Amorphous Carbon-Induced Surface Defect Repair for Reinforcing the Mechanical Properties of Carbon Fiber
Abstract
:1. Introduction
2. The Defect Theory of CF
3. Experimental
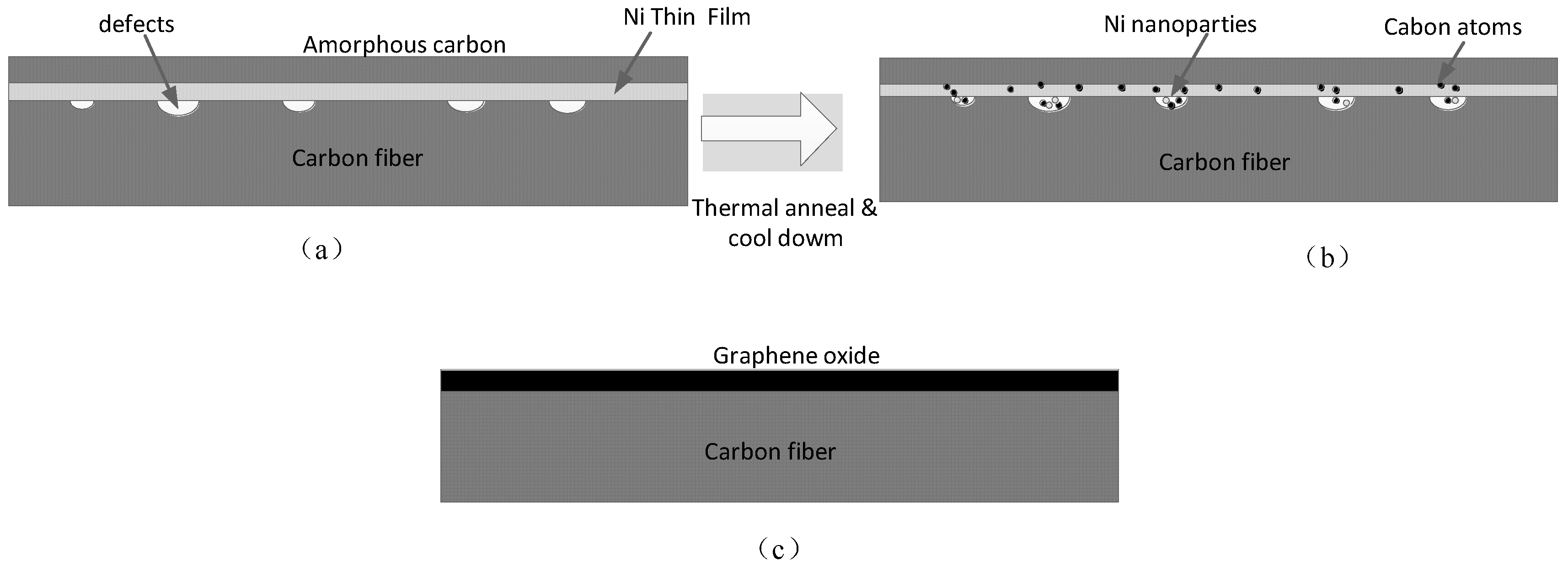
3.1. The Defect Repair Process of CF
3.2. Microstructural Analysis of CF Surface
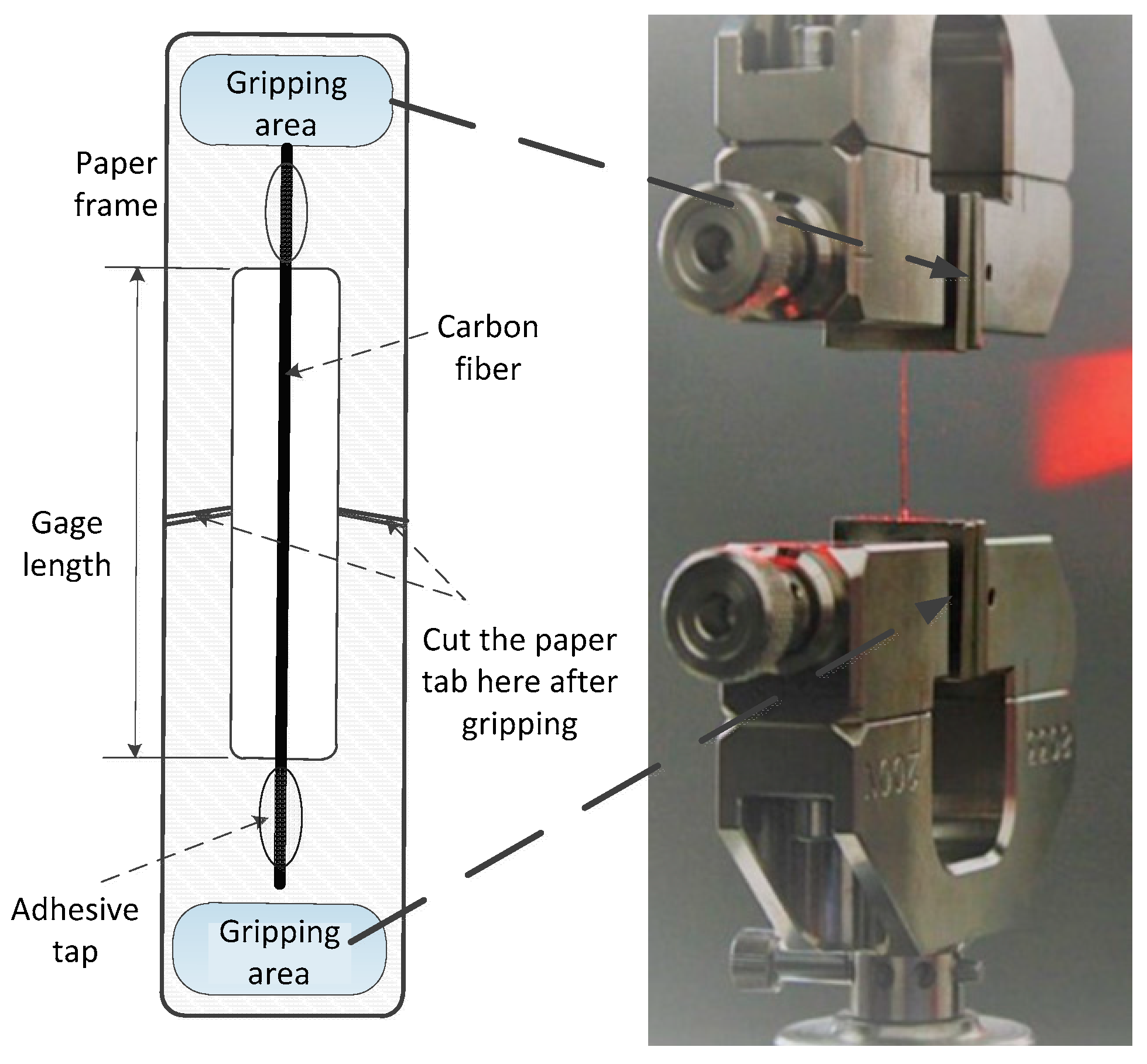
3.3. Single Fiber Tensile Test of CFs
