The Microstructure, Electric, Optical and Photovoltaic Properties of BiFeO3 Thin Films Prepared by Low Temperature Sol–Gel Method
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- López, N.; Reichertz, L.A.; Yu, K.M.; Campman, K.; Walukiewicz, W. Engineering the Electronic Band Structure for Multiband Solar Cells. Phys. Rev. Lett. 2011, 106, 028701. [Google Scholar] [CrossRef] [PubMed]
- Arnulf, J.W. Thin Film Photovoltaics: Markets and Industry. Int. J. Photoenergy 2012, 2012, 768368. [Google Scholar]
- Nie, C.; Zhao, S.; Bai, Y.; Lu, Q. The ferroelectric photovoltaic effect of BiCrO3/BiFeO3 bilayer composite films. Ceram. Int. 2016, 42, 14036–14040. [Google Scholar] [CrossRef]
- Fridkin, V.M. Bulk Photovoltaic Effect in Noncentrosymmetric Crystals. Crystallogr. Rep. 2001, 46, 654–658. [Google Scholar] [CrossRef]
- Katiyar, R.K.; Sharma, Y.; Misra, P.; Puli, V.S.; Sahoo, S.; Kumar, A.; Scott, J.F.; Morell, G.; Weiner, B.R.; Katiyar, R.S. Studies of the switchable photovoltaic effect in co-substituted BiFeO3 thin films. Appl. Phys. Lett. 2014, 105, 172904. [Google Scholar] [CrossRef]
