Three-Dimensional Nano-Morphology of Carbon Nanotube/Epoxy Filled Poly(methyl methacrylate) Microcapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microcapsules Preparation
2.2.1. Microencapsulation Parameters
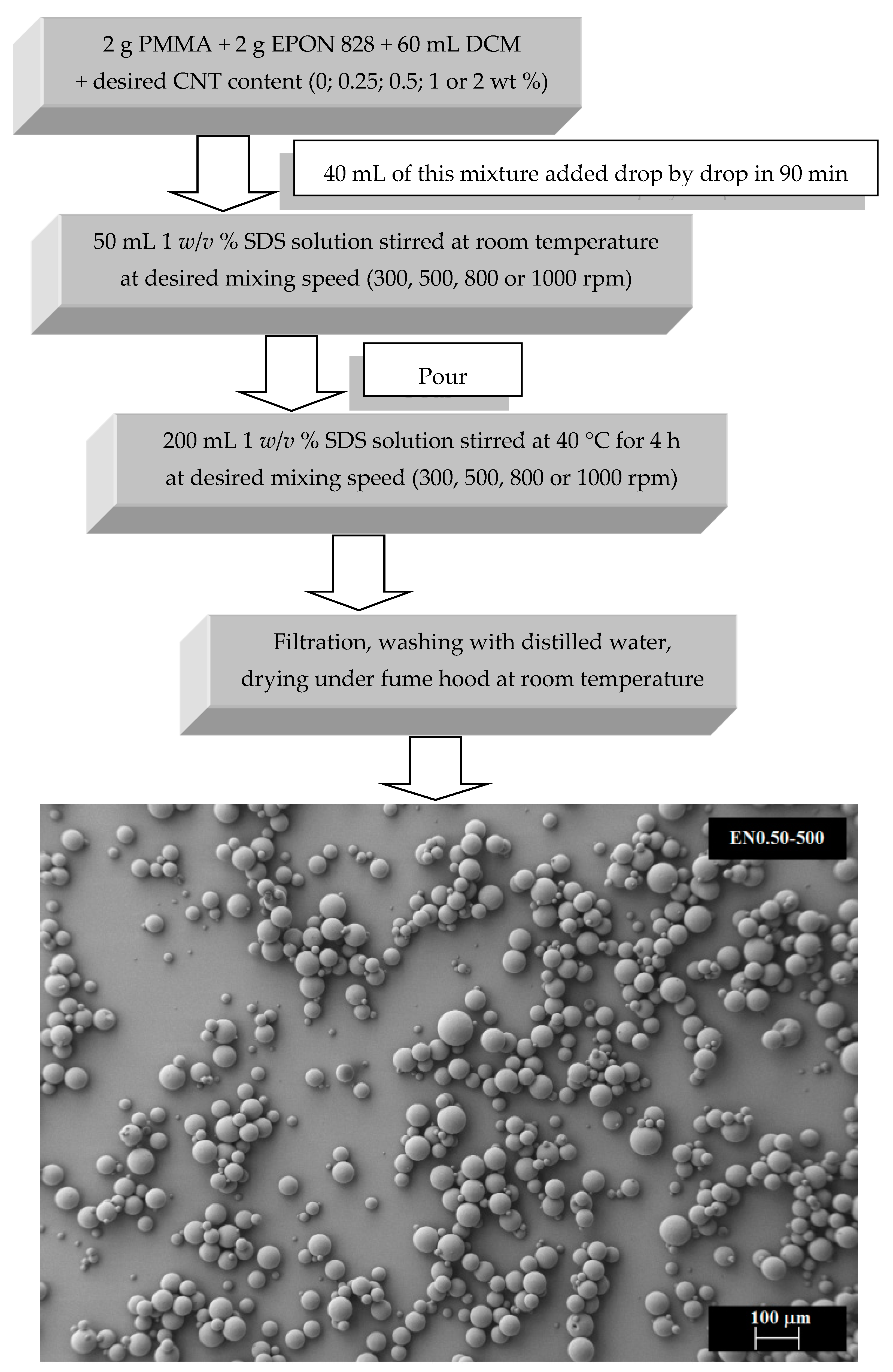
2.2.2. Microencapsulation Procedure
2.2.3. Microcapsules Characterization
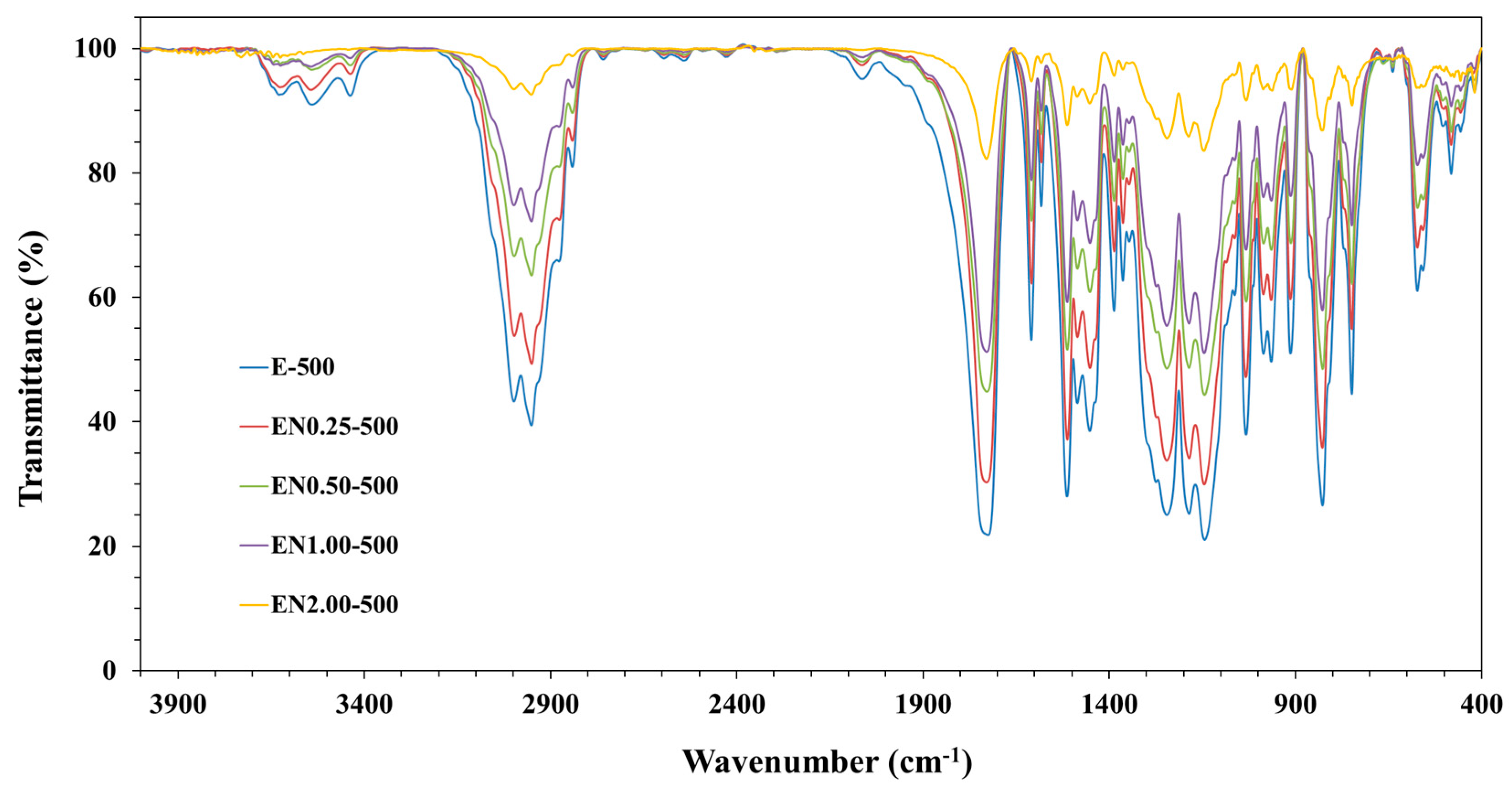
Fourier-Transform Infrared Characterization
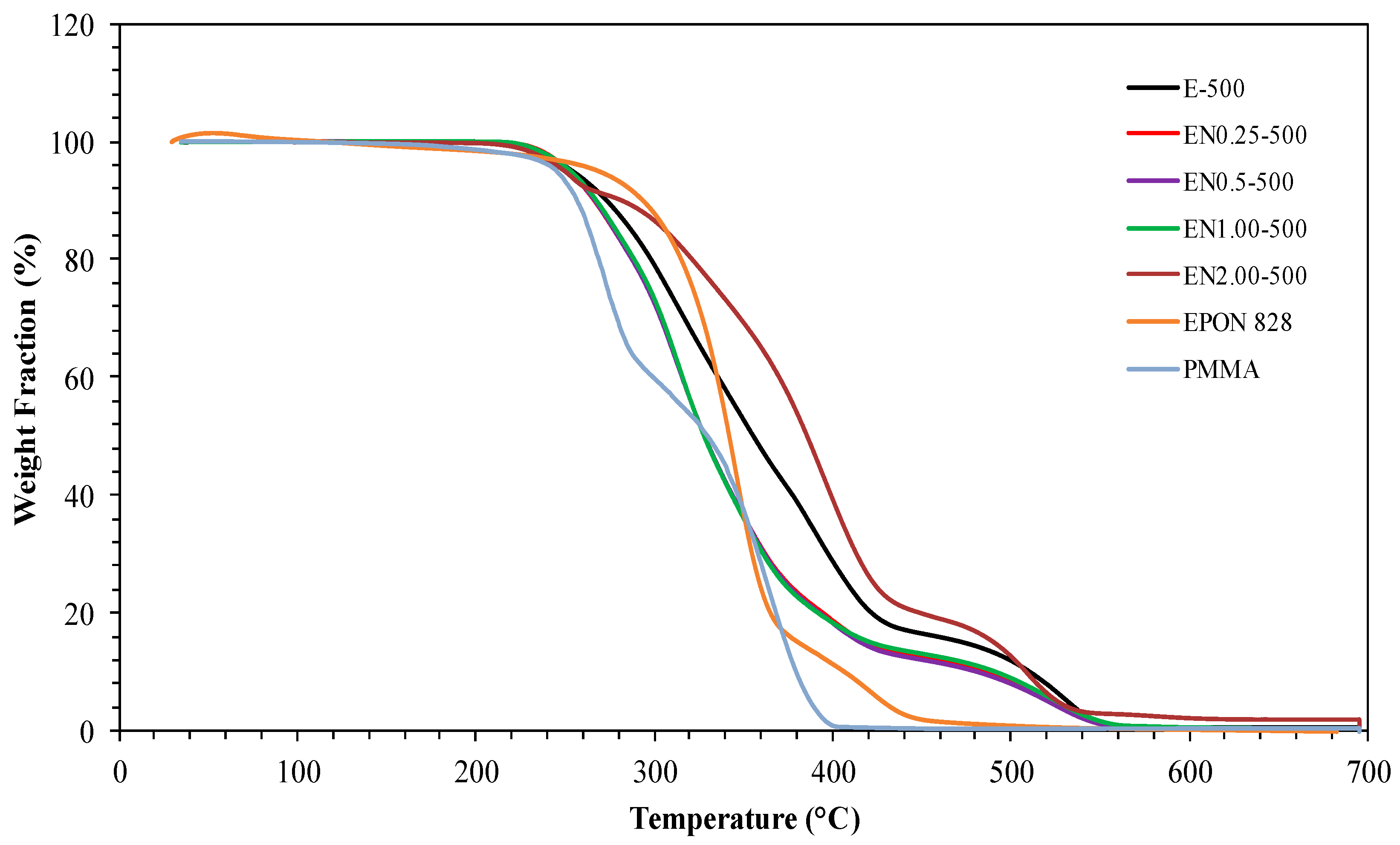
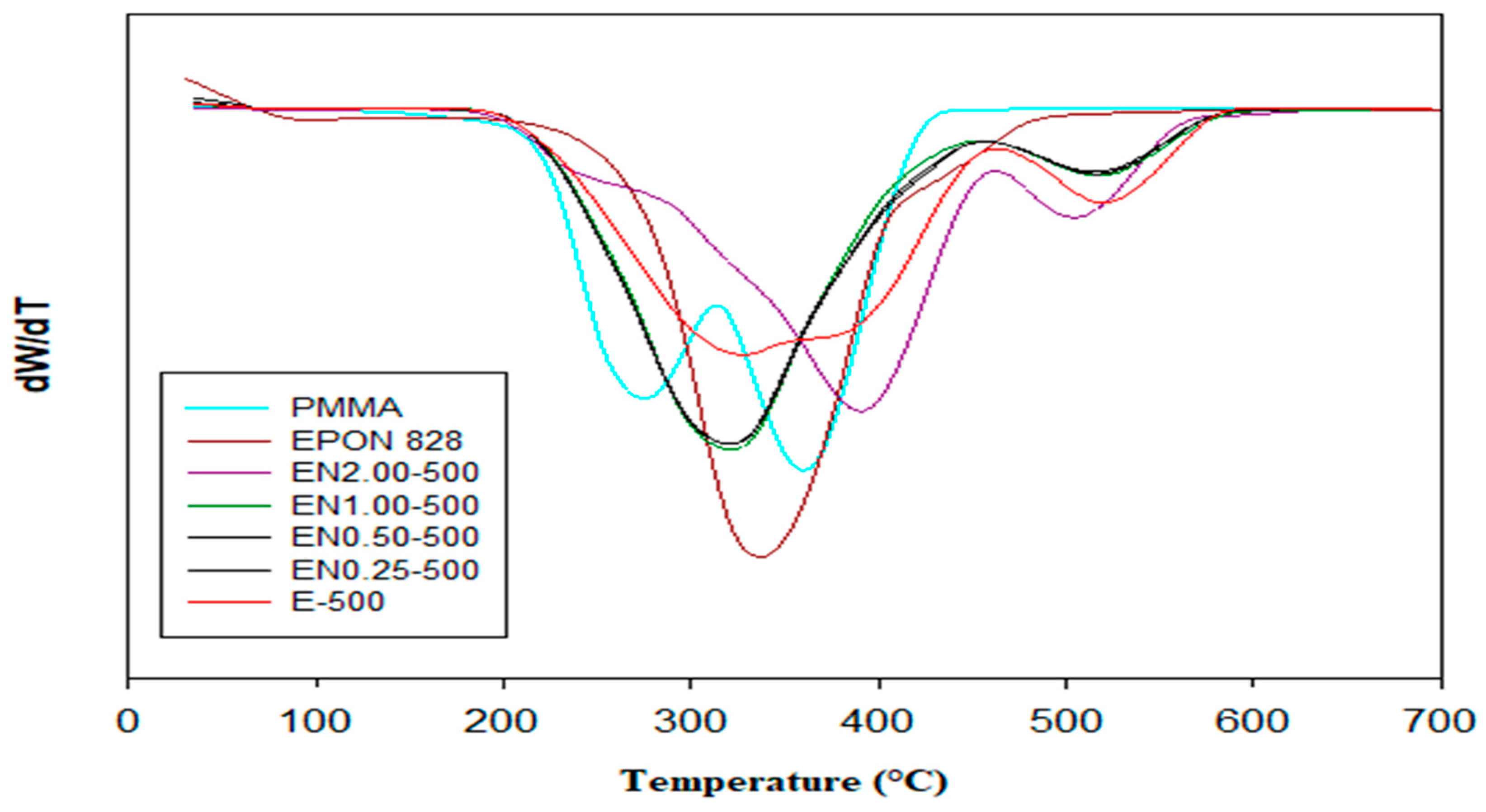
Thermal Stability
Scanning Electron Microscopy (SEM)
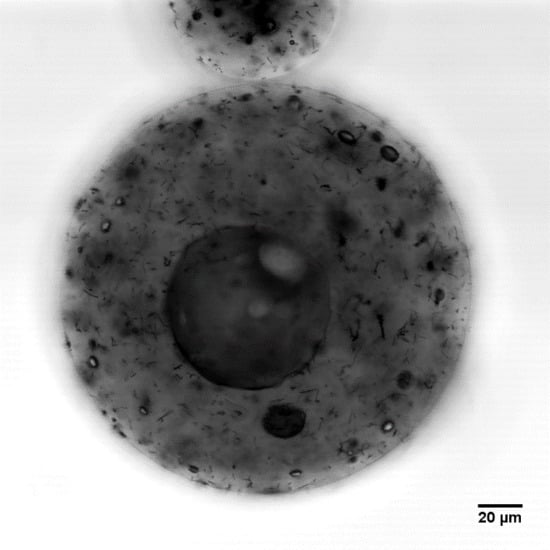
Laser Scanning Confocal Microscopy (LSCM)
3. Results and Discussion
3.1. Chemical Composition of Microcapsules
3.2. Effect of Carbon Nanotube Content on Thermal Stability of Microcapsules
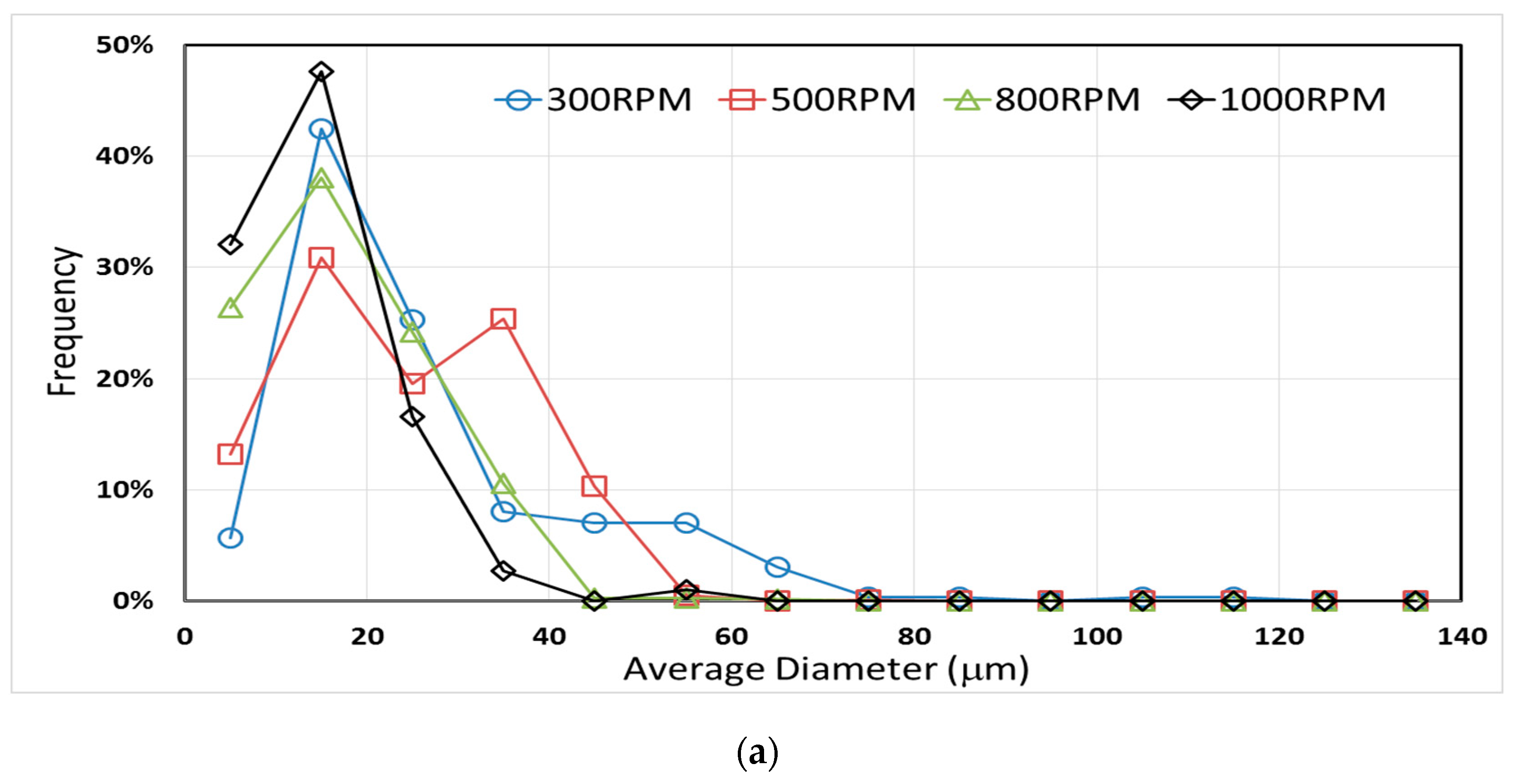
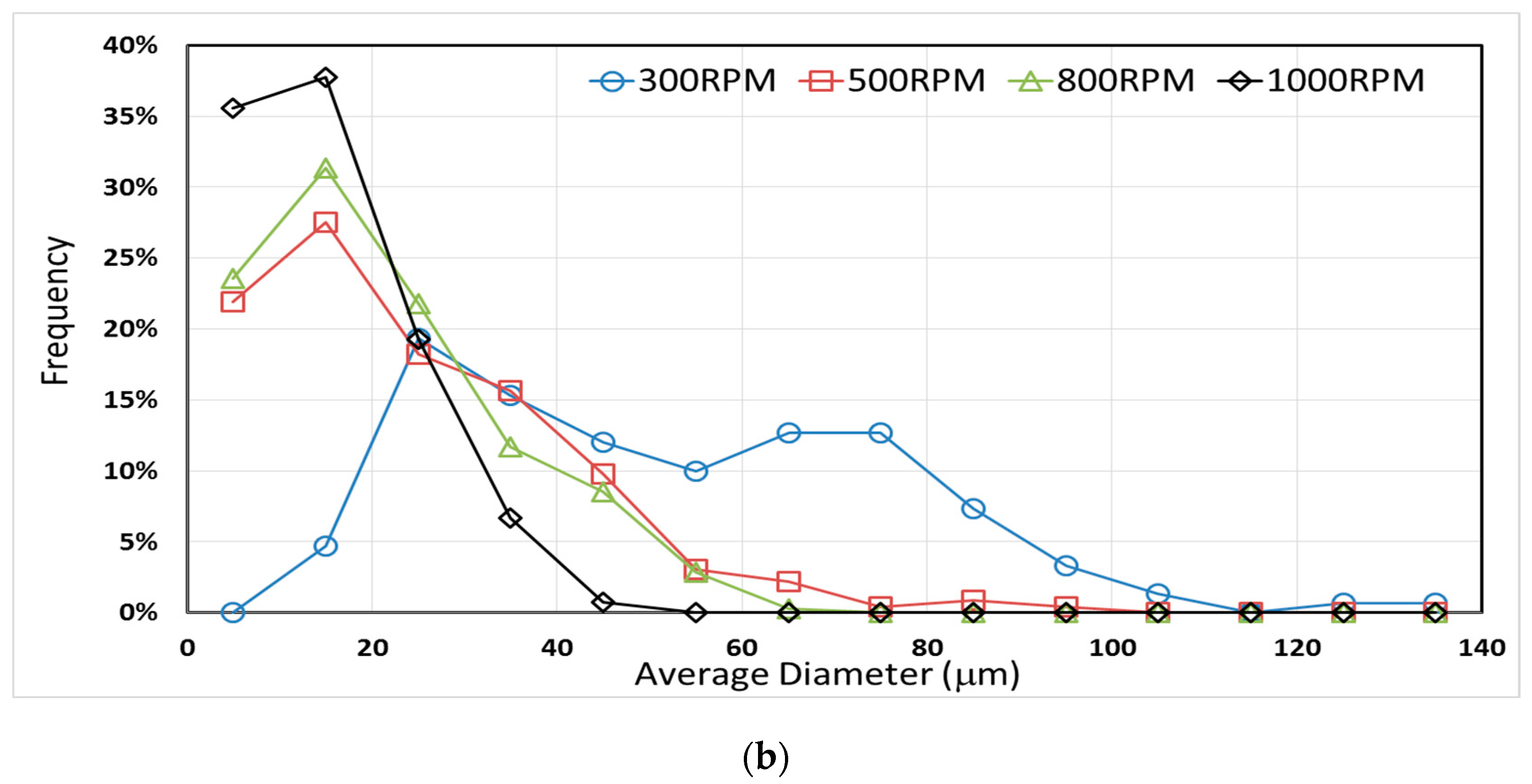
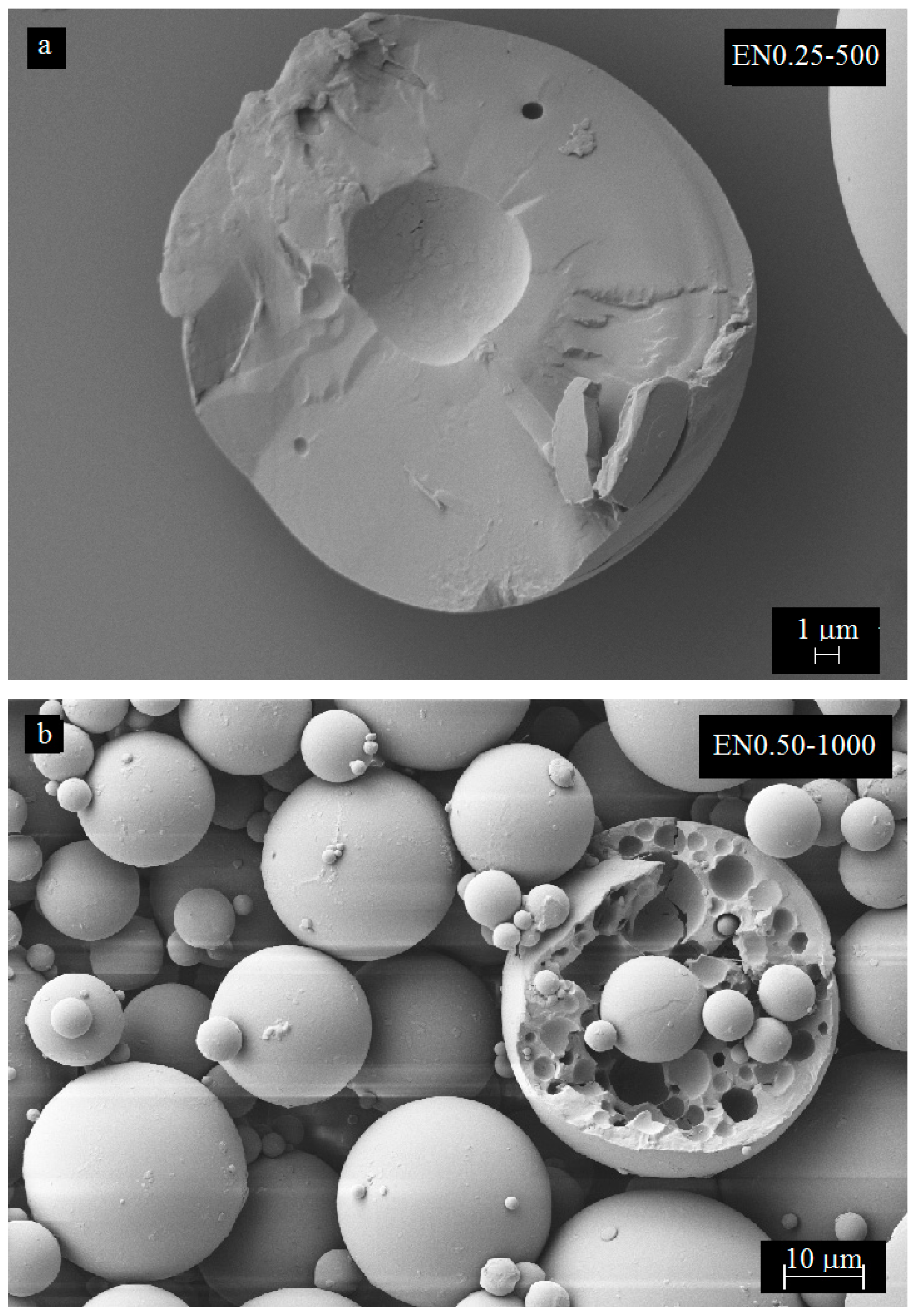
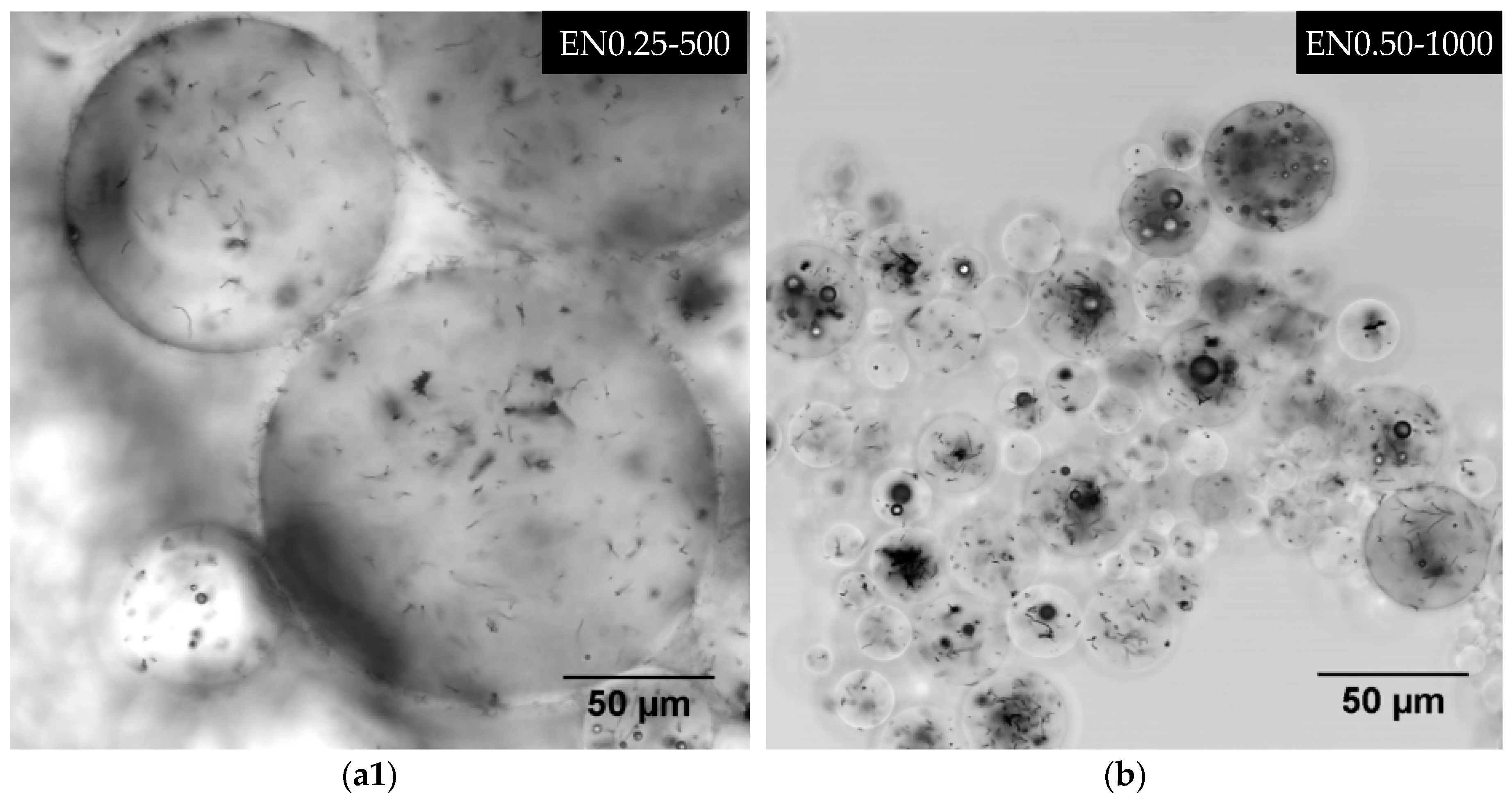
3.3. Effect of Mixing Speed and CNT Content on Microcapsule Size Distribution
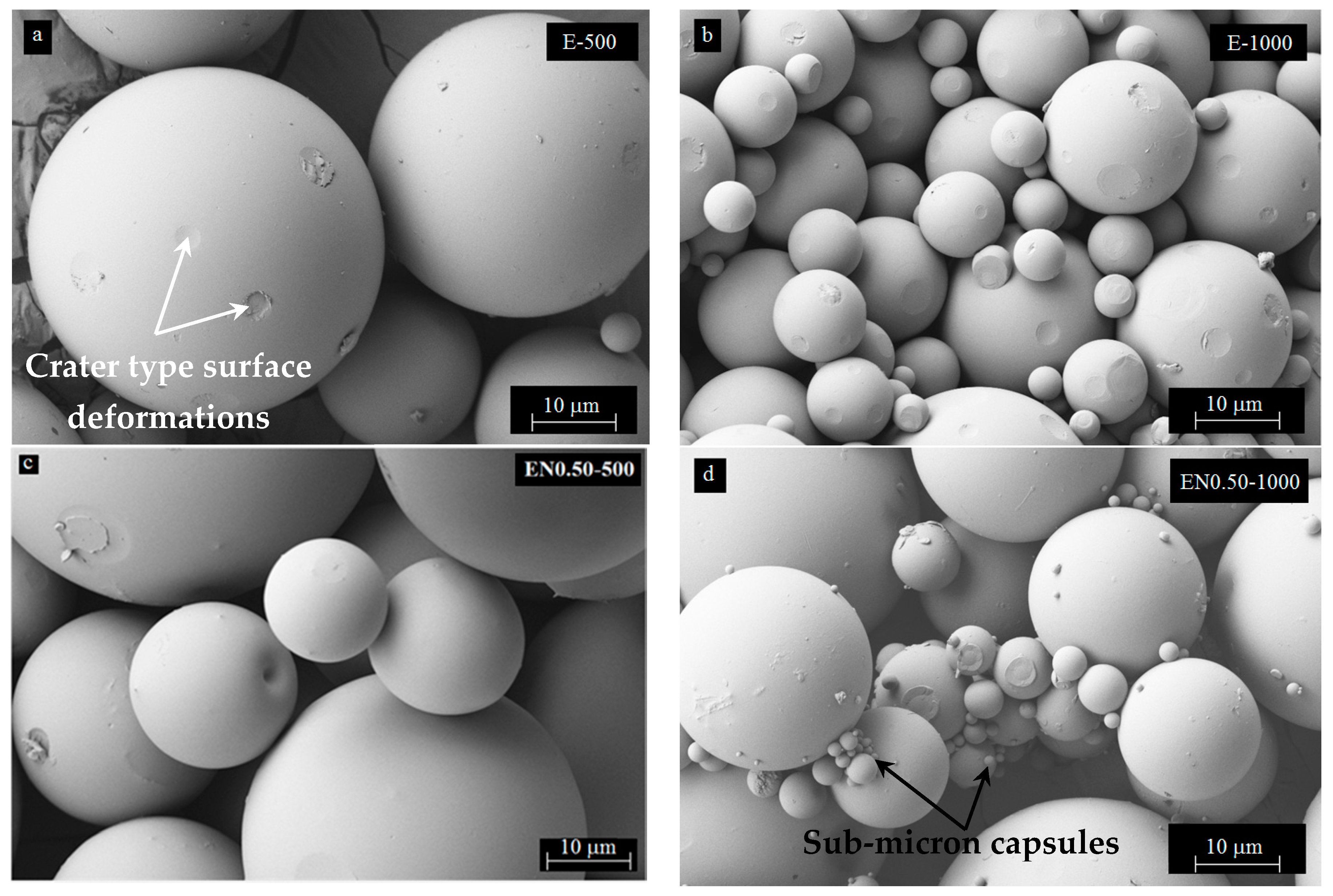
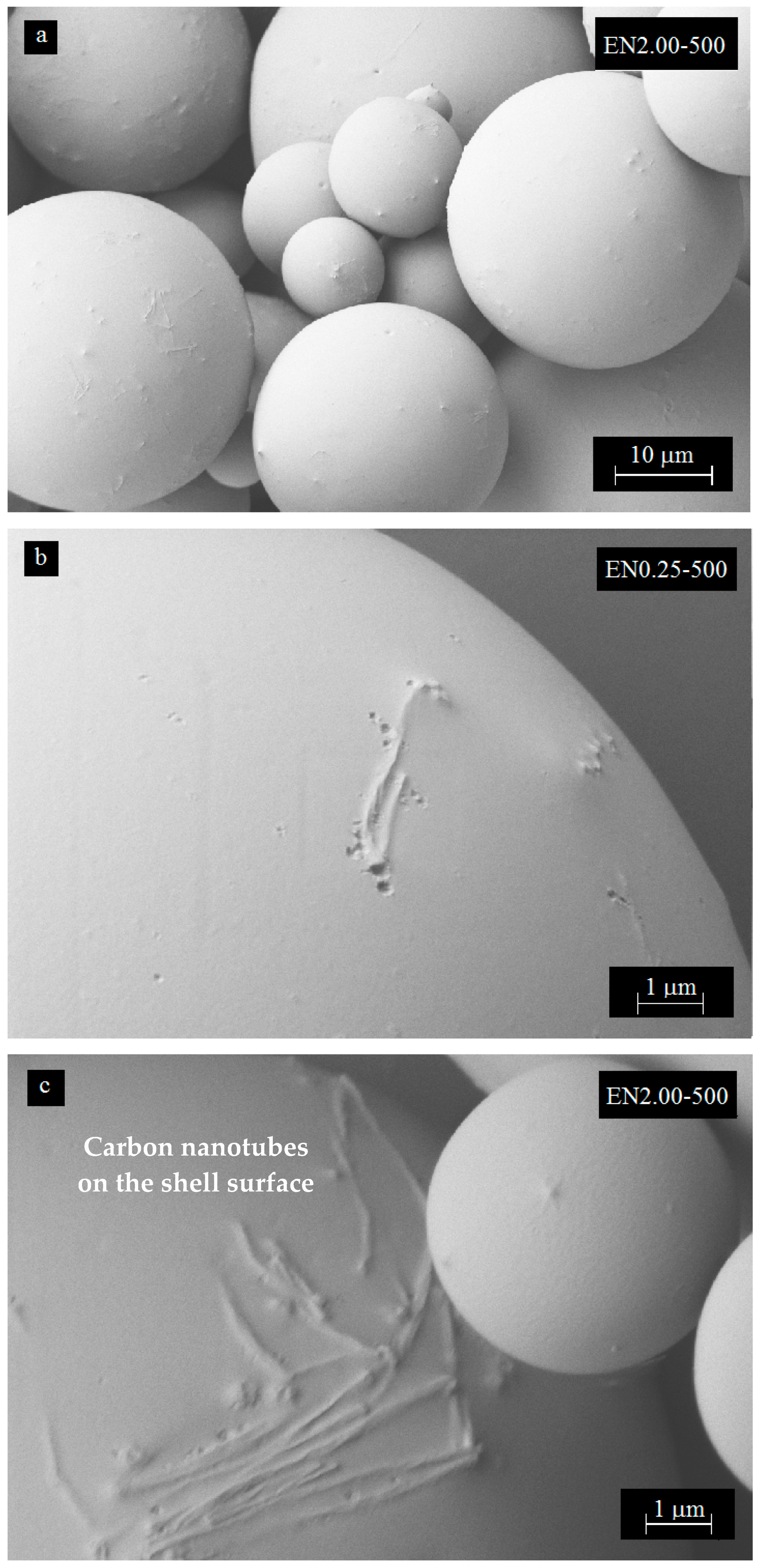
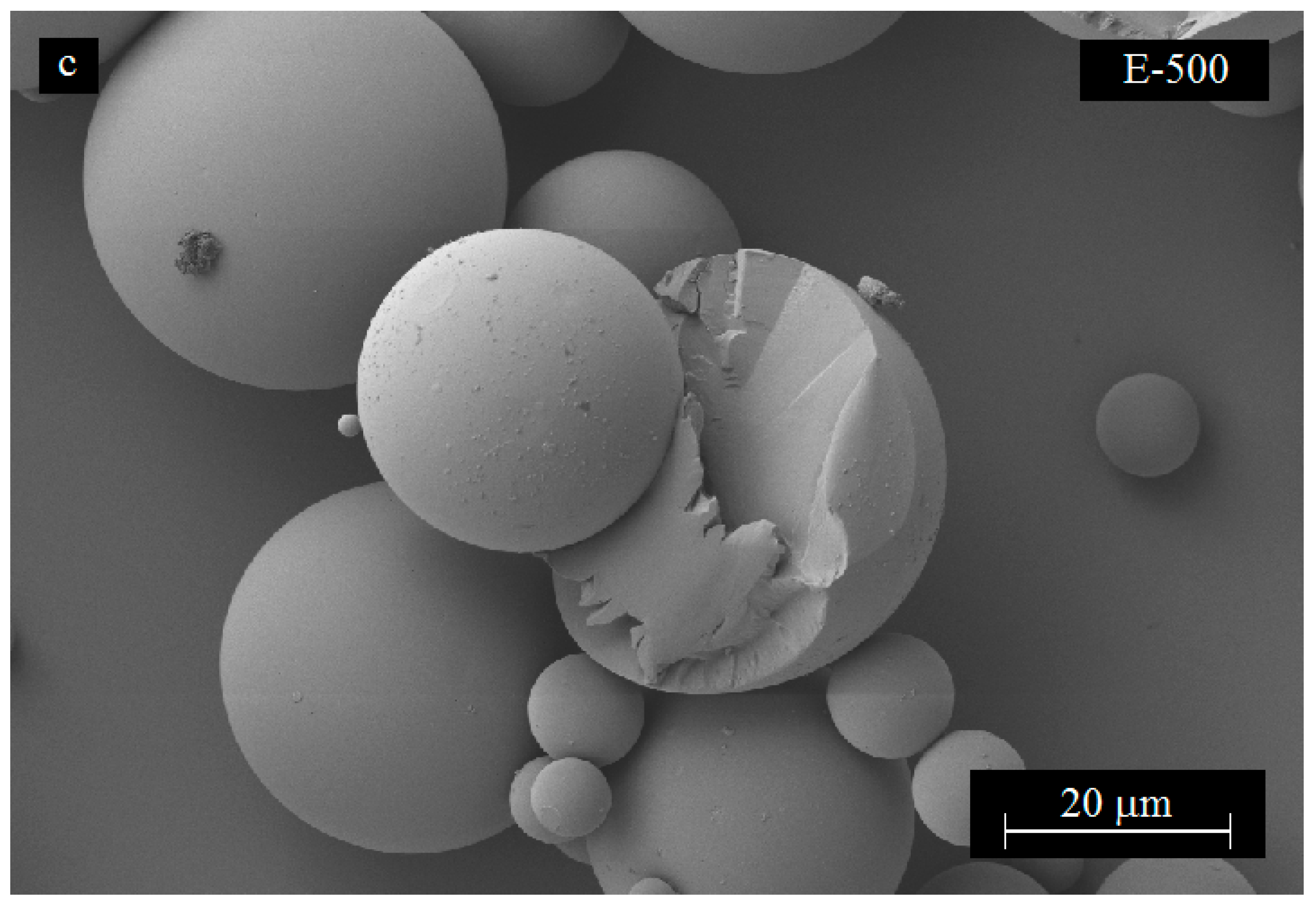
3.4. Effect of Mixing Speed on Microcapsule Morphology
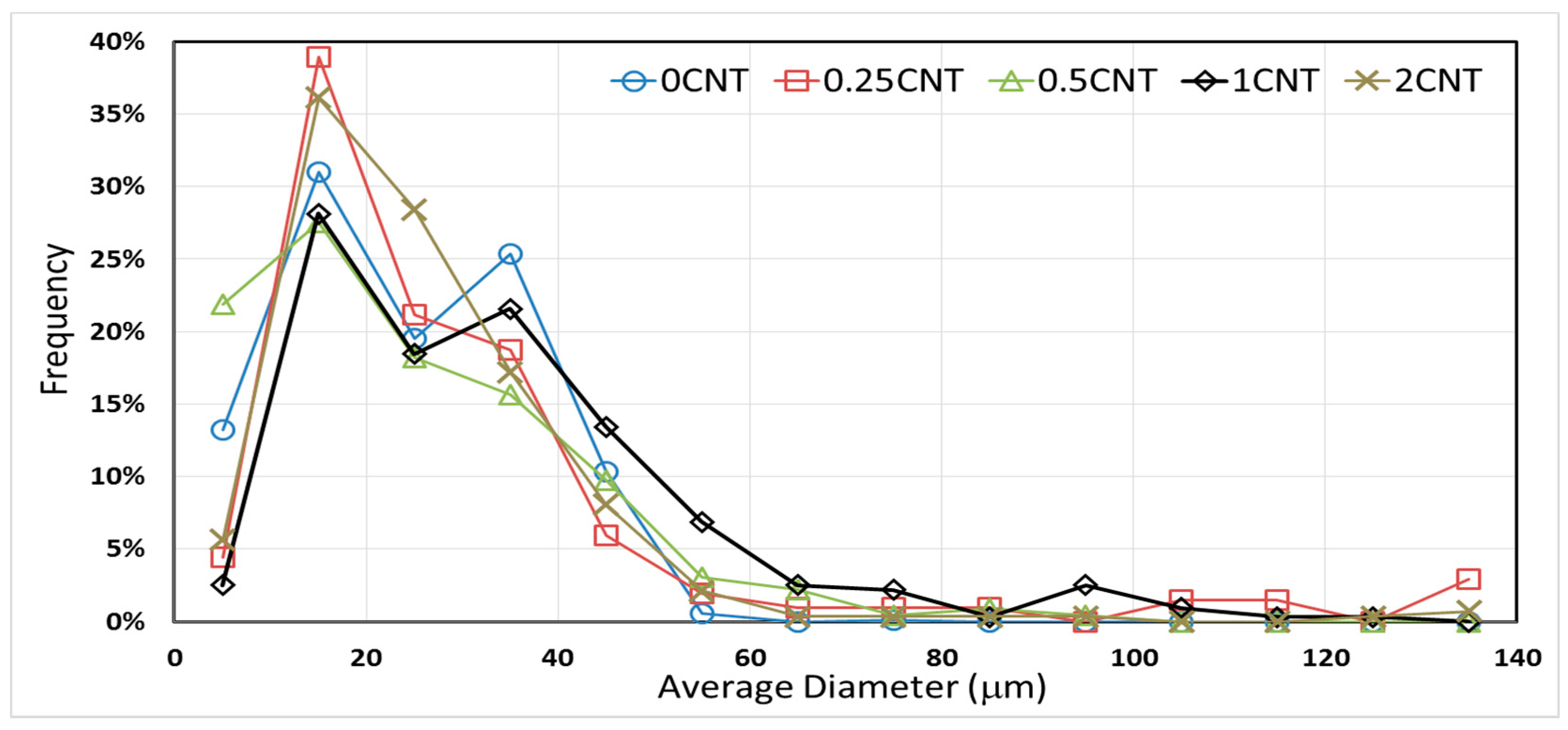
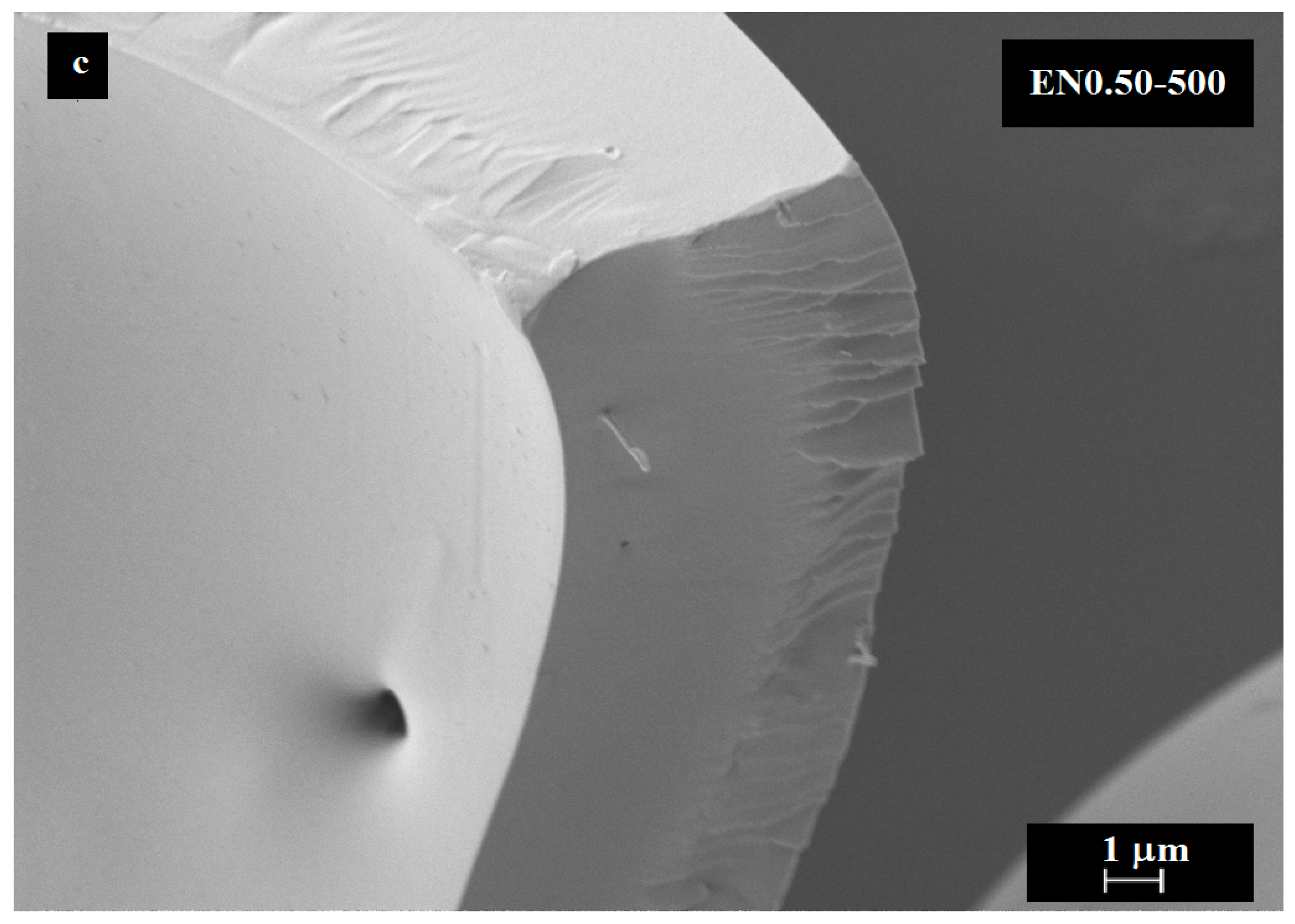
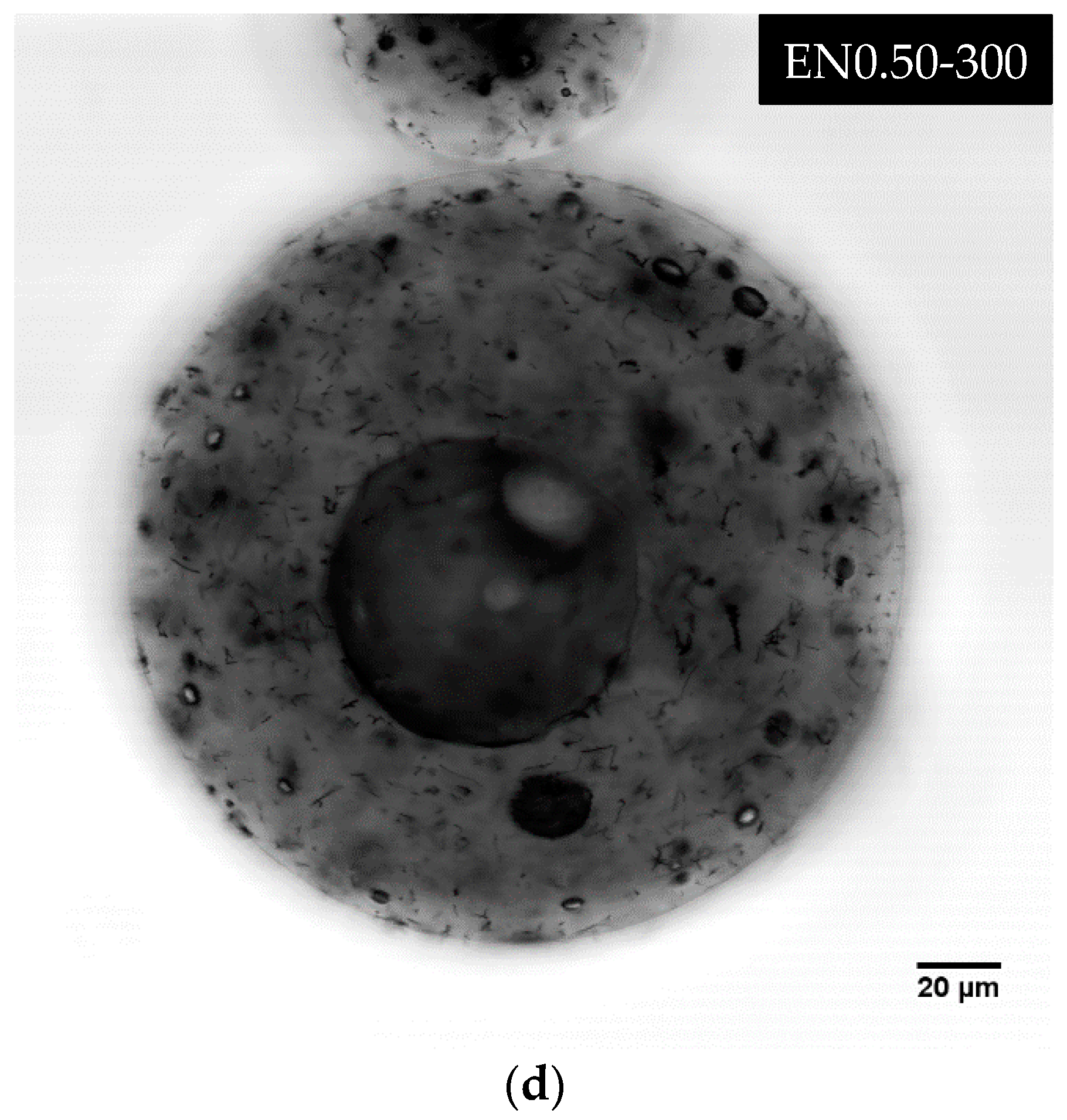
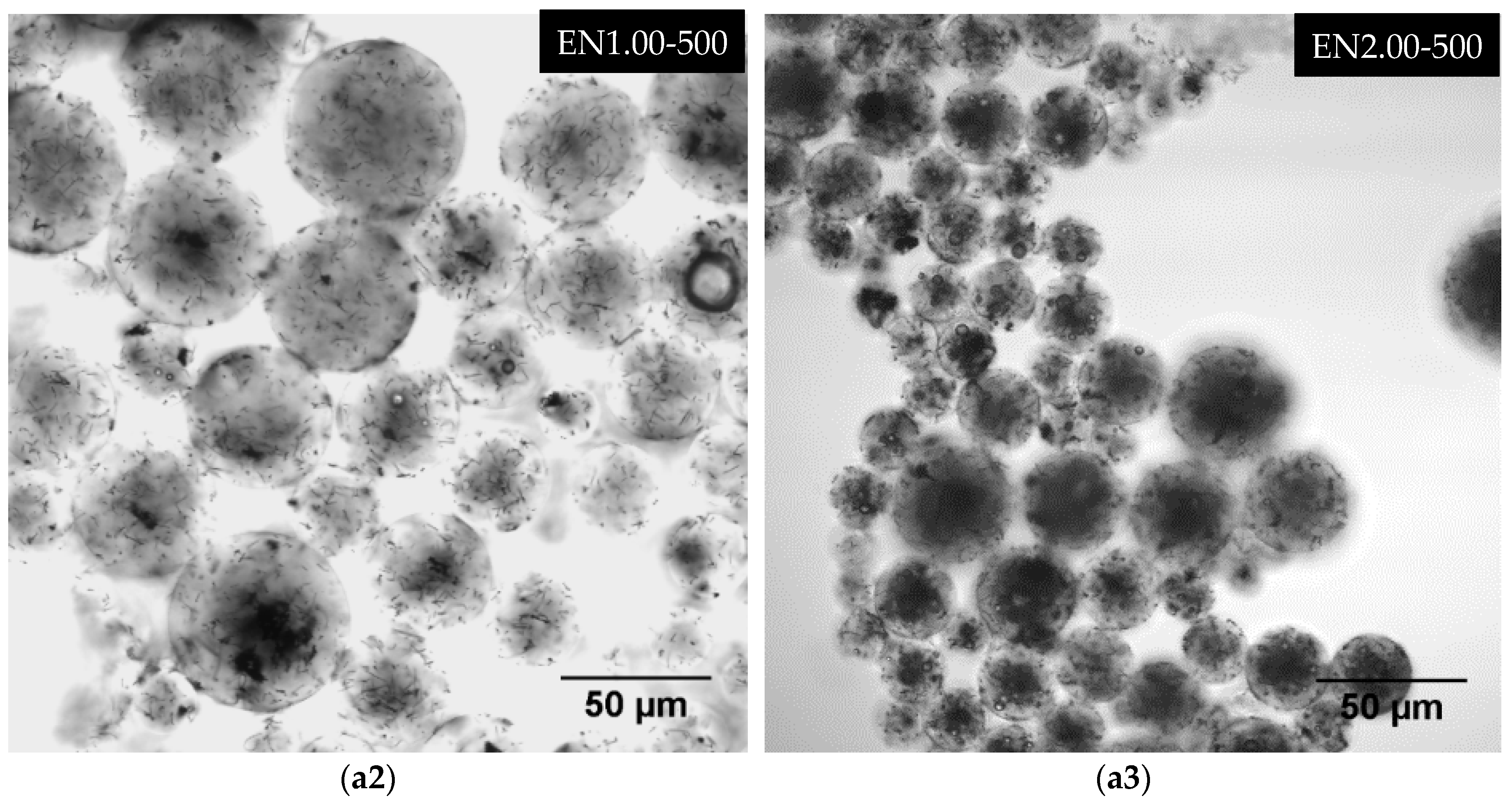
3.5. Effect of Carbon Nanotube Content on Microcapsule Morphology
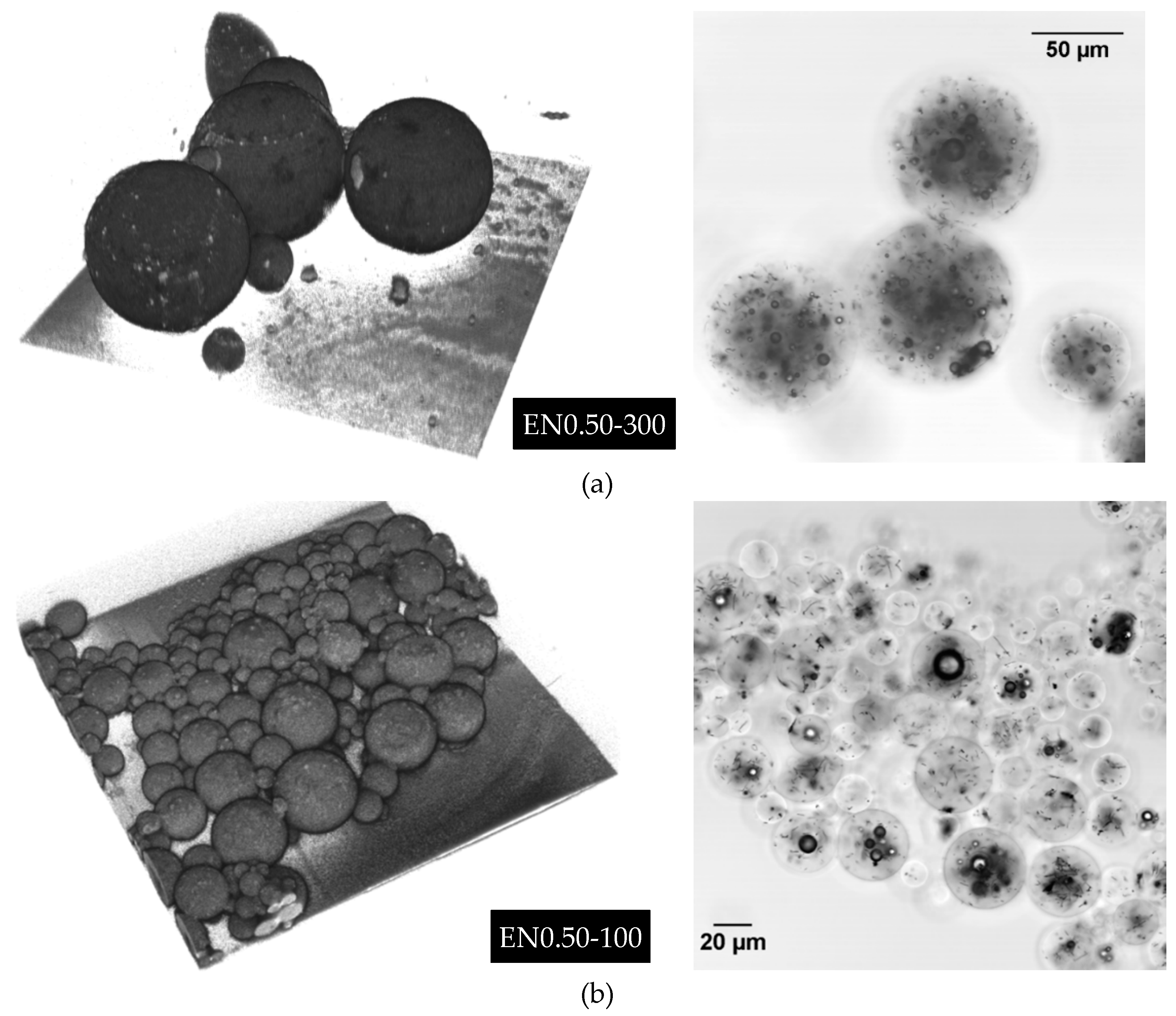
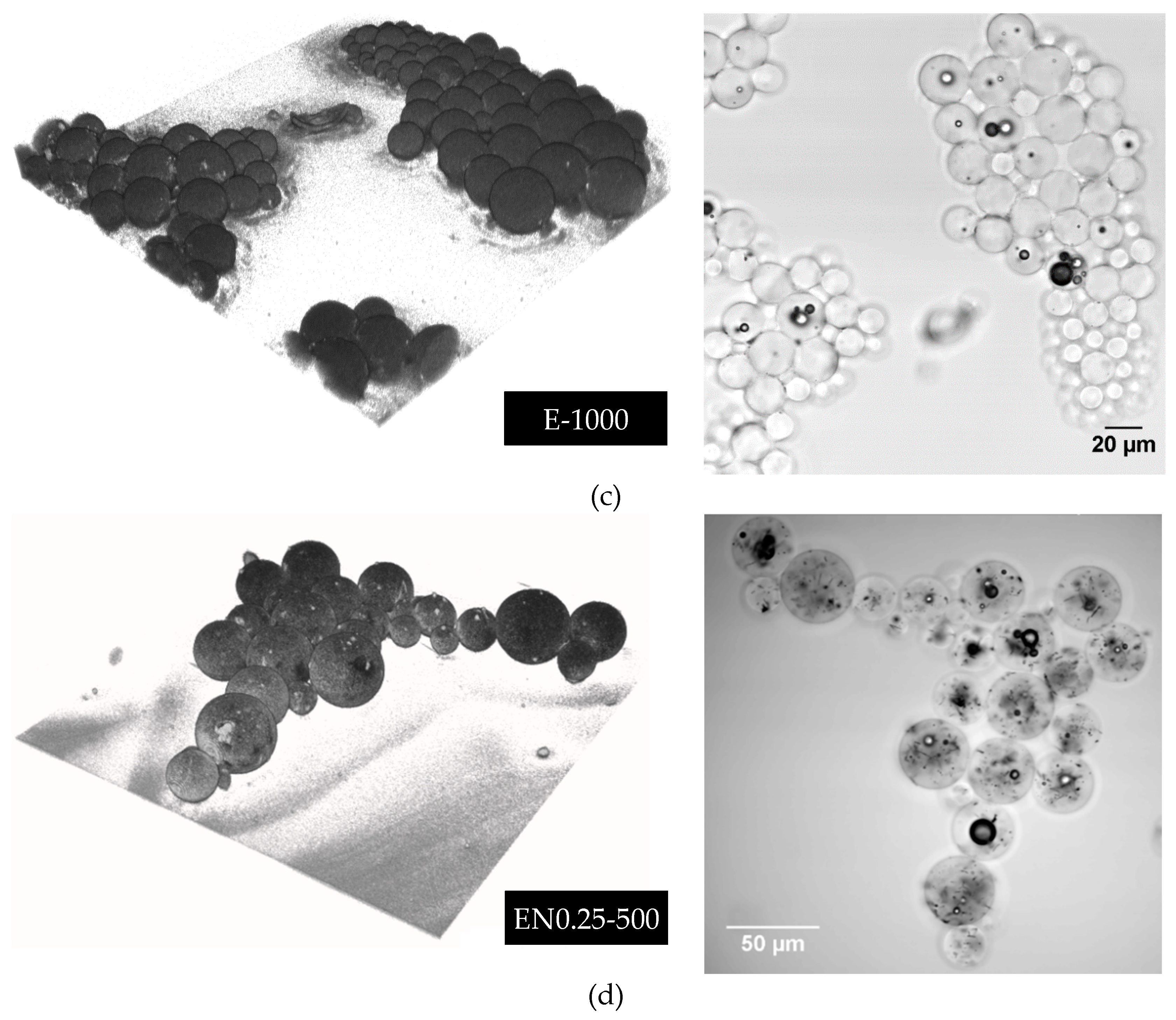
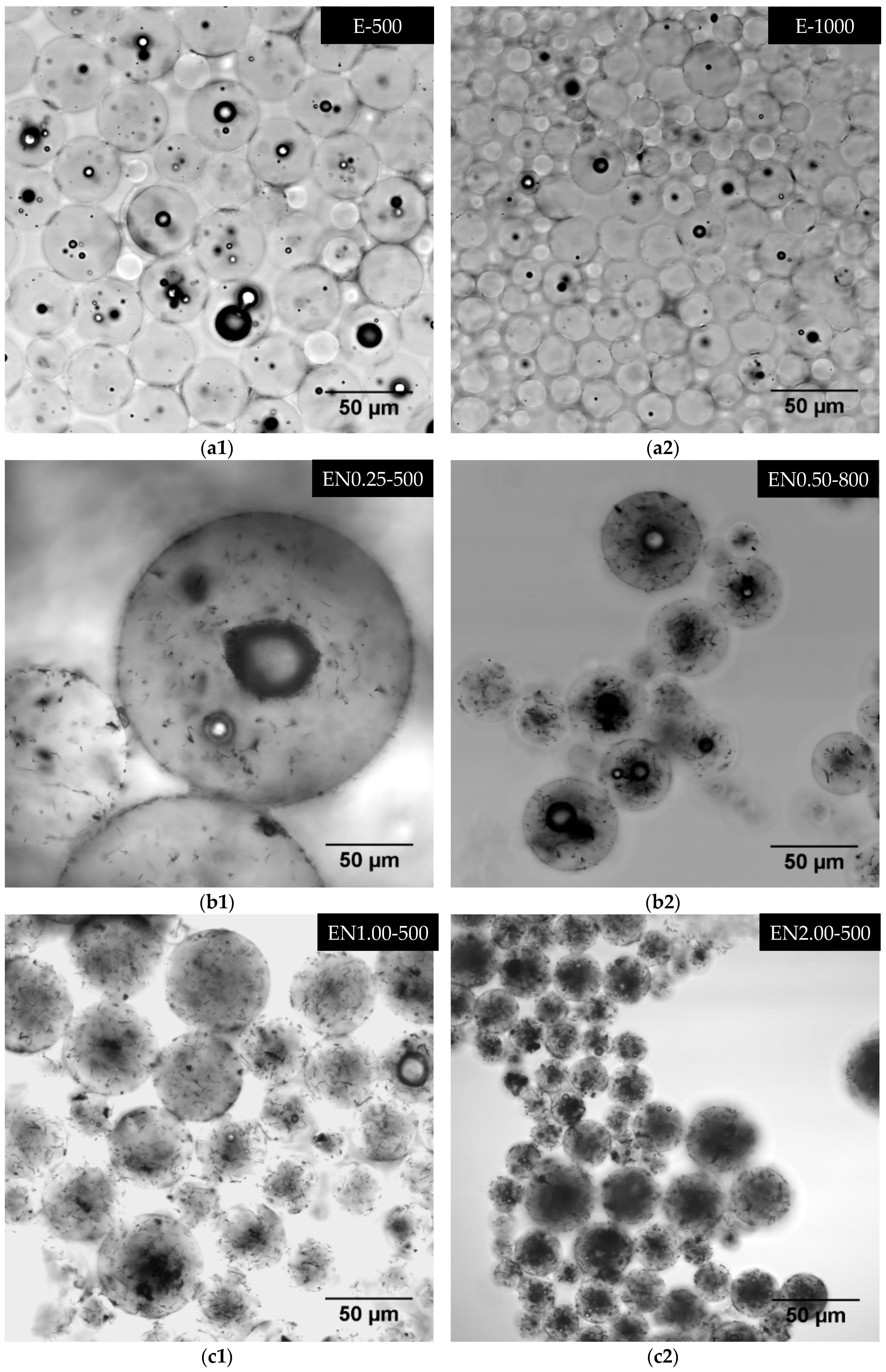
3.6. Three-Dimensional Morphology of CNT Dispersion in Microcapsules
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tewes, F.; Boury, F.; Benoit, J.P. Microencapsulation: Methods and Industrial Applications; Benita, S., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 1–54. [Google Scholar]
