Plasmonics with Metallic Nanowires
Abstract
1. Introduction
2. Plasmon Resonance
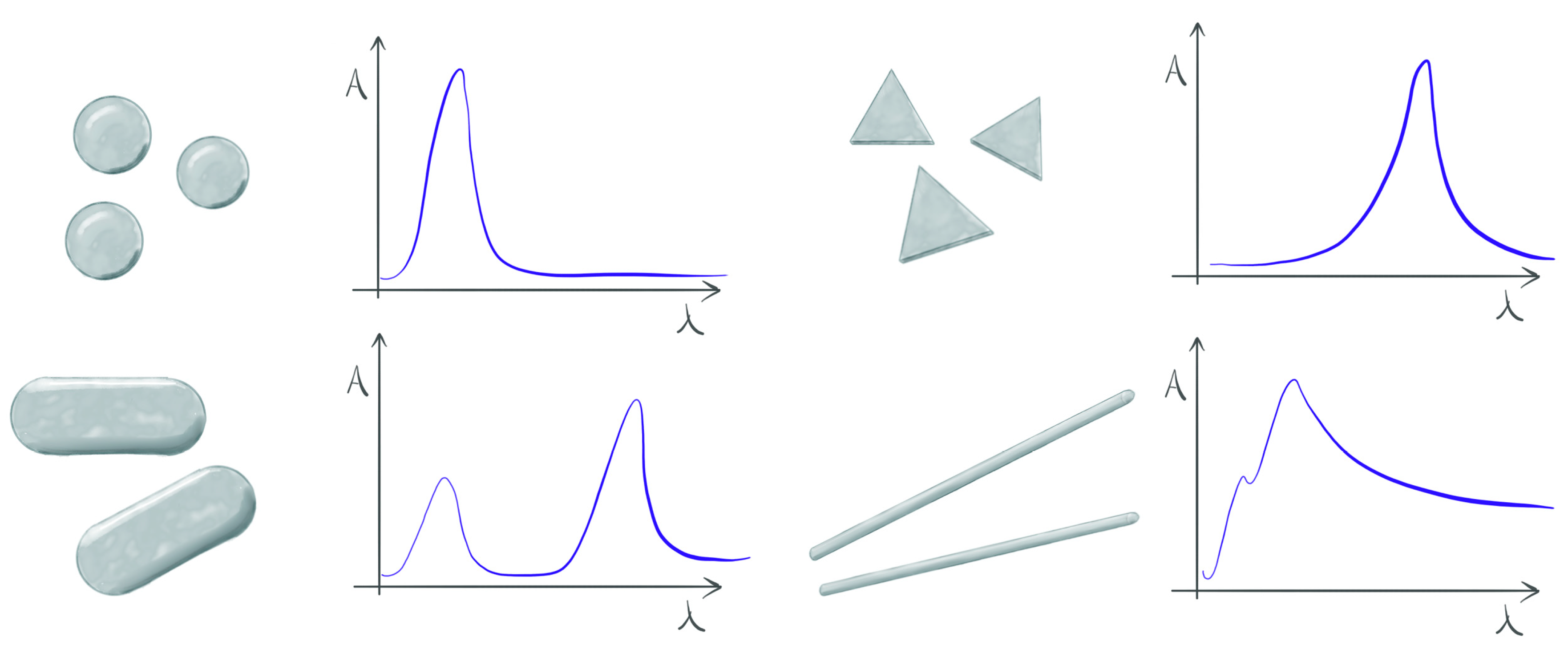
2.1. Metallic Nanoparticles
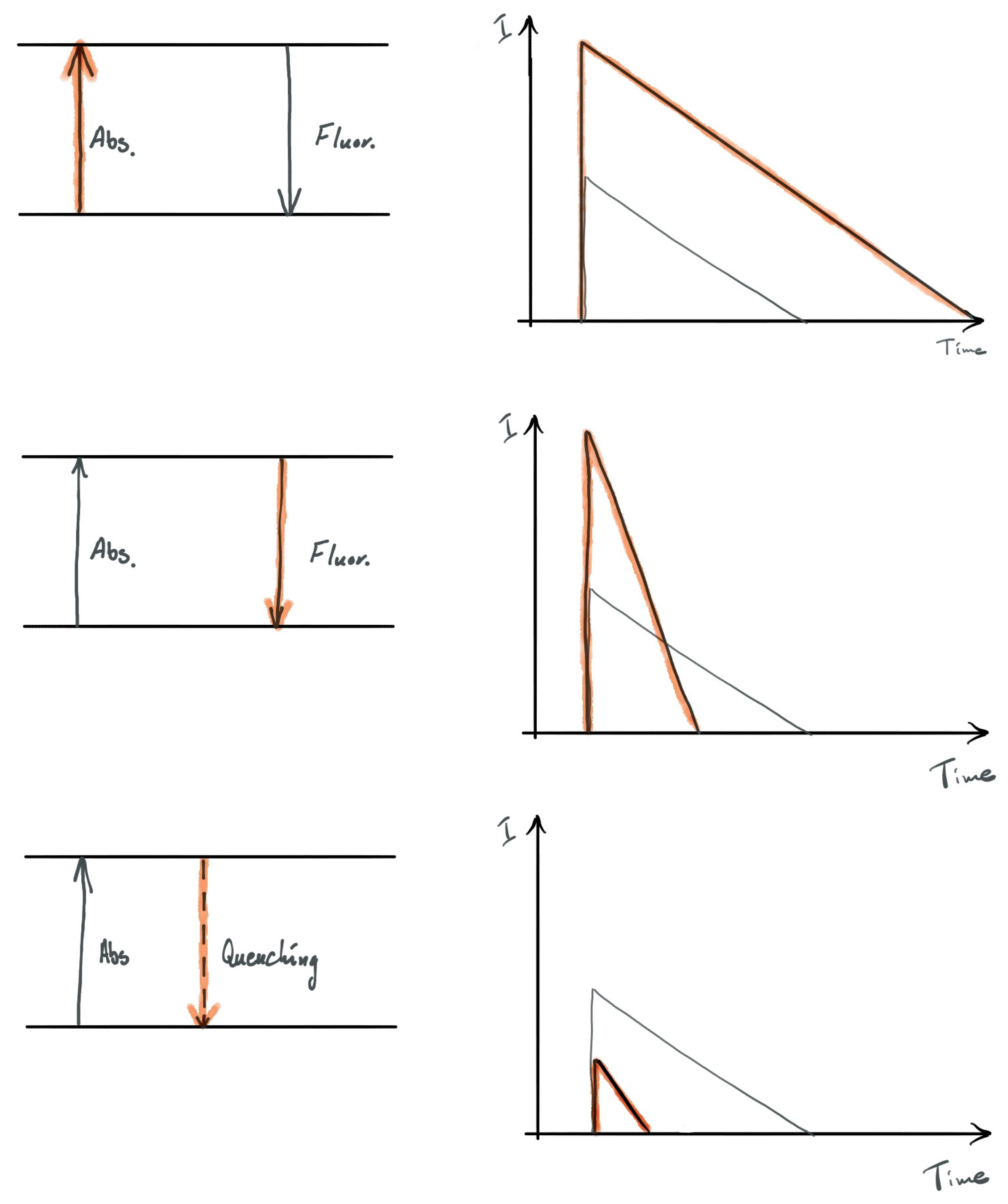
2.2. Interaction between Metallic Nanoparticles and Emitters
3. Fabrication of Metallic Nanowires
3.1. Lithography
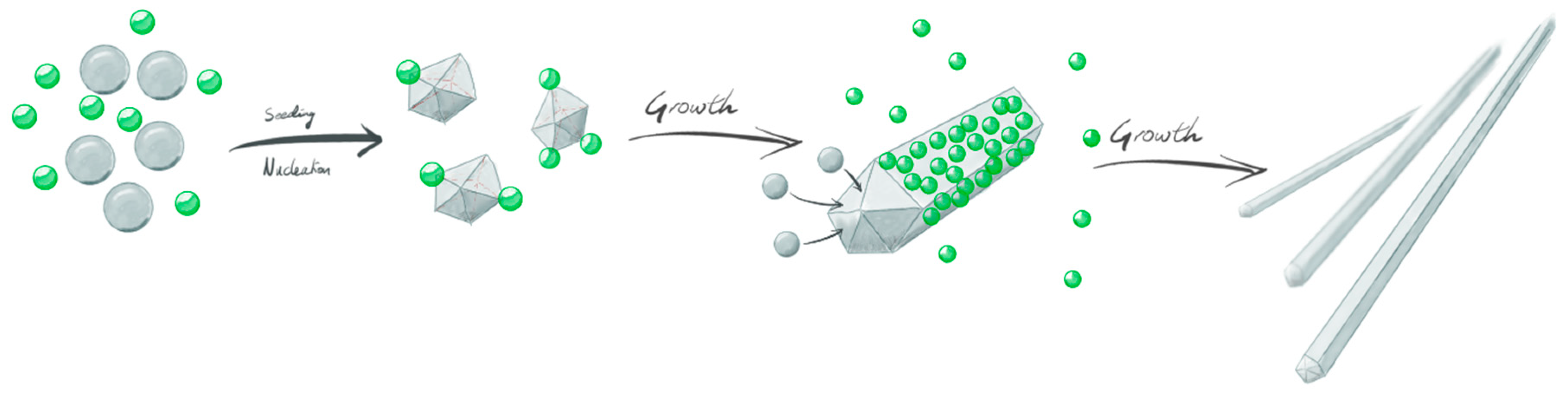
3.2. Wet-Chemistry Synthesis
3.3. Printing and Photochemical Methods
4. Metal-Enhanced Fluorescence with Silver Nanowires
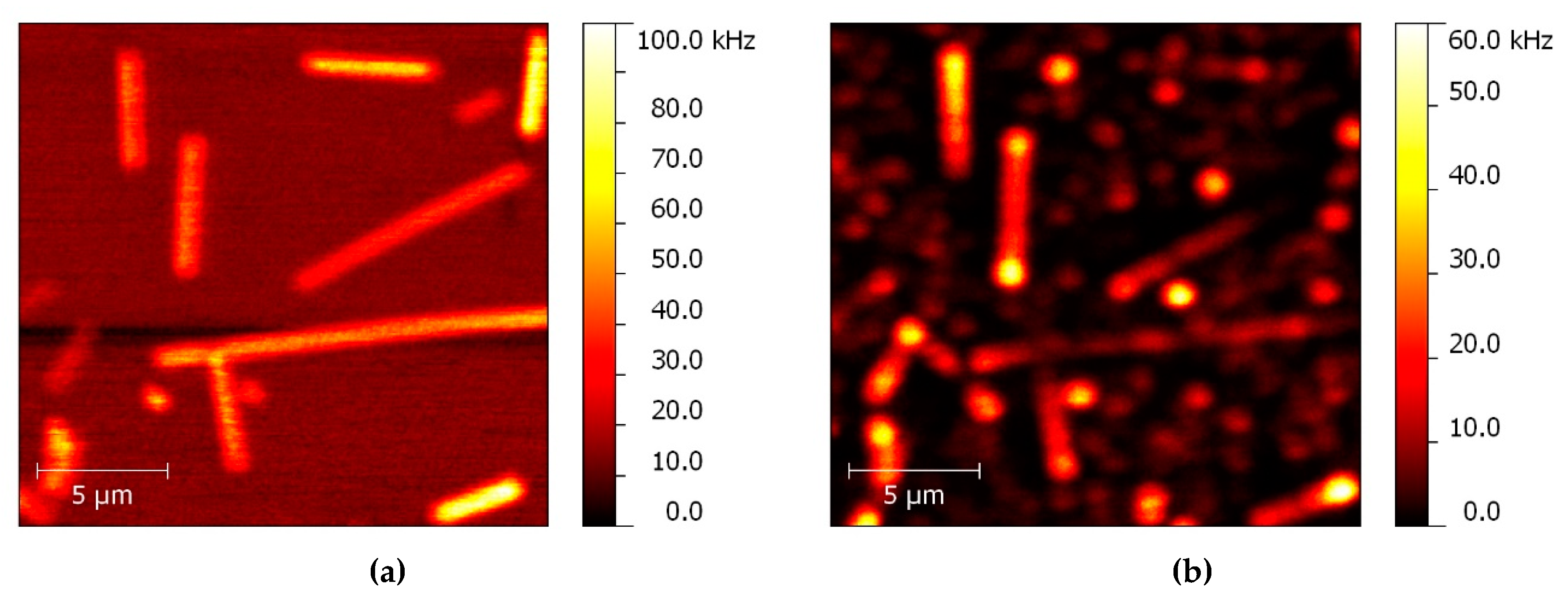
4.1. Planar Structures
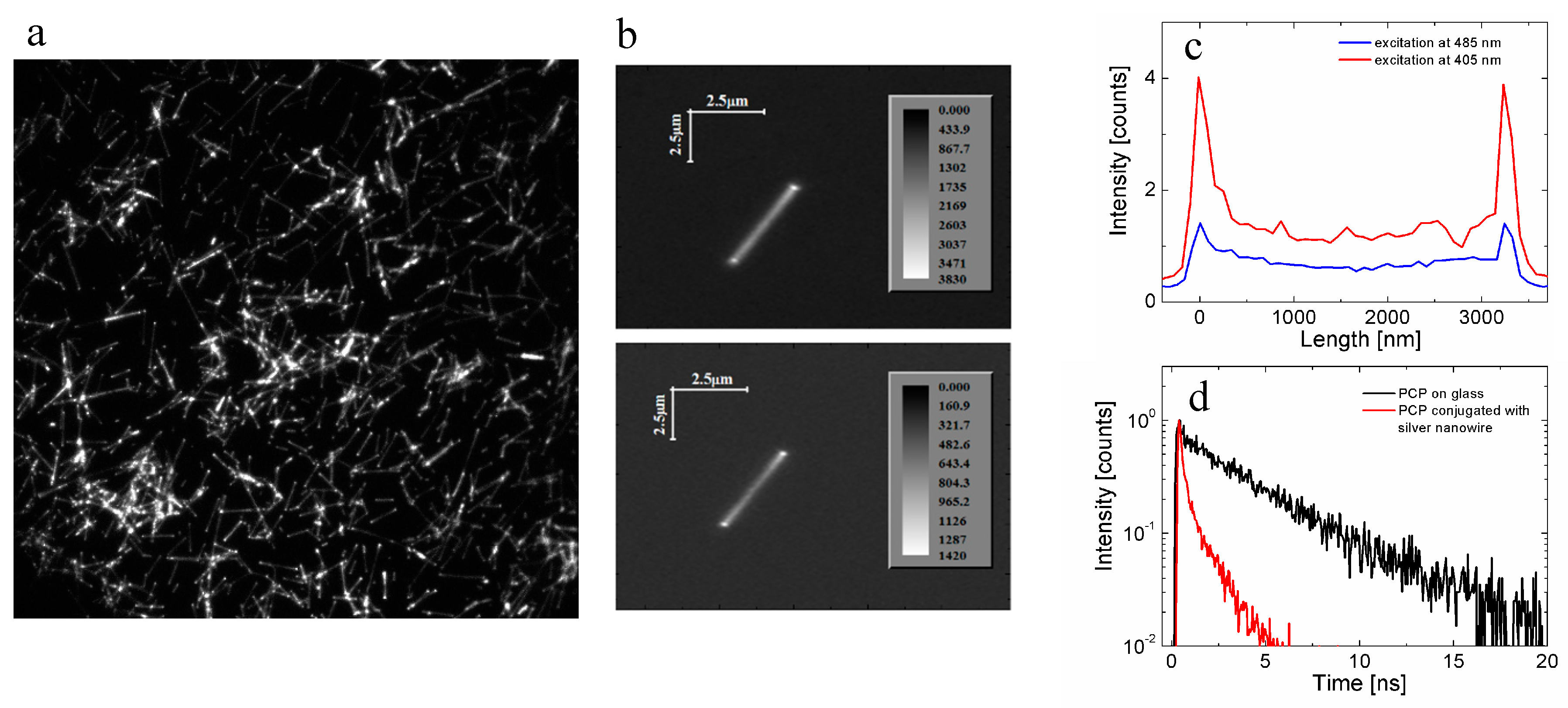
4.2. Bioconjugated Systems
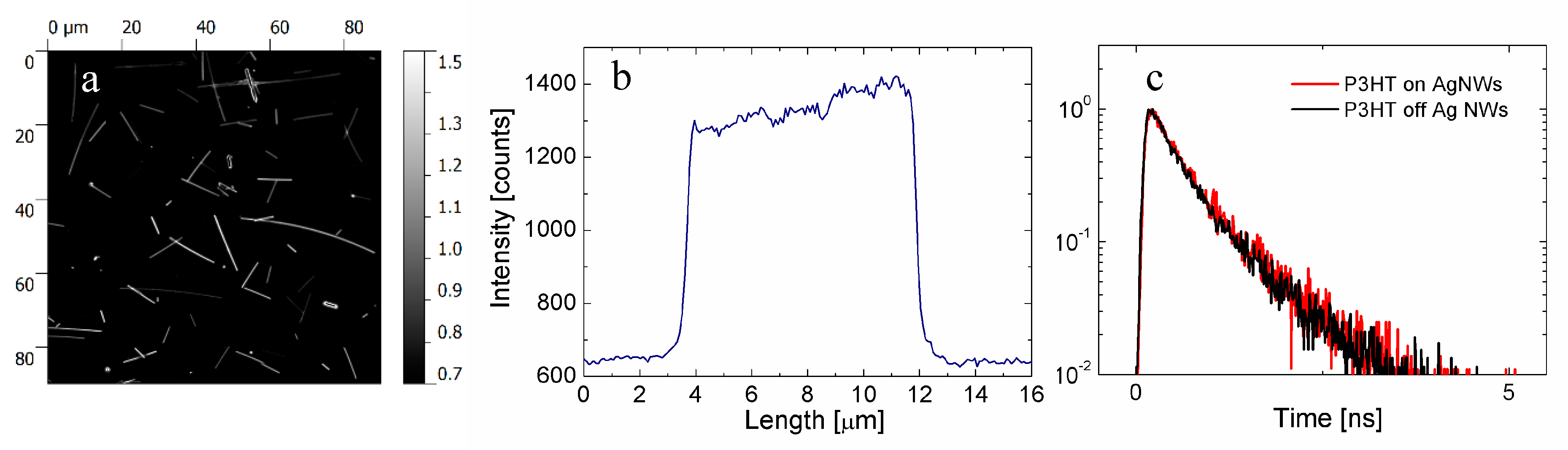
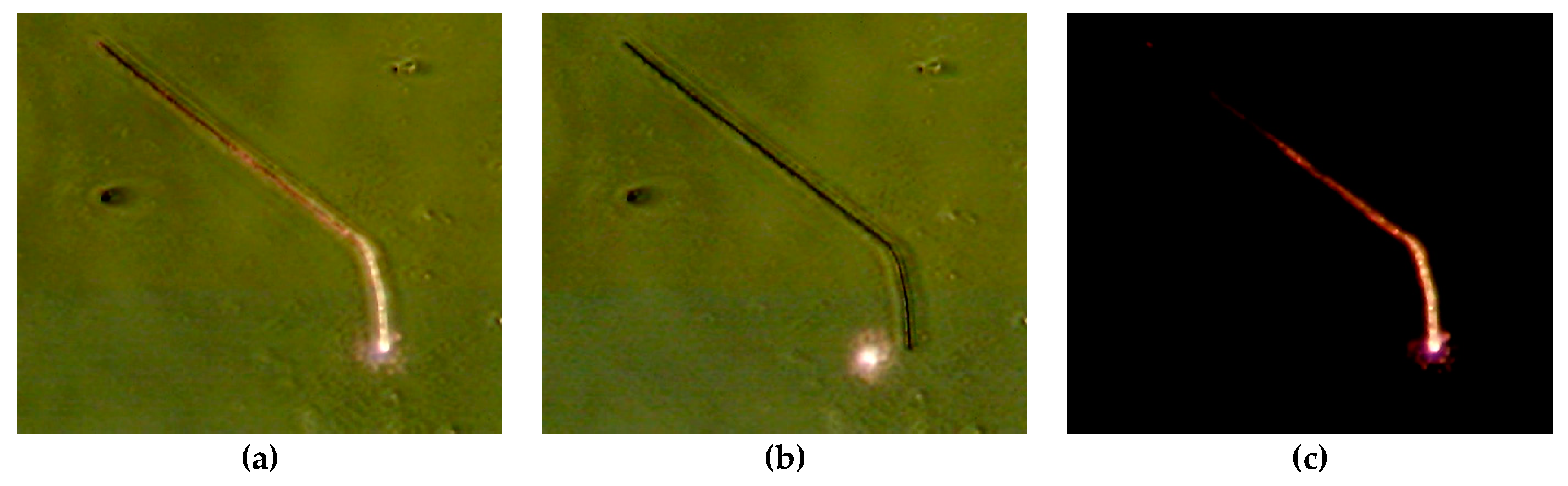
5. Propagation of Energy in Metallic Nanowires
6. Biochemical Sensing with Metallic Nanowires
7. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novotny, L.; Hecht, B. Principles of Nano-Optics; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Schuller, J.A.; Barnard, E.S.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef]
- Jiang, R.; Ming, T.; Chen, H.; Li, Q.; Wang, J. Plasmon-Controlled Fluorescence: Beyond the Intensity Enhancement. J. Phys. Chem. Lett. 2012, 3, 191–202. [Google Scholar]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007. [Google Scholar]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef]
- Aherne, D.; Ledwith, D.M.; Gara, M.; Kelly, J.M. Optical Properties and Growth Aspects of Silver Nanoprisms Produced by a Highly Reproducible and Rapid Synthesis at Room Temperature. Adv. Funct. Mater. 2008, 18, 2005–2016. [Google Scholar] [CrossRef]
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence Enhancement by Au Nanostructures: Nanoshells and Nanorods. ACS Nano 2009, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Bryant, G.W.; De Abajo, F.J.G.; Aizpurua, J. Mapping the Plasmon Resonances of Metallic Nanoantennas. Nano Lett. 2008, 8, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Novotny, L. Spectral dependence of single molecule fluorescence enhancement. Opt. Express 2007, 15, 14266. [Google Scholar] [CrossRef]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; van Veggel, F.C.J.M.