Revealing the Microstructure Evolution and Carbonation Hardening Mechanism of β-C2S Pastes by Backscattered Electron Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of β-C2S
2.2. Carbonation of β-C2S Pastes
2.3. Test Methods
3. Results and Discussion
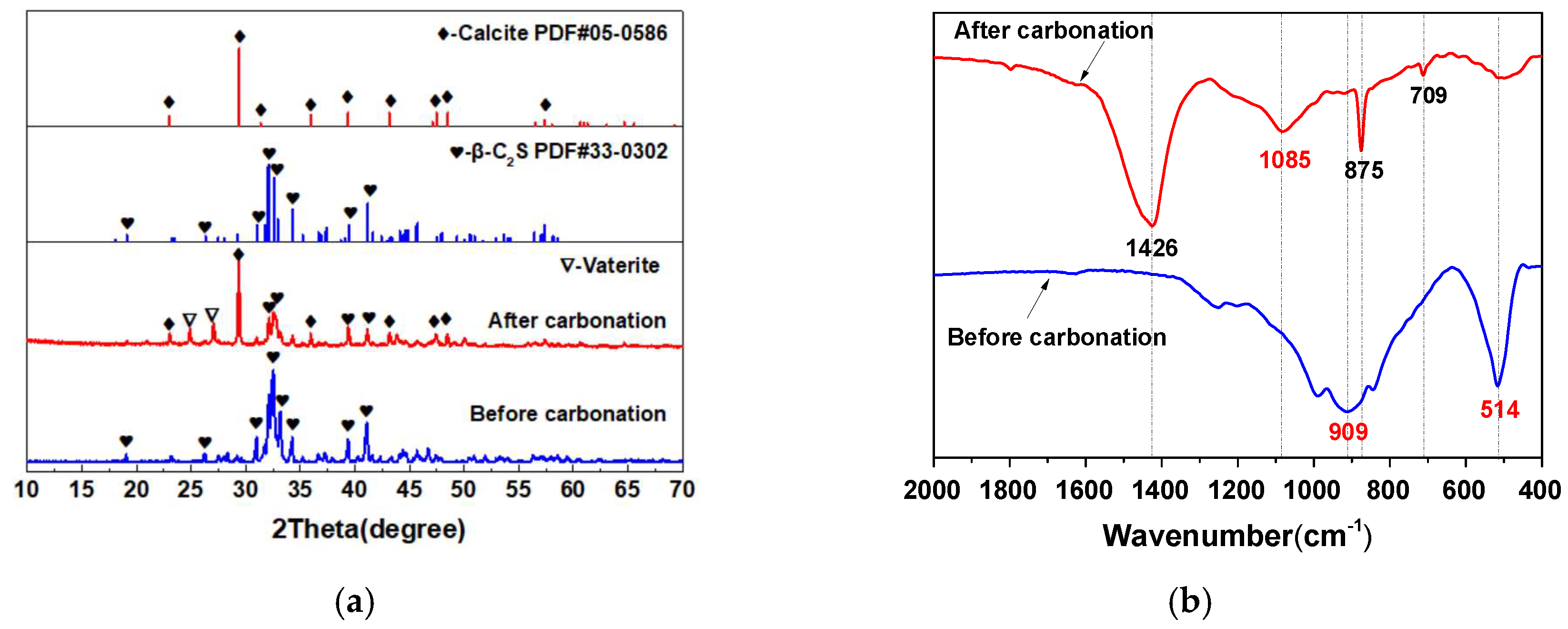
