Synthesis of Zeolite 4A from Kaolin and Its Adsorption Equilibrium of Carbon Dioxide
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization Techniques
2.3. Synthesis of Zeolite 4A
2.4. Carbon Dioxide Adsorption Equilibrium Experiment
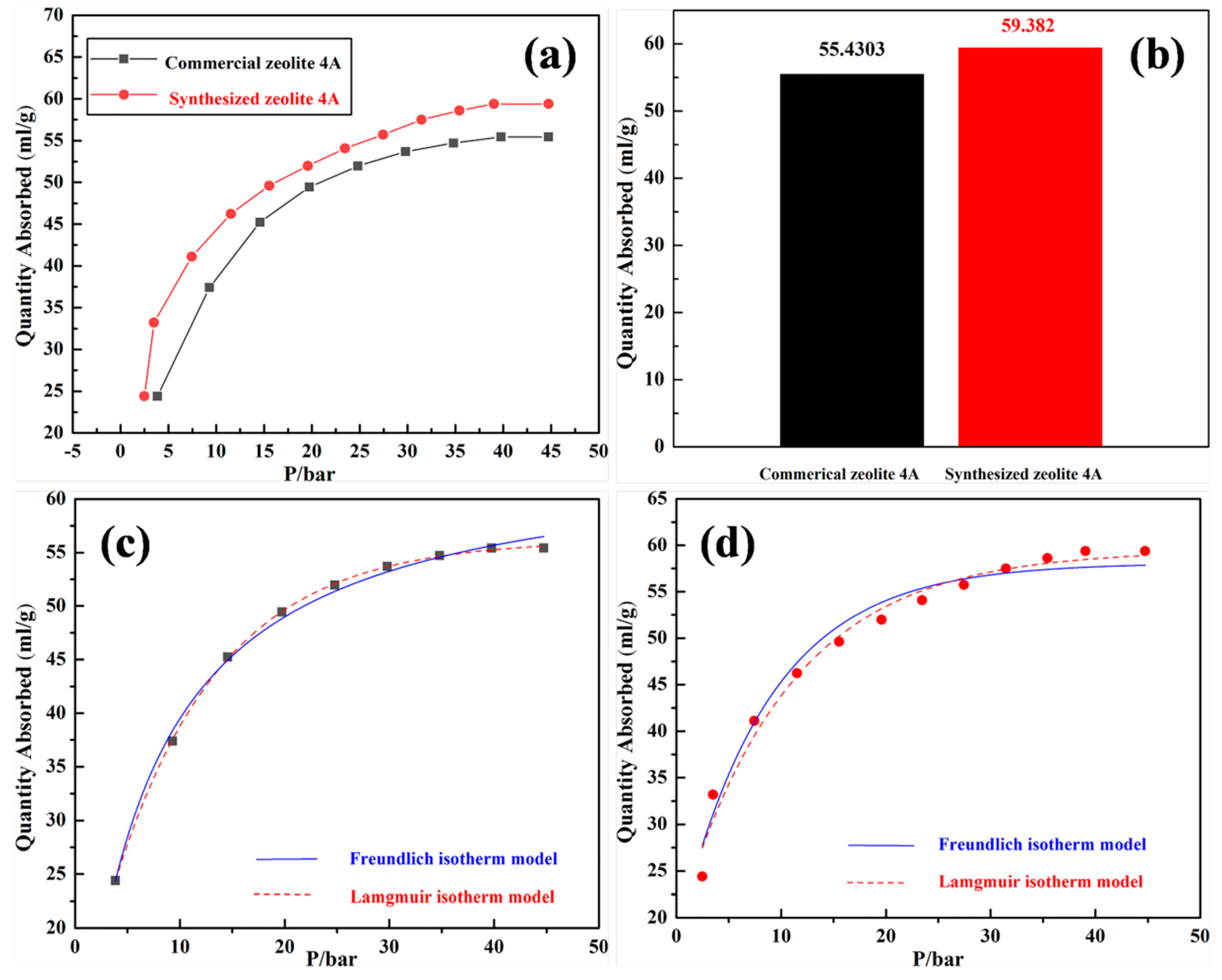
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jongseok, L.; Jonghwa, K.; Jintae, K. Adsorption Equilibria of CO2 on Zeolite 13X and Zeolite X/Activated Carbon Composite. J. Chem. Eng. Data 2002, 47, 1237–1242. [Google Scholar]
- Hwang, K.J.; Choi, W.S.; Jung, S.H.; Kwon, Y.J.; Hong, S.; Choi, C. Synthesis of zeolitic material from basalt rock and its adsorption properties for carbon dioxide. RSC Adv. 2018, 8, 9524–9529. [Google Scholar] [CrossRef]
- Oddy, S.; Poupore, J.; Tezel, F.H. Separation of CO2 and CH4 on CaX zeolite for use in Landfill gas separation. Can. J. Chem. Eng. 2013, 91, 1031–1039. [Google Scholar] [CrossRef]
