High Efficiency Mercury Sorption by Dead Biomass of Lysinibacillus sphaericus—New Insights into the Treatment of Contaminated Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Mercury Removal by Free Cells
2.2. Mercury Removal by Immobilized Dead Cells
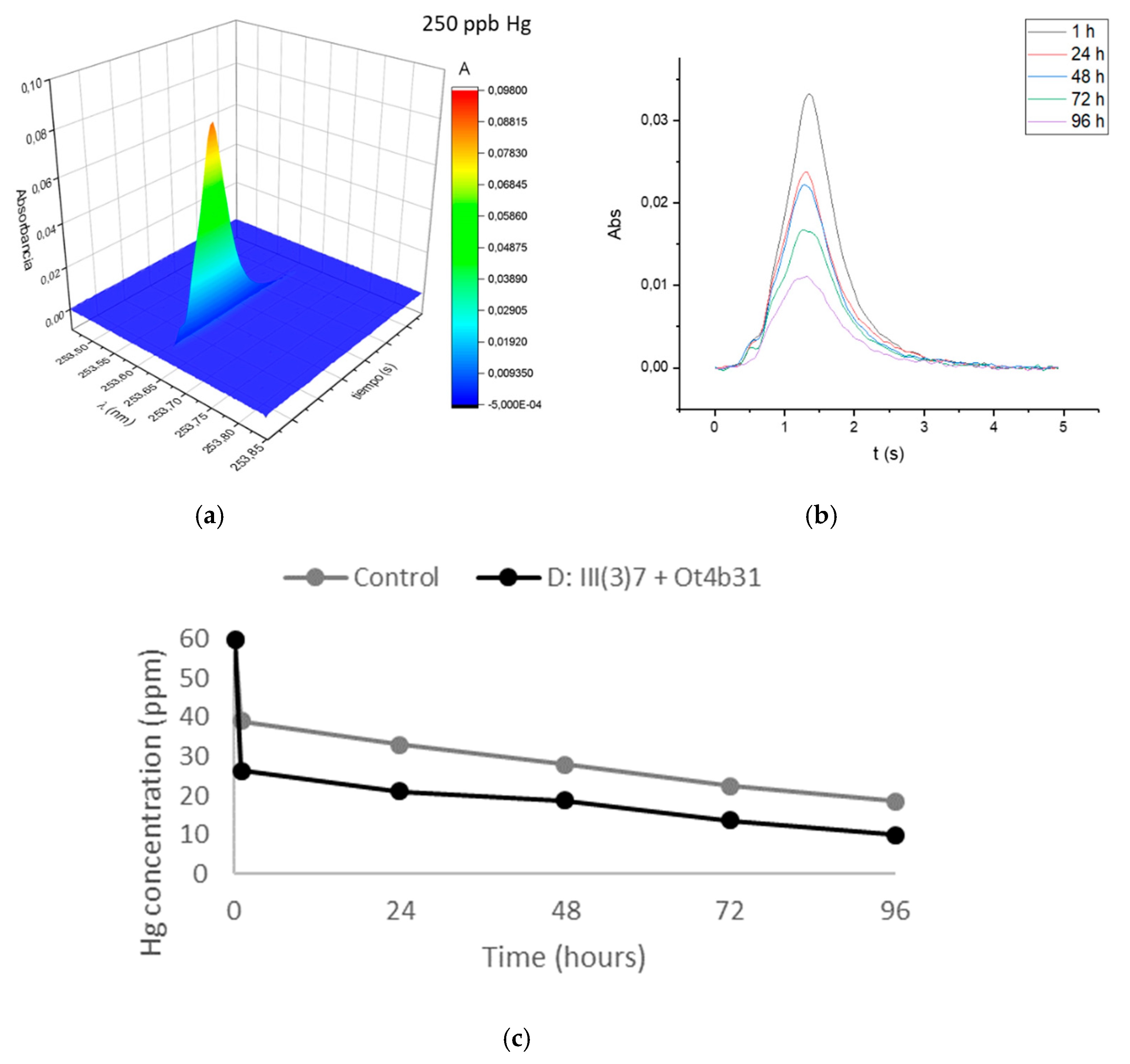
2.3. Mercury Quantification in Free Cells