- Gardner, G.L. Manufacturing encapsulated products. Chem. Eng. Prog. 1966, 62, 87–91. [Google Scholar]
- Fanger, G.O. What good are microcapsules? Chemtech 1974, 4, 397–405. [Google Scholar]
- Green, B.K.; Schleicher, L. Oil-containing microscopic capsules and method of making them. U.S. Patent 2,800,457, 23 July 1957. [Google Scholar]
- Li, M.; Zhang, G.H.; Su, Z.G. Dual gradient ion-exchange chromatography improved refolding yield of lysozyme. J. Chromatogr. A. 2002, 959, 113–120. [Google Scholar] [CrossRef]
- Tang, M.; Cao, X.; Liu, Z.; Wu, X.; Gance, D. Synthesis of glycidyl methacrylate-based matrix and its application in affinity chromatography of urokinase. Processes Biochem. 1999, 34, 857–862. [Google Scholar] [CrossRef]
- Kim, B.K.; Hwang, S.J.; Park, J.B.; Park, H.J. Preparation and characterization of drug-loaded polymethacrylate microspheres by an emulsion solvent evaporation method. J. Microencapsul. 2002, 19, 811–822. [Google Scholar] [CrossRef]
- Uchida, T.; Shinosaki, K.; Nakada, Y.; Fukada, K.; Eda, Y.; Tokiyoshi, S.; Nagareya, N.; Matsuyama, K. Microencapsulation of hepatitis B core antigen for vaccine preparation. Pharm. Res. 1998, 15, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.S.; Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q. Self-healing epoxy based on cationic chain polymerization. Polymer 2009, 50, 560–568. [Google Scholar] [CrossRef]
- Yi, H.; Deng, Y.; Wang, C. Pickering emulsion-based fabrication of epoxy and amine microcapsules for dual core self-healing coating. Compos. Sci. Technol. 2016, 133, 51–59. [Google Scholar] [CrossRef]
- Caruso, M.M.; Schelkopf, S.R.; Jackson, A.C.; Landry, A.M.; Braun, P.V.; Moore, J.S. Microcapsules containing suspensions of carbon nanotubes. J. Mater. Chem. 2009, 19, 6093–6096. [Google Scholar] [CrossRef]