- Gholipour, F.; Mofarahi, M. Adsorption equilibrium of methane and carbon dioxide on zeolite 13X: Experimental and thermodynamic modeling. J. Supercrit. Fluid 2016, 111, 47–54. [Google Scholar] [CrossRef]
- Gomes, V.G.; Yee, K.W.K. Pressure swing adsorption for carbon dioxide sequestration from exhaust gases. Sep. Purif. Technol. 2002, 28, 161–171. [Google Scholar] [CrossRef]
- Long, B.; Junli, L.I.; Wang, Q. CO2 removal by zeolite molecular sieves. J. Tsinghua Univ. Sci. Technol. 2016, 56, 824–829. [Google Scholar]
- Pires, J.; Saini, V.K.; Pinto, M.L. Studies on selective adsorption of biogas components on pillared clays: Approach for biogas improvement. Environ. Sci. Technol. 2008, 42, 8727–8732. [Google Scholar] [CrossRef]
- Garshasbi, V.; Jahangiri, M.; Anbia, M. Equilibrium CO2 adsorption on zeolite 13X prepared from natural clays. Appl. Surf. Sci. 2017, 393, 225–233. [Google Scholar] [CrossRef]
- Malek, A.M.; Jahangiri, M.; Rashidi, A.M. A novel selective H2S sensor using dodecylamine and ethylenediamine functionalized graphene oxide. J. Ind. Eng. Chem. 2015, 29, 97–103. [Google Scholar] [CrossRef]
