Evaluation of a Catalyst Durability in Absence and Presence of Toluene Impurity: Case of the Material Co2Ni2Mg2Al2 Mixed Oxide Prepared by Hydrotalcite Route in Methane Dry Reforming to Produce Energy
Abstract
1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
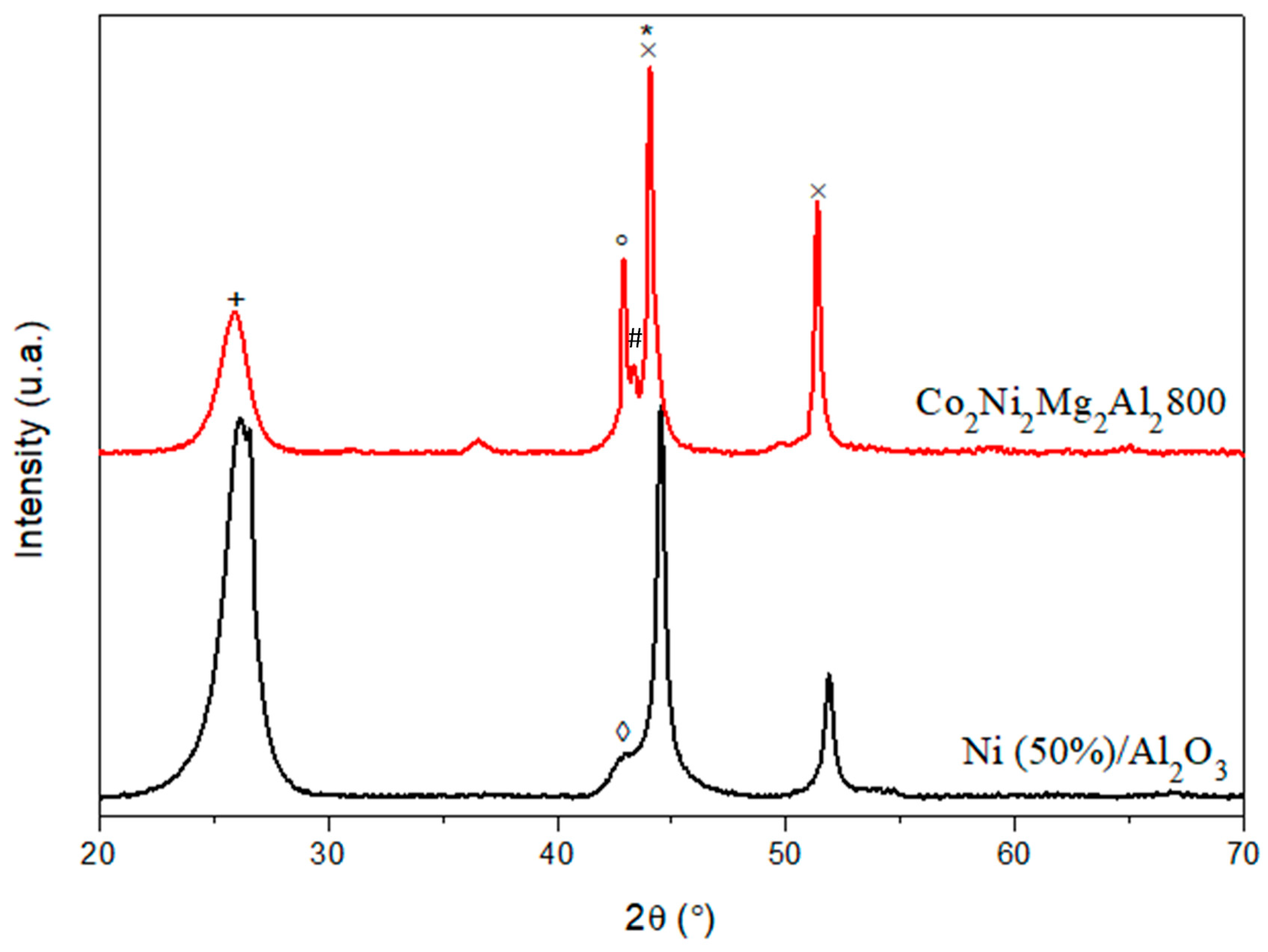
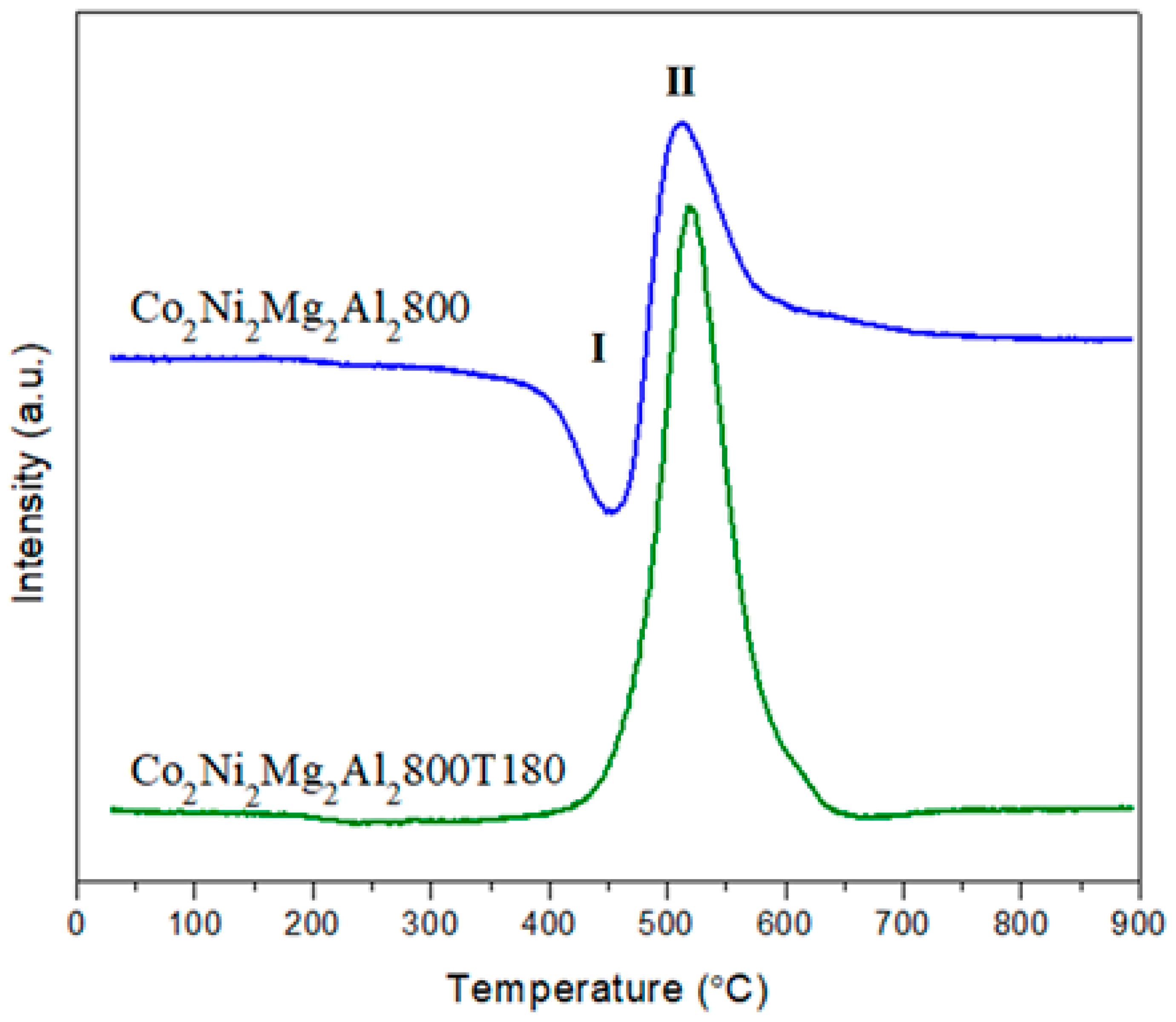
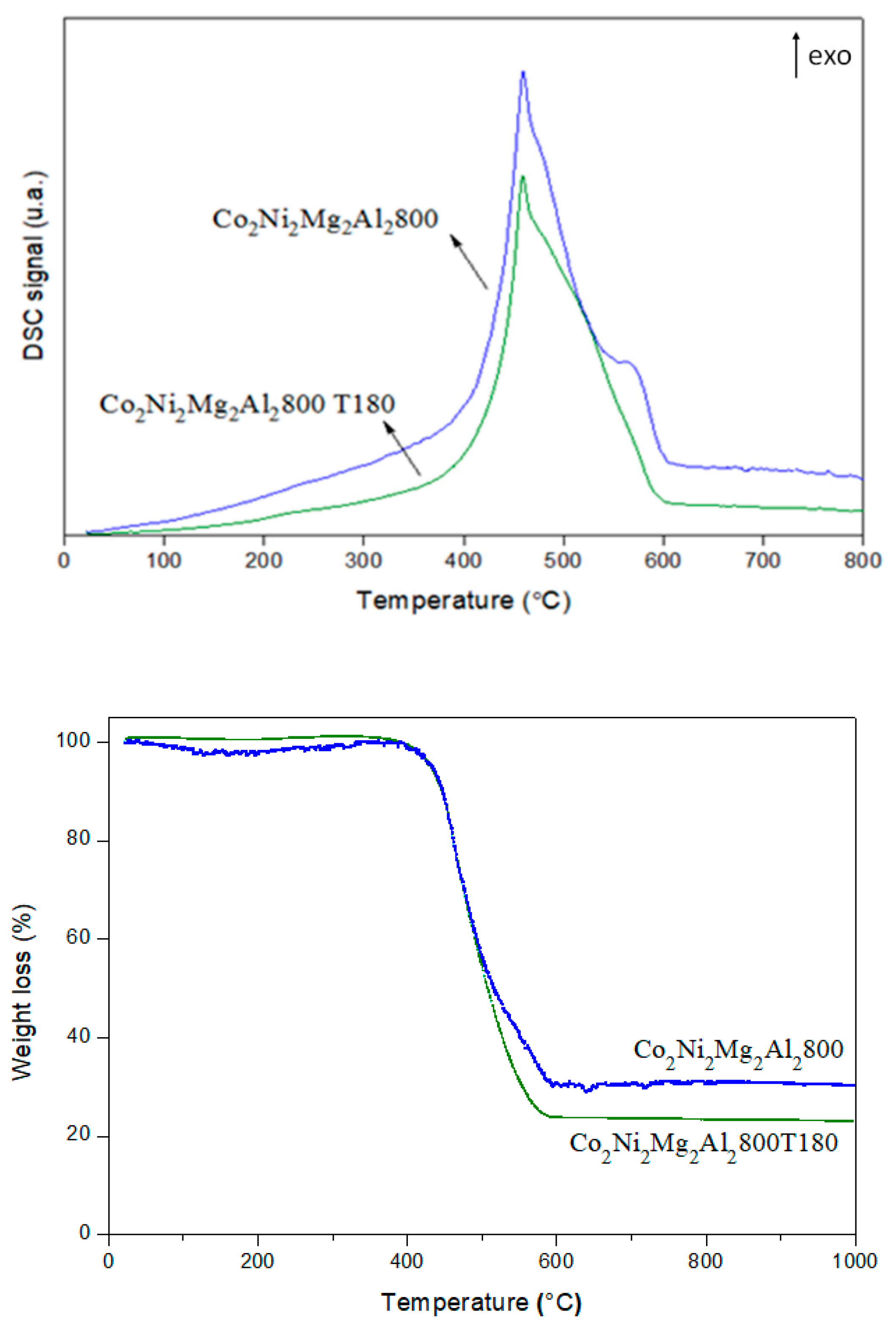
2.2. Characterization of the Solids
2.3. Experimental Conditions of the Test
3. Results
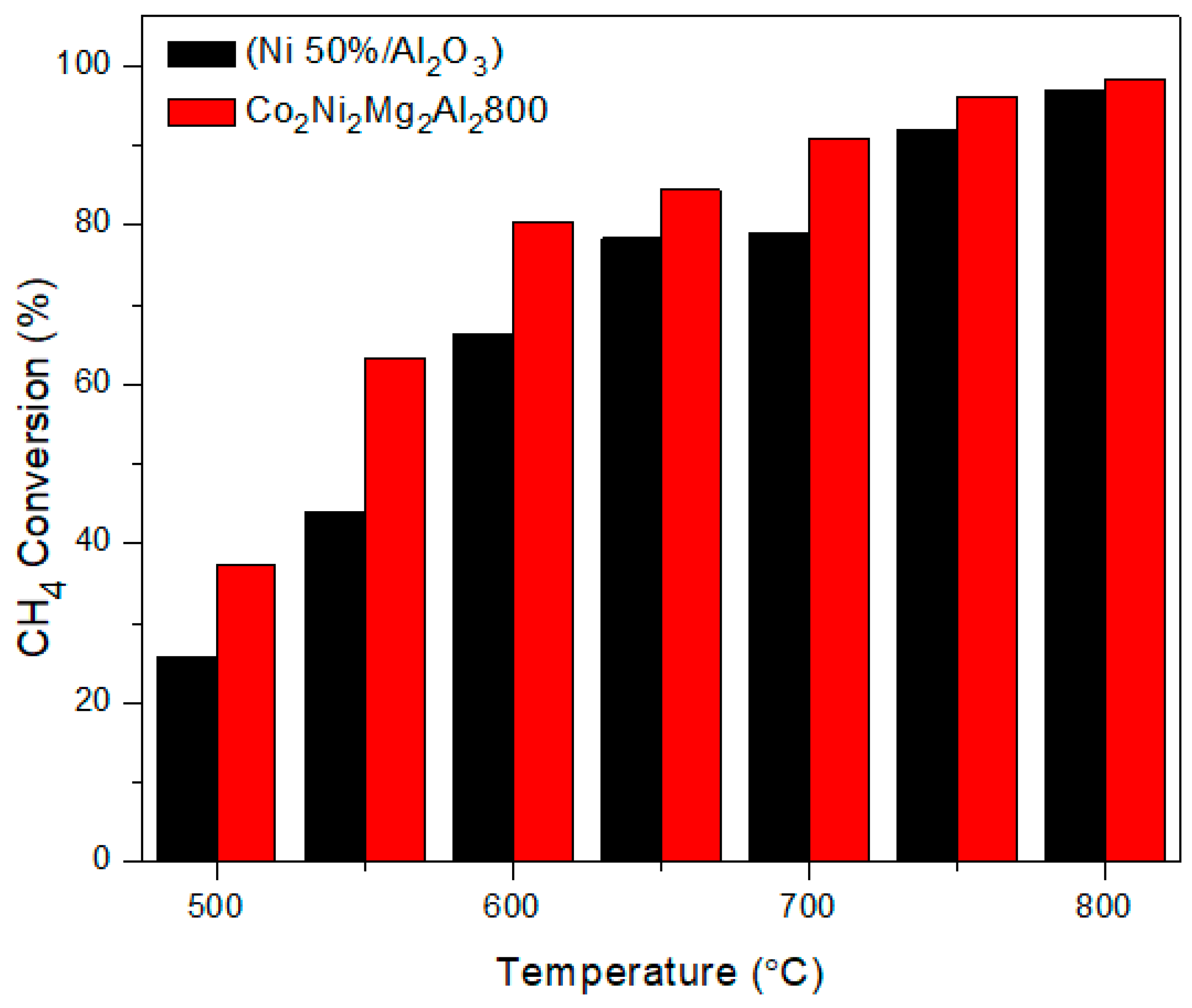
3.1. Comparison between Co2Ni2Mg2Al2800 and the Commercial Catalyst Ni (50%)/Al2O3
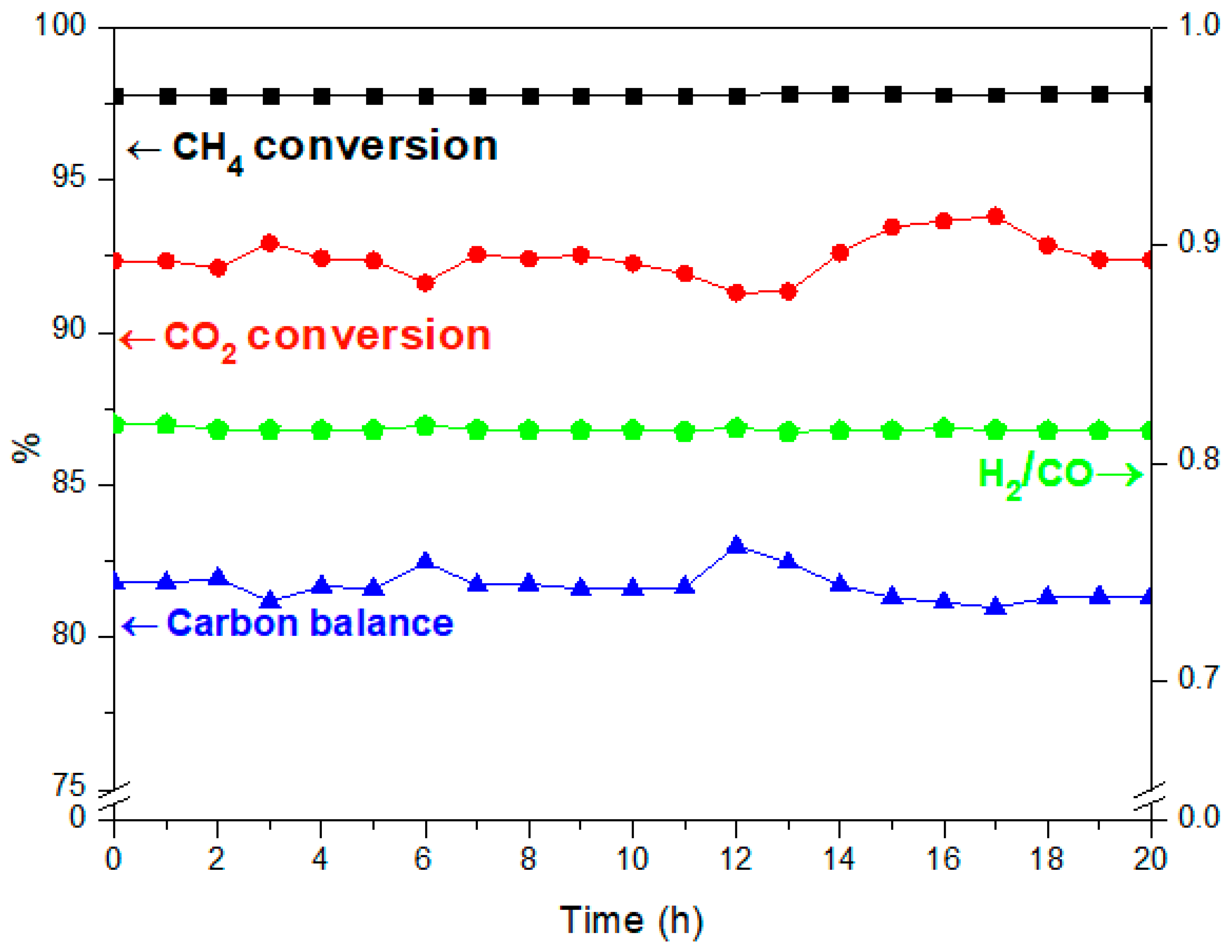
3.2. Stability Test for Co2Ni2Mg2Al2800
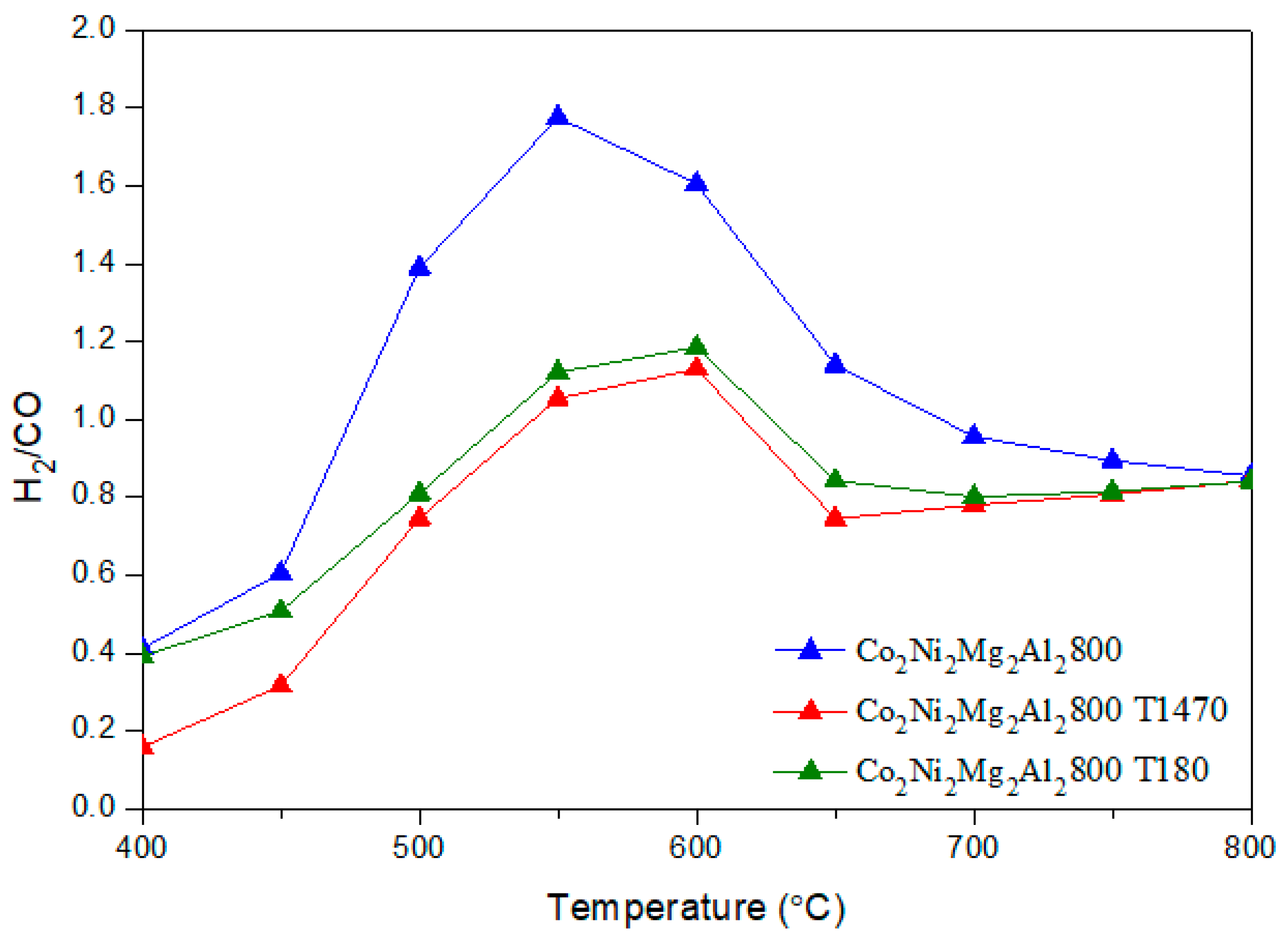
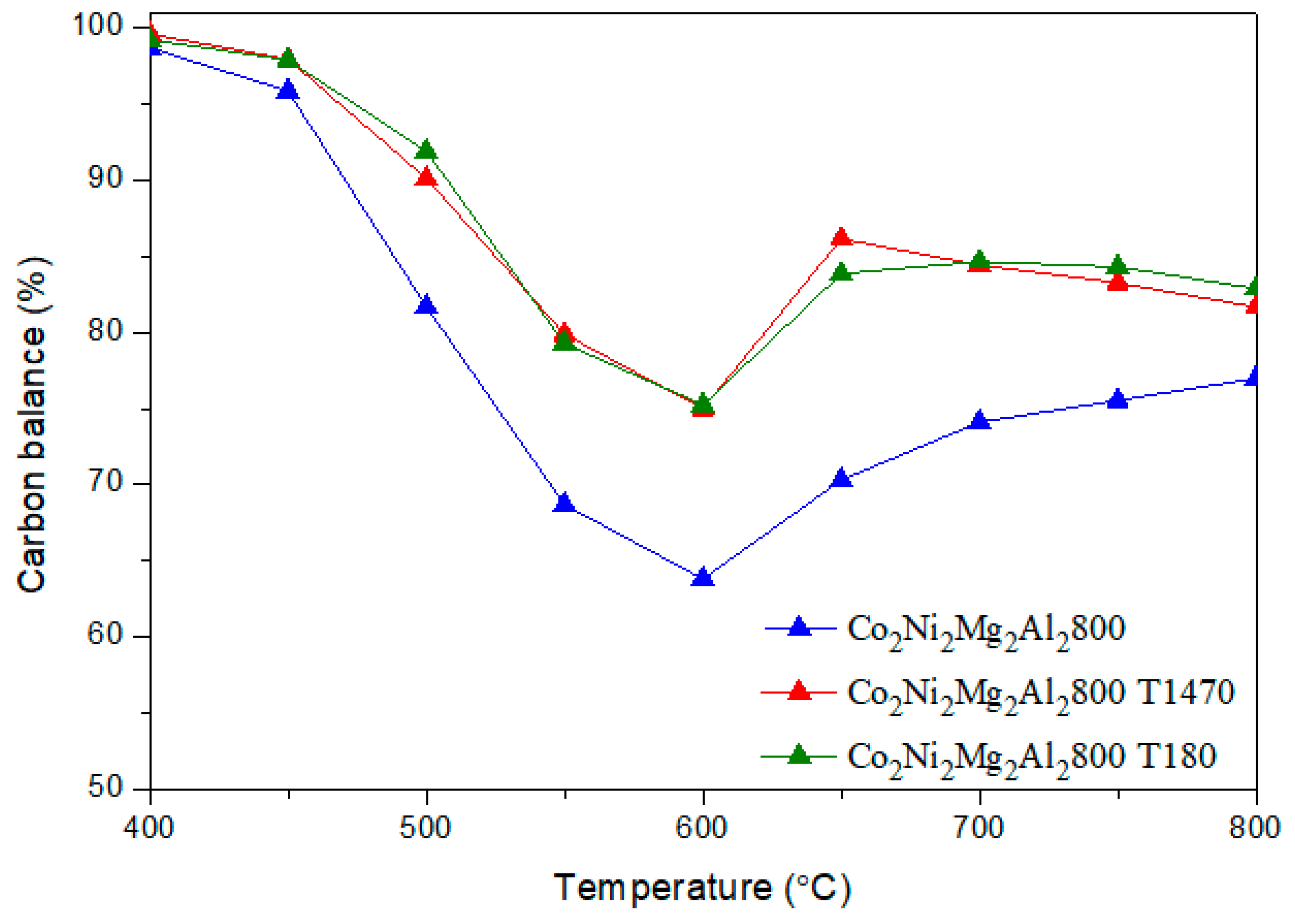
 CO + H2O) (b) and the methane decomposition (CH4 → C + 2 H2) (c) compete with the main DRM reaction. Indeed, during the reverse water gas shift (b), CO2 reacts with H2 to produce CO and H2O, thus leading to a ratio H2/CO < 1. In fact, small amounts of water were detected. In addition, the methane decomposition (c) produces C and H2. Therefore, higher CH4 conversion compared to CO2 as well as carbon deposition, which leads to a carbon balance lower than 100%, could be due to the occurrence of reaction (c).
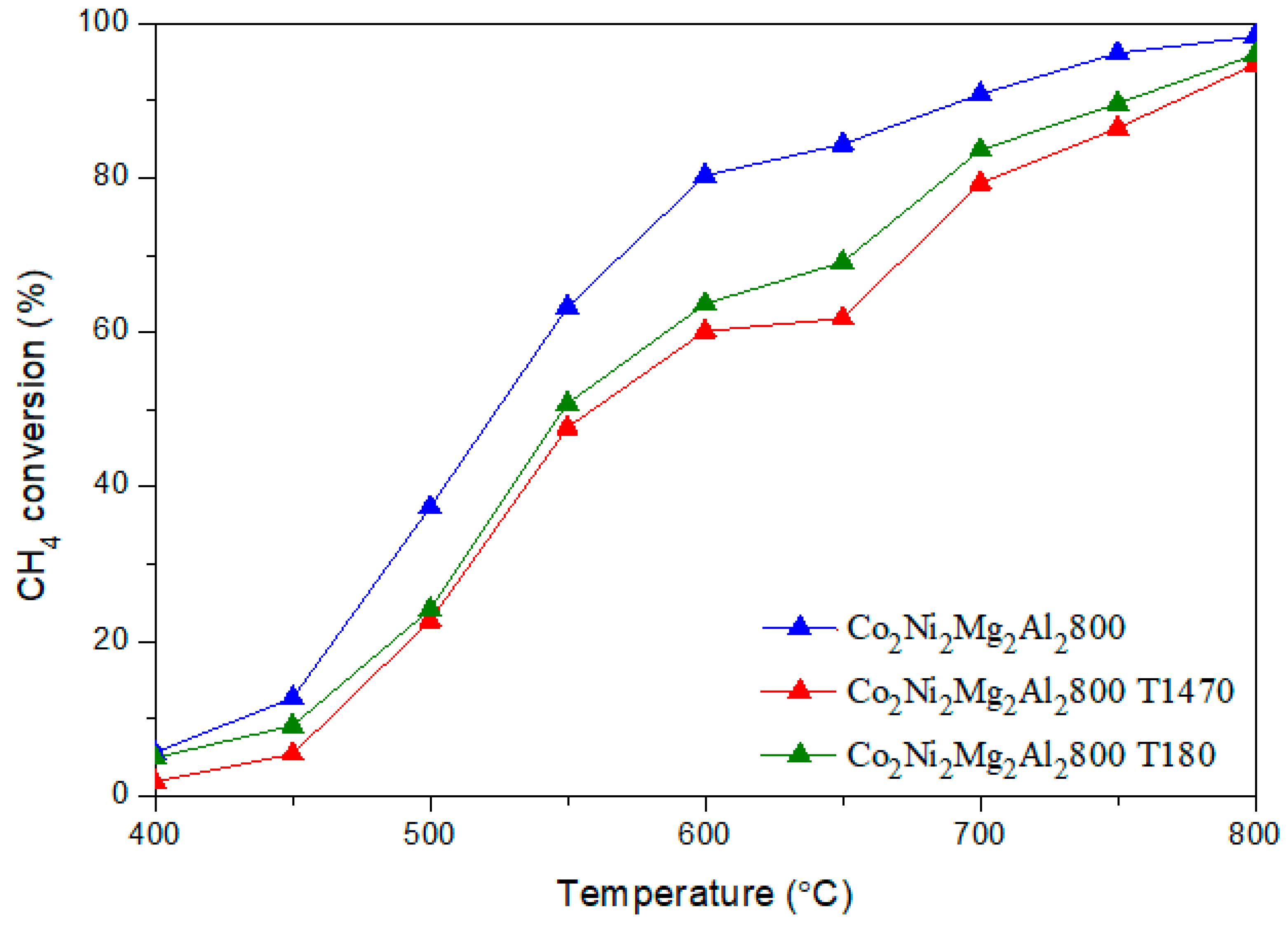
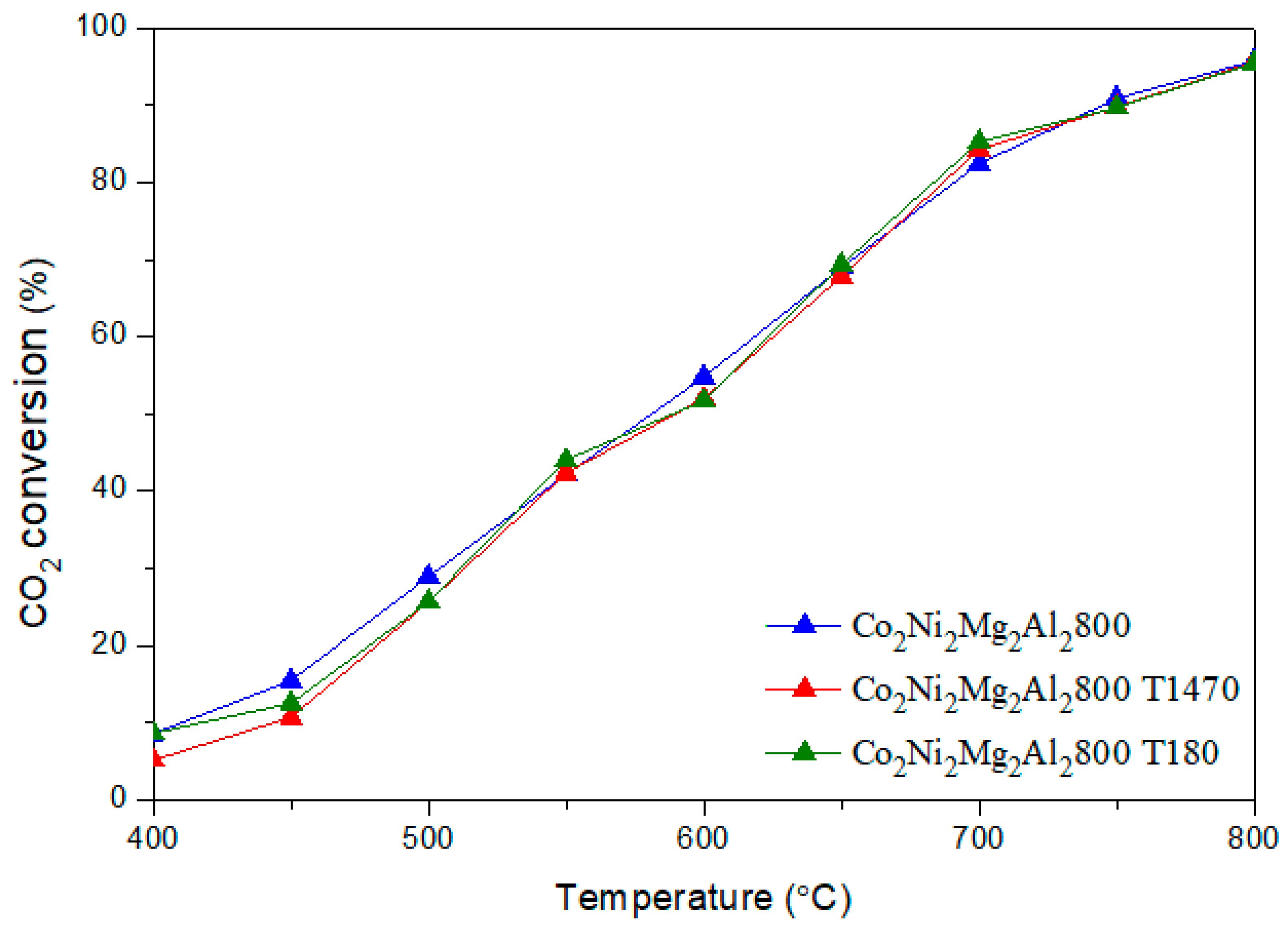
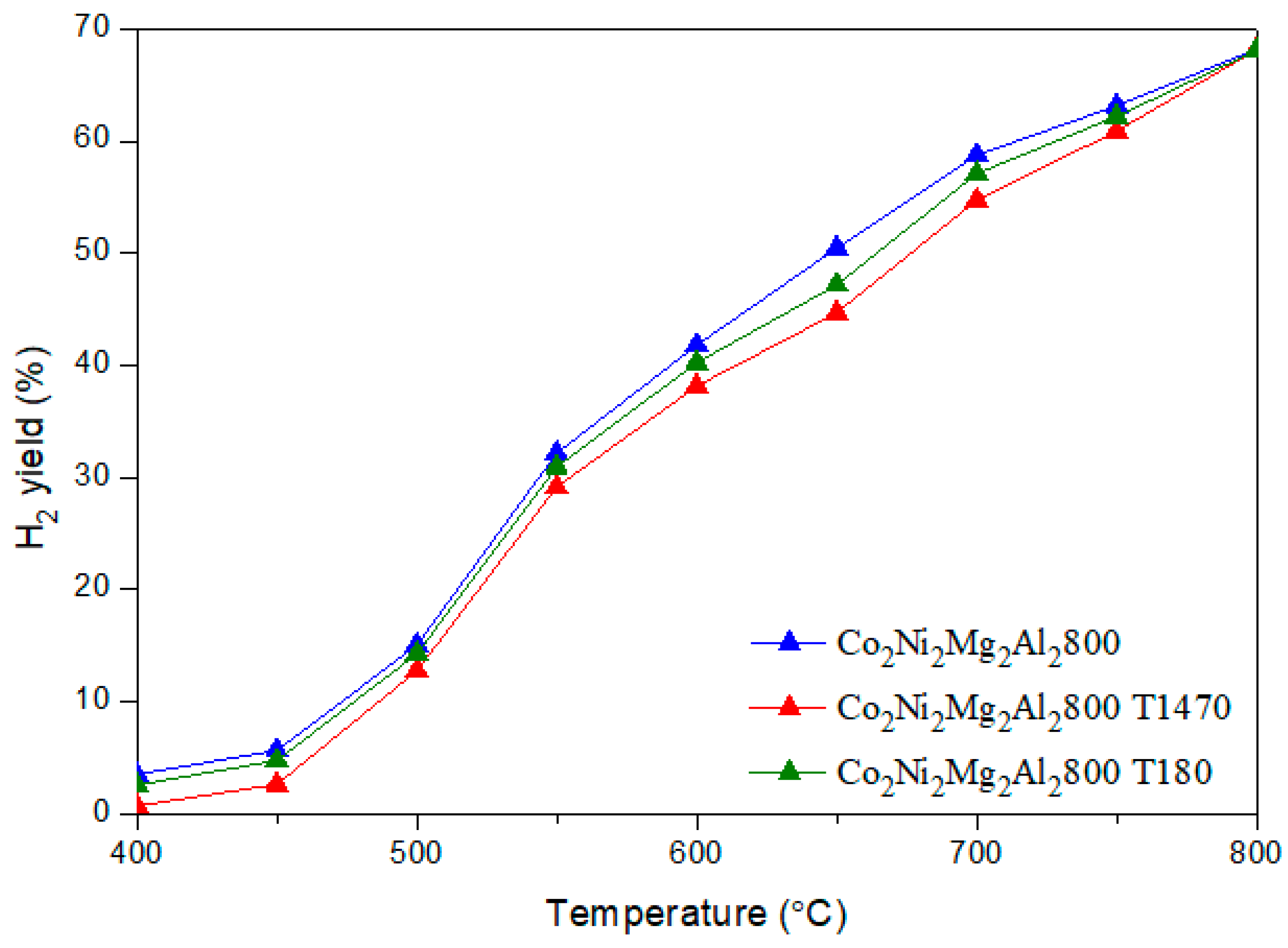
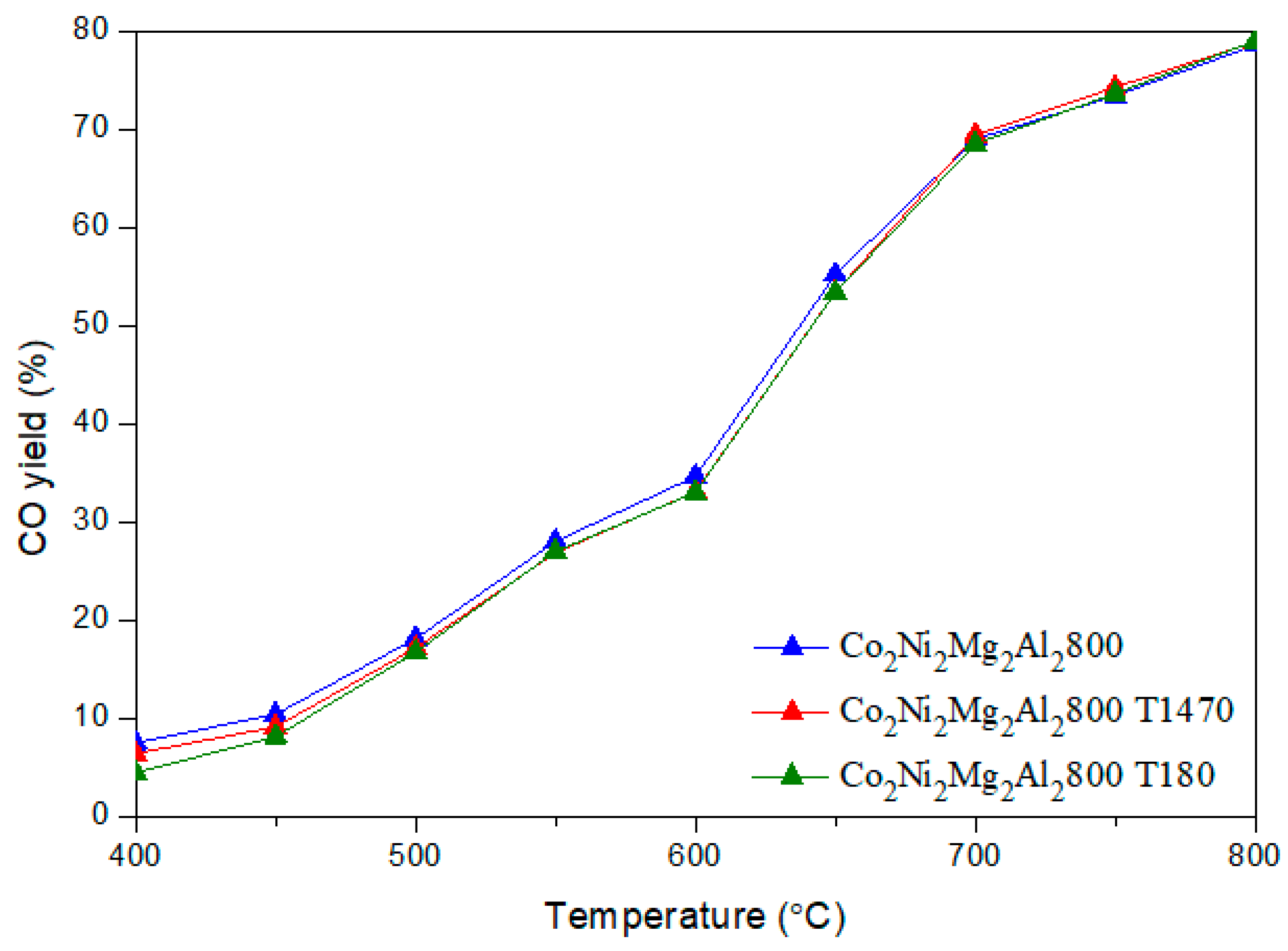
CO + H2O) (b) and the methane decomposition (CH4 → C + 2 H2) (c) compete with the main DRM reaction. Indeed, during the reverse water gas shift (b), CO2 reacts with H2 to produce CO and H2O, thus leading to a ratio H2/CO < 1. In fact, small amounts of water were detected. In addition, the methane decomposition (c) produces C and H2. Therefore, higher CH4 conversion compared to CO2 as well as carbon deposition, which leads to a carbon balance lower than 100%, could be due to the occurrence of reaction (c). 3.3. Dry Reforming of Methane in Presence of Impurities
