, and Wenzhuo Sun. 2020. "Prediction of Chloride Distribution for Offshore Concrete Based on Statistical Analysis" Materials 13, no. 1: 174. https://doi.org/10.3390/ma13010174
APA StyleLiu, Q.-f., Hu, Z., Lu, X.-y., Yang, J., Azim, I., & Sun, W. (2020). Prediction of Chloride Distribution for Offshore Concrete Based on Statistical Analysis. Materials, 13(1), 174. https://doi.org/10.3390/ma13010174






