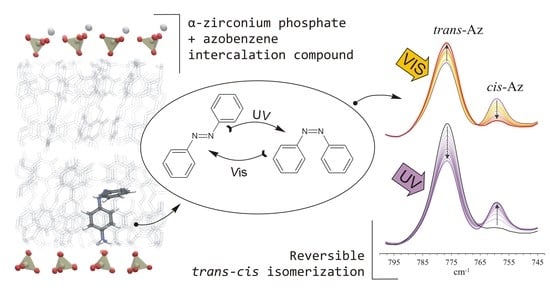
The Nature of Interactions and UV-Induced Response within α-Zirconium Phosphate Intercalation Compounds with Azobenzenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of a Layered α-zirconium Phosphate and the Modification Procedure
2.2. Characterization Methods
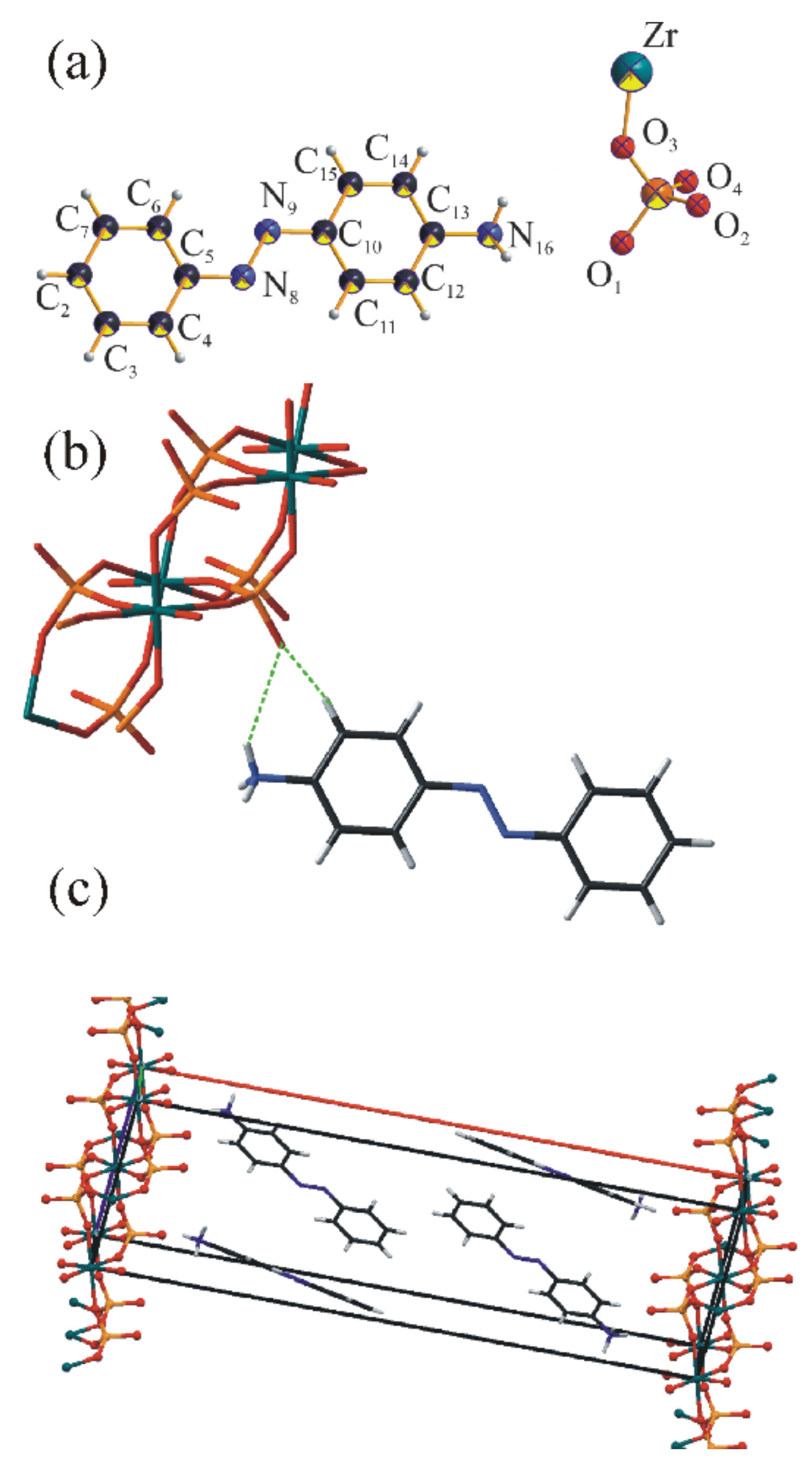
2.3. Structure Refinement of ZpA Sample
2.4. Examination of the Photo-Induced Behavior
2.5. Molecular Simulations
3. Results and Discussion
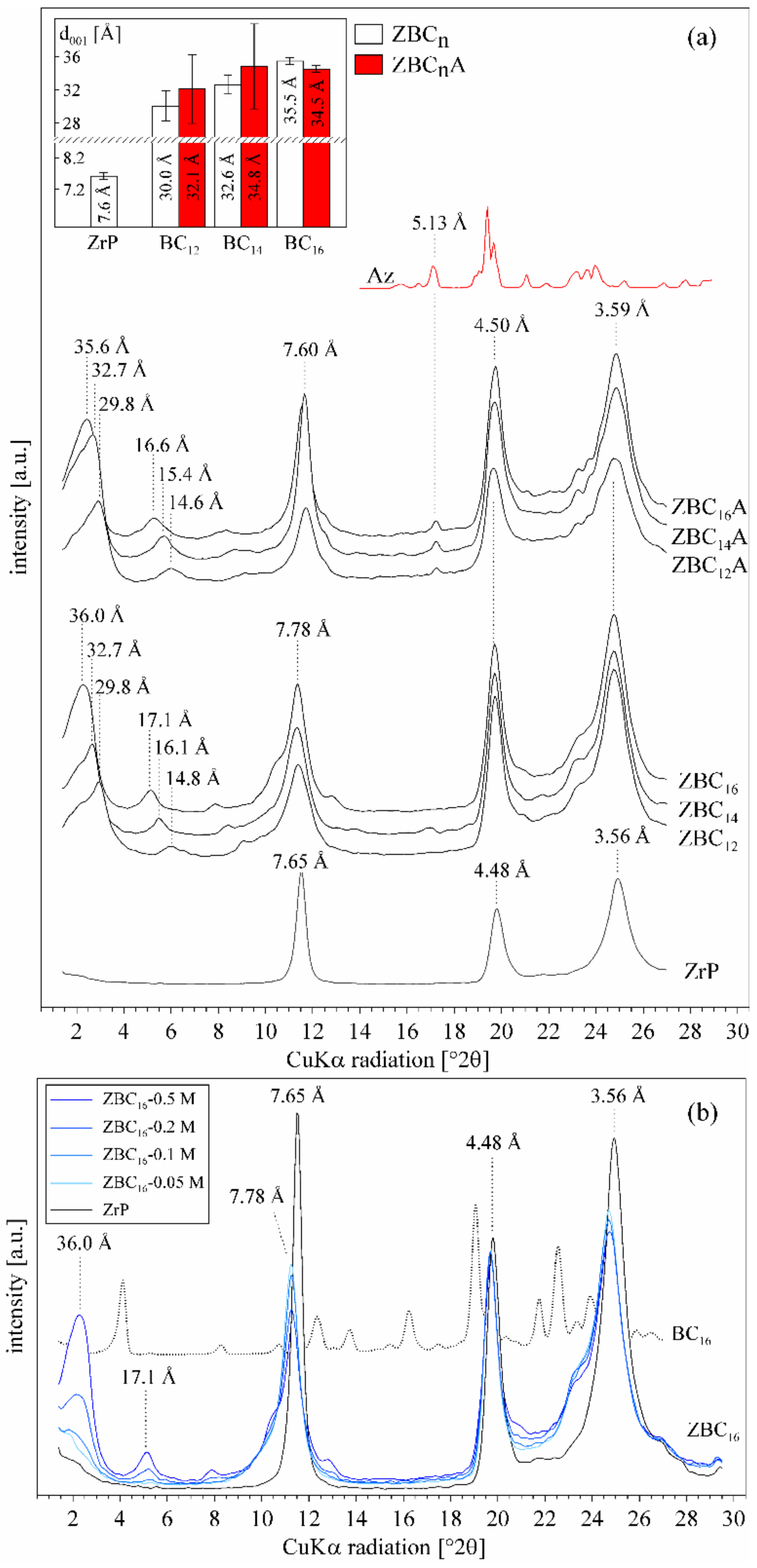
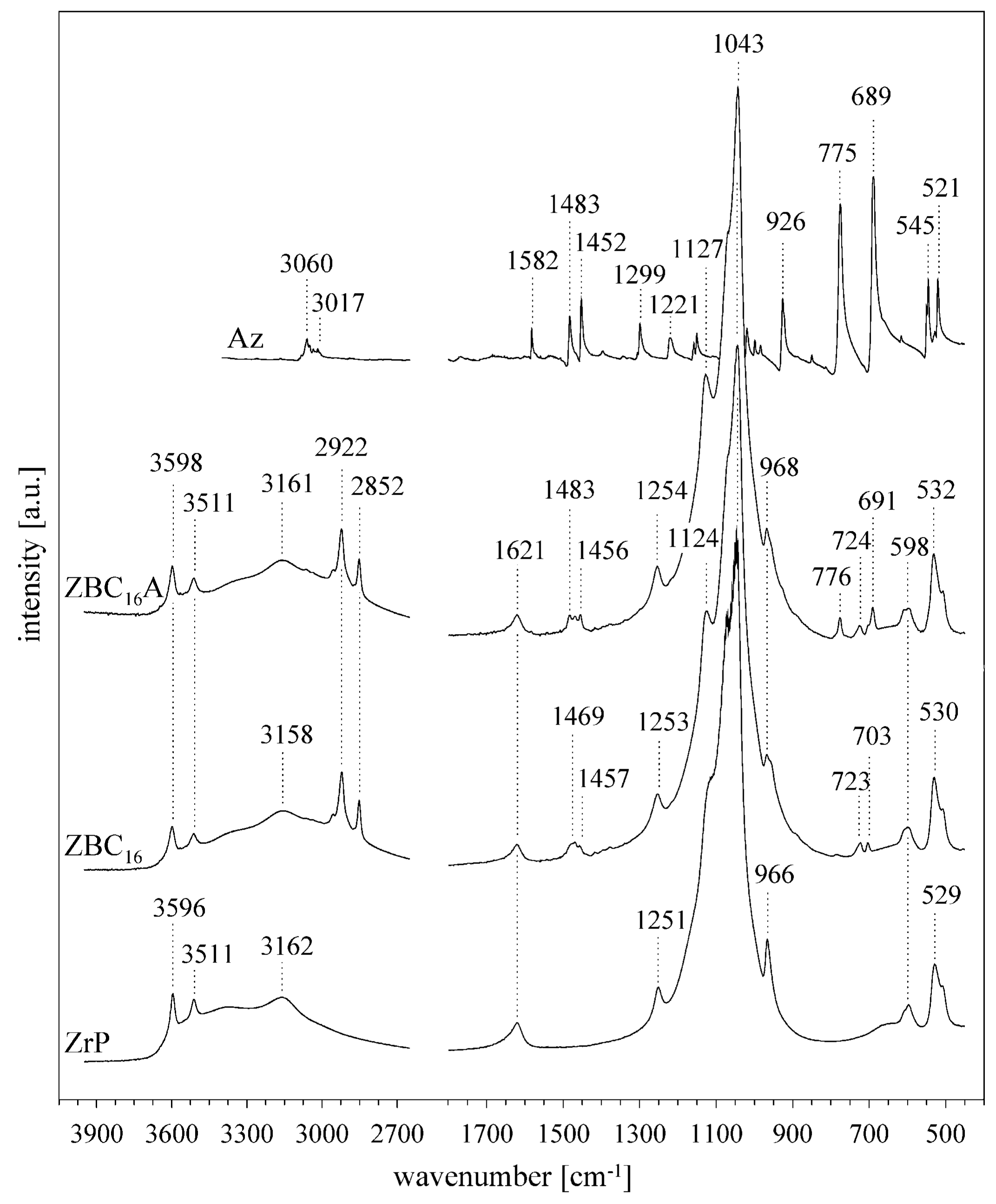
3.1. Characterization of the Synthesized α-ZrP
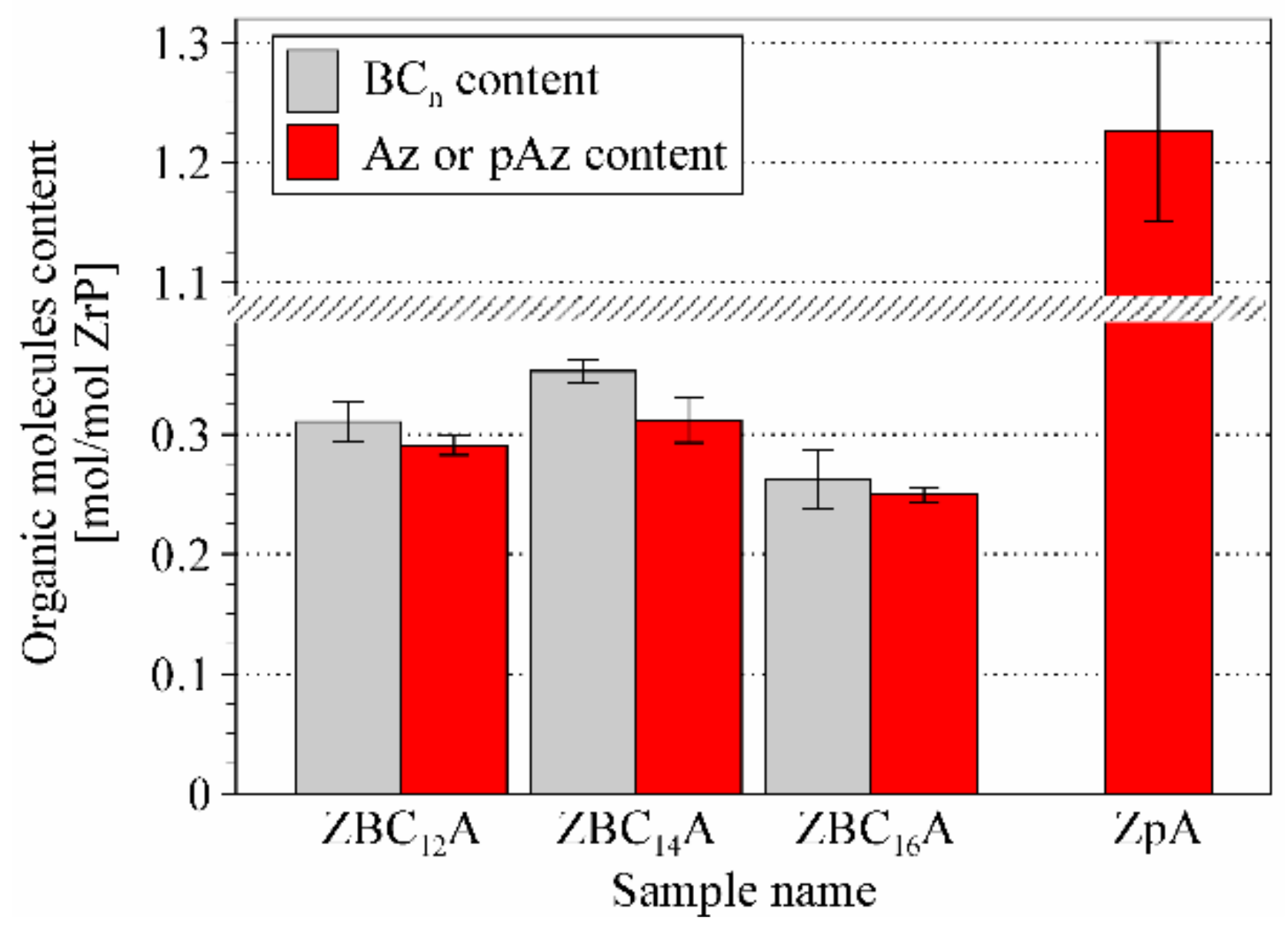
3.2. Effect of the Benzylalkylammonium Surfactants and Azobenzene Intercalation
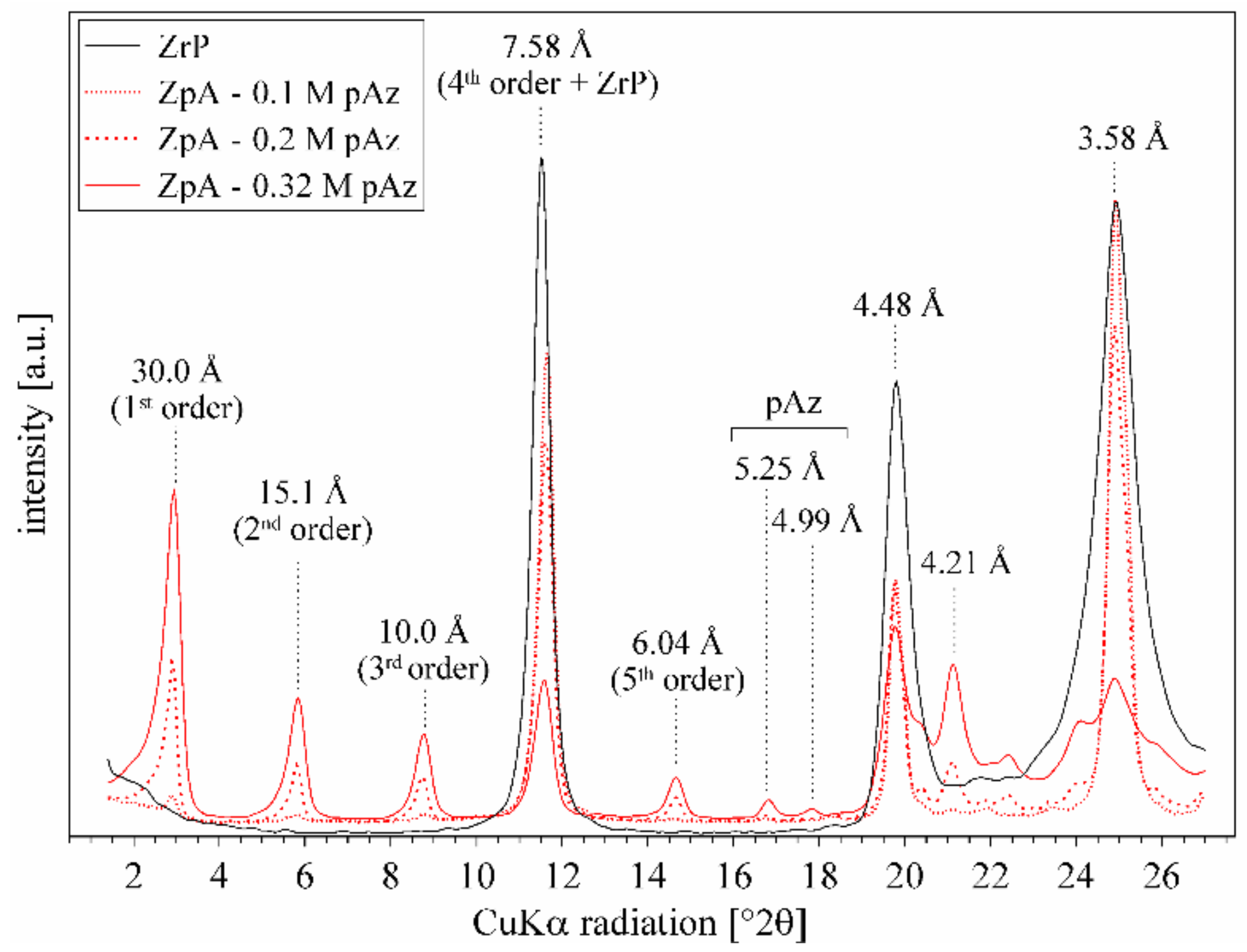
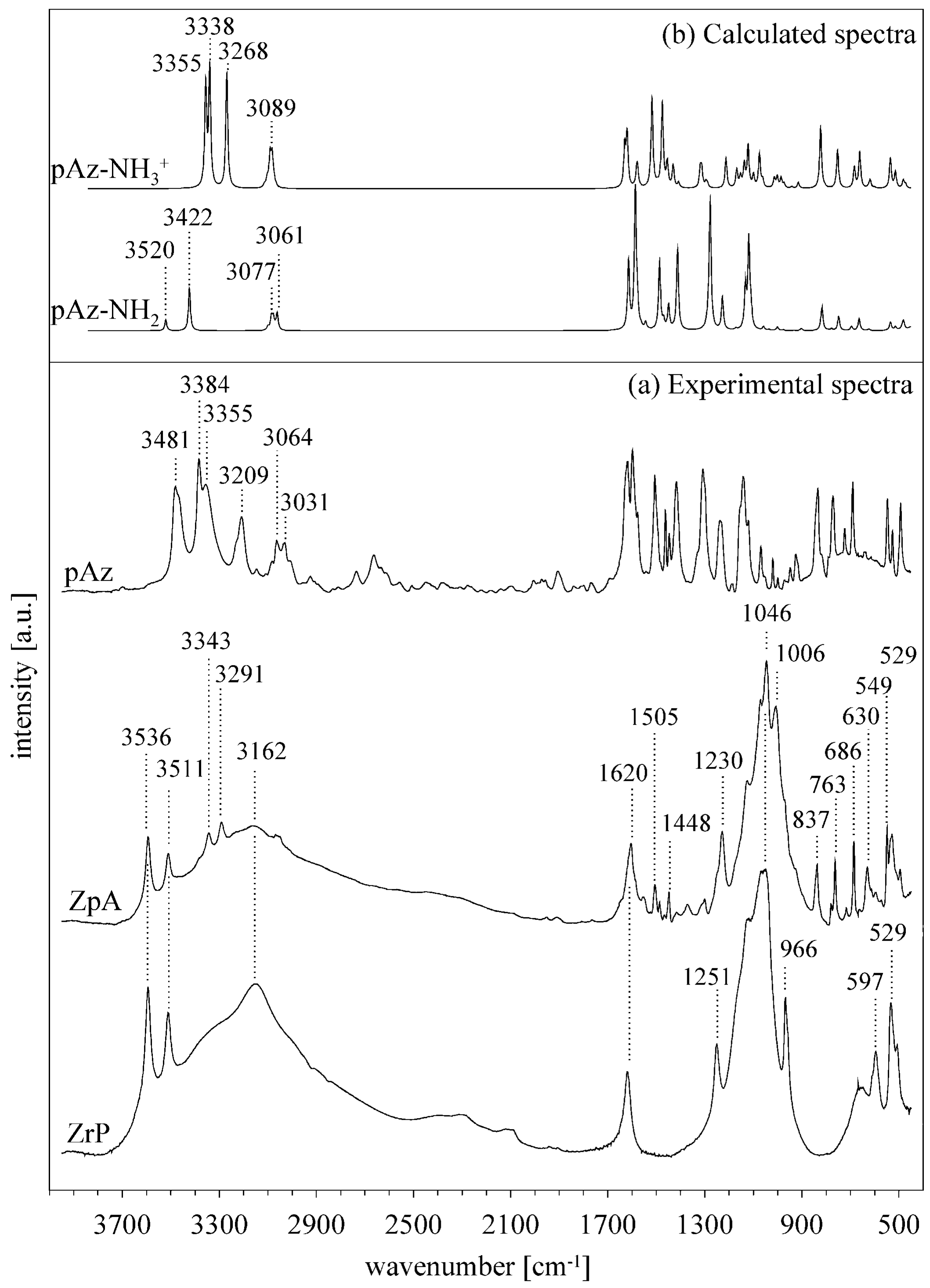
3.3. Effect of p-aminoazobenzene Intercalation
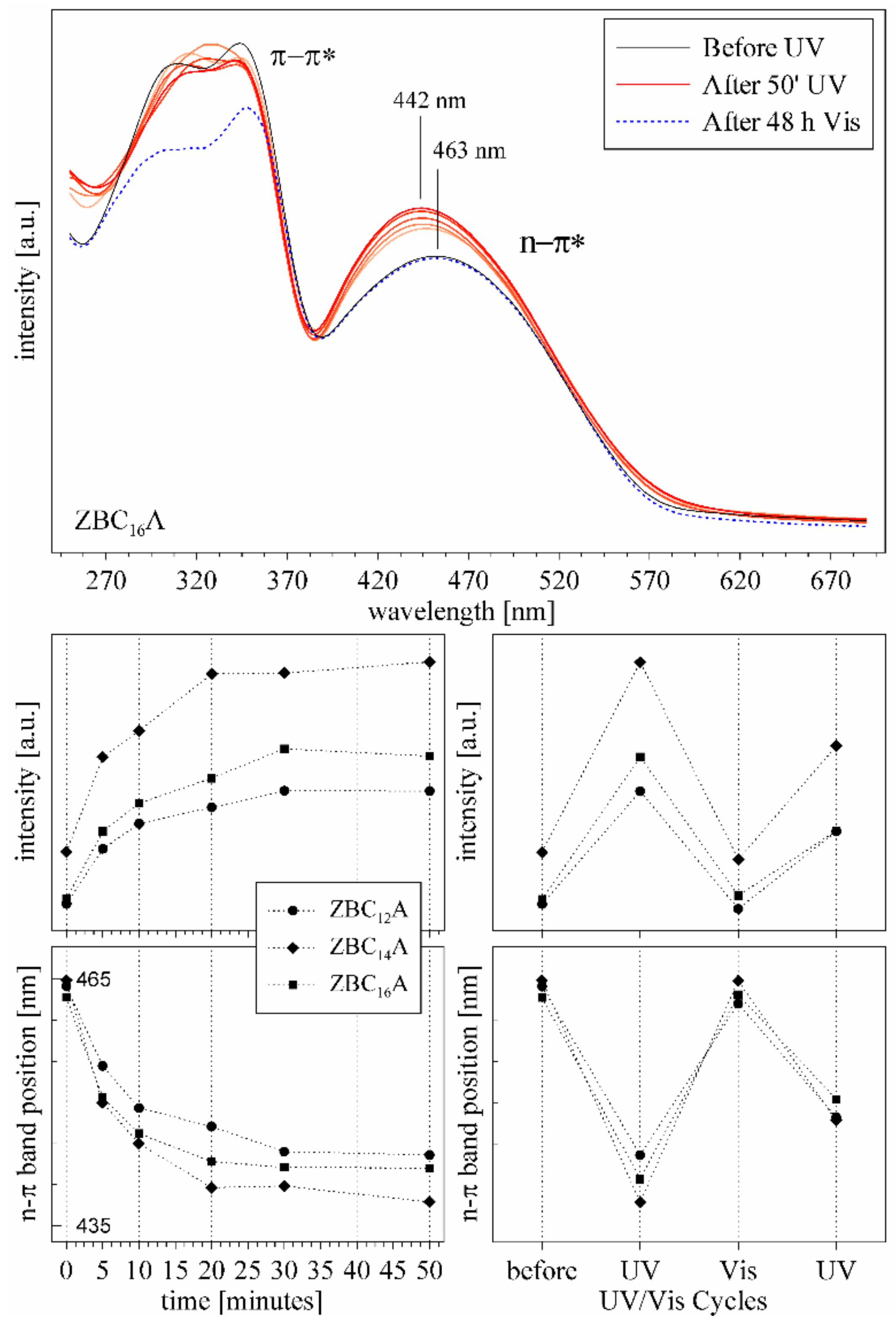
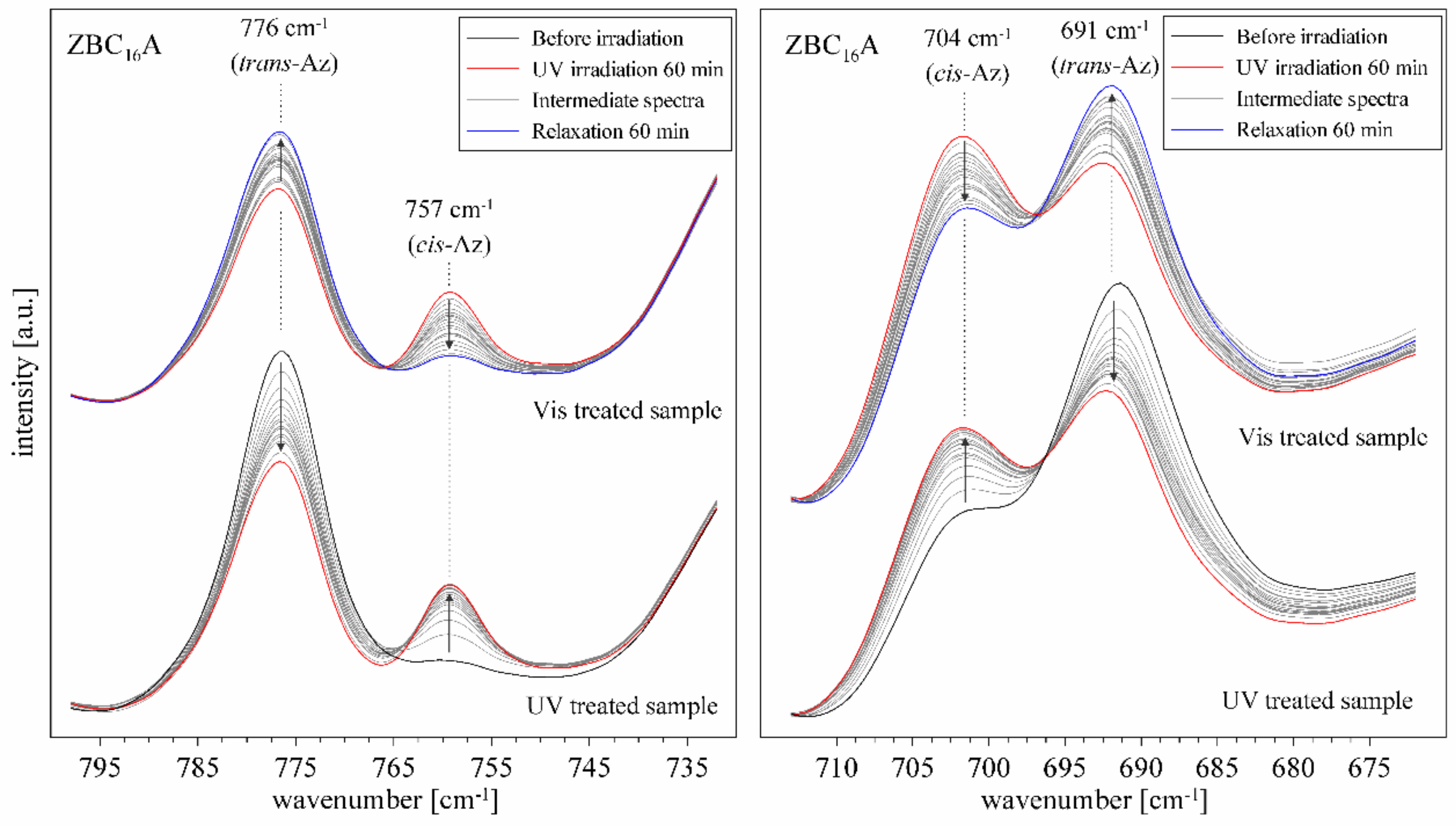
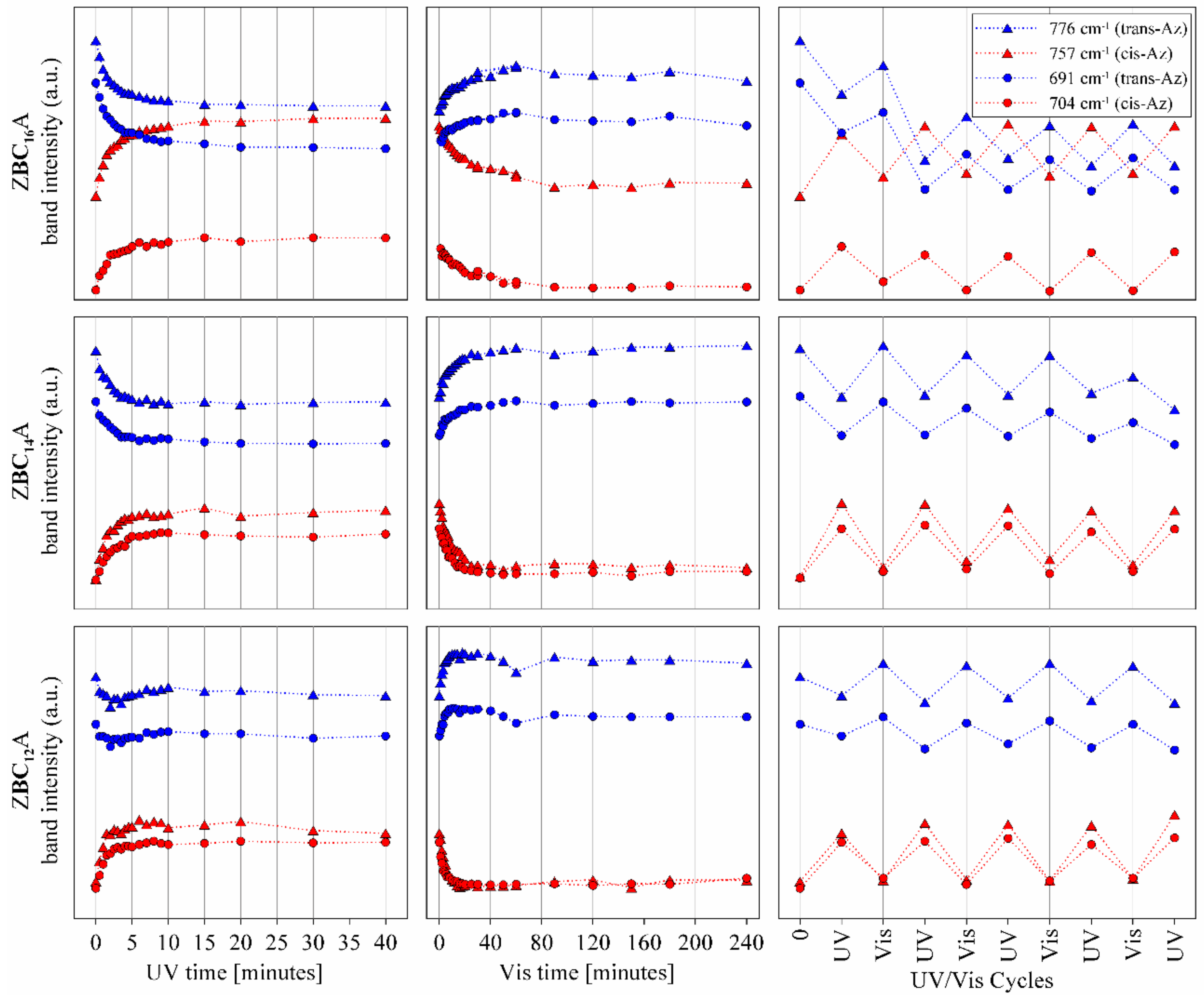
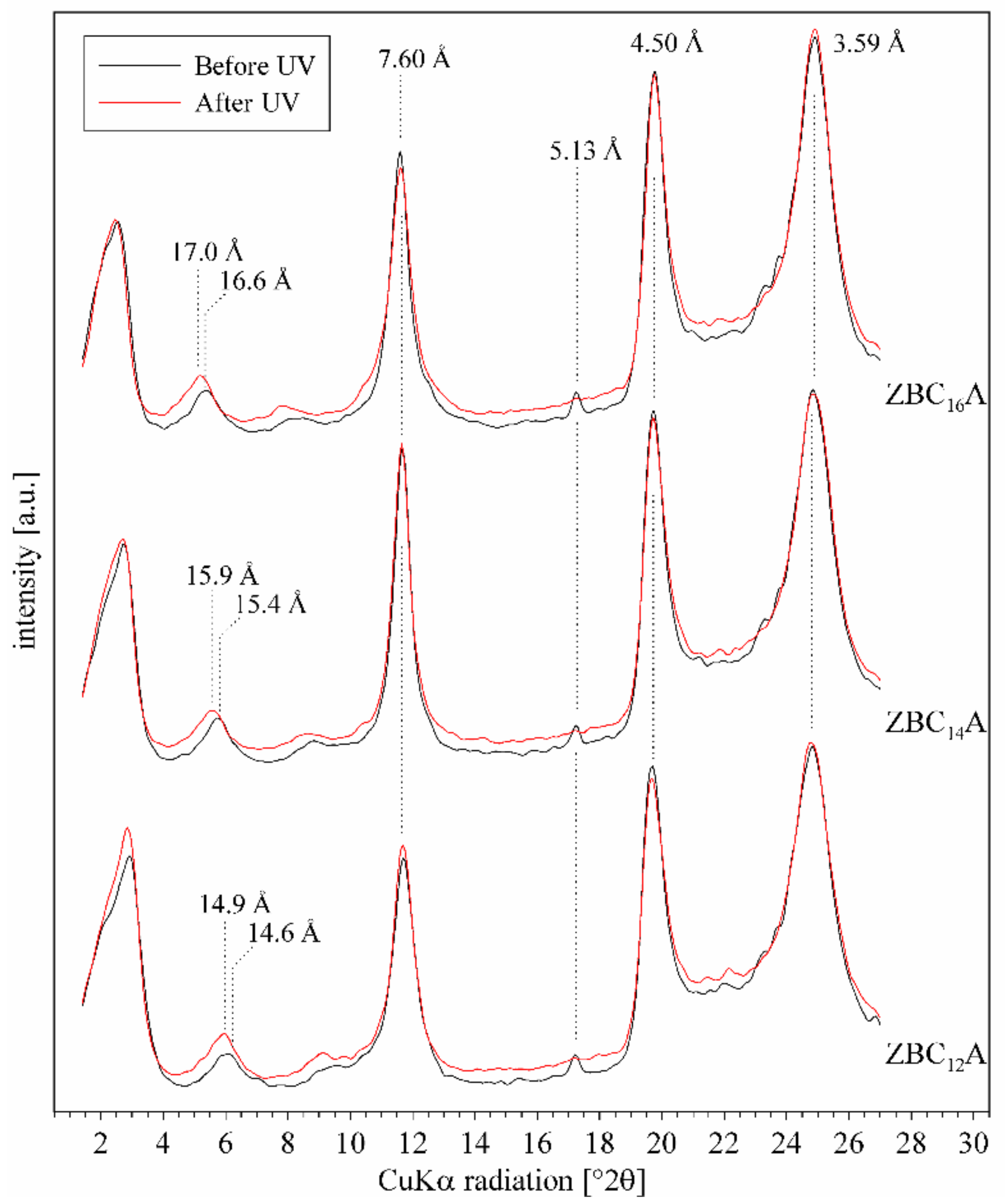
3.4. ZBCnA Samples—Reaction to the UV Irradiation
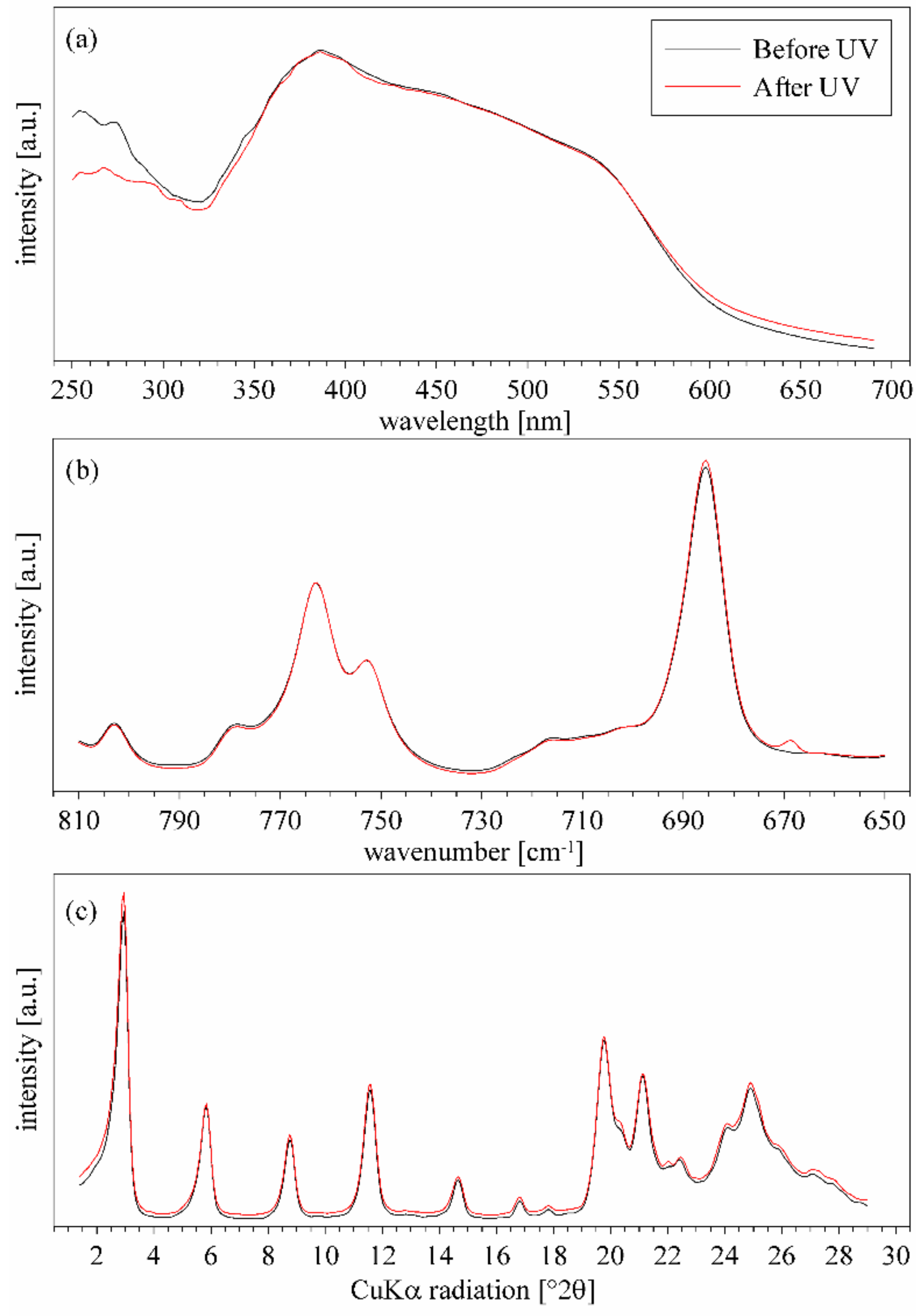
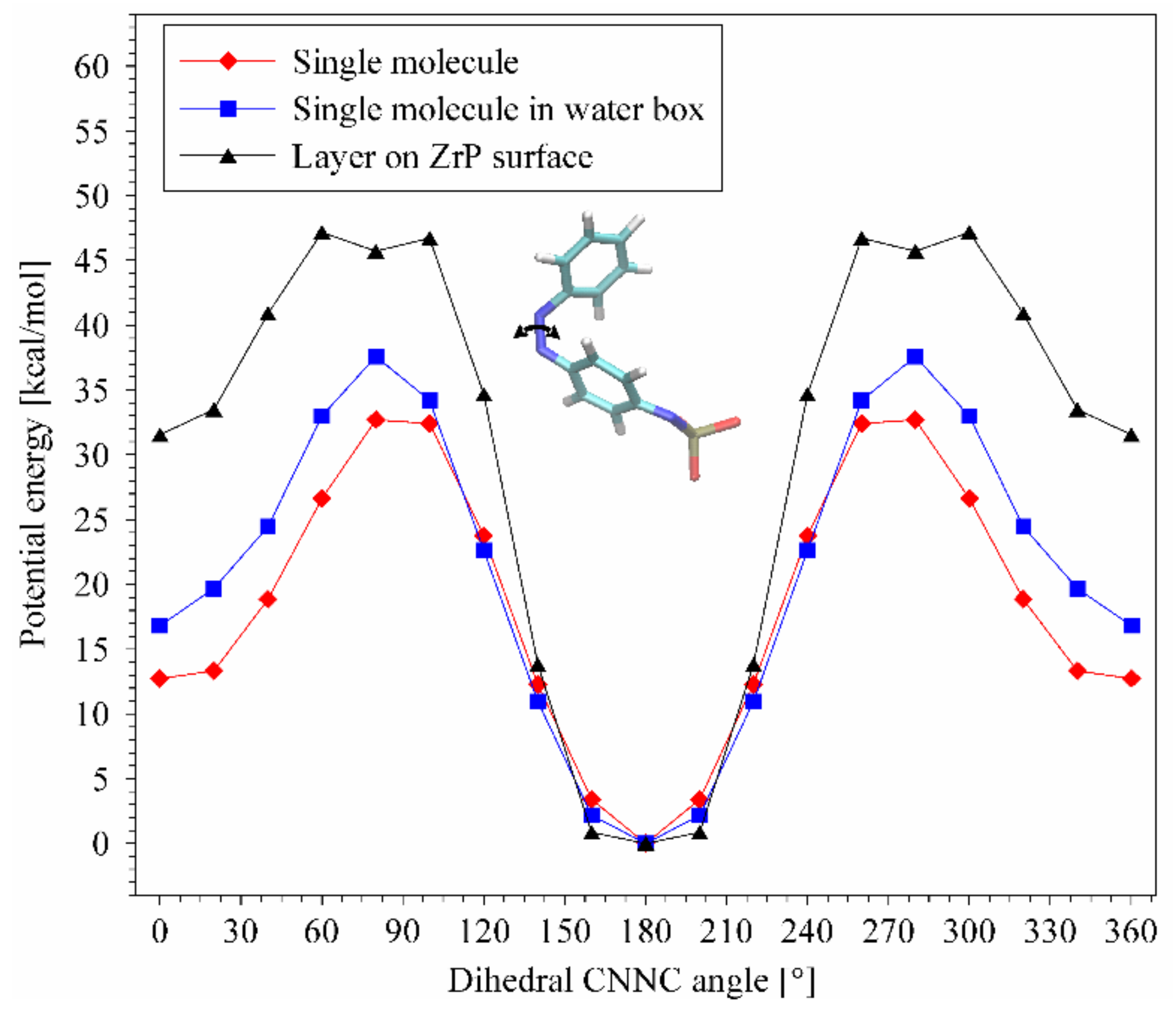
3.5. ZpA sample—Reaction to the UV Light and a Molecular Explanation for Lack of Isomerization Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Zhou, W.; Yang, Q.; Zhu, L.; Zhu, L. Sorption characteristics of nitrosodiphenylamine (NDPhA) and diphenylamine (DPhA) onto organo-bentonite from aqueous solution. Chem. Eng. J. 2014, 240, 487–493. [Google Scholar] [CrossRef]