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence Quenching of Dye Molecules near Gold Nanoparticles: Radiative and Nonradiative Effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative Decay Engineering: Biophysical and Biomedical Applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and Quenching of Single-Molecule Fluorescence. Phys. Rew. Lett. 2006, 96. [Google Scholar] [CrossRef]
- Bujak, Ł.; Czechowski, N.; Piatkowski, D.; Litvin, R.; Mackowski, S.; Brotosudarmo, T.H.P.; Cogdell, R.J.; Pichler, S.; Heiss, W. Fluorescence enhancement of light-harvesting complex 2 from purple bacteria coupled to spherical gold nanoparticles. Appl. Phys. Lett. 2011, 99, 173701. [Google Scholar] [CrossRef]
- Ray, K.; Badugu, R.; Lakowicz, J.R. Sulforhodamine Adsorbed Langmuir-Blodgett Layers on Silver Island Films: Effect of Probe Distance on the Metal-Enhanced Fluorescence. J. Phys. Chem. C 2007, 111, 7091–7097. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fan, D.; Ekinci, Y.; Padeste, C. Fabrication of ultrahigh resolution metal nanowires and nanodots through EUV interference lithography. Microelectron. Eng. 2015, 141, 32–36. [Google Scholar] [CrossRef]
- Doan, N.M.; Qiang, L.; Li, Z.; Vaddiraju, S.; Bishop, G.W.; Rusling, J.F.; Papadimitrakopoulos, F.; Kerman, K. Low-Cost Photolithographic Fabrication of Nanowires and Microfilters for Advanced Bioassay Devices. Sensors 2015, 15, 6091–6104. [Google Scholar] [CrossRef]