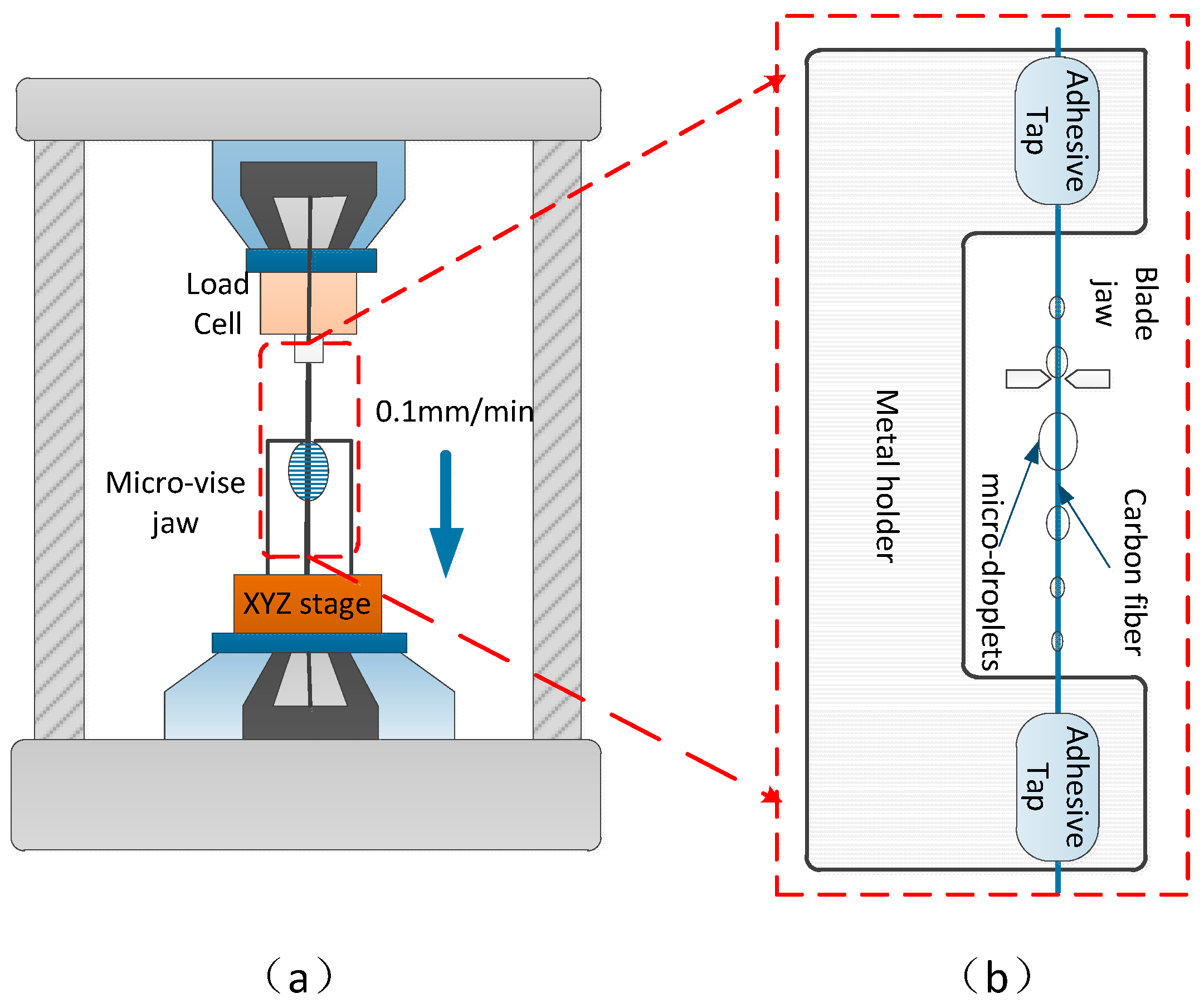
3.4. Interfacial Shear Strength Test of the CFs
4. Results and Discussion
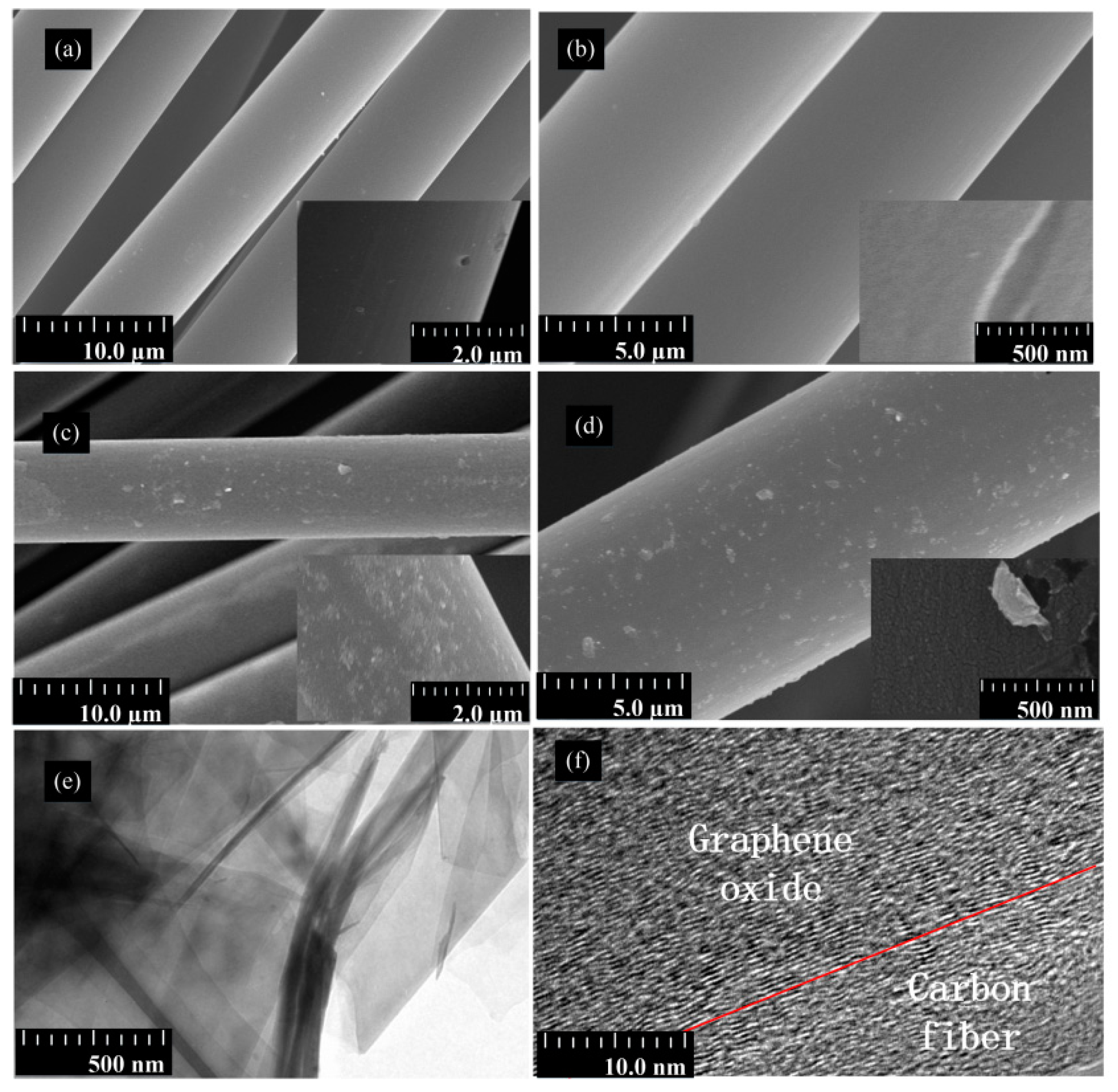
4.1. Surface Morphology of CF
4.2. Raman Characterization
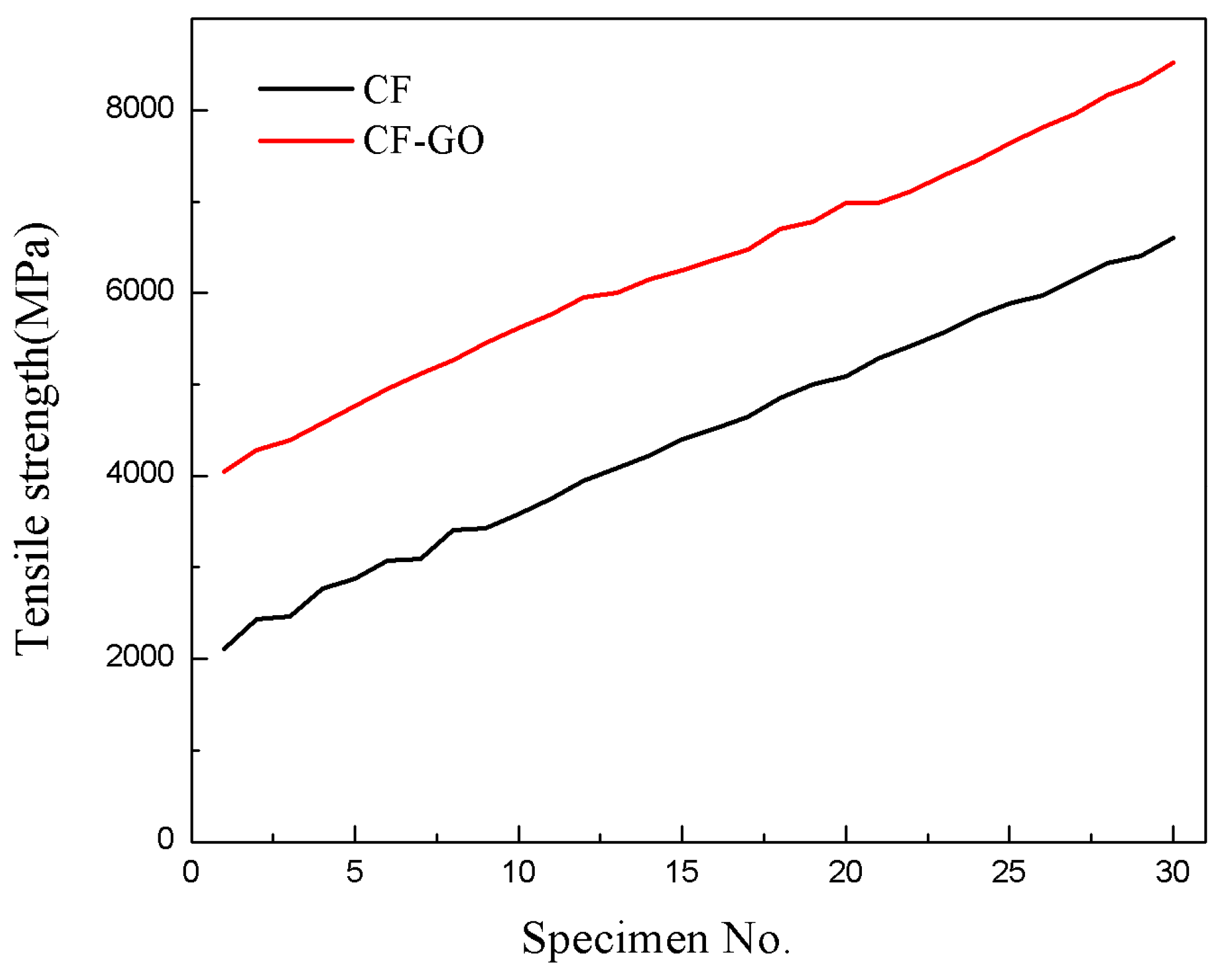
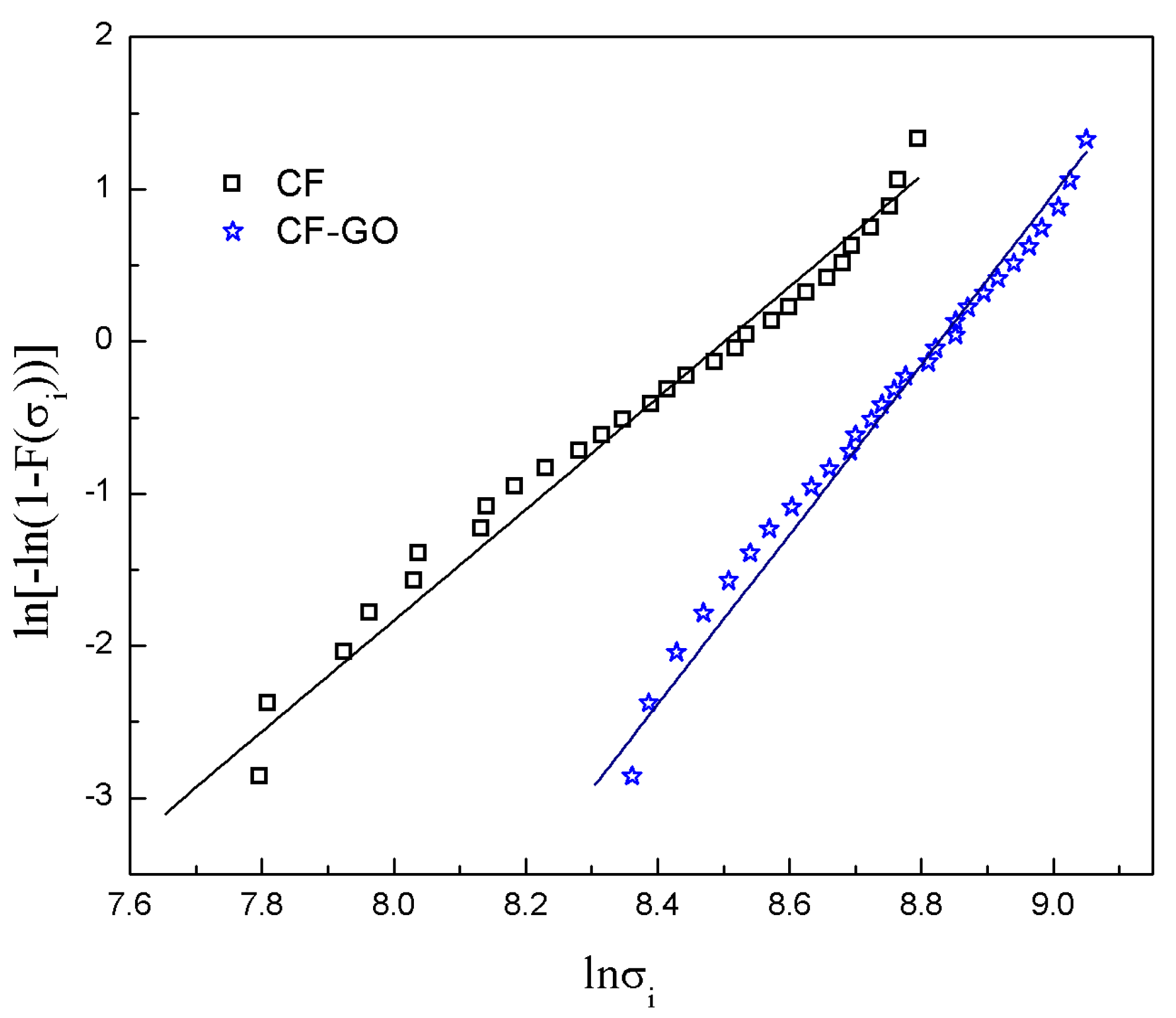
4.3. Tensile Strength of the CFs