- Delgado, J.A.; Uguina, M.A.; Gómez, J.M. Adsorption equilibrium of carbon dioxide, methane and nitrogen onto mordenite at high pressures. Sep. Purif. Technol. 2005, 48, 223–228. [Google Scholar] [CrossRef]
- Deng, Y.; Deng, C.; Qi, D. Synthesis of Core/Shell Colloidal Magnetic Zeolite Microspheres for the Immobilization of Trypsin. Adv. Mater. 2009, 21, 1377–1382. [Google Scholar] [CrossRef]
- Wang, L. Investigation and Application of CO2/N2 Separation by Adsorption Process using Zeolite 13XAPG; East China University of Science and Technology: ShangHai, China, 2013. [Google Scholar]
- Zhen, L.; Carlos, A.G.; Ping, L. Adsorption and Desorption of Carbon Dioxide and Nitrogen on Zeolite 5A. Sep. Sci. Technol. 2011, 46, 434–451. [Google Scholar]
- Cavenati, S.; And, C.A.G.; Rodrigues, A.E. Adsorption Equilibrium of Methane, Carbon Dioxide, and Nitrogen on Zeolite 13X at High Pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y. Synthesis of zeolite A from Ethiopian kaolin 2015, 215, 29–36. Microporous Mesoporous Mater. 2015, 215, 29–36. [Google Scholar] [CrossRef]
- White, C.E.; Perander, L.M.; Provis, J.L. The use of XANES to clarify issues related to bonding environments in metakaolin: A discussion of the paper S. Sperinck et al. “Dehydroxylation of kaolinite to metakaolin-a molecular dynamics study”. J. Mater. Chem. 2011, 21, 2118–2125. [Google Scholar]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y. Conventional versus alkali fusion synthesis of zeolite A from low grade kaolin. Appl. Clay Sci. 2016, 132, 485–490. [Google Scholar] [CrossRef]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Magnetic zeolite naa: Synthesis, characterization based on metakaolin and its application for the removal of cu2+, pb2+. Chemosphere 2013, 91, 1539–1546. [Google Scholar] [CrossRef]