- Koteja, A.; Matusik, J. Di- and triethanolamine grafted kaolinites of different structural order as adsorbents of heavy metals. J. Colloid Interface Sci. 2015, 455, 83–92. [Google Scholar] [CrossRef]
- Knebel, A.; Sundermann, L.; Mohmeyer, A.; Strauß, I.; Friebe, S.; Behrens, P.; Caro, J. Azobenzene guest molecules as light-switchable co2 valves in an ultrathin uio-67 membrane. Chem. Mater. 2017, 29, 3111–3117. [Google Scholar] [CrossRef]
- Candu, N.; Paul, D.; Marcu, I.-C.; Tudorache, M.; Parvulescu, V.I.; Coman, S.M. New organic-inorganic LDH composites: Synthesis, characterization and catalytic behavior in the green epoxidation of α, β-unsaturated esters. Inorg. Chim. Acta 2018, 475, 127–132. [Google Scholar] [CrossRef]
- Matusik, J.; Stodolak, E.; Bahranowski, K. Synthesis of polylactide/clay composites using structurally different kaolinites and kaolinite nanotubes. Appl. Clay Sci. 2011, 51, 102–109. [Google Scholar] [CrossRef]
- Mansa, R.; Dzene, L.; Quintela, A.; Rocha, F.; Detellier, C. Preparation and characterization of novel clay/scleroglucan nanocomposites. Appl. Clay Sci. 2016, 126, 235–244. [Google Scholar] [CrossRef]
- Wuttke, S.; Lismont, M.; Escudero, A.; Rungtaweevoranit, B.; Parak, W.J. Positioning metal-organic framework nanoparticles within the context of drug delivery—A comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials 2017, 123, 172–183. [Google Scholar] [CrossRef]