2.4. Mercury Quantification in Immobilized Cells
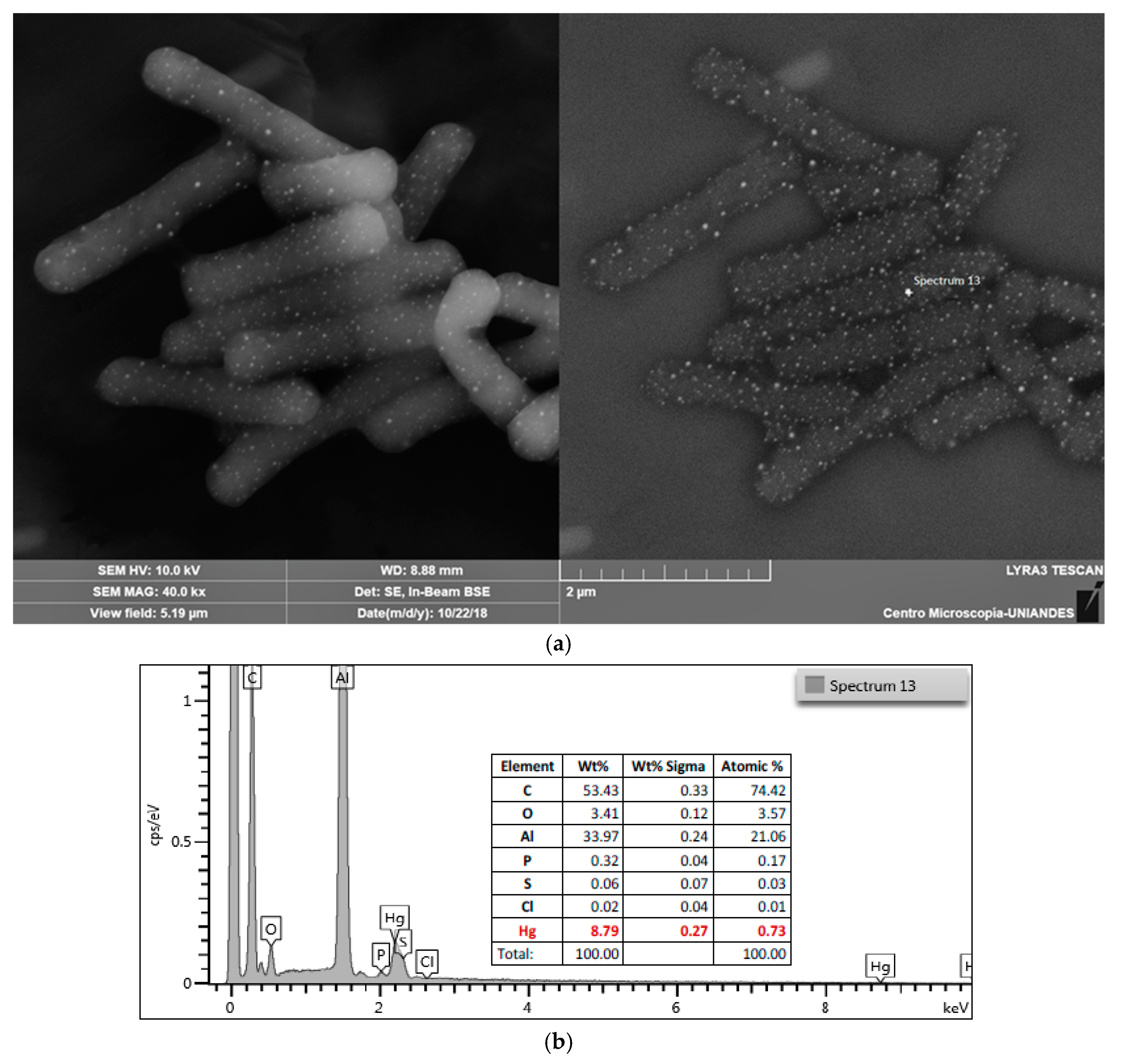
2.5. EDS-SEM Analysis
2.6. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Growth Concentration (MIC)
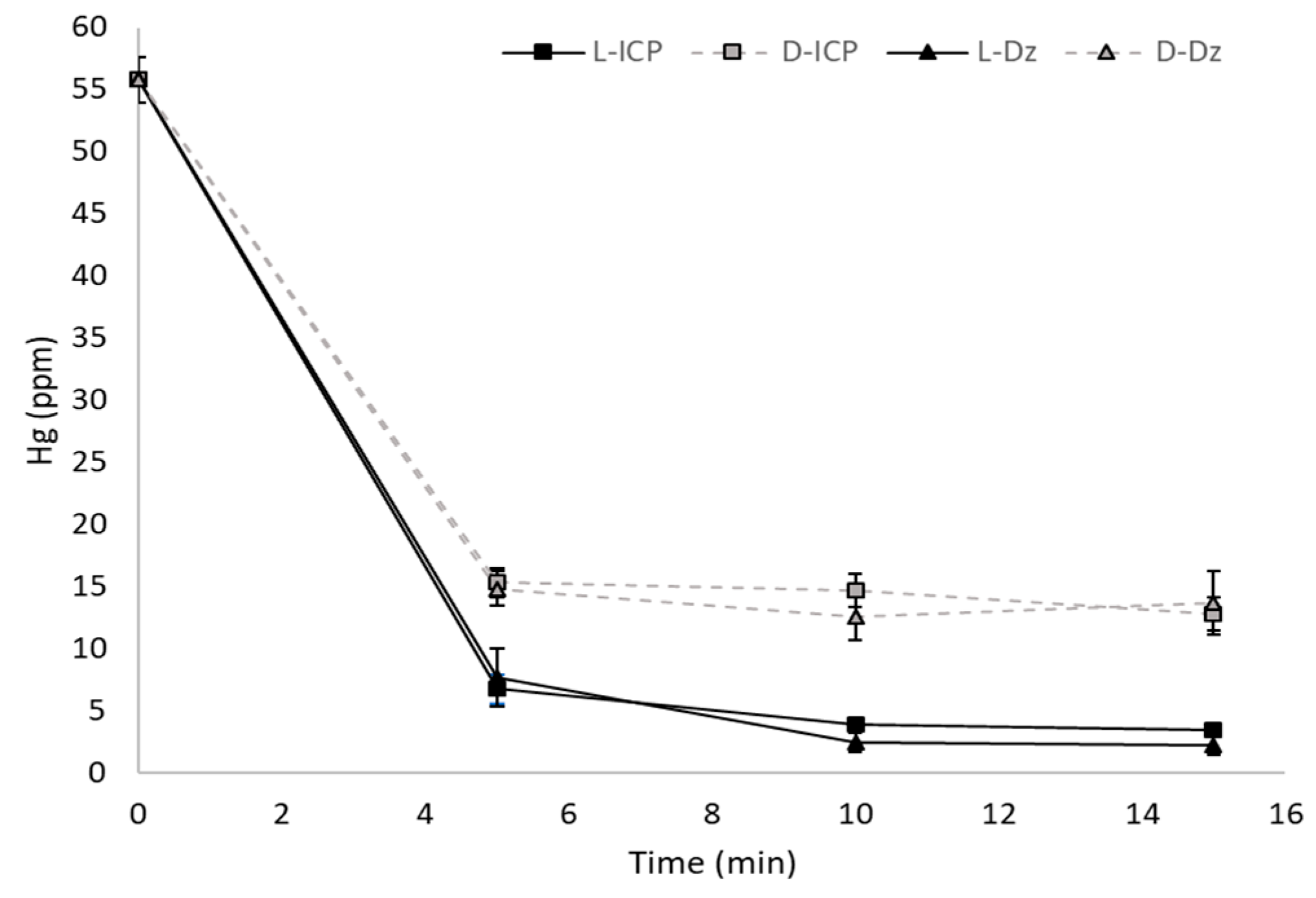
3.2. Mercury Removal by Non-Immobilized L. sphaericus Cells
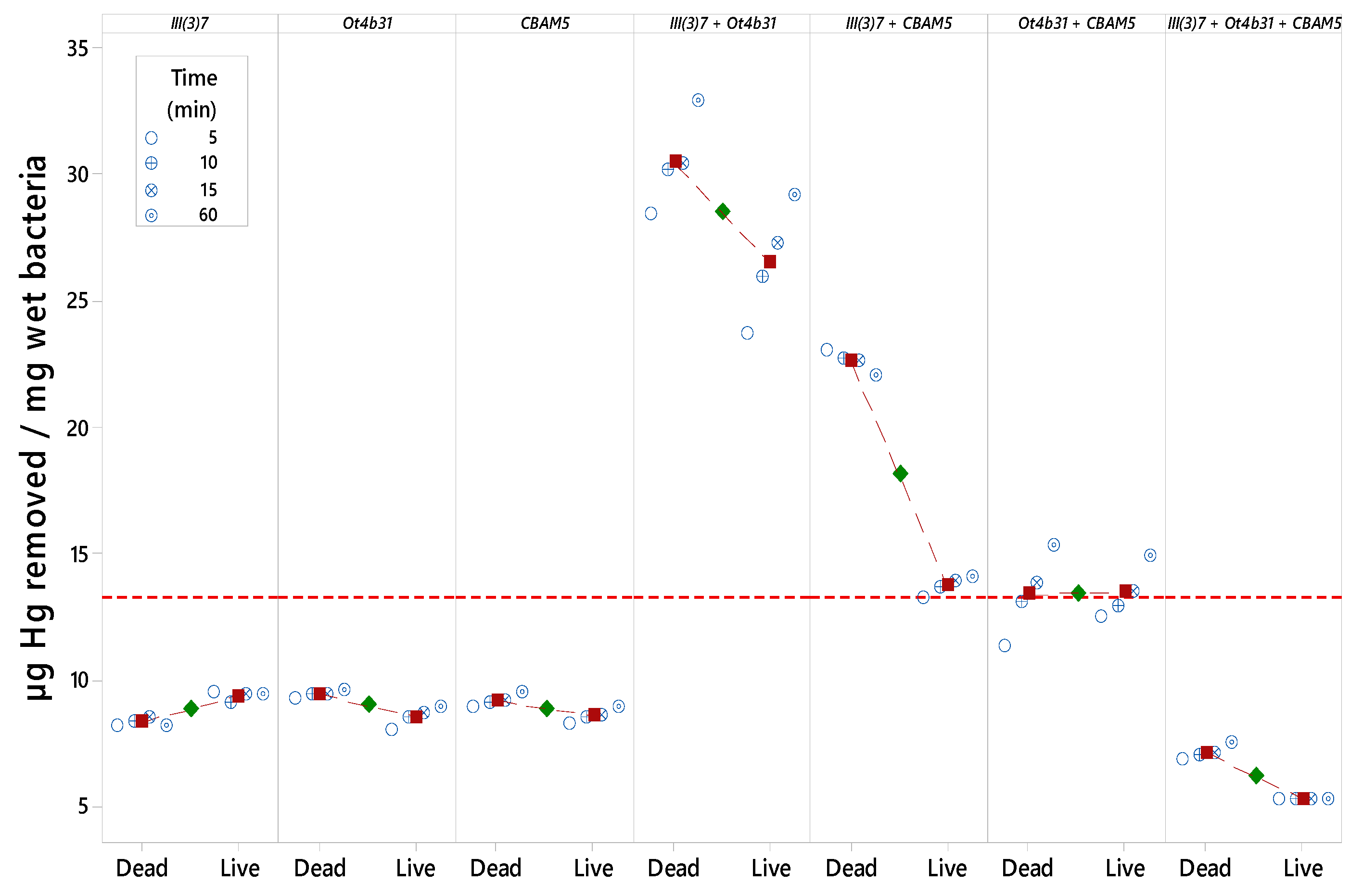