- Zhang, P.; Wyman, I.; Hu, J.; Lin, S.; Zhong, Z.; Tu, Y.; Huang, Z.; Wei, Y. Silver nanowires: Synthesis technologies, growth mechanism and multifunctional applications. Mater. Sci. Eng. B 2017, 223, 1–23. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A Membrane-Based Synthetic Approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem. Commun. 2001, 617–618. [Google Scholar] [CrossRef]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline Silver Nanowires by Soft Solution Processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Im, S.H.; Lee, Y.T.; Wiley, B.; Xia, Y. Large-Scale Synthesis of Silver Nanocubes: The Role of HCl in Promoting Cube Perfection and Monodispersity. Angew. Chem. Int. Ed. 2005, 44, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Song, J.H.; Yang, P. Photochemical Synthesis of Gold Nanorods. J. Am. Chem. Soc. 2002, 124, 14316–14317. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Huitink, D.; Wang, K.; Liang, H. Photochemical formation of electrically conductive silver nanowires on polymer scaffolds. J. Colloid Interface Sci. 2010, 344, 334–342. [Google Scholar] [CrossRef] [PubMed]
- He, G.-C.; Zheng, M.-L.; Dong, X.-Z.; Jin, F.; Liu, J.; Duan, X.-M.; Zhao, Z.-S. The Conductive Silver Nanowires Fabricated by Two-beam Laser Direct Writing on the Flexible Sheet. Sci. Rep. 2017, 7, 41757. [Google Scholar] [CrossRef] [PubMed]
- Fedutik, Y.; Temnov, V.; Schöps, O.; Woggon, U.; Artemyev, M.; Temnov, V.V.; Artemyev, M.V. Exciton-Plasmon-Photon Conversion in Plasmonic Nanostructures. Phys. Lett. 2007, 99, 136802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Y.; Hu, C.; Zhang, Y.; Chen, X.; Zhang, M.Q. Effective excitation and control of guided surface plasmon polaritons in a conjugated polymer–silver nanowire composite system. J. Mater. Chem. C 2013, 1, 1265–1271. [Google Scholar] [CrossRef]
- Shegai, T.; Huang, Y.; Xu, H.; Käll, M. Coloring fluorescence emission with silver nanowires. Appl. Phys. Lett. 2010, 96, 103114. [Google Scholar] [CrossRef]