- Chi, H.; Gu, Y.; Xu, T.; Cao, F. Multifunctional organic-inorganic hybrid nanoparticles and nanosheets based on chitosan derivative and layered double hydroxide: Cellular uptake mechanism and application for topical ocular drug delivery. Int. J. Nanomed. 2017, 12, 1607–1620. [Google Scholar] [CrossRef]
- Nguelo, B.B.; Dedzo, G.K.; Tonle, I.K.; Detellier, C.; Ngameni, E. Sensitive amperometric determination of thiocyanates at ionic liquid nanohybrid kaolinite modified glassy carbon electrode. Electroanalysis 2018, 30, 543–550. [Google Scholar] [CrossRef]
- Webb, J.D.; Neidlinger, H.H.; Connoly, J.S. An infrared study of azobenzene photoisomerization in a polymer matrix. Polym. Photochem. 1986, 7, 503–513. [Google Scholar] [CrossRef]
- Lednev, I.K.; Ye, T.-Q.; Abbott, L.C.; Hester, R.E.; Moore, J.N. Photoisomerization of a capped azobenzene in solution probed by ultrafast time-resolved electronic absorption spectroscopy. J. Phys. Chem. A 1998, 102. [Google Scholar] [CrossRef]
- Yu, Y.; Nakano, M.; Ikeda, T. Photomechanics: Directed bending of a polymer film by light. Nature 2003, 425, 145. [Google Scholar] [CrossRef]
- Yamada, M.; Kondo, M.; Mamiya, J.-I.; Yu, Y.; Kinoshita, M.; Barrett, C.J.; Ikeda, T. Photomobile polymer materials: Towards light-driven plastic motors. Angew. Chem. Int. Ed. 2008, 47, 4986–4988. [Google Scholar] [CrossRef]
- Okada, T.; Sakai, H.; Ogawa, M. The effect of the molecular structure of a cationic azo dye on the photoinduced intercalation of phenol in a montmorillonite. Appl. Clay Sci. 2008, 40, 187–192. [Google Scholar] [CrossRef]