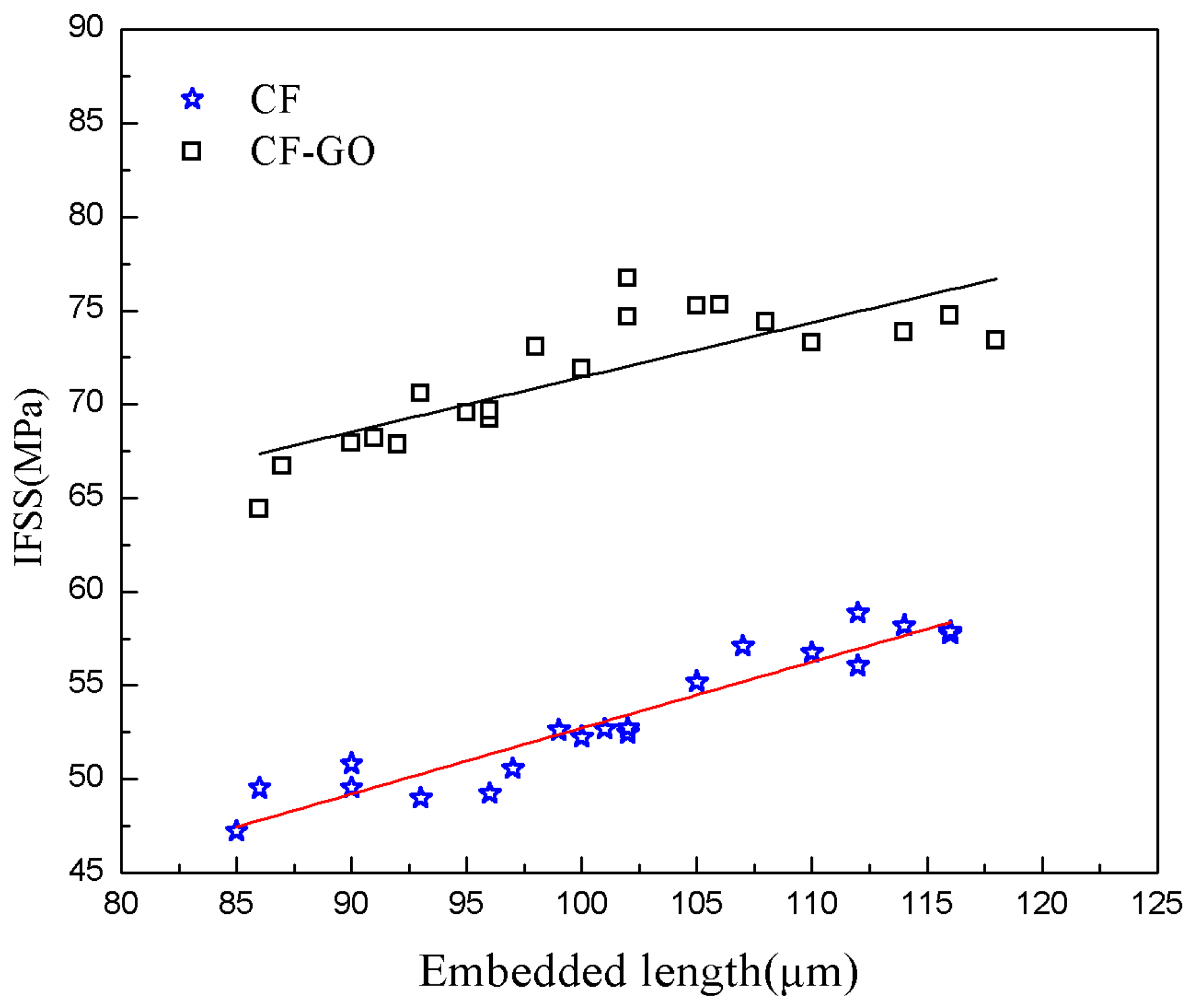
4.4. Interfacial Shear Strength of CFs
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Zhang, Y.; Wu, G. Interlaminar shear properties of z-pinned carbon fiber reinforced aluminum matrix composites by short-beam shear test. Materials 2018, 11, 1874. [Google Scholar] [CrossRef]