3.3. Mercury Removal by Immobilized Mixture of Dead L. sphaericus Strains III(3)7 and Ot4b31 on RH
3.4. EDS-SEM
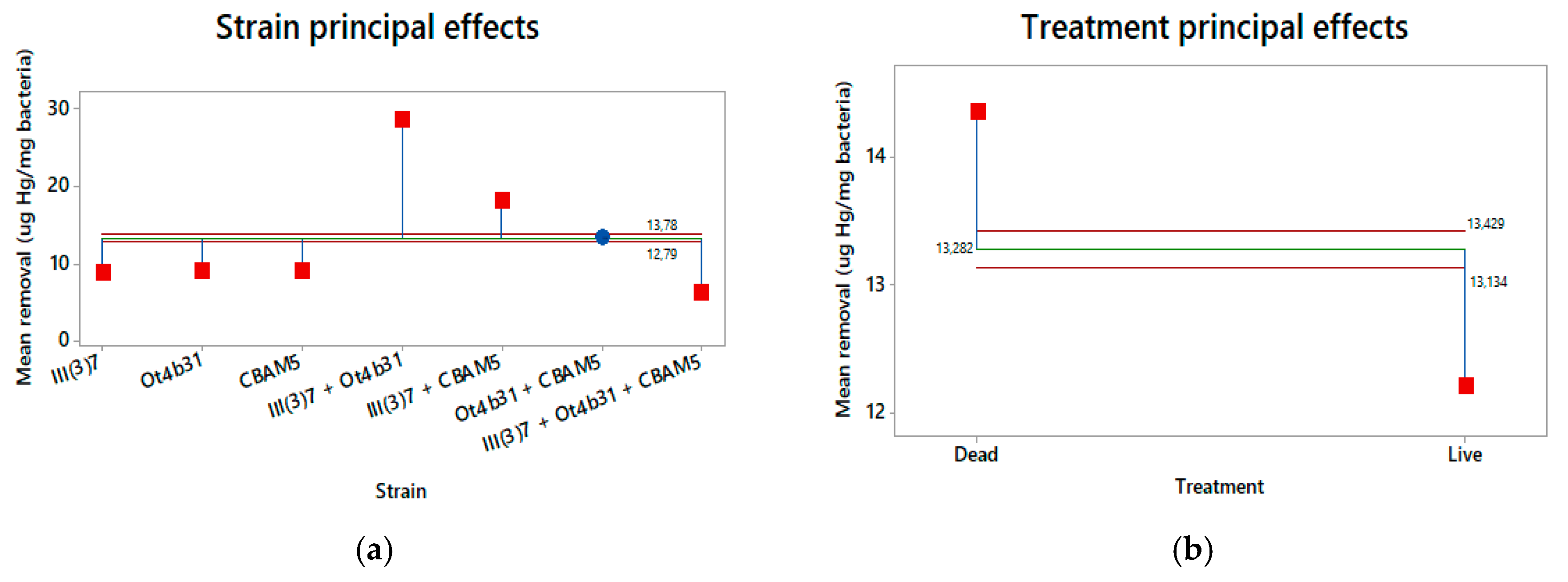
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Geochemistry of mercury in tropical swamps impacted by gold mining. Chemosphere 2015, 134, 44–51. [Google Scholar] [CrossRef]
- Ha, E.; Basu, N.; Bose-O’Reilly, S.; Dórea, J.G.; McSorley, E.; Sakamoto, M.; Chan, H.M. Current progress on understanding the impact of mercury on human health. Environ. Res. 2017, 152, 419–433. [Google Scholar] [CrossRef]
- UNEP. Global Mercury Assessment 2013 Sources, Emissions, Releases and Environmental Transport. UN Environment. 2013. Available online: http://www.unenvironment.org/es/node/15600 (accessed on 21 September 2018).