3.1. The Carbonation Products of β-C2S Pastes
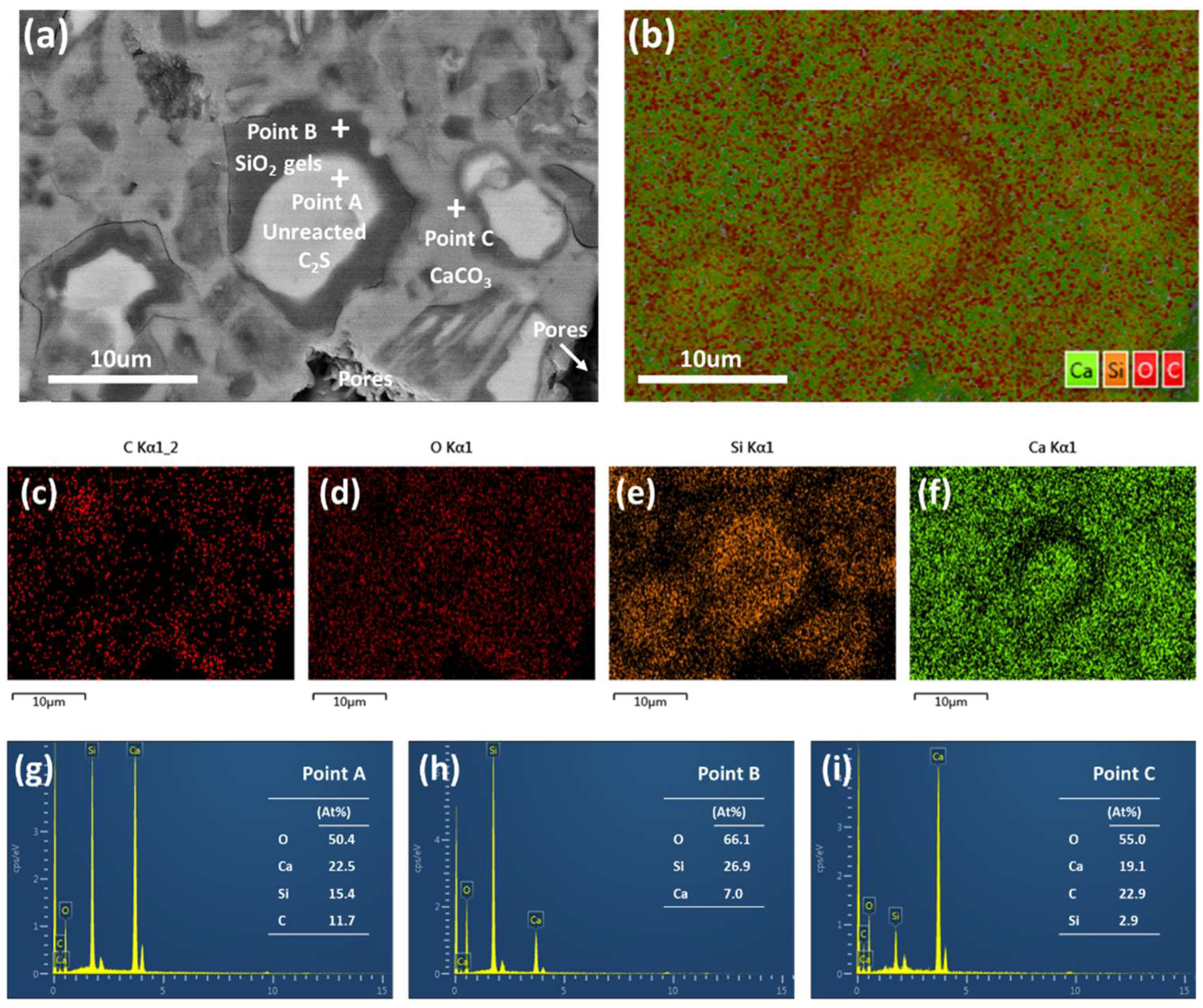
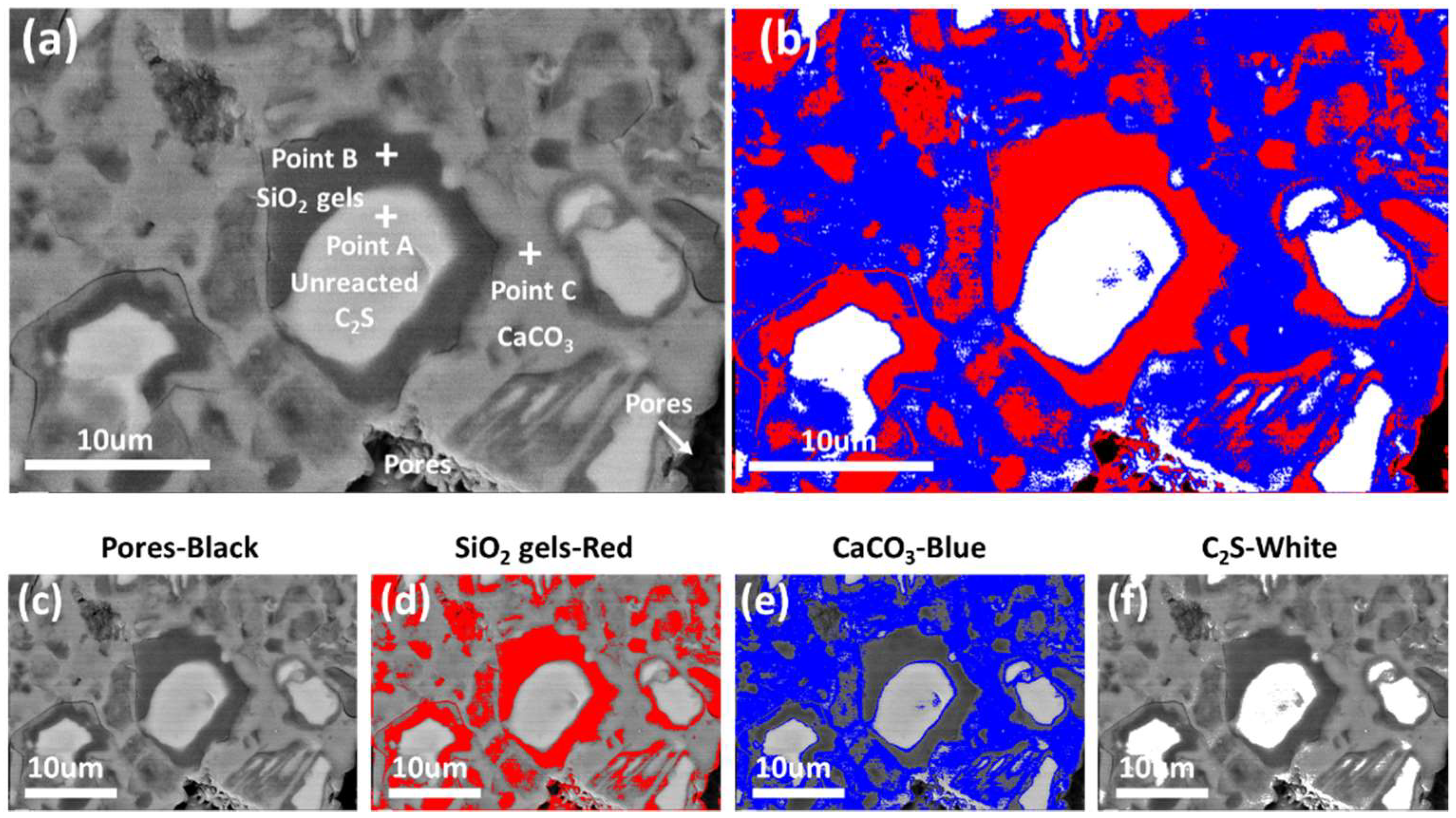
3.2. Microstructure and Distribution of the Carbonation Products
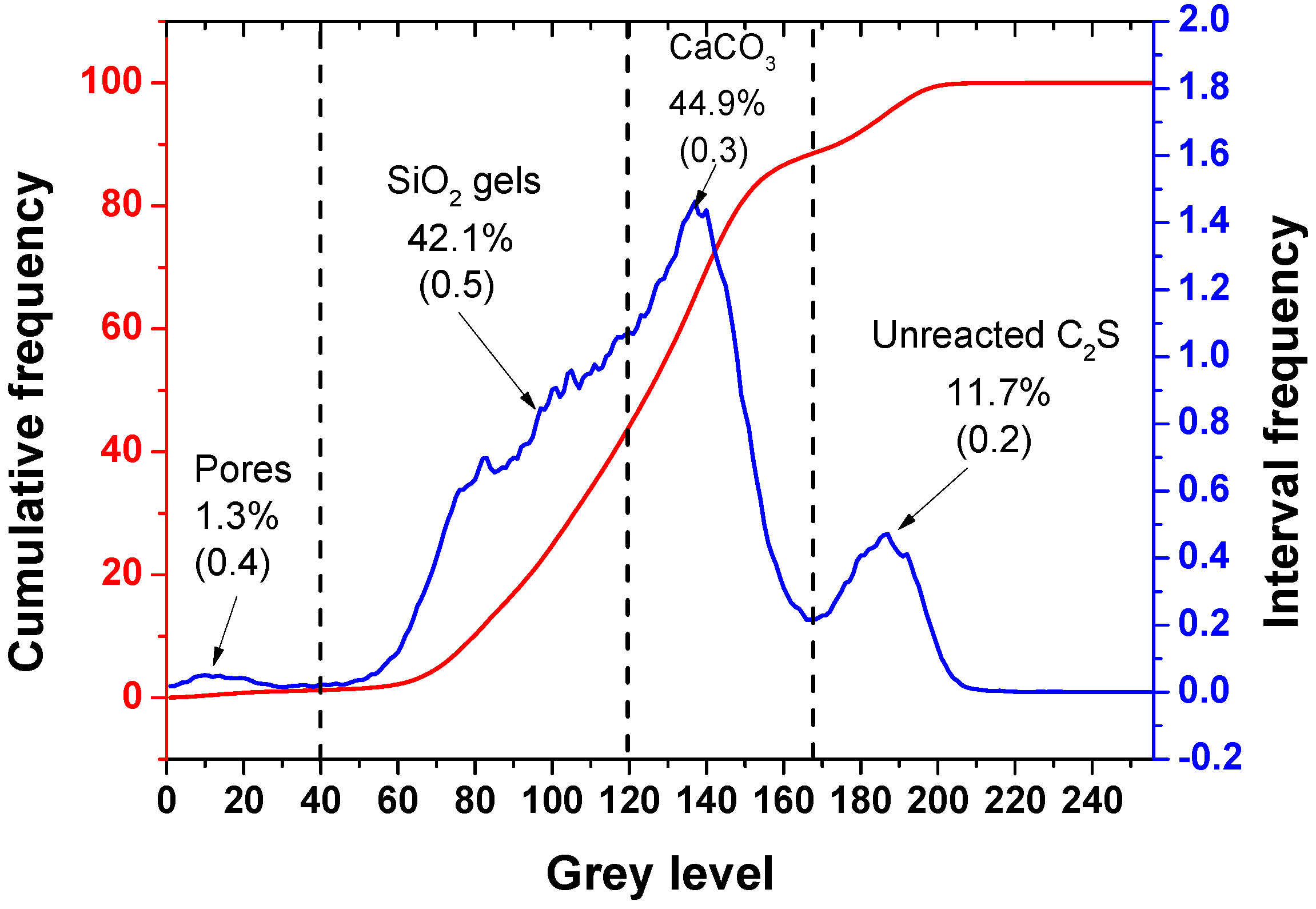
3.3. Quantitative Analysis of the Carbonation Products