3.3.1. Influence of Catalyst Shaping
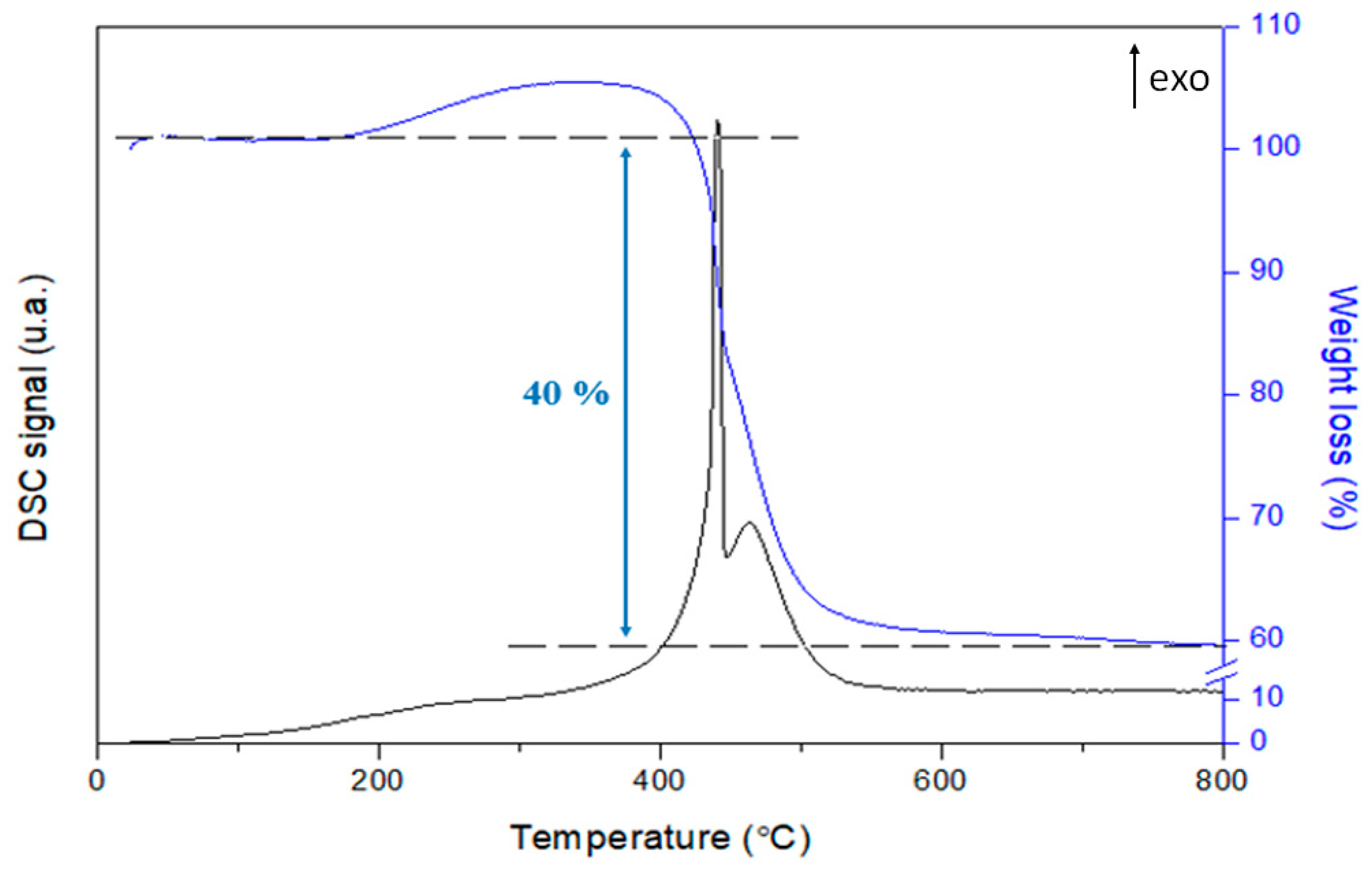
3.3.2. Influence of Adding Toluene
- Reverse water gas shift reaction (RWGS): CO2 + H2
CO + H2O (b) favorable at T > 600 °C
- Methane decomposition: CH4 → C + 2 H2 (c) favorable at T > 500 °C
- Boudouard reaction: 2 CO → C + CO2 (d) favorable at T < 750 °C
- Methanation: CO2 + 4 H2 → CH4 + 2 H2O (e) favorable at T < 750 °C
- Reverse of carbon gasification: CO + H2 → C + H2O (f) favorable at T < 750 °C
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Temperature-phased anaerobic digestion of Industrial Organic Fraction of Municipal Solid Waste: A batch study. Chem. Eng. J. 2015, 270, 597–604. [Google Scholar] [CrossRef]
- Gulhane, M.; Khardenavis, A.A.; Karia, S.; Pandit, P.; Kanade, G.S.; Lokhande, S.; Vaidya, A.N.; Purohit, H.J. Biomethanation of vegetable market waste in an anaerobic baffled reactor: Effect of effluent recirculation and carbon mass balance analysis. Bioresour. Technol. 2016, 215, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ye, L.; Zhang, L.; Ren, Y.; Yue, B.; Chen, X.; He, H. Highly Dispersed Nickel-Containing Mesoporous Silica with Superior Stability in Carbon Dioxide Reforming of Methane: The Effect of Anchoring. Materials 2014, 7, 2340–2355. [Google Scholar] [CrossRef] [PubMed]
- Acar, C.; Dincer, I. Review and Evaluation of Hydrogen Production Options for Better Environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrog. Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Li, P.; Yu, F.; Altaf, N.; Zhu, M.; Li, J.; Dai, B.; Wang, Q. Two-Dimensional Layered Double Hydroxides for Reactions of Methanation and Methane Reforming in C1 Chemistry. Materials 2018, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Sumrunronnasak, S.; Tantayanon, S.; Kiatgamolchai, S.; Sukonket, T. Improved hydrogen production from dry reforming reaction using a catalytic packed-bed membrane reactor with Ni-based catalyst and dense PdAgCu alloy membrane. Int. J. Hydrog. Energy 2016, 41, 2621–2630. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Daza, L. Long-term stability test of Ni-based catalyst in carbon dioxide reforming of methane. Appl. Catal. Gen. 2014, 474, 107–113. [Google Scholar] [CrossRef]
- Al-Fatesh, A. Suppression of carbon formation in CH4–CO2 reforming by addition of Sr into bimetallic Ni–Co/γ-Al2O3 catalyst. J. King Saud Univ. Eng. Sci. 2015, 27, 101–107. [Google Scholar]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Chattanathan, S.A.; Adhikari, S.; McVey, M.; Fasina, O. Hydrogen production from biogas reforming and the effect of H2S on CH4 conversion. Int. J. Hydrog. Energy 2014, 39, 19905–19911. [Google Scholar] [CrossRef]
- Chiodo, V.; Maisano, S.; Zafarana, G.; Urbani, F. Effect of pollutants on biogas steam reforming. Int. J. Hydrog. Energy 2017, 42, 1622–1628. [Google Scholar] [CrossRef]
- Appari, S.; Janardhanan, V.M.; Bauri, R.; Jayanti, S.; Deutschmann, O. A detailed kinetic model for biogas steam reforming on Ni and catalyst deactivation due to sulfur poisoning. Appl. Catal. Gen. 2014, 471, 118–125. [Google Scholar] [CrossRef]
- Jablonski, W.S.; Villano, S.M.; Dean, A.M. A comparison of H2S, SO2, and COS poisoning on Ni/YSZ and Ni/K2O-CaAl2O4 during methane steam and dry reforming. Appl. Catal. Gen. 2015, 502, 399–409. [Google Scholar] [CrossRef]
- Freije-Carrelo, L.; Moldovan, M.; Ignacio García Alonso, J.; Encinar, J.R. Methods for the Analysis of Key Organic Impurities in Biogas. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Arrhenius, K.; Yaghooby, H.; Rosell, L.; Büker, O.; Culleton, L.; Bartlett, S.; Murugan, A.; Brewer, P.; Li, J.; Van Der Veen, A.M.H.; et al. Suitability of vessels and adsorbents for the short-term storage of biogas/biomethane for the determination of impurities—Siloxanes, sulfur compounds, halogenated hydrocarbons, BTEX. Biomass Bioenergy 2017, 105, 127–135. [Google Scholar] [CrossRef]