- Sun, Q. Synthesis and Characterization of Zeolite and Magnetic Zeolite from Kaolin and Fly Ash. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2015. [Google Scholar]
- Bessa, R.D.A.; Costa, L.D.S.; Oliveira, C.P.; Bohn, F.; Nascimento, R.F.D.; José, M.S. Kaolin-based magnetic zeolites A and P as water softeners. Microporous Mesoporous Mater. 2017, 245, 64–72. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, G.; Liu, H.; Wu, J.; Mei, J. Crystallization mechanism of zeolite a from coal kaolin using a two-step method. Appl. Clay Sci. 2014, 97, 110–114. [Google Scholar] [CrossRef]
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8. [Google Scholar] [CrossRef]
- Yao, G.Y.; Lei, J.J.; Zhang, X.Y.; Sun, Z.M.; Zheng, S.L.; Sridhar, K. Mechanism of zeolite X crystallization from diatomite. Mater. Res. Bull. 2018, 107, 132–138. [Google Scholar] [CrossRef]
- Su, S.; Ma, H.; Chuan, X. Hydrothermal synthesis of zeolite a from k-feldspar and its crystallization mechanism. Adv. Powder Technol. 2016, 27, 139–144. [Google Scholar] [CrossRef]
- Huo, Z.; Xu, X.; Zhi, L.; Song, J.; He, M.; Li, Z. Synthesis of zeolite NaP with controllable morphologies. Microporous Mesoporous Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Sivamani, S.; Sujit, S. Optimization of synthesis parameters and characterization of coal fly ash derived microporous zeolite X. Appl. Surf. Sci. 2018, 455, 903–910. [Google Scholar]
- Liu, Y.; Yan, C.J. Synthesis of zeolite P1 from fly ash under solvent-free conditions for ammonium removal from water. J. Clean. Prod. 2018, 202, 11–22. [Google Scholar] [CrossRef]
- Ariful, I.S.; Adisorn, A.; Amornvadee, V. Equilibrium and Kinetic Behaviour of CO2 Adsorption onto Zeolites, Carbon Molecular Sieve and Activated Carbons. Energy Procedia 2017, 114, 2450–2459. [Google Scholar]
- Keller, J.; Staudt, R. Gas Adsorption Equilibria: Experimental Methods and Adsorption Isotherms; Springer Science & Business Media: Siegen, Germany, 2004. [Google Scholar]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