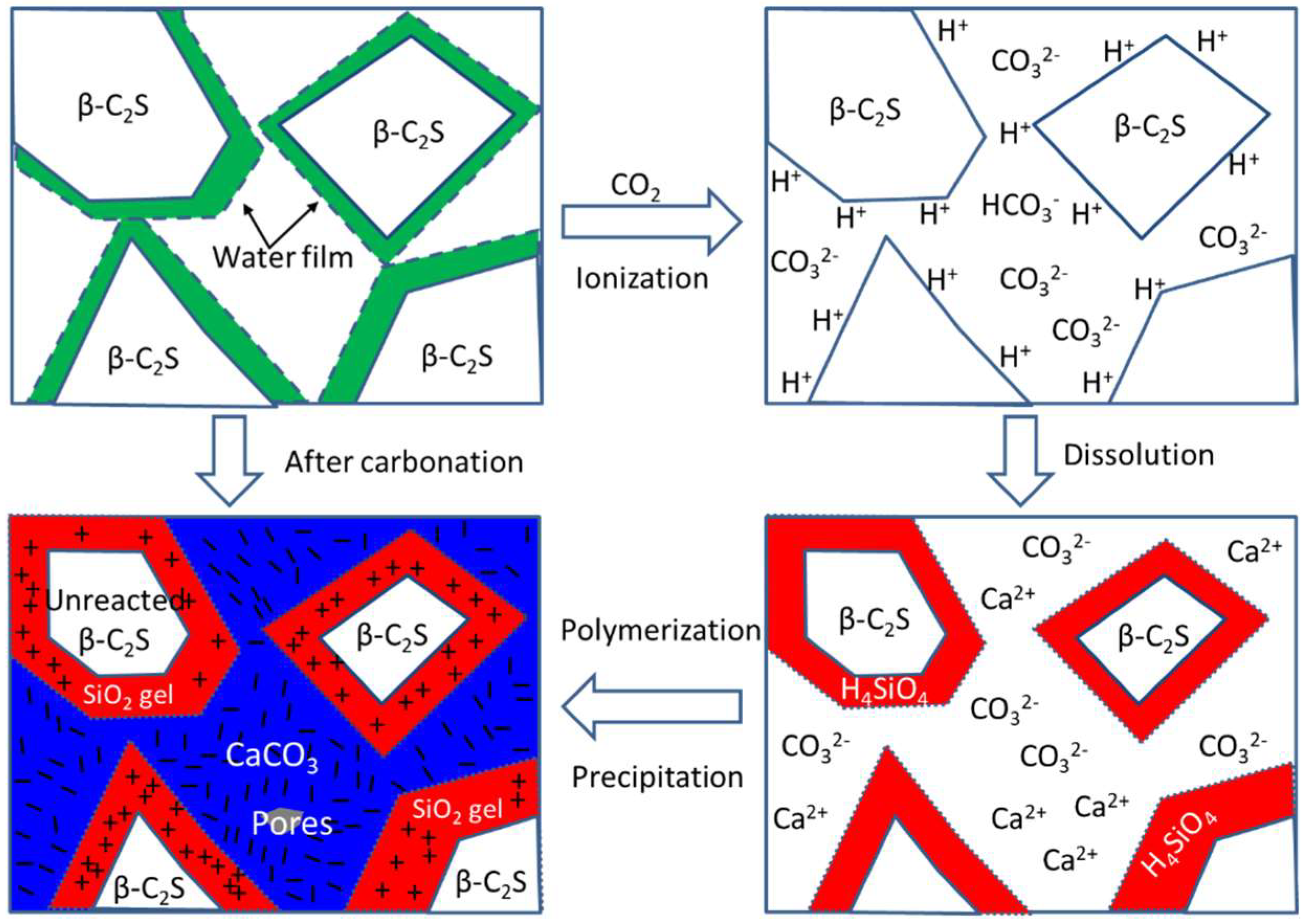
3.4. The Carbonation Hardening Mechanism of β-C2S Pastes
4. Conclusions
- After the carbonation curing of β-C2S for 24 h, a dense hardened paste was obtained. The hardened pastes are composed of pores, silica gel, calcium carbonate, and unreacted dicalcium silicate, with relative volume fractions of 1.3%, 42.1%, 44.9%, and 11.7%, respectively.
- The unreacted β-C2S center is coated with a layer of SiO2 gel, and the pores between the original β-C2S particles are filled with CaCO3.
- Rapid generation of a sufficient number of SiO2 gels and CaCO3 crystals, and the formation of network structures of these carbonation products are responsible for the carbonation hardening of the β-C2S mineral.
- These results obtained may provide a profound understanding of the carbonation reaction process and hardening mechanism of low-calcium silicate-based cementitious materials.