- Odom, S.A.; Tyler, T.P.; Caruso, M.M.; Ritchey, J.A.; Schulmerich, M.V.; Robinson, S.J.; Bhargava, R.; Sottos, N.R.; White, S.R.; Hersam, M.C.; et al. Autonomic restoration of electrical conductivity using polymer-stabilized carbon nanotube and graphene microcapsules. Appl. Phys. Lett. 2012, 101, 043106. [Google Scholar] [CrossRef][Green Version]
- Wei, B.; Zhang, L.; Chen, G. A multi-walled carbon nanotube/poly(urea-formaldehyde) composite prepared by in situ polycondensation for enhanced electrochemical sensing. New J. Chem. 2010, 34, 453–457. [Google Scholar] [CrossRef]
- Fereidoon, A.; Ahangari, M.G.; Jahanshahi, M. Effect of nanoparticles on the morphology and thermal properties of self-healing poly(urea-formaldehyde) microcapsules. J. Polym. Res. 2013, 20, 1–8. [Google Scholar] [CrossRef]
- Jung, D.; Hegeman, A.; Sottos, N.R.; Geubelle, P.H.; White, S.R. Self-healing composites using embedded microspheres. Am. Soc. Mech. Eng. Mat. Dev. 1997, 80, 265–275. [Google Scholar]
- Brown, E.N.; Kessler, M.R.; Sottos, N.R.; White, S.R.J. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. J. Microencapsul. 2003, 20, 719–730. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Kessler, M.R.; Sottos, N.R.; White, S.R. Self-healing structural composite materials. Compos. Part A Appl. Sci. Manuf. 2003, 34, 743–753. [Google Scholar] [CrossRef]
- Jin, H.; Mangun, C.L.; Stradley, D.S.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing thermoset using encapsulated epoxy-amine healing chemistry. Polymer 2012, 53, 581–587. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Pircheraghi, G.; Monemian, S.A.; Manas-Zloczower, I. Epoxy composites with carbon nanotubes and graphene nanoplatelets—Dispersion and synergy effects. Carbon 2014, 78, 268–278. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nuesch, F.A.; Chu, B.T.T. Comparing carbon nanotubes and graphene nanoplatelets as reinforcements in polyamide 12 composites. Nanotechnology 2011, 22, 1–8. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Abdul Kudus, M.H.; Akil, H.M.; Thirmizir, M.Z.M. Comparative study of graphene nanoparticle and multiwall carbon nanotube filled epoxy nanocomposites based on mechanical, thermal and dielectric properties. Compos. B Eng. 2017, 119, 57–66. [Google Scholar] [CrossRef]
- Ye, X.J.; Zhu, Y.; Yuan, Y.C.; Song, Y.X.; Yang, G.C.; Rong, M.Z.; Zhang, M.Q. Improvement of fatigue resistance of epoxy composite with microencapsulated epoxy-SbF5 self-healing system. Express Polym. Lett. 2017, 11, 853–862. [Google Scholar] [CrossRef]
- Li, C.; Tan, J.; Gu, J.; Qiao, L.; Zhang, B.; Zhang, Q. Rapid and efficient synthesis of isocyanate microcapsules via thiol-ene photopolymerization in Pickering emulsion and its application in self-healing coating. Compos. Sci. Tech. 2016, 123, 250–258. [Google Scholar] [CrossRef]
- Su, J.F.; Zha, Y.H.; Wang, X.Y.; Dong, H.; Wang, S.B. Effect of interface debonding on the thermal conductivity of microencapsulated-paraffin filled epoxy matrix composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 325–332. [Google Scholar] [CrossRef]
- Ullah, H.; Azizli, K.A.M.; Man, Z.B.; Ismail, M.B.C.; Khan, M.I. the potential of microencapsulated self-healing materials for microcracks recovery in self-healing systems: a review. Polym. Rev. 2016, 56, 429–485. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Caruso, M.M.; McIlroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Evaluation of peroxide initiators for radical polymerization-based self-healing applications. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Jiang, X.B.; Rong, M.Z.; Zhang, M.Q. Free radical polymerization aided self-healing. J. Intell. Mater. Syst. Struct. 2014, 25, 31–39. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Rong, M.; Zhang, M. Microencapsulation of styrene with melamine-formaldehyde resin. Coll. Polym. Sci. 2009, 287, 1089–1097. [Google Scholar] [CrossRef]