- Gupta, R.C.; Milatovic, D.; Lall, R.; Srivastava, A. Mercury. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 455–462. [Google Scholar]
- Branco, V.; Caito, S.; Farina, M.; da Rocha, J.T.; Aschner, M.; Carvalho, C. Biomarkers of mercury toxicity: Past, present, and future trends. J. Toxicol. Environ. Health Part B 2017, 20, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.F.; Lamborg, C.H. Geochemistry of Mercury in the Environment. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 91–129. [Google Scholar]
- Richard, J.-H.; Bischoff, C.; Ahrens, C.G.M.; Biester, H. Mercury (II) reduction and co-precipitation of metallic mercury on hydrous ferric oxide in contaminated groundwater. Sci. Total Environ. 2016, 539, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, J.; Horvat, M.; Jereb, V. Modelling of mercury geochemical cycle in Lake Velenje, Slovenia. Environ. Model. Softw. 2002, 17, 593–611. [Google Scholar] [CrossRef]
- Watras, C.J.; Morrison, K.A.; Rubsam, J.L.; Rodger, B. Atmospheric mercury cycles in northern Wisconsin. Atmos. Environ. 2009, 43, 4070–4077. [Google Scholar] [CrossRef]
- Wells, E.M.; Herbstman, J.B.; Lin, Y.H.; Hibbeln, J.R.; Halden, R.U.; Witter, F.R.; Goldman, L.R. Methyl mercury, but not inorganic mercury, associated with higher blood pressure during pregnancy. Environ. Res. 2017, 154, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.W.M.; Bodaly, R.A.; Fisher, N.S.; Kelly, C.A.; Kopec, D.; Whipple, C. Fifty years after its discharge, methylation of legacy mercury trapped in the Penobscot Estuary sustains high mercury in biota. Sci. Total Environ. 2018, 642, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, S.; Anglen, J.; Turyk, M. Methyl mercury exposure in populations at risk: Analysis of NHANES 2011–2012. Environ. Res. 2015, 140, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Suda, I.; Takahashi, H. Degradation of methyl and ethyl mercury into inorganic mercury by other reactive oxygen species besides hydroxyl radical. Arch. Toxicol. 1992, 66, 34–39. [Google Scholar] [CrossRef]
- Nordberg, G.; Fowler, B.; Nordberg, M. (Eds.) Handbook on the Toxicology of Metals, 4th ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- UNEP. Parties and Signatories. Minamata Convention on Mercury. 2018. Available online: http://www.mercuryconvention.org/Countries/Parties/tabid/3428/language/en-US/Default.aspx (accessed on 28 December 2018).
- Gao, Z.; Ying, X.; Yan, J.; Wang, J.; Cai, S.; Yan, C. Acute mercury vapor poisoning in a 3-month-old infant: A case report. Clin. Chim. Acta 2017, 465, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Ori, M.R.; Larsen, J.B.; Shirazi, F.M. Mercury Poisoning in a Toddler from Home Contamination due to Skin-Lightening Cream. J. Pediatr. 2018, 196, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Eqani, S.A.; Bhowmik, A.K.; Qamar, S.; Shah, S.T.; Sohail, M.; Mulla, S.I.; Fasola, M.; Shen, H. Mercury contamination in deposited dust and its bioaccumulation patterns throughout Pakistan. Sci. Total Environ. 2016, 569–570, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Budnik, L.T.; Casteleyn, L. Mercury pollution in modern times and its socio-medical consequences. Sci. Total Environ. 2019, 654, 720–734. [Google Scholar] [CrossRef]
- Betancur-Corredor, B.; Loaiza-Usuga, J.C.; Denich, M.; Borgemeister, C. Gold mining as a potential driver of development in Colombia: Challenges and opportunities. J. Clean. Prod. 2018, 199, 538–553. [Google Scholar] [CrossRef]
- Pinedo-Hernández, J.; Marrugo-Negrete, J.; Díez, S. Speciation and bioavailability of mercury in sediments impacted by gold mining in Colombia. Chemosphere 2015, 119, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Webster, P.C. Not all that glitters: Mercury poisoning in Colombia. Lancet 2012, 379, 1379–1380. [Google Scholar] [CrossRef]