Author Contributions
Funding
Conflicts of Interest
References
- Lu, B.; Shi, C.; Hou, G. Strength and microstructure of CO2 cured low-calcium clinker. Constr. Build. Mater. 2018, 188, 417–423. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M. Mechanical performance and microstructure of the calcium carbonate binders produced by carbonating steel slag paste under CO2 curing. Cement Concrete Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cement Concrete Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 2016, 51, 6173–6191. [Google Scholar] [CrossRef]
- Chang, J.; Fang, Y.; Shang, X. The role of β-C2S and γ-C2S in carbon capture and strength development. Mater. Struct. 2016, 49, 4417–4424. [Google Scholar] [CrossRef]
- Qian, B.; Li, X.; Shen, X. Preparation and accelerated carbonation of low temperature sintered clinker with low Ca/Si ratio. J. Clean. Prod. 2016, 120, 249–259. [Google Scholar] [CrossRef]
- Guan, X.; Liu, S.; Feng, C.; Qiu, M. The hardening behavior of γ-C2S binder using accelerated carbonation. Constr. Build. Mater. 2016, 114, 204–207. [Google Scholar] [CrossRef]
- Li, Z.; He, Z.; Shao, Y. Early Age Carbonation Heat and Products of Tricalcium Silicate Paste Subject to Carbon Dioxide Curing. Materials 2018, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Liu, Z.; Wang, F.; Huang, X. Carbonation characteristics of γ-dicalcium silicate for low-carbon building material. Constr. Build. Mater. 2018, 177, 322–331. [Google Scholar] [CrossRef]
- Igarashi, S.; Kawamura, M.; Watanabe, A. Analysis of cement pastes and mortars by a combination of backscatter-based SEM image analysis and calculations based on the Powers model. Cement Concrete Compos. 2004, 26, 977–985. [Google Scholar] [CrossRef]
- Li, Y.; Guo, W.; Li, H. Quantitative analysis on ground blast furnace slag behavior in hardened cement pastes based on backscattered electron imaging and image analysis technology. Constr. Build. Mater. 2016, 110, 48–53. [Google Scholar] [CrossRef]
- Wong, H.S.; Head, M.K.; Buenfeld, N.R. Pore segmentation of cement-based materials from backscattered electron images. Cement Concrete Res. 2006, 36, 1083–1090. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Mons, Y.A.; Isachsen, K.; Detwiler, R.J. Preparation of flat-polished specimens for SEM-backscattered electron imaging and X-ray microanalysis-importance of epoxy impregnation. Cement Concrete Res. 2003, 33, 611–616. [Google Scholar] [CrossRef]
- Scrivener, K.L. Backscattered electron imaging of cementitious microstructures: understanding and quantification. Cement Concrete Compos. 2004, 26, 935–945. [Google Scholar] [CrossRef]
- Stutzman, P. Scanning electron microscopy imaging of hydraulic cement microstructure. Cement Concrete Compos. 2004, 26, 957–966. [Google Scholar] [CrossRef]
- Wesselsky, A.; Jensen, O.M. Synthesis of pure Portland cement phases. Cement Concrete Res. 2009, 39, 973–980. [Google Scholar] [CrossRef]
- Liu, S.; Dou, Z.; Zhang, S.; Zhang, H.; Guan, X.; Feng, C.; Zhang, J. Effect of sodium hydroxide on the carbonation behavior of γ-dicalcium silicate. Constr. Build. Mater. 2017, 150, 591–594. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J.; Tian, N. Multiscale characterization of carbonated wollastonite paste and application of homogenization schemes to predict its effective elastic modulus. Cement Concrete Compos. 2016, 72, 284–298. [Google Scholar] [CrossRef]
- Okada, Y.; Masuda, T.; Takada, M.; Xu, L.; Mitsuda, T. Relationship between NMR 29Si Chemical Shifts and FT-IR Wave Numbers in Calcium Silicates. In Nuclear Magnetic Resonance Spectroscopy of Cement-Based Materials; Colombet, P., Grimmer, A.R., et al., Eds.; Springer: Berlin, Germany, 1998. [Google Scholar]
- Park, S.; Jang, J.; Chae, S.; Lee, H. An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment. Materials 2016, 9, 308. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Guan, X.; Zhang, H.; Wang, Y.; Gou, M. Revealing the Microstructure Evolution and Carbonation Hardening Mechanism of β-C2S Pastes by Backscattered Electron Images. Materials 2019, 12, 1561. https://doi.org/10.3390/ma12091561
Liu S, Guan X, Zhang H, Wang Y, Gou M. Revealing the Microstructure Evolution and Carbonation Hardening Mechanism of β-C2S Pastes by Backscattered Electron Images. Materials. 2019; 12(9):1561. https://doi.org/10.3390/ma12091561
Chicago/Turabian StyleLiu, Songhui, Xuemao Guan, Haibo Zhang, Yuli Wang, and Mifeng Gou. 2019. "Revealing the Microstructure Evolution and Carbonation Hardening Mechanism of β-C2S Pastes by Backscattered Electron Images" Materials 12, no. 9: 1561. https://doi.org/10.3390/ma12091561
APA StyleLiu, S., Guan, X., Zhang, H., Wang, Y., & Gou, M. (2019). Revealing the Microstructure Evolution and Carbonation Hardening Mechanism of β-C2S Pastes by Backscattered Electron Images. Materials, 12(9), 1561. https://doi.org/10.3390/ma12091561