- Tanios, C. Caractérisation, Évaluation de la Toxicité du Biogaz issu de Déchets Ménagers et Valorisation par Reformage Catalytique. Ph.D. Thesis, Université du Littoral Côte d’Opale, Dunkerque, France, The Lebanese University, Beirut, Lebanon, 2017. [Google Scholar]
- Tanios, C.; Bsaibes, S.; Gennequin, C.; Labaki, M.; Cazier, F.; Billet, S.; Tidahy, H.L.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Syngas production by the CO2 reforming of CH4 over Ni–Co–Mg–Al catalysts obtained from hydrotalcite precursors. Int. J. Hydrog. Energy 2017, 42, 12818–12828. [Google Scholar] [CrossRef]
- Azároff, L.V.; Buerger, M.J. The Powder Method in X-ray Crystallography; McGraw-Hill: New York, NY, USA, 1958; p. 342. [Google Scholar]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Pachatouridou, E.; Iliopoulou, E. Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction: Influence of the synthesis method. Int. J. Hydrog. Energy 2015, 40, 9183–9200. [Google Scholar] [CrossRef]
- Dahdah, E.; Abou Rached, J.; Aouad, S.; Gennequin, C.; Tidahy, H.L.; Estephane, J.; Aboukaïs, A.; Abi Aad, E. CO2 reforming of methane over NixMg6−xAl2 catalysts: Effect of lanthanum doping on catalytic activity and stability. Int. J. Hydrog. Energy 2017, 42, 12808–12817. [Google Scholar] [CrossRef]
- Long, H.; Xu, Y.; Zhang, X.; Hu, S.; Shang, S.; Yin, Y.; Dai, X. Ni-Co/Mg-Al catalyst derived from hydrotalcite-like compound prepared by plasma for dry reforming of methane. J. Energy Chem. 2013, 22, 733–739. [Google Scholar] [CrossRef]
- Mao, S.; Tan, Z.; Zhang, L.; Huang, Q. Plasma-assisted biogas reforming to syngas at room temperature condition. J. Energy Inst. 2018, 91, 172–183. [Google Scholar] [CrossRef]
- Abou Rached, J. Production d’Hydrogène par Reformage Catalytique du Toluène sur des Oxydes Mixtes Ni-Mg-Al. Effet de l’Ajout de Cérium ou de Lanthane. Ph.D. Thesis, Université du Littoral Côte d’Opale, Dunkerque, France, The University of Balamand, Beirut, Lebanon, 2017. [Google Scholar]