- Xing, S.; Yang, J.; Huang, Y.; Zheng, Q.; Zeng, J. Preparation and characterization of a novel microcapsule-type latent curing agent for epoxy resin. Mater. Des. 2015, 85, 661–670. [Google Scholar] [CrossRef]
- Prasertmanakit, S.; Praphairaksit, N.; Chiangthong, W.; Muangsin, N. Ethyl cellulose microcapsules for protecting and controlled release of folic acid. AAPS Pharmac. Sci. Tech. 2009, 10, 1104–1112. [Google Scholar] [CrossRef]
- Wilson, G.O.; Henderson, J.W.; Caruso, M.M.; Blaiszik, B.J.; McIntire, P.J.; Sottos, N.R.; White, S.R.; Moore, J.S. Evaluation of peroxide initiators for radical polymerization-based self-healing applications. J. Polym. Sci. Part A 2010, 48, 2698–2708. [Google Scholar] [CrossRef]
- Xu, H.; Fang, Z.; Tong, L. Effect of microencapsulated curing agents on the curing behavior for diglycidyl ether of bisphenol A epoxy resin systems. J. Appl. Polym. Sci. 2008, 107, 1661–1669. [Google Scholar] [CrossRef]
- Vijayan, P.; AlMaadeed, M.A. ‘Containers’ for self-healing epoxy composites and coating: Trends and advances. Express Polym. Lett. 2016, 10, 506–524. [Google Scholar] [CrossRef]
- Li, Q.; Mishra, A.K.; Kim, N.H.; Kuila, T.; Lau, K.; Lee, J.H. Effects of processing conditions of poly(methylmethacrylate) encapsulated liquid curing agent on the properties of self-healing composites. Compos. B Eng 2013, 49, 6–15. [Google Scholar] [CrossRef]