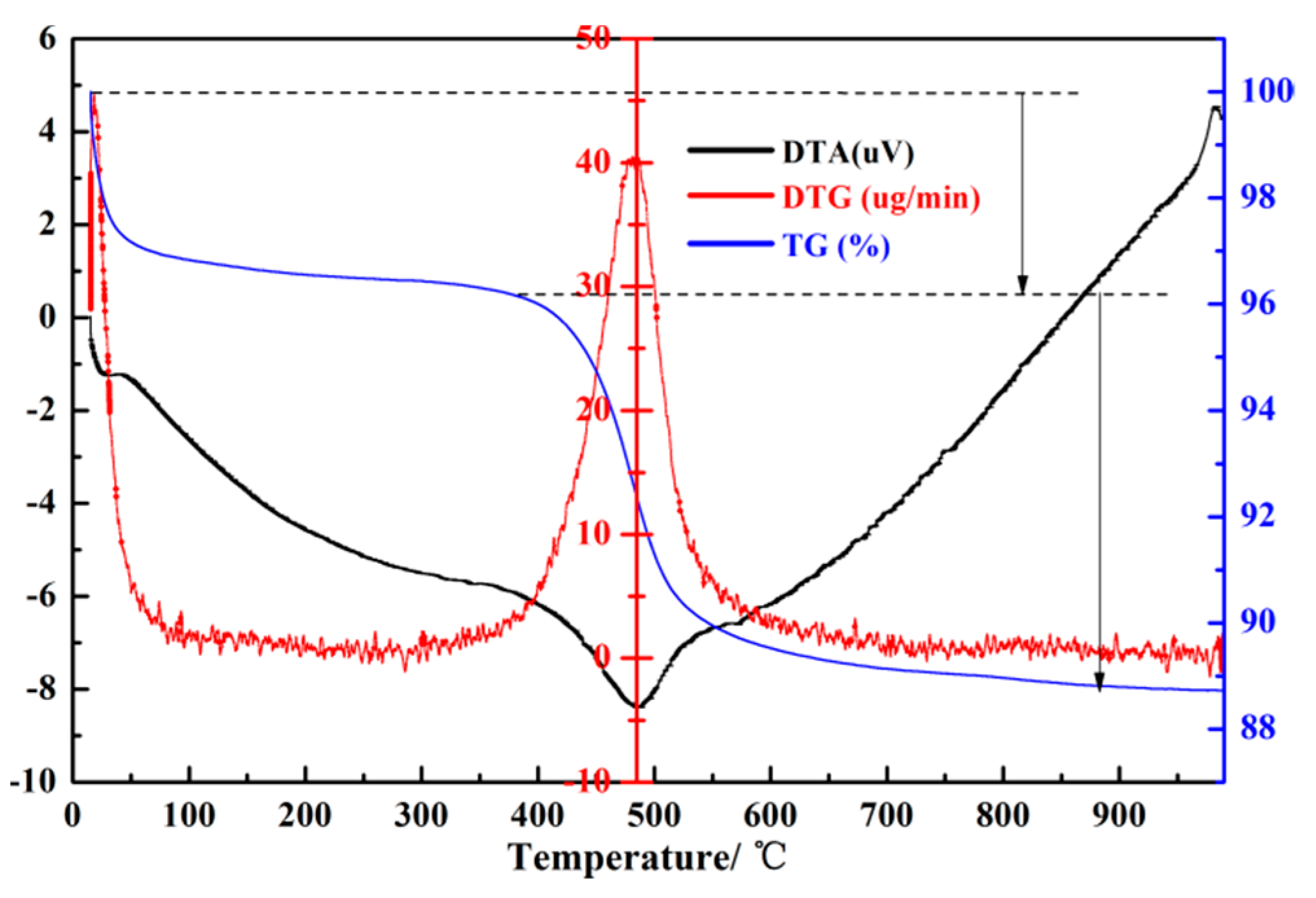

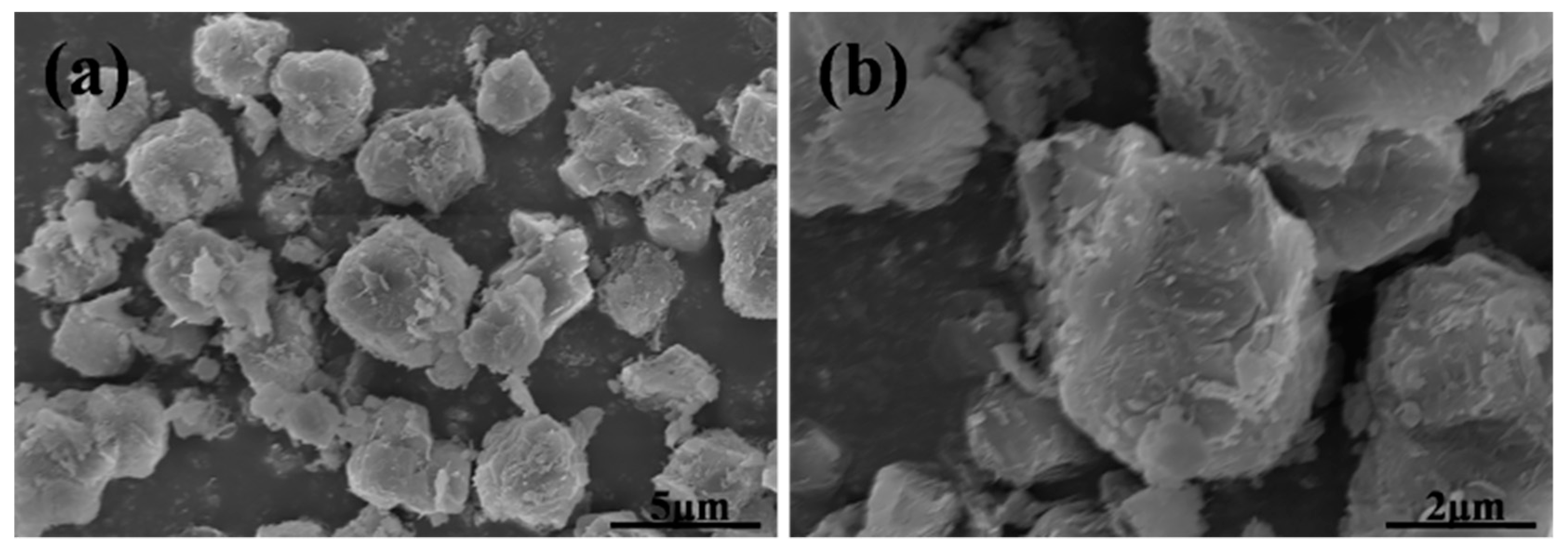
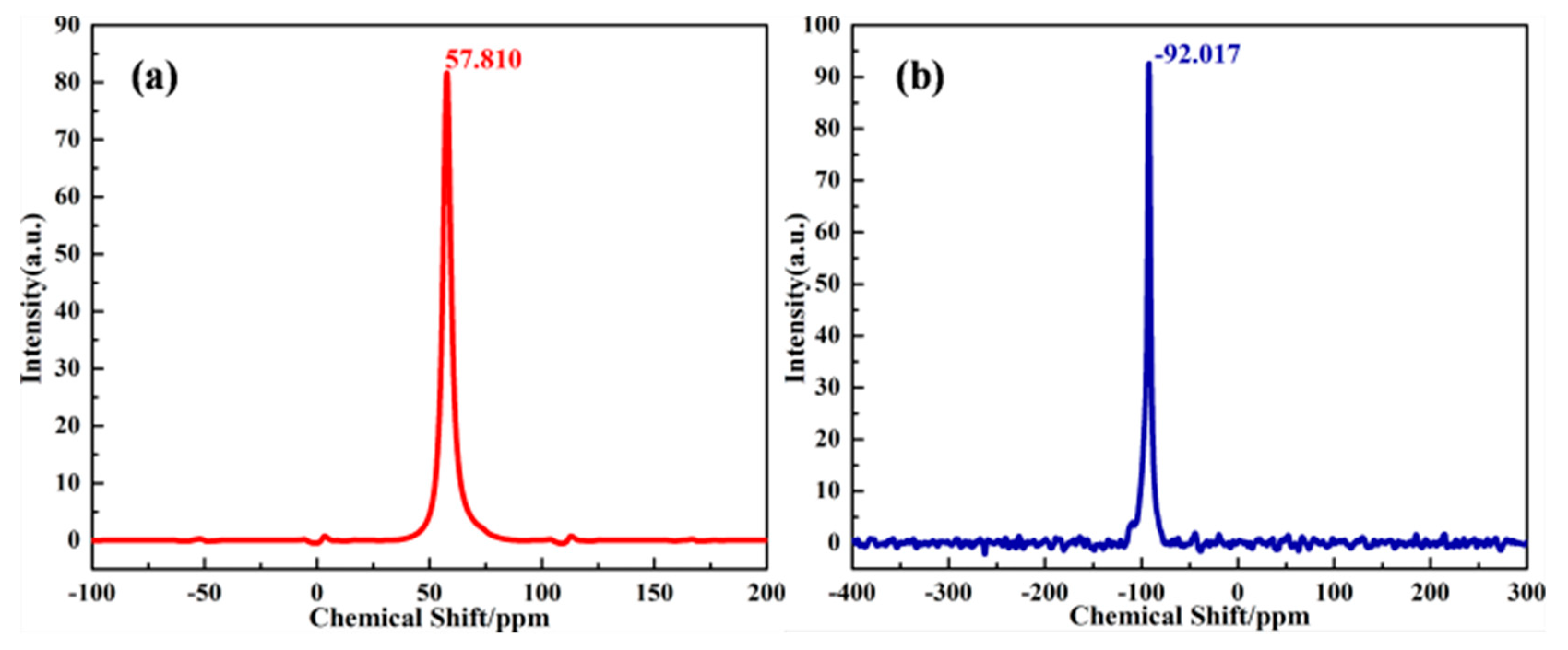
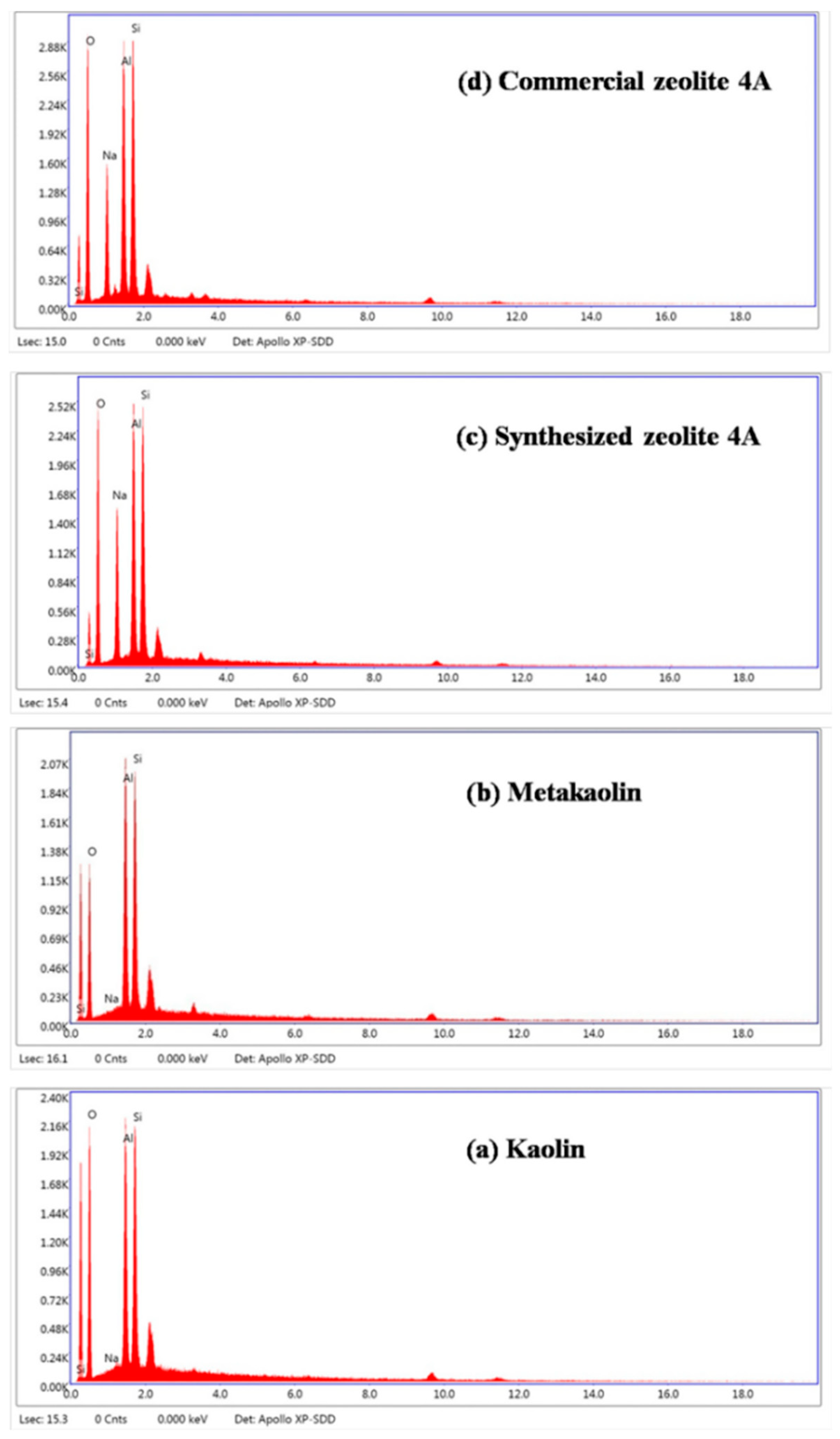
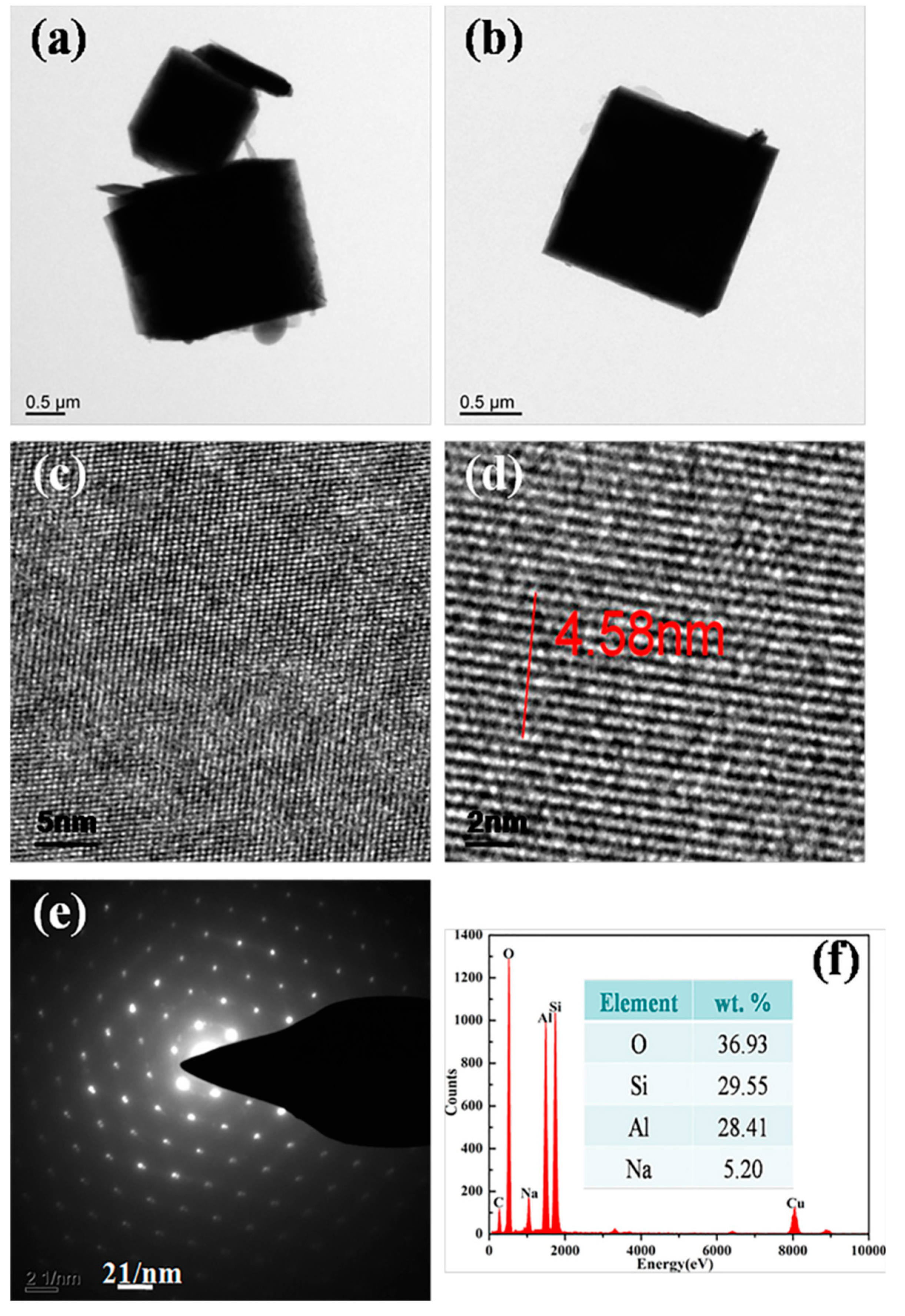
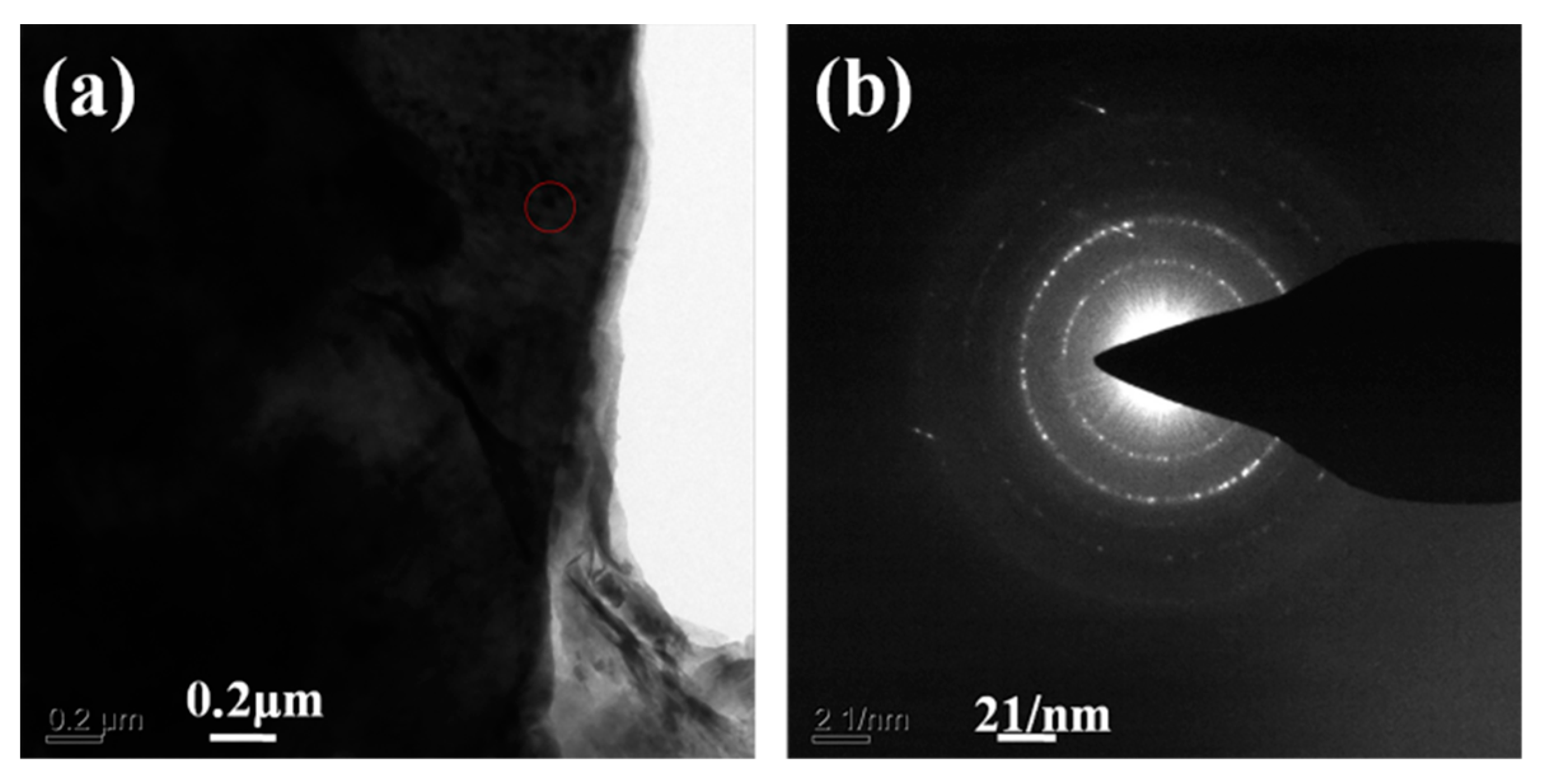
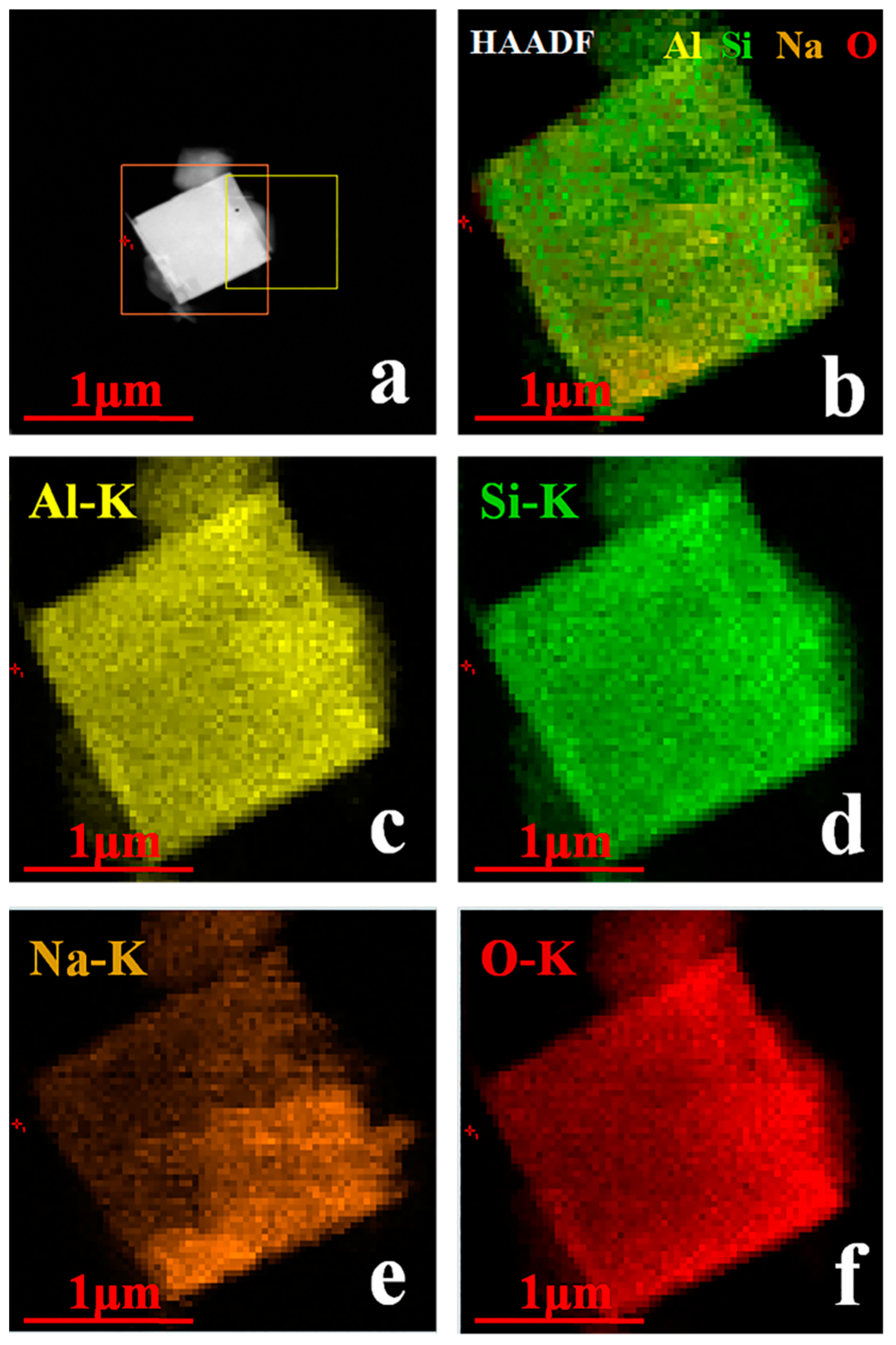

| Formula (wt %) | Raw Kaolin | Metakaolin | Synthesized Zeolite 4A | Commercial Zeolite 4A |
|---|---|---|---|---|
| SiO2 | 55.466 | 56.268 | 46.730 | 46.220 |
| Al2O3 | 39.240 | 38.735 | 33.135 | 29.720 |
| Fe2O3 | 1.415 | 1.339 | 1.0849 | 2.090 |
| Na2O | 0.054 | 0.069 | 16.350 | 15.740 |
| SiO2/Al2O3 | 2.4 | 2.5 | 2.4 | 2.6 |
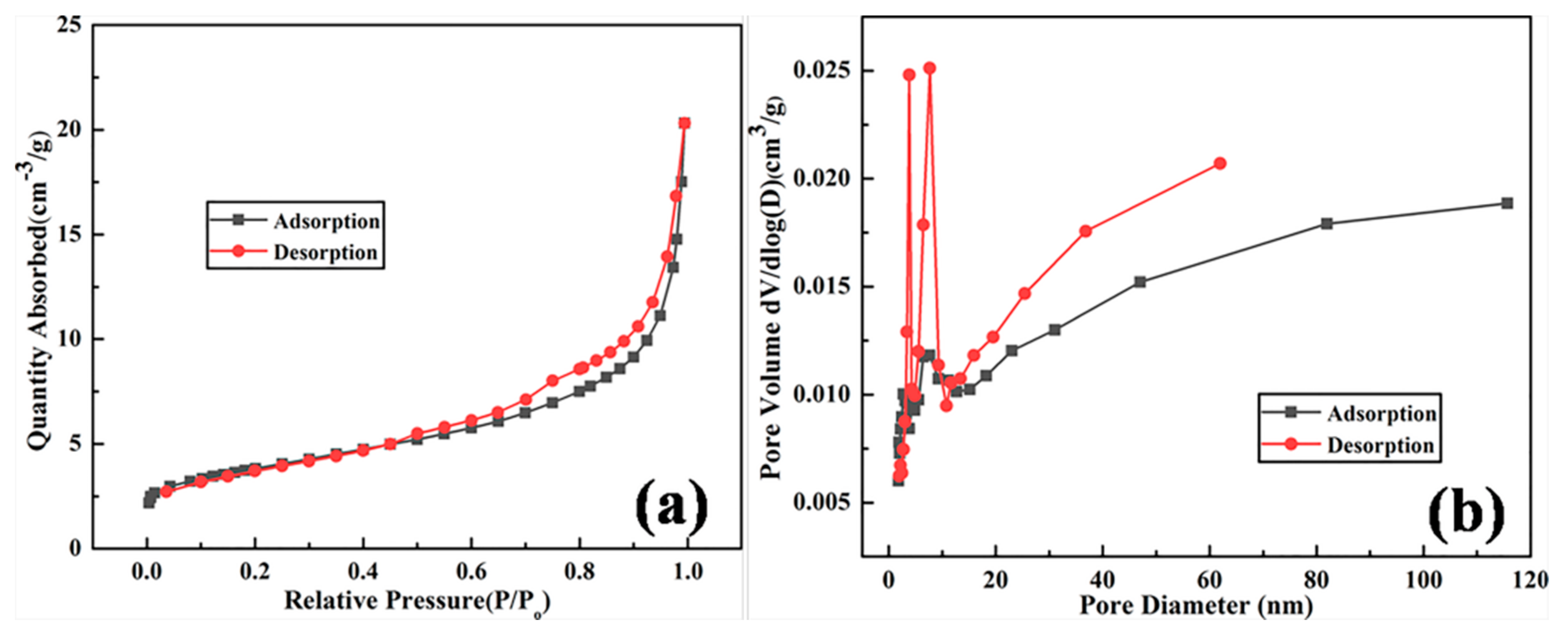
| Samples | BET Surface Area (m2/g) | Micropore Surface Area (m2/g) | Micropore Volume (cm3/g) |
|---|---|---|---|
| Synthesized zeolite 4A | 13.3723 | 2.4624 | 0.001141 |
| Commercial zeolite 4A | 7.3738 | 0.6201 | 0.000227 |
| Adsorbents | Correlation Coefficient (R2) | |
|---|---|---|