- Tan, Z.; Tian, J.; Fan, Z.; Lu, Z.; Zhang, L.; Zheng, D.; Wang, Y.; Chen, D.; Qin, M.; Zeng, M.; et al. Polarization imprint effects on the photovoltaic effect in Pb(Zr,Ti)O3 thin films. Appl. Phys. Lett. 2018, 112, 152905. [Google Scholar] [CrossRef]
- Su, R.; Zhang, D.; Wu, M. Plasmonic-enhanced ferroelectric photovoltaic effect in 0–3 type BaTiO3-Au ceramics. J. Alloys Compd. 2019, 785, 584–589. [Google Scholar] [CrossRef]
- Ma, N.; Yang, Y. Boosted photocurrent in ferroelectric BaTiO3 materials via two dimensional planar-structured contact configurations. Nano Energy 2018, 50, 417–424. [Google Scholar] [CrossRef]
- Machado, P.; Scigaj, M.; Gazquez, J.; Rueda, E.; Sanchez Díaz, A.; Fina, I.; Giber Roca, M.; Puig, T.; Obradors, X.; Campoy Quiles, M.; et al. Band gap tuning of solution processed ferroelectric perovskite BiFe1−xCoxO3 thin Films. Chem. Mater. 2019, 31, 947–954. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Pei, W.; Lu, Y.; Zhang, Q.; Zhang, Q.; He, Y. Bismuth ferritematerials for solar cells: Current status and prospects. Mater. Res. Bull. 2019, 110, 39–49. [Google Scholar] [CrossRef]