- Palacios-Torres, Y.; Caballero-Gallardo, K.; Olivero-Verbel, J. Mercury pollution by gold mining in a global biodiversity hotspot, the Choco biogeographic region, Colombia. Chemosphere 2018, 193, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Narváez, D.M.; Groot, H.; Diaz, S.M.; Palma, R.M.; Muñoz, N.; Cros, M.P.; Hernández-Vargas, H. Oxidative stress and repetitive element methylation changes in artisanal gold miners occupationally exposed to mercury. Heliyon 2017, 3, e00400. [Google Scholar] [CrossRef]
- Salazar-Camacho, C.; Salas-Moreno, M.; Marrugo-Madrid, S.; Marrugo-Negrete, J.; Díez, S. Dietary human exposure to mercury in two artisanal small-scale gold mining communities of northwestern Colombia. Environ. Int. 2017, 107, 47–54. [Google Scholar] [CrossRef]
- Gutiérrez-Mosquera, H.; Sujitha, S.B.; Jonathan, M.P.; Sarkar, S.K.; Medina-Mosquera, F.; Ayala-Mosquera, H.; Morales-Mira, G.; Arreola-Mendoza, L. Mercury levels in human population from a mining district in Western Colombia. J. Environ. Sci. 2018, 68, 83–90. [Google Scholar] [CrossRef]
- Zolnikov, T.R.; Ortiz, D.R. A systematic review on the management and treatment of mercury in artisanal gold mining. Sci. Total Environ. 2018, 633, 816–824. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H. Nanomaterials for the adsorptive treatment of Hg(II) ions from water. Chem. Eng. J. 2019, 358, 264–282. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.; Tang, L.; Chen, Y.; Zhang, Y.; Wu, M.; Song, Y.; Wen, S. Electrospun nanofibrous mercury filter: Efficient concentration and determination of trace mercury in water with high sensitivity and tunable dynamic range. Anal. Chim. Acta 2017, 982, 96–103. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mercury in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Rüdisüli, M.; Schildhauer, T.J.; Biollaz, S.M.A.; van Ommen, J.R. Scale-up of bubbling fluidized bed reactors—A review. Powder Technol. 2012, 217, 21–38. [Google Scholar] [CrossRef]
- Mack, C.; Burgess, J.; Duncan, J. Membrane bioreactors for metal recovery from wastewater: A review. Water SA 2004, 30, 521–532. [Google Scholar] [CrossRef]
- Anderson, E.L. Analysis of Various Bioreactor Configurations for Heavy Metal Removal Using the Fungus Penicillium ochro-chloron; Worcester Polytechnic Institute: Worcester, MA, USA, 1999. [Google Scholar]
- Lafrance-Vanasse, J.; Lefebvre, M.; di Lello, P.; Sygusch, J.; Omichinski, J.G. Crystal Structures of the Organomercurial Lyase MerB in Its Free and Mercury-bound Forms: Insights into the mechanism of methylmercury degradation. J. Biol. Chem. 2009, 284, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Distefano, M.D.; Zydowsky, L.D.; Cummings, R.T.; Walsh, C.T. Organomercurial lyase and mercuric ion reductase: Nature’s mercury detoxification catalysts. Acc. Chem. Res. 1990, 23, 301–308. [Google Scholar] [CrossRef]
- Hassen, A.; Saidi, N.; Cherif, M.; Boudabous, A. Effects of heavy metals on Pseudomonas aeruginosa and Bacillus thuringiensis. Bioresour. Technol. 1998, 65, 73–82. [Google Scholar] [CrossRef]
- Al Rmalli, S.W.; Dahmani, A.A.; Abuein, M.M.; Gleza, A.A. Biosorption of mercury from aqueous solutions by powdered leaves of castor tree Ricinus communis L. J. Hazard. Mater. 2008, 152, 955–959. [Google Scholar] [CrossRef]
- Amin, F.; Talpur, F.N.; Balouch, A.; Chandio, Z.A.; Surhio, M.A.; Afridi, H.I. Biosorption of mercury(II) from aqueous solution by fungal biomass Pleurotus eryngii: Isotherm, kinetic, and thermodynamic studies: Remediation/Treatment. Environ. Prog. Sustain. Energy 2016, 35, 1274–1282. [Google Scholar] [CrossRef]
- Yu, J.G.; Yue, B.Y.; Wu, X.W.; Liu, Q.; Jiao, F.P.; Jiang, X.Y.; Chen, X.Q. Removal of mercury by adsorption: A review. Environ. Sci. Pollut. Res. 2016, 23, 5056–5076. [Google Scholar] [CrossRef]
- Castillo González, C.; Dussán Garzón, J. Caracterización Molecular de la Resistencia a Mercurio Mercúrico de Bacterias Nativas Colombianas. Ph.D. Dissertation, University of Los Andes, Bogota, Colombia, 2007. [Google Scholar]
- Eom, Y.; Won, J.H.; Ryu, J.-Y.; Lee, T.G. Biosorption of mercury(II) ions from aqueous solution by garlic Allium sativum L. powder. Korean J. Chem. Eng. 2011, 28, 1439–1443. [Google Scholar] [CrossRef]