- Knebel, A.; Zhou, C.; Huang, A.; Zhang, J.; Kustov, L.; Caro, J. Smart metal-organic frameworks: Switching gas permeation through MOF membranes by external stimuli. Chem. Eng. Technol. 2017, 42. [Google Scholar] [CrossRef]
- Okada, T.; Nozaki, N.; Seo, J.; Kwon, J.E.; Park, S.Y.; Hashizume, H.; Sasaki, T.; Ogawa, M. Photoinduced structural changes of cationic azo dyes confined in two dimensional nanospace by two different mechanisms. RSC Adv. 2017, 7, 8077–8081. [Google Scholar] [CrossRef]
- Ogawa, M.; Ishii, T.; Miyamoto, N.; Kuroda, K. Intercalation of a cationic azobenzene into montmorillonite. Appl. Clay Sci. 2003, 22, 179–185. [Google Scholar] [CrossRef]
- Koteja, A.; Szczerba, M.; Matusik, J. Smectites intercalated with azobenzene and aminoazobenzene: Structure changes at nanoscale induced by UV light. J. Phys. Chem. Solids 2017, 111, 294–303. [Google Scholar] [CrossRef]
- Fujita, T.; Iyi, N.; Klapyta, Z. Optimum conditions for photoresponse of azobenzene-organophilic tetrasilicic mica complexes. Mater. Res. Bull. 2001, 36, 557–571. [Google Scholar] [CrossRef]
- Fujita, T.; Iyi, N.; Klapyta, Z.; Fujii, K.; Kaneko, Y.; Kitamura, K. Photomechanical response of azobenzene/organophilic mica complexes. Mater. Res. Bull. 2003, 38, 2009–2017. [Google Scholar] [CrossRef]
- Ogawa, M.; Ishii, T.; Miyamoto, N.; Kuroda, K. photocontrol of the basal spacing of azobenzene-magadiite intercalation compound. Adv. Mater. 2001, 13, 1107–1109. [Google Scholar] [CrossRef]