- Teh, Y.S.; Bhattacharya, K. Photovoltaic effect in multi-domain ferroelectric perovskite oxides. J. Appl. Phys. 2019, 125, 064103. [Google Scholar] [CrossRef]
- Bandara, T.M.; Knee, C.S.; Furlani, M.; Albinsson, I.; Mellander, B.E. Novel photo-voltaic device based on Bi1−xLaxFeO3 perovskite films with higher efficiency. J. Mater. Sci. Mater. Electron. 2019, 30, 1654–1662. [Google Scholar] [CrossRef]
- Choi, T.; Lee, S.; Choi, Y.J.; Kiryukhin, V.; Cheong, S.W. Switchable ferroelectric diode and photovoltaic effect in BiFeO3. Science 2009, 324, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Fu, C.; Cai, W.; Chen, G.; Deng, X.; Zhang, H.; Sun, J.; Shen, B. Electric Control of the Hall effect in Pt/Bi0.9La0.1FeO3 bilayers. Sci. Rep. UK 2016, 6, 20330. [Google Scholar] [CrossRef]
- Katiyar, R.K.; Sharma, Y.; Barrionuevo, D.; Kooriyattil, S.; Pavunny, S.P.; Young, J.S.; Morell, G.; Weiner, B.R.; Katiyar, R.S.; Scott, J.F. Ferroelectric photovoltaic properties in doubly substituted (Bi0.9La0.1)(Fe0.97Ta0.03)O3 thin films. Appl. Phys. Lett. 2015, 106, 082903. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Fan, L.; Pan, Z.; Wang, J.; Ibrahim, K.; Tian, J.; Xing, X. Enhanced switchable photovoltaic response and ferromagnetic of Co-doped BiFeO3 based ferroelectric thin films. J. Alloys Compd. 2018, 742, 351–355. [Google Scholar] [CrossRef]
- Li, J.; Sha, N.; Zhao, Z. Effect of annealing atmosphere on the ferroelectric properties of inkjet printed BiFeO3 thin films. Appl. Surf. Sci. 2018, 454, 233–238. [Google Scholar] [CrossRef]
- Lvuca, G.D.; Schoenherr, P.; Mendil, J.; Meier, D.; Fiebig, M.; Trassin, M. Domain-Pattern Transfer across an Artificial Magnetoelectric Interface. Phys. Rev. Appl. 2018, 10, 054030. [Google Scholar] [CrossRef]
- Sharma, S.; Tomar, M.; Gupta, V. Effect of top metal contact on the ferroelectric photovoltaic response of BFO thin film capacitors. Vacuum 2018, 158, 117–120. [Google Scholar] [CrossRef]
- Wang, H.; Khatkhatay, F.; Jian, J.; Huang, J.; Fan, M.; Wang, H. Strain tuning of ferroelectric and optical properties of rhombohedral-like BiFeO3 thin films on SrRuO3-buffered substrates. Mater. Res. Bull. 2019, 110, 120–125. [Google Scholar] [CrossRef]