- Yuan, L.; Liang, G.; Xie, J.; Li, L.; Guo, J. Preparation and characterization of poly(urea-formaldehyde) microcapsules filled with epoxy resins. Polymer 2006, 47, 5338–5349. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Wang, L.; Hao, D.; Ma, G. Fabrication strategy for amphiphilic microcapsules with narrow size distribution by premix membrane emulsification. Colloids Surf. B Biointerfaces 2011, 87, 399–408. [Google Scholar] [CrossRef]
- Tadros, T.F.; Vandamme, A.; Levecke, B.; Booten, K.; Steven, C.V. Stabilization of emulsions using polymeric surfactants based on inulin. Adv. Colloid Interface Sci. 2004, 108–109, 207–226. [Google Scholar] [CrossRef]
- Li, Q.; Siddaramaiah; Kim, N.H.; Hui, D.; Lee, J.H. Effects of dual component microcapsules of resin and curing agent on the self-healing efficiency of epoxy. Compos. B Eng. 2013, 55, 79–85. [Google Scholar] [CrossRef]
- Loxley, A.; Vincent, B. Preparation of Poly(methylmethacrylate) Microcapsules with Liquid Cores. J. Colloid Interface Sci. 1998, 208, 49–62. [Google Scholar] [CrossRef]
- Torza, S.; Mason, S.G. Three-phase interactions in shear and electrical fields. J. Colloid Interface Sci. 1970, 33, 67–83. [Google Scholar] [CrossRef]
- Phamduy, P.; Kim, B. Microcapsules containing solvent and epoxy with multi-walled carbon nanotubes for self-healing. In Proceedings of the ASME 2014 International Mechanical Engineering Congress, Montreal, QC, Canada, 14–20 November 2014. [Google Scholar] [CrossRef]
- Cheng, Q.H.; Debnath, S.; Gregan, E.; Byrne, H.J. Ultrasound-assisted SWNTs dispersion: Effects of sonication parameters and solvent properties. J. Phys. Chem. C 2010, 114, 8821–8827. [Google Scholar] [CrossRef]
- Aktas, L.; Bauman, D.P.; Bowen, S.T.; Saha, M.C.; Altan, M.C. Effect of Distribution Media Length and Multiwalled Carbon Nanotubes on the Formation of Voids in VARTM Composites. J. Eng. Mater. Technol. 2011, 133, 1–9. [Google Scholar] [CrossRef]