- Guo, S.-H.; Britti, D.G.; Heetderks, J.J.; Kan, H.-C.; Phaneuf, R.J. Spacer Layer Effect in Fluorescence Enhancement from Silver Nanowires over a Silver Film; Switching of Optimum Polarization. Nano Lett. 2009, 9, 2666–2670. [Google Scholar] [CrossRef]
- Kowalska, D.; Krajnik, B.; Olejnik, M.; Twardowska, M.; Czechowski, N.; Hofmann, E.; Mackowski, S. Metal-Enhanced Fluorescence of Chlorophylls in Light-Harvesting Complexes Coupled to Silver Nanowires. Sci. World. J. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Smolarek, K.; Ebenhoch, B.; Czechowski, N.; Prymaczek, A.; Twardowska, M.; Samuel, I.D.W.; Mackowski, S. Silver nanowires enhance absorption of poly(3-hexylthiophene). Appl. Phys. Lett. 2013, 103, 203302. [Google Scholar] [CrossRef]
- Szalkowski, M.; Olmos, J.D.J.; Buczyńska, D.; Maćkowski, S.; Kowalska, D.; Kargul, J. Plasmon-induced absorption of blind chlorophylls in photosynthetic proteins assembled on silver nanowires. Nanoscale 2017, 9, 10475–10486. [Google Scholar] [CrossRef]
- Ćwik, M.; Buczyńska, D.; Sulowska, K.; Roźniecka, E.; Mackowski, S.; Niedziółka-Jönsson, J. Optical Properties of Submillimeter Silver Nanowires Synthesized Using the Hydrothermal Method. Materials 2019, 12, 721. [Google Scholar] [CrossRef]
- Kowalska, D.; Szalkowski, M.; Ashraf, K.; Grzelak, J.; Lokstein, H.; Niedziolka-Jonsson, J.; Cogdell, R.; Mackowski, S. Spectrally selective fluorescence imaging of Chlorobaculum tepidum reaction centers conjugated to chelator-modified silver nanowires. Photosynth. Res. 2018, 135, 329. [Google Scholar] [CrossRef] [PubMed]