- Takahashi, Y.; Châtellier, X.; Hattori, K.H.; Kato, K.; Fortin, D. Adsorption of rare earth elements onto bacterial cell walls and its implication for REE sorption onto natural microbial mats. Chem. Geol. 2005, 219, 53–67. [Google Scholar] [CrossRef]
- Gómez-Garzón, C.; Hernández-Santana, A.; Dussán, J. A genome-scale metabolic reconstruction of Lysinibacillus sphaericus unveils unexploited biotechnological potentials. PLoS ONE 2017, 12, e0179666. [Google Scholar] [CrossRef]
- Vargas, J.E.; Dussan, J. Adsorption of Toxic Metals and Control of Mosquitos-borne Disease by Lysinibacillus sphaericus: Dual Benefits for Health and Environment. Biomed. Environ. Sci. BES 2016, 29, 187–196. [Google Scholar]
- Shaw, D.R.; Dussan, J. Transcriptional analysis and molecular dynamics simulations reveal the mechanism of toxic metals removal and efflux pumps in Lysinibacillus sphaericus OT4b.31. Int. Biodeterior. Biodegrad. 2018, 127, 46–61. [Google Scholar] [CrossRef]
- Shaw, D.R.; Dussan, J. Mathematical Modelling of Toxic Metal Uptake and Efflux Pump in Metal-Resistant Bacterium Bacillus cereus Isolated From Heavy Crude Oil. Water Air Soil Pollut. 2015, 226, 112. [Google Scholar] [CrossRef]
- Bustos, M.; Ibarra, H.; Dussán, J. The Golden Activity of Lysinibacillus sphaericus: New Insights on Gold Accumulation and Possible Nanoparticles Biosynthesis. Materials 2018, 11, 1587. [Google Scholar] [CrossRef]
- Velásquez, L.; Dussan, J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J. Hazard. Mater. 2009, 167, 713–716. [Google Scholar] [CrossRef]
- Merroun, M.L.; Raff, J.; Rossberg, A.; Hennig, C.; Reich, T.; Selenska-Pobell, S. Complexation of Uranium by Cells and S-Layer Sheets of Bacillus sphaericus JG-A12. Appl. Environ. Microbiol. 2005, 71, 5532–5543. [Google Scholar] [CrossRef]
- Ilk, N.; Egelseer, E.M.; Sleytr, U.B. S-layer fusion proteins—Construction principles and applications. Curr. Opin. Biotechnol. 2011, 22, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Allievi, M.C. Metal Biosorption by Surface-Layer Proteins from Bacillus Species. J. Microbiol. Biotechnol. 2011, 21, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci. Technol. 2014, 69, 1775–1787. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Andleeb, S.; Batool, I.; Ali, S.; Akhtar, K.; Ali, N.M. Accumulation of Heavy Metals by Living and Dead Bacteria as Biosorbents: Isolated from Waste Soil. Biol. Sci. PJSIR 2017, 60, 102–111. [Google Scholar]
- Lozano, L.C.; Dussán, J. Metal tolerance and larvicidal activity of Lysinibacillus sphaericus. World J. Microbiol. Biotechnol. 2013, 29, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; Silva-Quintero, L.; Dussán, J. Complete genome sequencing and comparative genomic analysis of functionally diverse Lysinibacillus sphaericus III(3)7. Genom. Data 2016, 9, 78–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peña-Montenegro, T.D.; Dussán, J. Genome sequence and description of the heavy metal tolerant bacterium Lysinibacillus sphaericus strain OT4b.31. Stand. Genom. Sci. 2013, 9, 42–56. [Google Scholar] [CrossRef]
- Peña-Montenegro, T.D.; Lozano, L.; Dussán, J. Genome sequence and description of the mosquitocidal and heavy metal tolerant strain Lysinibacillus sphaericus CBAM5. Stand. Genom. Sci. 2015, 10, 2. [Google Scholar] [CrossRef]
- Chao, Y.; Zhan, T.G. Optimization of fixation methods for observation of bacterial cell morphology and surface ultrastructures by atomic force microscopy. Appl. Microbiol. Biotechnol. 2011, 92, 381–392. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Orozco, J.M.G.; Cañizares Macías, M.D.P. Determinación de mercurio en formulaciones farmacéuticas utilizando un sistema de flujo continuo y ditizona en medio micelar. Rev. Mex. Cienc. Farm. 2010, 41, 37–43. [Google Scholar]
- Suárez, L.G.; Aristizabal, J.D. Mercury and gold mining in Colombia: A failed state. Univ. Sci. 2013, 18, 33–49. [Google Scholar]
- WWF. Congreso Aprobó Vinculación de Colombia al Convenio de Minamata. Available online: http://www.wwf.org.co/?uNewsID=325172 (accessed on 8 April 2019).