- Troup, J.M.; Clearfield, A. On the mechanism of ion exchange in zirconium phosphates. 20. Refinement of the crystal structure of α-zirconium phosphate. Inorg. Chem. 1977, 16, 3311–3314. [Google Scholar] [CrossRef]
- Tang, M.; Yang, T.; Zhang, Y. A brief review on α-zirconium phosphate intercalation compounds and nano-composites. Sci. China Technol. Sci. 2015, 59, 436–441. [Google Scholar] [CrossRef]
- Mosby, B.M.; Díaz, A.; Clearfield, A. Surface modification of layered zirconium phosphates: A novel pathway to multifunctional materials. Dalton T. 2014, 43. [Google Scholar] [CrossRef]
- Gentili, P.L.; Costantino, U.; Vivani, R.; Latterini, L.; Nocchetti, M.; Aloisi, G.G. Preparation and characterization of zirconium phosphonate–azobenzene intercalation compounds. A structural, photophysical and photochemical study. J. Mater. Chem. 2004, 14, 1656–1662. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Černý, R. FOX, ’freeobjects for crystallography’: A modular approach to ab initio structure determination from powder diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Dollase, W.A. Correction of intensities for preferred orientation in powder diffractometry: Application of the March Model. J. Appl. Crystallogr. 1986, 19, 267–272. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Montgomery, J.; Vreven, J.; Kudin, K.; Burant, J.; et al. Gaussian-94, Revision, C.3.; Gaussian, Inc.: Pittsburgh, PA, USA, 2004. [Google Scholar]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard III, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114. [Google Scholar] [CrossRef]