- Szalkowski, M.; Sulowska, K.; Grzelak, J.; Niedziółka-Jönsson, J.; Roźniecka, E.; Kowalska, D.; Mackowski, S. Wide-Field Fluorescence Microscopy of Real-Time Bioconjugation Sensing. Sensors 2018, 18, 290. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Wrench, P.M.; Sharples, F.P.; Hiller, R.G.; Welte, W.; Diederichs, K. Structural Basis of Light Harvesting by Carotenoids: Peridinin-Chlorophyll-Protein from Amphidinium carterae. Science 1996, 272, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.; Johanning, S.; Hofmann, E. Structure and function of native and refolded peridinin-chlorophyll-proteins from dinoflagellates. Eur. J. Cell Boil. 2010, 89, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Kleima, F.J.; Hofmann, E.; Gobets, B.; Van Stokkum, I.H.; Van Grondelle, R.; Diederichs, K.; Van Amerongen, H. Förster excitation energy transfer in peridinin-chlorophyll-a-protein. Biophys. J. 2000, 78, 344–353. [Google Scholar] [CrossRef]
- Mackowski, S.; Wörmke, S.; Brotosudarmo, T.H.P.; Jung, C.; Hiller, R.G.; Scheer, H.; Bräuchle, C. Energy Transfer in Reconstituted Peridinin-Chlorophyll-Protein Complexes: Ensemble and Single-Molecule Spectroscopy Studies. Biophys. J. 2007, 93, 3249–3258. [Google Scholar] [CrossRef]
- Wörmke, S.; Maćkowski, S.; Brotosudarmo, T.; Jung, C.; Zumbusch, A.; Ehrl, M.; Scheer, H.; Hofmann, E.; Hiller, R.; Bräuchle, C. Monitoring fluorescence of individual chromophores in peridinin–chlorophyll–protein complex using single molecule spectroscopy. Biochim. et Biophys. Acta (BBA) - Gen. Sub. 2007, 1767, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform Silver Nanowires Synthesis by Reducing AgNO3 with Ethylene Glycol in the Presence of Seeds and Poly(Vinyl Pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Ozbay, E. Plasmonics: Merging Photonics and Electronics at Nanoscale Dimensions. Science 2006, 311, 189–193. [Google Scholar] [CrossRef]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895. [Google Scholar] [CrossRef]
- Kim, C.-H.; Cha, S.-H.; Kim, S.C.; Song, M.; Lee, J.; Shin, W.S.; Moon, S.-J.; Bahng, J.H.; Kotov, N.A.; Jin, S.-H. Silver Nanowire Embedded in P3HT:PCBM for High-Efficiency Hybrid Photovoltaic Device Applications. ACS Nano 2011, 5, 3319–3325. [Google Scholar] [CrossRef]