| Langmuir Isotherm Model | Freundlich Isotherm Model | |
| Synthesized zeolite 4A | 0.99921 | 0.97560 |
| Commercial zeolite 4A | 0.99958 | 0.97034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Sun, Q.; Zhang, Y.; Cao, J. Synthesis of Zeolite 4A from Kaolin and Its Adsorption Equilibrium of Carbon Dioxide. Materials 2019, 12, 1536. https://doi.org/10.3390/ma12091536
Wang P, Sun Q, Zhang Y, Cao J. Synthesis of Zeolite 4A from Kaolin and Its Adsorption Equilibrium of Carbon Dioxide. Materials. 2019; 12(9):1536. https://doi.org/10.3390/ma12091536
Chicago/Turabian StyleWang, Peng, Qi Sun, Yujiao Zhang, and Jun Cao. 2019. "Synthesis of Zeolite 4A from Kaolin and Its Adsorption Equilibrium of Carbon Dioxide" Materials 12, no. 9: 1536. https://doi.org/10.3390/ma12091536
APA StyleWang, P., Sun, Q., Zhang, Y., & Cao, J. (2019). Synthesis of Zeolite 4A from Kaolin and Its Adsorption Equilibrium of Carbon Dioxide. Materials, 12(9), 1536. https://doi.org/10.3390/ma12091536