- Nawfal, M. Valorisation Catalytique du Biogaz pour une Énergie Propre et Renouvelable. Ph.D. Thesis, Université du Littoral Côte d’Opale, Dunkerque, France, 2015. [Google Scholar]
- Sharifi, M.; Haghighi, M.; Rahmani, M.; Karimipour, S. Syngas production via dry reforming of CH4 over Co- and Cu-promoted Ni/Al2O3–ZrO2 nanocatalysts synthesized via sequential impregnation and sol–gel methods. J. Nat. Gas Sci. Eng. 2014, 21, 993–1004. [Google Scholar] [CrossRef]
- Phongaksorn, M.; Tungkamani, S.; Dharmasaroja, N.; Sornchamni, T.; Chuvaree, R.; Kanjanabat, N.; Siri-Nguan, N. Elucidation of the Influence of Ni-Co Catalytic Properties on Dry Methane Reforming Performance. Chem. Eng. Trans. 2015, 43, 925–930. [Google Scholar]
- Arbag, H.; Yasyerli, S.; Yasyerli, N.; Dogu, G.; Dogu, T. Enhancement of catalytic performance of Ni based mesoporous alumina by Co incorporation in conversion of biogas to synthesis gas. Appl. Catal. B Environ. 2016, 198, 254–265. [Google Scholar] [CrossRef]
- Sidik, S.M.; Triwahyono, S.; Jalil, A.A.; Majid, Z.A.; Salamun, N.; Talib, N.B.; Abdullah, T.A.T. CO2 reforming of CH4 over Ni–Co/MSN for syngas production: Role of Co as a binder and optimization using RSM. Chem. Eng. J. 2016, 295, 1–10. [Google Scholar] [CrossRef]
- Rakib, A. Valorisation du Méthane en Hydrogène par Reformage Catalytique. Ph.D. Thesis, Université du Littoral Côte d’Opale, Dunkerque, France, 2012. [Google Scholar]
- Estephane, J.; Aouad, S.; Hany, S.; El Khoury, B.; Gennequin, C.; El Zakhem, H.; El Nakat, J.; Aboukaïs, A.; Abi Aad, E. CO2 reforming of methane over Ni–Co/ZSM5 catalysts. Aging and carbon deposition study. Int. J. Hydrog. Energy 2015, 40, 9201–9208. [Google Scholar] [CrossRef]