- Chen, C.-C.; Dou, L.; Zhu, R.; Chung, C.-H.; Song, T.-B.; Zheng, Y.B.; Hawks, S.; Li, G.; Weiss, P.S.; Yang, Y. Visibly transparent polymer solar cells produced by solution processing. ACS Nano 2012, 6, 7185–7190. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Plasmonics in Biology and Plasmon-Controlled Fluorescence. Plasmonics 2006, 1, 5–33. [Google Scholar] [CrossRef] [PubMed]
- Smolarek, K. Oddziaływania Plazmonowe w Polimerowych Układach Hybrydowych dla Zastosowań w Optoelektronice. Ph.D. Thesis, Nicolaus Copernicus University, Torun, Poland, 2017. [Google Scholar]
- Beyer, S.R.; Ullrich, S.; Kudera, S.; Gardiner, A.T.; Cogdell, R.J.; Köhler, J. Hybrid Nanostructures for Enhanced Light-Harvesting: Plasmon Induced Increase in Fluorescence from Individual Photosynthetic Pigment–Protein Complexes. Nano Lett. 2011, 11, 4897–4901. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, I.; Lieberman, I.; Kraversky, L.; Fan, Z.; Govorov, A.O.; Markovich, G.; Richter, S. Broad Band Enhancement of Light Absorption in Photosystem I by Metal Nanoparticle Antennas. Nano Lett. 2010, 10, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Nieder, J.B.; Bittl, R.; Brecht, M. Fluorescence Studies into the Effect of Plasmonic Interactions on Protein Function. Angew. Chem. Int. Ed. 2010, 49, 10217–10220. [Google Scholar] [CrossRef]
- Kim, I.; Bender, S.L.; Hranisavljevic, J.; Utschig, L.M.; Huang, L.; Wiederrecht, G.P.; Tiede, D.M. Metal Nanoparticle Plasmon-Enhanced Light-Harvesting in a Photosystem I Thin Film. Nano Lett. 2011, 11, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Govorov, A.O.; Dulka, J.; Kotov, N.A. Bioconjugates of CdTe Nanowires and Au Nanoparticles: Plasmon−Exciton Interactions, Luminescence Enhancement, and Collective Effects. Nano Lett. 2004, 4, 2323–2330. [Google Scholar] [CrossRef]