- Tang, L.; Dang, J.; He, M.; Li, J.; Kong, J.; Tang, Y.; Gu, J. Preparation and properties of cyanate-based wave-transparent laminated composites reinforced by dopamine/POSS functionalized Kevlar cloth. Compos. Sci. Technol. 2019, 169, 120–126. [Google Scholar] [CrossRef]
- Knipprath, C.; McCombe, G.P.; Trask, R.S.; Bond, I.P. Predicting self-healing strength recovery using a multi-objective genetic algorithm. Compos. Sci. Tech. 2012, 72, 752–759. [Google Scholar] [CrossRef]
- Khun, N.W.; Zhang, H.; Yang, J.; Liu, E. Mechanical and tribological properties of epoxy matrix composites modified with microencapsulated mixture of wax lubricant and multi-walled carbon nanotubes. Friction 2013, 1, 341–349. [Google Scholar] [CrossRef]
- Duan, G.; Zhang, C.; Li, A.; Yang, X.; Lu, L.; Wang, X. Preparation and characterization of mesoporous zirconia made by using a poly (methyl methacrylate) template. Nanoscale Res. Lett. 2008, 3, 118–122. [Google Scholar] [CrossRef]
- Eissa, M.M.; Youssef, M.S.A.; Ramadan, A.M.; Amin, A. Poly(ester-amine) hyperbranched polymer as toughening and co-curing agent for epoxy/clay nanocomposites. Polym. Eng. Sci. 2013, 53, 1011–1020. [Google Scholar] [CrossRef]


| Sample Code | CNT Content (wt %) | Mixing Speed (rpm) |
|---|---|---|
| E-300 | 0 | 300 |
| E-500 | 0 | 500 |
| E-800 | 0 | 800 |
| E-1000 | 0 | 1000 |
| EN0.25-500 | 0.25 | 500 |
| EN0.50-300 | 0.50 | 300 |
| EN0.50-500 | 0.50 | 500 |
| EN0.50-800 | 0.50 | 800 |
| EN0.50-1000 | 0.50 | 1000 |
| EN1.00-500 | 1.00 | 500 |
| EN2.00-500 | 2.00 | 500 |
| Sample Code | T5 (°C) | T30 (°C) | THRI (°C) |
|---|---|---|---|
| PMMA | 245.37 | 279.32 | 130.21 |
| EPON 828 | 269.38 | 327.82 | 149.17 |
| E-500 | 254.87 | 317.36 | 143.26 |
| EN0.25-500 | 252.71 | 304.15 | 138.95 |
| EN0.50-500 | 252.86 | 303.89 | 138.90 |
| EN1.00-500 | 253.69 | 304.88 | 139.36 |
| EN2.00-500 | 249.80 | 348.63 | 151.46 |