- Addicoat, M.A.; Vankova, N.; Akter, I.F.; Heine, T. Extension of the Universal Force Field to Metal–Organic Frameworks. J. Chem. Theory Comput. 2014, 10, 880–891. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-rage molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- LAMMPS Molecular Dynamics Simulator. Available online: http://lammps.sandia.gov (accessed on 19 March 2018).
- Alberti, G.; Torracca, E. Crystalline insoluble salts of polybasic metals—II. synthesis of crystalline zirconium or titanium phosphate by direct precipitation. J. Inorg. Nucl. Chem. 1968, 30, 317–318. [Google Scholar] [CrossRef]
- Clearfield, A.; Stynes, J.A. The preparation of crystalline zirconium phosphate and some observations on its ion exchange behaviour. J. Inorg. Nucl. Chem. 1964, 26, 117–129. [Google Scholar] [CrossRef]
- Shuai, M.; Mejia, A.F.; Chang, Y.-W.; Cheng, Z. Hydrothermal synthesis of layered α-zirconium phosphate disks: control of aspect ratio and polydispersity for nano-architecture. CrystEngComm 2013, 15, 1970–1977. [Google Scholar] [CrossRef]
- Horsley, S.E.; Nowell, D.V.; Stewart, D.T. The infrared and Raman spectra of α-zirconium phosphate. Spectrochim. Acta 1974, 30A, 535–541. [Google Scholar] [CrossRef]
- Kurazhkovskaya, V.S.; Orlova, A.I.; Petkov, V.I.; Kemenov, D.V.; Kaplunnik, L.N. IR study of the structure of rhombohedral zirconium and alkali metal orthophosphates. J. Struct. Chem. 2000, 41, 61–66. [Google Scholar] [CrossRef]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer Structure and Molecular Environment of Alkylammonium Layered Silicates. Chem. Mater. 1994, 6, 1017–1022. [Google Scholar] [CrossRef]