- Lee, J.; Javed, T.; Skeini, T.; Govorov, A.O.; Bryant, G.W.; Kotov, N.A. Bioconjugated Ag Nanoparticles and CdTe Nanowires: Metamaterials with Field-Enhanced Light Absorption. Angew. Chem. 2006, 118, 4937–4941. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor Nanocrystals: Structure, Properties, and Band Gap Engineering. Accounts Chem. 2010, 43, 190–200. [Google Scholar] [CrossRef]
- Pompa, P.P.; Martiradonna, L.; Della Torre, A.; Della Sala, F.; Manna, L.; De Vittorio, M.; Calabi, F.; Cingolani, R.; Rinaldi, R. Metal-enhanced fluorescence of colloidal nanocrystals with nanoscale control. Nat. Nanotechnol. 2006, 1, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, S.; Tian, X.; Xu, H. Highly tunable propagating surface plasmons on supported silver nanowires. Proc. Acad. Sci. 2013, 110, 4494–4499. [Google Scholar] [CrossRef]
- De Torres, J.; Ferrand, P.; Francs, G.C.D.; Wenger, J. Coupling Emitters and Silver Nanowires to Achieve Long-Range Plasmon-Mediated Fluorescence Energy Transfer. ACS Nano 2016, 10, 3968–3976. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Q.; Fan, F.; Xu, H.; Wang, Z.L. Light Propagation in Curved Silver Nanowire Plasmonic Waveguides. Nano Lett. 2011, 11, 1603–1608. [Google Scholar] [CrossRef]
- Johns, P.; Beane, G.; Yu, K.; Hartland, G.V. Dynamics of Surface Plasmon Polaritons in Metal Nanowires. J. Phys. Chem. C 2017, 121, 5445–5459. [Google Scholar] [CrossRef]
- Dickson, R.M.; Lyon, L.A. Unidirectional Plasmon Propagation in Metallic Nanowires. J. Phys. Chem. B 2000, 104, 6095–6098. [Google Scholar] [CrossRef]
- Hartmann, N.; Piatkowski, D.; Ciesielski, R.; Mackowski, S.; Hartschuh, A. Radiation Channels Close to a Plasmonic Nanowire Visualized by Back Focal Plane Imaging. ACS Nano 2013, 7, 10257–10262. [Google Scholar] [CrossRef] [PubMed]