- Raksa, P.; Pinitsoontorn, S.; Maensiri, S. Structural, Magnetic Properties and Dye-Sensitized Solar Cells Application of Pure and La Doped BiFeO3 Powders Prepared by Sol-Gel. Ferroelectrics 2016, 492, 150–158. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Pugh, D.; Blackman, C.S.; Tang, J.; Carmalt, C.J. Photocatalytic Oxygen Evolution from Cobalt-Modified Nanocrystalline BiFeO3 Films Grown via Low-Pressure Chemical Vapor Deposition from β-Diketonate Precursors. Cryst. Growth Des. 2016, 16, 3818–3825. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, W.; Jiang, W.; Fu, C.; Li, C.; Song, Y. Effects of annealing temperature on the microstructure, optical, ferroelectric and photovoltaic properties of BiFeO3 thin films prepared by sol-gel method. Ceram. Int. 2013, 39, 8729–8736. [Google Scholar] [CrossRef]
- Singh, M.K.; Jang, H.M.; Ryu, S.; Jo, M.H. Polarized Raman scattering of multiferroic BiFeO 3 epitaxial films with rhombohedral R3c symmetry. Appl. Phys. Lett. 2006, 88, 042907. [Google Scholar] [CrossRef]
- Hermet, P.; Goffinet, M.; Kreisel, J.; Ghosez, P. Raman and infrared spectra of multiferroic bismuth ferrite from first principles. Phys. Rev. B 2007, 75, 220102(R). [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.Q.; Wang, H.; Jia, Q.X. Oxygen concentration and its effect on the leakage current in BiFeO3 thin films. Appl. Phys. Lett. 2010, 96, 012909. [Google Scholar] [CrossRef]
- Singh, D.; Tabari, T.; Ebadi, M.; Trochowski, M.; Yagci, M.B. Efficient synthesis of BiFeO3 by the microwave-assisted sol-gel method: “A”site influence on the photoelectrochemical activity of perovskites. Appl. Surf. Sci. 2019, 471, 1017–1027. [Google Scholar] [CrossRef]
- Lu, Y.W.; Qi, X. Hydrothermal synthesis of pure and Sb-doped BiFeO3 with the typical hysteresis loops of ideal ferroelectrics. J. Alloys Compd. 2019, 774, 386–395. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, Y.; Wang, Y.; Liu, Y.; Wei, M.; Zhang, J.; Feng, M.; Yang, J. Effect of Cr doping on the phase structure, surface appearance and magnetic property of BiFeO3 thin films prepared via sol–gel technology. J. Mater. Sci. Mater. Electron. 2017, 28, 17490–17498. [Google Scholar] [CrossRef]