- Beneš, L.; Melánová, K.; Svoboda, J.; Zima, V.; Kincl, M. Intercalation of aminonaphthalenes into α-zirconium hydrogenphosphate. J. Phys. Chem. Solids 2007, 68, 803–807. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Ogawa, M.; Hama, M.; Kuroda, K. Photochromism of azobenzene in the hydrophobic interlayer spaces of dialkyldimethylammonium-fluor-tetrasilicic mica films. Clay Miner. 1999, 34, 213–220. [Google Scholar] [CrossRef]
- Koteja, A.; Matusik, J. Preparation of azobenzene intercalated kaolinite and monitoring its photo-induced activity. Clay Miner. 2019, in press. [Google Scholar] [CrossRef]
- Beharry, A.A.; Sadovski, O.; Woolley, G.A. Azobenzene photoswitching without ultraviolet light. J. Am. Chem. Soc. 2011, 133, 19684–19687. [Google Scholar] [CrossRef]
| Reagent name | Chemical formula | Symbol |
| Zirconyl chloride a | ZrOCl2 8H2O | - |
| Phosphoric acid b | H3PO4 | - |
| Dodecyl benzyldimethylammonium chloride a | CH3(CH2)11N+(CH3)2C7H5 Cl- | BC12 |
| Tetradecyl benzyldimethylammonium chloride d | CH3(CH2)13N+(CH3)2C7H5 Cl- | BC14 |
| Hexadecyl benzyldimethylammonium bromide a | CH3(CH2)15N+(CH3)2C7H5 Br- | BC16 |
| Azobenzene a | C6H5–N=N–C6H5 | Az |
| p-aminoazobenzenee | C6H5–N=N–C6H4–NH2 | pAz |
| Material description | Symbol | |
| α-zirconium phosphate | ZrP | |
| ZrP modified with p-aminoazobenzene | ZpA | |