- Piatkowski, D.; Hartmann, N.; Macabelli, T.; Nyk, M.; Mackowski, S.; Hartschuh, A. Silver nanowires as receiving-radiating nanoantennas in plasmon-enhanced up-conversion processes. Nanoscale 2015, 7, 1479–1484. [Google Scholar] [CrossRef]
- Nyk, M.; Kumar, R.; Ohulchanskyy, T.Y.; Bergey, E.J.; Prasad, P.N. High Contrast in Vitro and in Vivo Photoluminescence Bioimaging Using Near Infrared to Near Infrared Up-Conversion in Tm3+ and Yb3+ Doped Fluoride Nanophosphors. Nano Lett. 2008, 8, 3834–3838. [Google Scholar] [CrossRef]
- Dempsey, G.T.; Bates, M.; Kowtoniuk, W.E.; Liu, D.R.; Tsien, R.Y.; Zhuang, X. Photoswitching Mechanism of Cyanine Dyes. J. Am. Chem. Soc. 2009, 131, 18192–18193. [Google Scholar] [CrossRef] [PubMed]
- Shuang, S.; Lv, R.; Xie, Z.; Zhang, Z. Surface Plasmon Enhanced Photocatalysis of Au/Pt-decorated TiO2 Nanopillar Arrays. Sci. Rep. 2016, 6, 26670. [Google Scholar] [CrossRef]
- Grzelak, J.; Sulowska, K.; Leśniewski, A.; Rozniecka, E.; Janczuk-Richter, M.; Richter, Ł.; Łoś, M.; Jönsson-Niedziółka, M.; Maćkowski, S.; Niedziółka-Jönsson, J. Capturing fluorescing viruses with silver nanowires. Sensors Actuators B Chem. 2018, 273, 689–695. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedziółka-Jönsson, J.; Mackowski, S. Plasmonics with Metallic Nanowires. Materials 2019, 12, 1418. https://doi.org/10.3390/ma12091418
Niedziółka-Jönsson J, Mackowski S. Plasmonics with Metallic Nanowires. Materials. 2019; 12(9):1418. https://doi.org/10.3390/ma12091418
Chicago/Turabian StyleNiedziółka-Jönsson, Joanna, and Sebastian Mackowski. 2019. "Plasmonics with Metallic Nanowires" Materials 12, no. 9: 1418. https://doi.org/10.3390/ma12091418
APA StyleNiedziółka-Jönsson, J., & Mackowski, S. (2019). Plasmonics with Metallic Nanowires. Materials, 12(9), 1418. https://doi.org/10.3390/ma12091418