- Sotero-Martins, A.; de Jesus, M.S.; Lacerda, M.; Moreira, J.C.; Filgueiras, A.L.L.; Barrocas, P.R.G. A conservative region of the mercuric reductase gene (mera) as a molecular marker of bacterial mercury resistance. Braz. J. Microbiol. 2008, 39, 307–310. [Google Scholar] [CrossRef]
- Pedrero, Z.; Bridou, R.; Mounicou, S.; Guyoneaud, R.; Monperrus, M.; Amouroux, D. Transformation, localization, and biomolecular binding of Hg species at subcellular level in methylating and nonmethylating sulfate-reducing bacteria. Environ. Sci. Technol. 2012, 46, 11744–11751. [Google Scholar] [CrossRef] [PubMed]
- Ndu, U.; Christensen, G.A.; Rivera, N.A.; Gionfriddo, C.M.; Deshusses, M.A.; Elias, D.A.; Hsu-Kim, H. Quantification of Mercury Bioavailability for Methylation Using Diffusive Gradient in Thin-Film Samplers. Environ. Sci. Technol. 2018, 52, 8521–8529. [Google Scholar] [CrossRef]
- Volesky, B. Sorption and Biosorption; BV Sorbex: Montreal, QC, Canada, 2003. [Google Scholar]
- Fourest, E.; Roux, J.-C. Heavy metal biosorption by fungal mycelial by-products: Mechanisms and influence of pH. Appl. Microbiol. Biotechnol. 1992, 37, 399–403. [Google Scholar] [CrossRef]
- Schaefer, J.K.; Rocks, S.S.; Zheng, W.; Liang, L.; Gu, B.; Morel, F.M.M. Active transport, substrate specificity, and methylation of Hg(II) in anaerobic bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 8714–8719. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Podar, M.; Bullock, A.L.; Graham, A.M.; Brown, S.D.; Somenahally, A.C.; Johs, A.; Hurt, R.A., Jr.; Bailey, K.L.; Elias, D.A. Mercury Methylation by Novel Microorganisms from New Environments. Environ. Sci. Technol. 2013, 47, 11810–11820. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liang, L.; Gu, B. Mercury Reduction and Oxidation by Reduced Natural Organic Matter in Anoxic Environments. Environ. Sci. Technol. 2012, 46, 292–299. [Google Scholar] [CrossRef]
- François, F.; Lombard, C.; Guigner, J.M.; Soreau, P.; Brian-Jaisson, F.; Martino, G.; Vandervennet, M.; Garcia, D.; Molinier, A.L.; Pignol, D.; et al. Isolation and Characterization of Environmental Bacteria Capable of Extracellular Biosorption of Mercury. Appl. Environ. Microbiol. 2012, 78, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, R.A. Modern Instrumental Methods of Elemental Analysis of Petroleum Products and Lubricants; ASTM International: West Conshohocken, PA, USA, 1991. [Google Scholar]
- Lal, D.; Lal, R. Evolution of mercuric reductase (merA) gene: A case of horizontal gene transfer. Mikrobiologiia 2010, 79, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Venkova, T.; Yeo, C.C.; Espinosa, M. Editorial: The Good, the Bad, and the Ugly: Multiple Roles of Bacteria in Human Life. Front. Microbiol. 2018, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Ashford, M. Could Humans Live without Bacteria? Live Science. Available online: https://www.livescience.com/32761-good-bacteria-boost-immune-system.html (accessed on 8 April 2019).

| Strain | Origin | Ref.Seq Assembly Accession Number | Metals Removal References |
|---|---|---|---|
| III(3)7 | Soil sample from oak forest | GCF_001598075.1 | [44,46,55,56] |
| Ot4b31 | Larvae, Beetles | GCF_000392615.1 | [47,48,57] |
| CBAM5 | Subsurface soil sample of petroleum exploration area | GCF_000568835.1 | [47,55,58] |
| L. sphaericus Strain | Liquid Culture Media (MSM/NB) | |||||
|---|---|---|---|---|---|---|
| 5 ppm | 10 ppm | 20 ppm | 40 ppm | 60 ppm | 80 ppm | |
| III(3)7 | +/+ | +/+ | −/+ | −/+ | −/+ | −/− |
| Ot4b31 | +/+ | −/+ | −/+ | −/+ | −/+ | −/− |
| CBAM5 | +/+ | +/+ | −/+ | −/+ | −/+ | −/− |
| Treatment | Mercury Removal Efficiency (µg Hg/mg bacteria) |
|---|---|
| Non-escalated mixture | 32.85 |
| Mixture with RH | 71.76 |
| Mixture without RH effect (normalized) | 27.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Páez, J.D.; Rivas, R.E.; Dussán-Garzón, J. High Efficiency Mercury Sorption by Dead Biomass of Lysinibacillus sphaericus—New Insights into the Treatment of Contaminated Water. Materials 2019, 12, 1296. https://doi.org/10.3390/ma12081296
Vega-Páez JD, Rivas RE, Dussán-Garzón J. High Efficiency Mercury Sorption by Dead Biomass of Lysinibacillus sphaericus—New Insights into the Treatment of Contaminated Water. Materials. 2019; 12(8):1296. https://doi.org/10.3390/ma12081296
Chicago/Turabian StyleVega-Páez, J. David, Ricardo E. Rivas, and Jenny Dussán-Garzón. 2019. "High Efficiency Mercury Sorption by Dead Biomass of Lysinibacillus sphaericus—New Insights into the Treatment of Contaminated Water" Materials 12, no. 8: 1296. https://doi.org/10.3390/ma12081296
APA StyleVega-Páez, J. D., Rivas, R. E., & Dussán-Garzón, J. (2019). High Efficiency Mercury Sorption by Dead Biomass of Lysinibacillus sphaericus—New Insights into the Treatment of Contaminated Water. Materials, 12(8), 1296. https://doi.org/10.3390/ma12081296