- Tathod, A.P.; Hayek, N.; Shpasser, D.; Simakov, D.S.A.; Gazit, O.M. Mediating interaction strength between nickel and zirconia using a mixed oxide nanosheets interlayer for methane dry reforming. Appl. Catal. B Environ. 2019, 249, 106–115. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Tzounis, L.; Sebastian, V.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Goula, M.A. Investigating the correlation between deactivation and the carbon deposited on the surface of Ni/Al2O3 and Ni/La2O3-Al2O3 catalysts during the biogas reforming reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef]
- Debek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Promotion effect of zirconia on Mg(Ni,Al)O mixed oxides derived from hydrotalcites in CO2 methane reforming. Appl. Catal. B Environ. 2018, 23, 36–46. [Google Scholar] [CrossRef]
- Dalin, L.; Xu, S.; Song, K.; Chen, C.; Zhan, Y.; Jiang, L. Hydrotalcite-derived Co/Mg(Al)O as a stable and coke-resistant catalyst for low-temperature carbon dioxide reforming of methane. Appl. Catal. Gen. 2018, 552, 21–29. [Google Scholar]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrog. Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Baklavaridis, A.; Papadakis, V.G.; Goula, M.A. Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al2O3 and Ni/CaO-Al2O3 catalysts. Waste Biomass Valori. 2016, 7, 725–736. [Google Scholar] [CrossRef]
- Debek, R.; Zubek, K.; Motak, M.; Galvez, M.E.; Da Costa, P.; Grzybek, T. Ni–Al hydrotalcite-like material as the catalyst precursors for the dry reforming of methane at low temperature. C. R. Chim. 2015, 18, 1205–1210. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Rodríguez, L.; Muñoz, G.; Daza, L. Biogas reforming on La-promoted NiMgAl catalysts derived from hydrotalcite-like precursors. J. Power Sources 2011, 196, 4404–4410. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Martin, A.J.; Folgado, M.A.; Daza, L. Dry reforming of methane to syngas over La-promoted hydrotalcite clay-derived catalysts. Int. J. Hydrog. Energy 2012, 37, 12342–12350. [Google Scholar] [CrossRef]