| ZrP modified with alkylbenzyldimethylammonium chlorides | ZBCn | |
| ZBCn modified with azobenzene | ZBCnA | |
| Compound | Zr0.5(HPO4)C12N3H11 |
|---|---|
| Empirical formula | Zr0.5(HPO4)C12N3H11 |
| Formula weight /g·mol–1 | 338.8 |
| Crystal system | monoclinic |
| Space group | P21/c |
| a/Å | 30.172(3) |
| b/Å | 5.250(3) |
| c/Å | 8.9401(16) |
| β [º] | 94.45(5) |
| V/Å3 | 1411.8(8) |
| Rp | 4.98 |
| Rwp | 6.60 |
| Colour | yellow |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koteja, A.; Matusik, J.; Luberda-Durnaś, K.; Szczerba, M. The Nature of Interactions and UV-Induced Response within α-Zirconium Phosphate Intercalation Compounds with Azobenzenes. Materials 2019, 12, 1436. https://doi.org/10.3390/ma12091436
Koteja A, Matusik J, Luberda-Durnaś K, Szczerba M. The Nature of Interactions and UV-Induced Response within α-Zirconium Phosphate Intercalation Compounds with Azobenzenes. Materials. 2019; 12(9):1436. https://doi.org/10.3390/ma12091436
Chicago/Turabian StyleKoteja, Anna, Jakub Matusik, Katarzyna Luberda-Durnaś, and Marek Szczerba. 2019. "The Nature of Interactions and UV-Induced Response within α-Zirconium Phosphate Intercalation Compounds with Azobenzenes" Materials 12, no. 9: 1436. https://doi.org/10.3390/ma12091436
APA StyleKoteja, A., Matusik, J., Luberda-Durnaś, K., & Szczerba, M. (2019). The Nature of Interactions and UV-Induced Response within α-Zirconium Phosphate Intercalation Compounds with Azobenzenes. Materials, 12(9), 1436. https://doi.org/10.3390/ma12091436