| Sample Code | Average Diameter (μm) ± 95% Confidence Interval | Dmax (μm) |
|---|---|---|
| E-300 | 26.1 ± 1.9 | 119.4 |
| E-500 | 24.2 ± 0.8 | 75.8 |
| E-800 | 17.5 ± 0.7 | 62.5 |
| E-1000 | 14.7 ± 0.9 | 57.1 |
| E-300 | 26.1 ± 1.9 | 119.4 |
| Sample Code | Average Diameter (μm) ± 95% Confidence Interval | Dmax (μm) |
|---|---|---|
| EN0.50-300 | 52.2 ± 4.0 | 149.3 |
| EN0.50-500 | 24.6 ± 1.5 | 95.5 |
| EN0.50-800 | 21.2 ± 1.3 | 69.6 |
| EN0.50-1000 | 15.6 ± 1.0 | 42.8 |
| Sample Code | Average Diameter (μm) ± 95% Confidence Interval | Dmax (μm) |
|---|---|---|
| E-500 | 24.2 ± 0.8 | 75.8 |
| EN0.25-500 | 31.8 ± 4.4 | 215.3 |
| EN0.50-500 | 24.6 ± 1.5 | 95.5 |
| EN1.00-500 | 34.0 ± 2.0 | 127.0 |
| EN2.00-500 | 26.0 ± 2.3 | 154.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Icduygu, M.G.; Asilturk, M.; Yalcinkaya, M.A.; Hamidi, Y.K.; Altan, M.C. Three-Dimensional Nano-Morphology of Carbon Nanotube/Epoxy Filled Poly(methyl methacrylate) Microcapsules. Materials 2019, 12, 1387. https://doi.org/10.3390/ma12091387
Icduygu MG, Asilturk M, Yalcinkaya MA, Hamidi YK, Altan MC. Three-Dimensional Nano-Morphology of Carbon Nanotube/Epoxy Filled Poly(methyl methacrylate) Microcapsules. Materials. 2019; 12(9):1387. https://doi.org/10.3390/ma12091387
Chicago/Turabian StyleIcduygu, M. Galip, Meltem Asilturk, M. Akif Yalcinkaya, Youssef K. Hamidi, and M. Cengiz Altan. 2019. "Three-Dimensional Nano-Morphology of Carbon Nanotube/Epoxy Filled Poly(methyl methacrylate) Microcapsules" Materials 12, no. 9: 1387. https://doi.org/10.3390/ma12091387
APA StyleIcduygu, M. G., Asilturk, M., Yalcinkaya, M. A., Hamidi, Y. K., & Altan, M. C. (2019). Three-Dimensional Nano-Morphology of Carbon Nanotube/Epoxy Filled Poly(methyl methacrylate) Microcapsules. Materials, 12(9), 1387. https://doi.org/10.3390/ma12091387