- Yu, X.; Wang, N.; Chu, W.; Liu, M. Carbon dioxide reforming of methane for syngas production over La-promoted NiMgAl catalysts derived from hydrotalcites. Chem. Eng. J. 2012, 209, 623–632. [Google Scholar] [CrossRef]
- Wei, J.M.; Xu, B.Q.; Li, J.L.; Cheng, Z.X.; Zhu, Q.M. Highly active and stable Ni/ZrO2 catalyst for syngas production by CO2 reforming of methane. Appl. Catal. Gen. 2000, 196, L167–L172. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.; Kawi, S. Kinetic and mechanistic aspects for CO2 reforming of methane over Ni based catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Laprune, D.; Theodoridi, C.; Tuel, A.; Farrusseng, D.; Meunier, F.C. Effect of polyaromatic tars on the activity for methane steam reforming of nickel particles embedded in silicalite-1. Appl. Catal. B Environ. 2017, 204, 515–524. [Google Scholar] [CrossRef]
- Dagle, V.L.; Dagle, R.D.; Kovarik, L.; Genc, A.; Wang, Y.G.; Bowden, M.; Wan, H.; Flake, M.; Glezakou, V.A.; King, D.L.; et al. Steam reforming of hydrocarbons from biomass-derived syngas over MgAl2O4-supported transition metals and bimetallic IrNi catalysts. Appl. Catal. B Environ. 2016, 184, 142–152. [Google Scholar] [CrossRef]
- Abou Rached, J.; Cesario, M.R.; Estephane, J.; Tidahy, H.L.; Gennequin, C.; Aouad, S.; Aboukaïs, A.; Abi Aad, E. Effects of cerium and lanthanum on Ni-based catalysts for CO2 reforming of toluene. J. Environ. Chem. Eng. 2018, 6, 4743–4754. [Google Scholar] [CrossRef]
- Abou Rached, J.; El Hayek, C.; Dahdah, E.; Gennequin, C.; Aouad, S.; Tidahy, H.L.; Estephane, J.; Nsouli, B.; Aboukaïs, A.; Abi Aad, E. Ni based catalysts promoted with cerium used in the steam reforming of toluene for hydrogen production. Int. J. Hydrog. Energy 2017, 42, 12829–12840. [Google Scholar] [CrossRef]
- Gac, W.; Greluk, M.; Słowik, G.; Turczyniak-Surdacka, S. Structural and surface changes of cobalt modified manganese oxide during activation and ethanol steam reforming reaction. Appl. Surf. Sci. 2018, 440, 1047–1062. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renew. Sustain. Energy Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Wang, Z.; Zhou, X.; Wang, Z.; Liu, C.J. Steam reforming of methane over Ni/SiO2 catalyst with enhanced coke resistance at low steam to methane ratio. Catal. Today 2015, 256, 130–136. [Google Scholar] [CrossRef]