- Scott, J.F. Ferroelectrics go bananas. J. Phys. Condens. Matter 2008, 20, 021001. [Google Scholar] [CrossRef]
- Ameer, S.; Jindal, K.; Sharma, S.; Jha, P.K.; Tomar, M.; Gupta, V. Structural, morphological and optical properties of BiFe0.99Cr0.01O3 thin films. Vacuum 2018, 158, 166–171. [Google Scholar] [CrossRef]

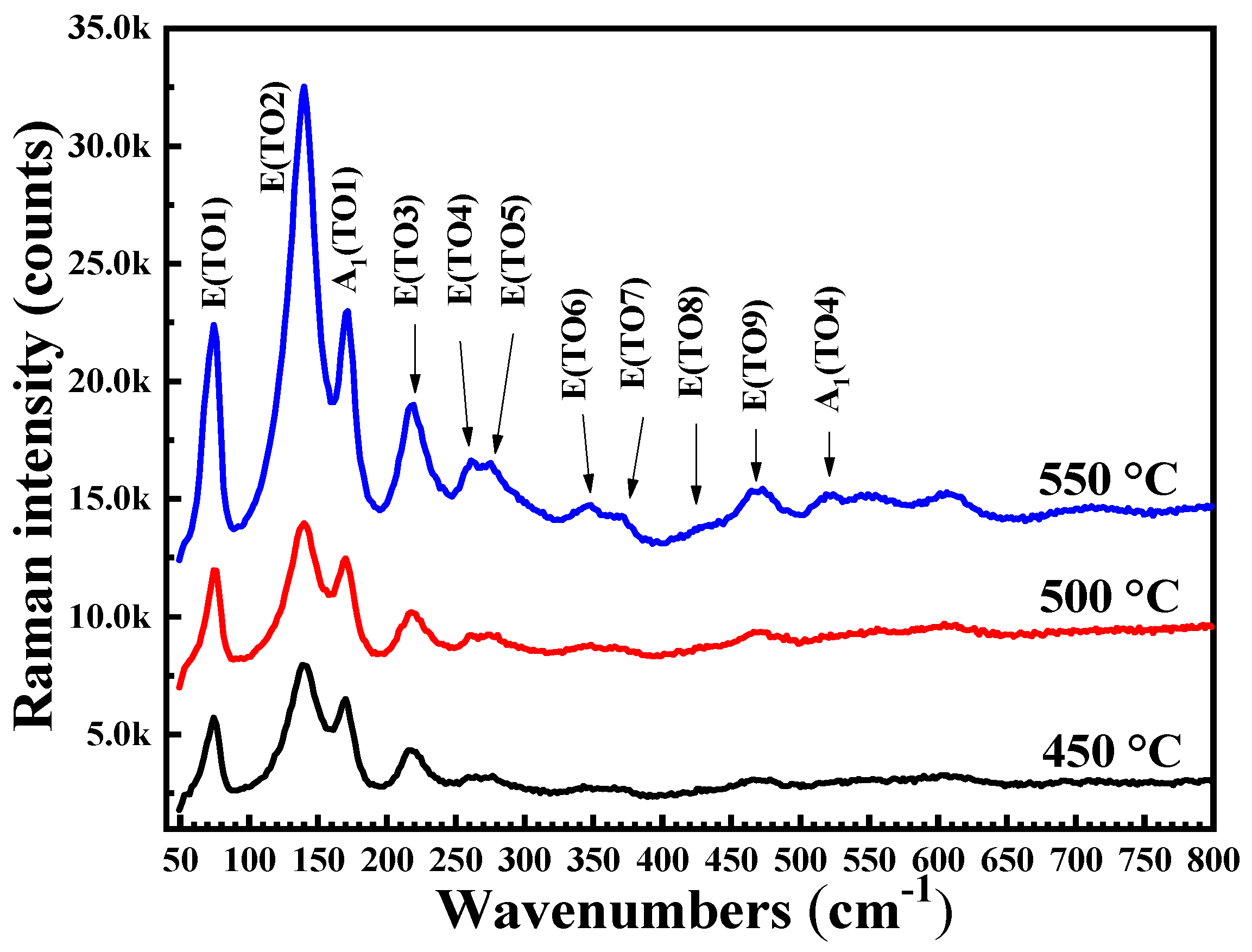
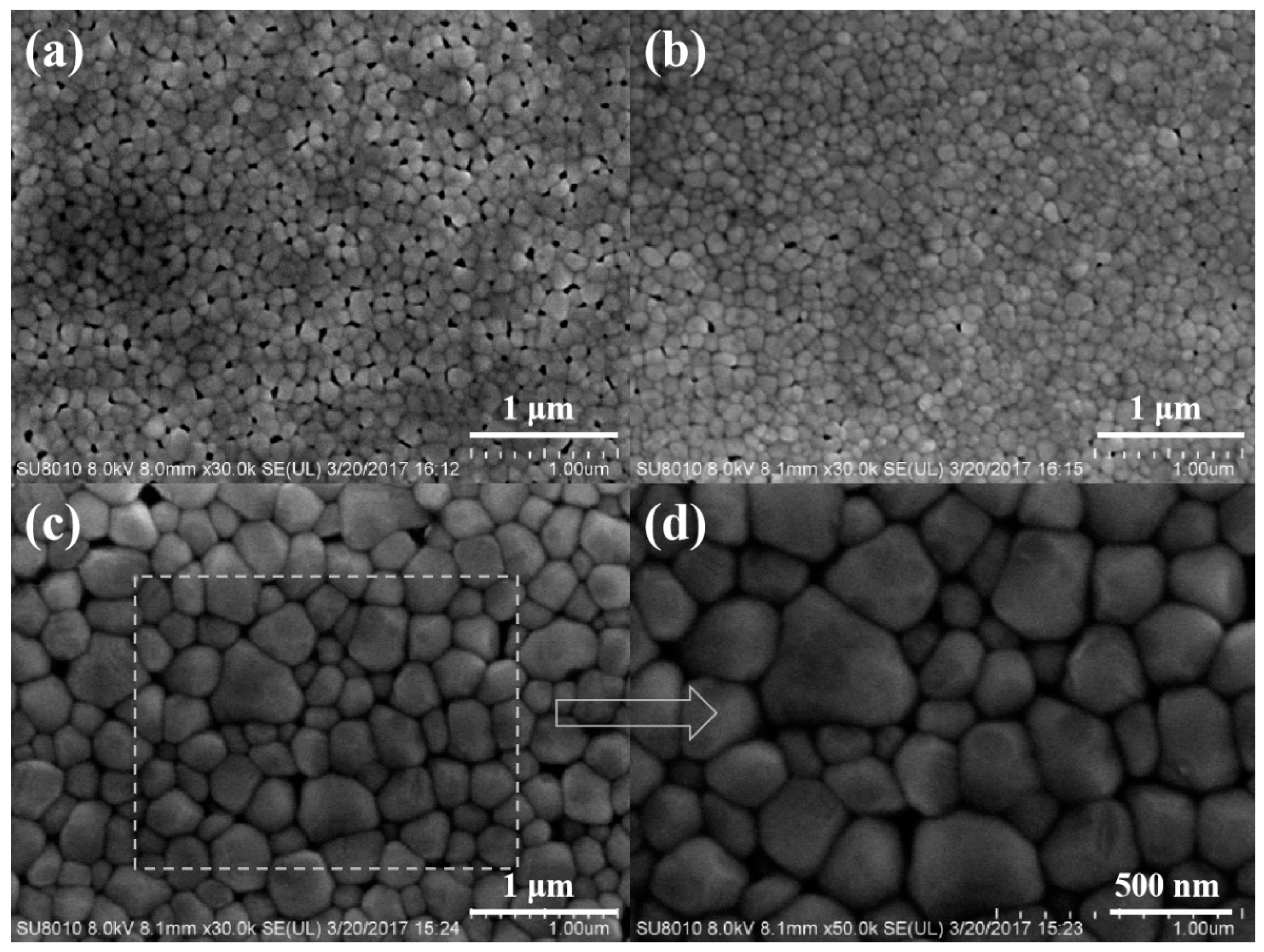
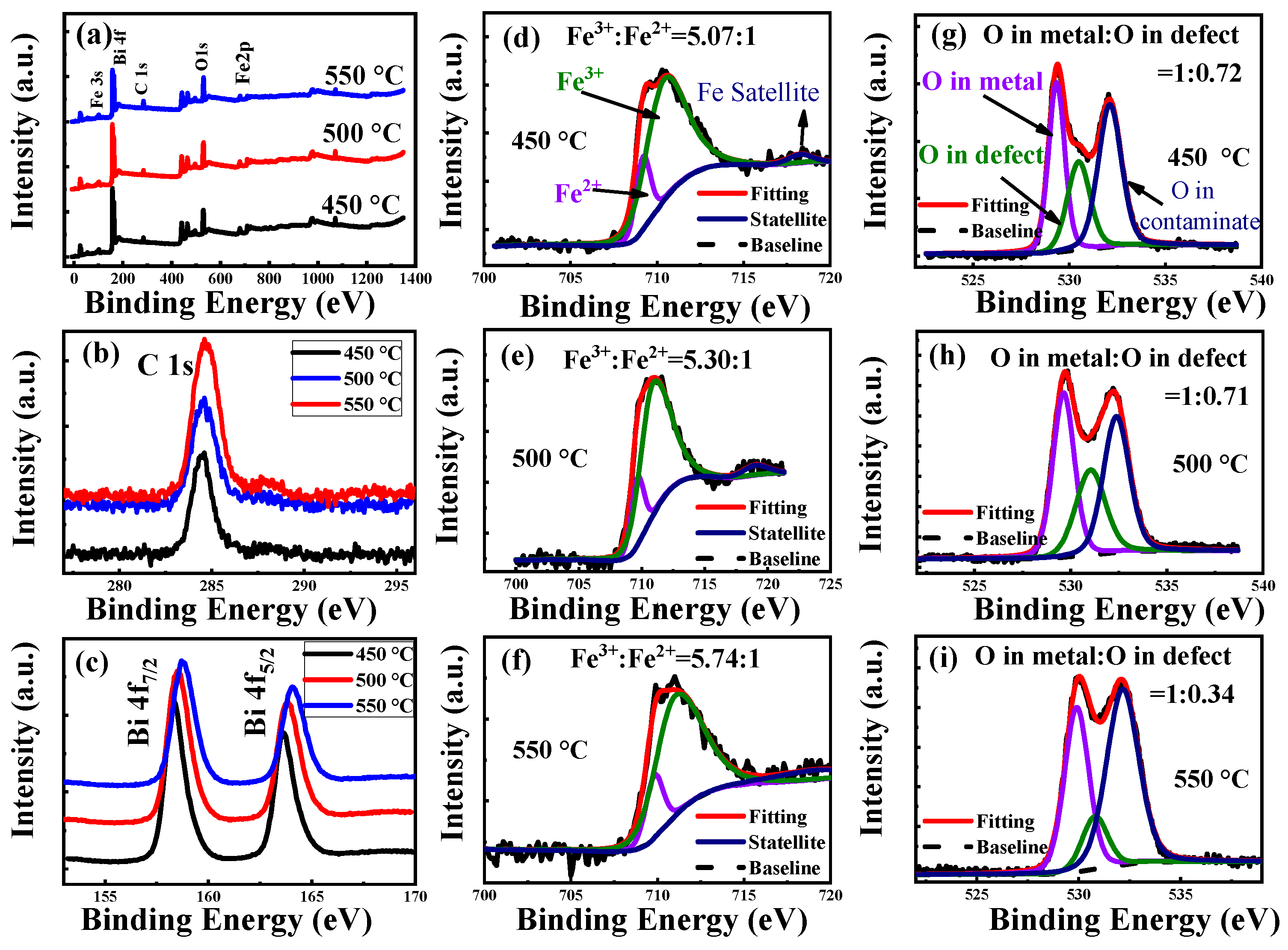
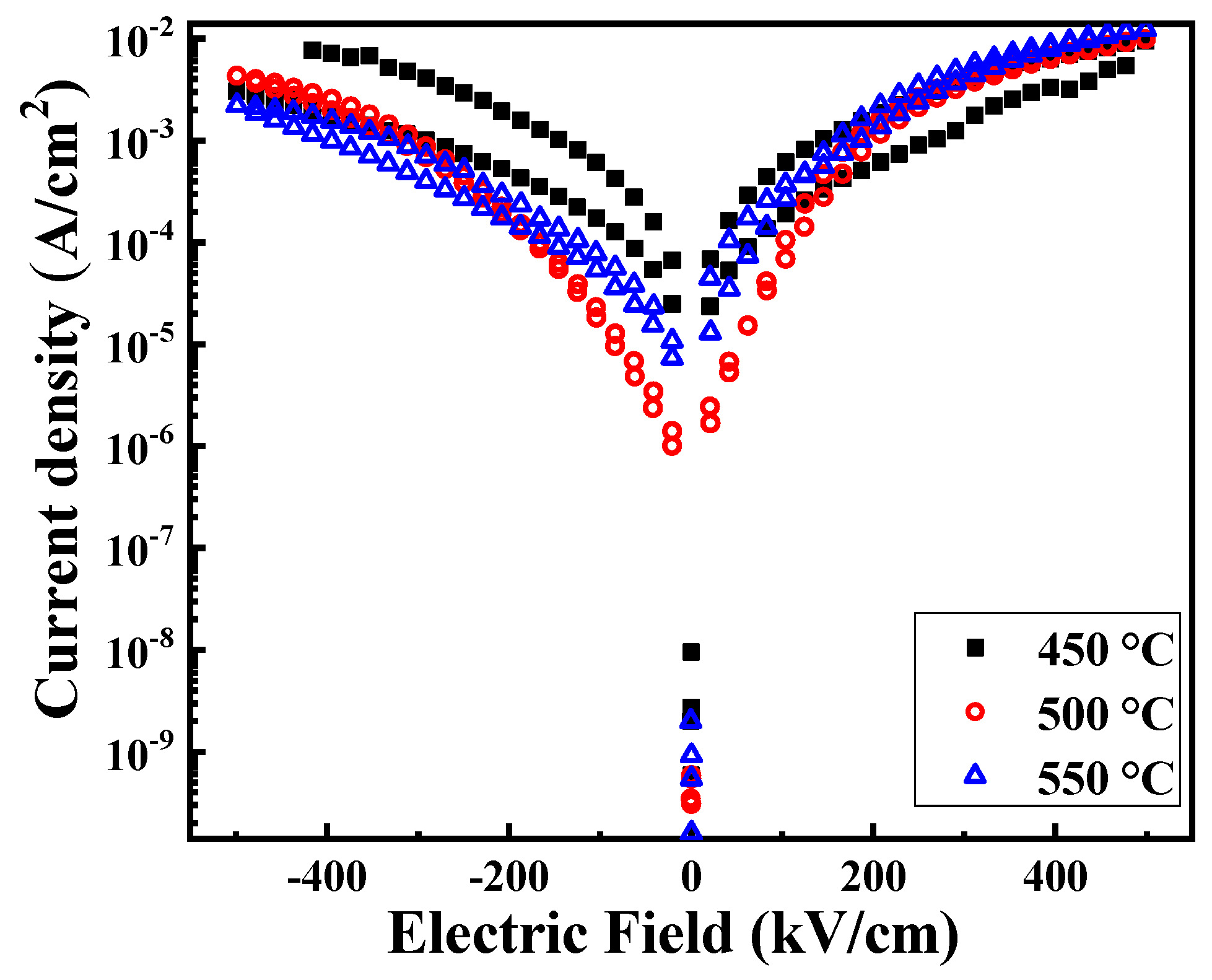
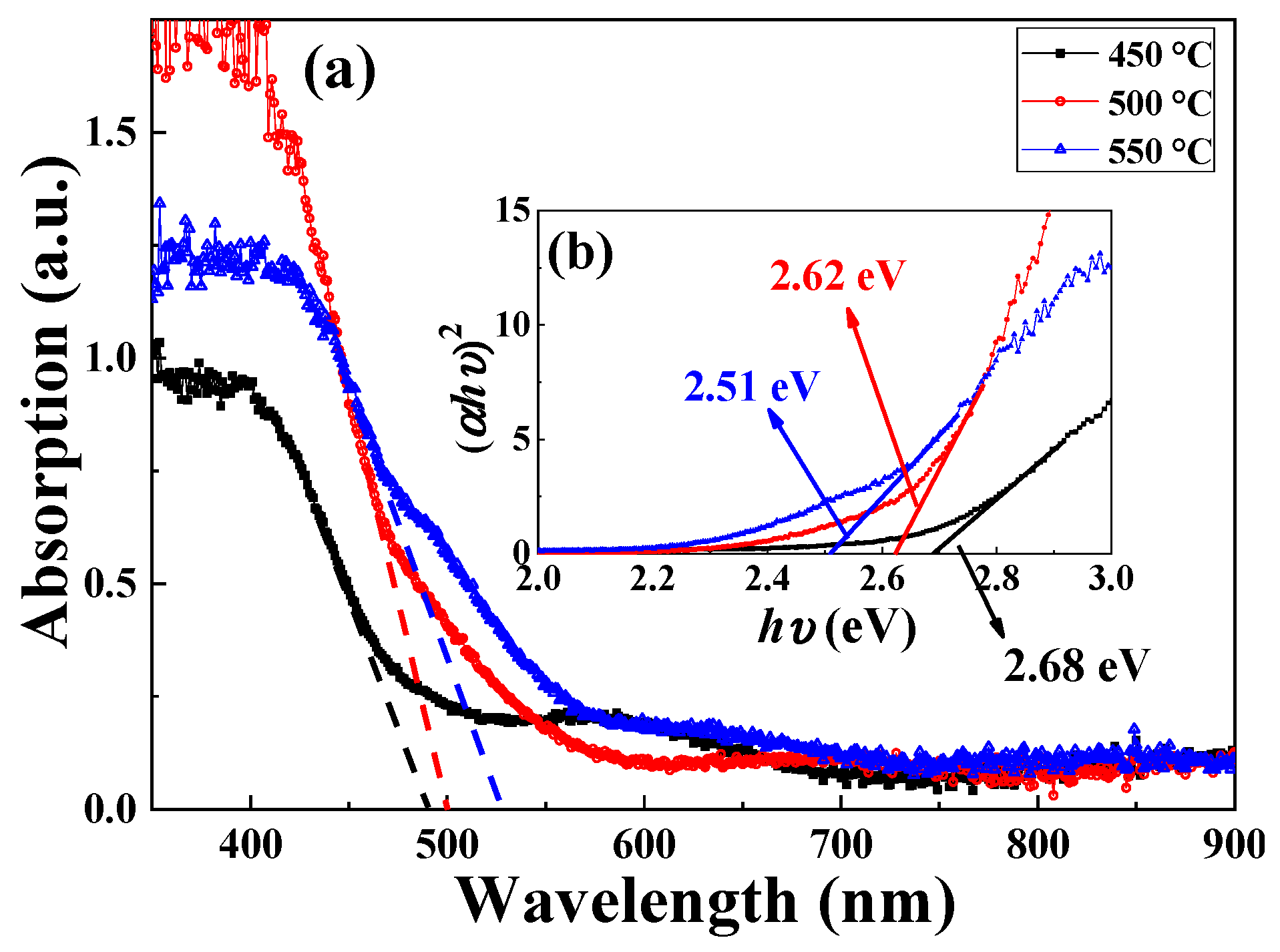
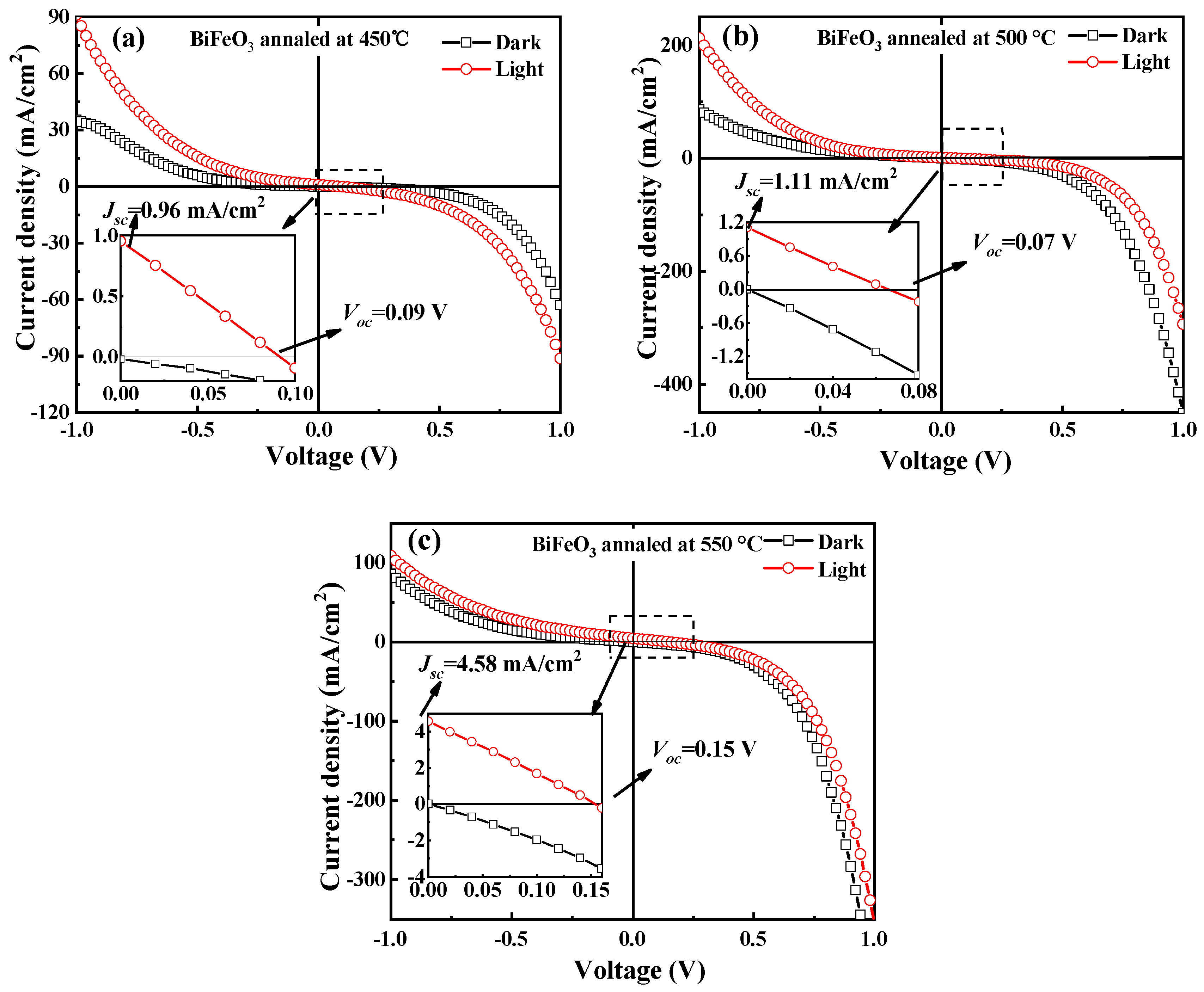
| Annealing Temperature (°C) | 450 | 500 | 550 |
|---|---|---|---|
| Grain size (nm) | 100–200 | 100–200 | 300–700 |
| Fe3+:Fe2+ | 5.07:1 | 5.30:1 | 5.74:1 |
| O in metal:O in defect | 1:0.72 | 1:0.71 | 1:0.34 |
| Eg (eV) | 2.70 | 2.62 | 2.54 |
| Jsc (mA/cm2) | 0.96 | 1.11 | 4.58 |
| Voc (V) | 0.09 | 0.07 | 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Luo, L.; Han, C.; Yun, R.; Tang, X.; Zhu, Y.; Nie, Z.; Zhao, W.; Feng, Z. The Microstructure, Electric, Optical and Photovoltaic Properties of BiFeO3 Thin Films Prepared by Low Temperature Sol–Gel Method. Materials 2019, 12, 1444. https://doi.org/10.3390/ma12091444
Wang J, Luo L, Han C, Yun R, Tang X, Zhu Y, Nie Z, Zhao W, Feng Z. The Microstructure, Electric, Optical and Photovoltaic Properties of BiFeO3 Thin Films Prepared by Low Temperature Sol–Gel Method. Materials. 2019; 12(9):1444. https://doi.org/10.3390/ma12091444
Chicago/Turabian StyleWang, Jiaxi, Li Luo, Chunlong Han, Rui Yun, Xingui Tang, Yanjuan Zhu, Zhaogang Nie, Weiren Zhao, and Zhechuan Feng. 2019. "The Microstructure, Electric, Optical and Photovoltaic Properties of BiFeO3 Thin Films Prepared by Low Temperature Sol–Gel Method" Materials 12, no. 9: 1444. https://doi.org/10.3390/ma12091444
APA StyleWang, J., Luo, L., Han, C., Yun, R., Tang, X., Zhu, Y., Nie, Z., Zhao, W., & Feng, Z. (2019). The Microstructure, Electric, Optical and Photovoltaic Properties of BiFeO3 Thin Films Prepared by Low Temperature Sol–Gel Method. Materials, 12(9), 1444. https://doi.org/10.3390/ma12091444