- Benito, M.; Ortiz, I.; Rodríguez, L.; Muñoz, G. Ni–Co bimetallic catalyst for hydrogen production in sewage treatment plants: Biogas reforming and tars removal. Int. J. Hydrog. Energy 2015, 40, 14456–14468. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanios, C.; Gennequin, C.; Labaki, M.; Tidahy, H.L.; Aboukaïs, A.; Abi-Aad, E. Evaluation of a Catalyst Durability in Absence and Presence of Toluene Impurity: Case of the Material Co2Ni2Mg2Al2 Mixed Oxide Prepared by Hydrotalcite Route in Methane Dry Reforming to Produce Energy. Materials 2019, 12, 1362. https://doi.org/10.3390/ma12091362
Tanios C, Gennequin C, Labaki M, Tidahy HL, Aboukaïs A, Abi-Aad E. Evaluation of a Catalyst Durability in Absence and Presence of Toluene Impurity: Case of the Material Co2Ni2Mg2Al2 Mixed Oxide Prepared by Hydrotalcite Route in Methane Dry Reforming to Produce Energy. Materials. 2019; 12(9):1362. https://doi.org/10.3390/ma12091362
Chicago/Turabian StyleTanios, Carole, Cédric Gennequin, Madona Labaki, Haingomalala Lucette Tidahy, Antoine Aboukaïs, and Edmond Abi-Aad. 2019. "Evaluation of a Catalyst Durability in Absence and Presence of Toluene Impurity: Case of the Material Co2Ni2Mg2Al2 Mixed Oxide Prepared by Hydrotalcite Route in Methane Dry Reforming to Produce Energy" Materials 12, no. 9: 1362. https://doi.org/10.3390/ma12091362
APA StyleTanios, C., Gennequin, C., Labaki, M., Tidahy, H. L., Aboukaïs, A., & Abi-Aad, E. (2019). Evaluation of a Catalyst Durability in Absence and Presence of Toluene Impurity: Case of the Material Co2Ni2Mg2Al2 Mixed Oxide Prepared by Hydrotalcite Route in Methane Dry Reforming to Produce Energy. Materials, 12(9), 1362. https://doi.org/10.3390/ma12091362