- Xu, P.; Yu, Y.; Liu, D.; He, M.; Li, G.; Yang, X. Enhanced interfacial and mechanical properties of high-modulus carbon fiber composites: Establishing modulus intermediate layer between fiber and matrix based on tailored-modulus epoxy. Compos. Sci. Technol. 2018, 163, 26–33. [Google Scholar] [CrossRef]
- Boehm, A.; Meininger, S.; Tesch, A.; Gbureck, U.; Müller, F.A. The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers. Materials 2018, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Unterweger, C.; Duchoslav, J.; Stifter, D.; Fürst, C. Characterization of carbon fiber surfaces and their impact on the mechanical properties of short carbon fiber reinforced polypropylene composites. Compos. Sci. Technol. 2015, 108, 41–47. [Google Scholar] [CrossRef]
- Johnson, D.J. Structure-property relationships in carbon fibers. J. Phys. D Appl. Phys. 1987, 20, 286. [Google Scholar] [CrossRef]
- Dobb, M.G.; Johnson, D.J.; Saville, B.P. Compressional behaviour of Kevlar fibres. Polymer 1981, 22, 960–965. [Google Scholar] [CrossRef]
- Zhang, R.L.; Gao, B.; Ma, Q.H.; Zhang, J.; Cui, H.Z.; Liu, L. Directly grafting graphene oxide onto carbon fiber and the effect on the mechanical properties of carbon fiber composites. Mater. Des. 2016, 93, 364–369. [Google Scholar] [CrossRef]
- Hassan, E.; Elagib, T.; Memon, H.; Yu, M.; Zhu, S. Surface Modification of Carbon Fibers by Grafting PEEK-NH2 for Improving Interfacial Adhesion with Polyetheretherketone. Materials 2019, 12, 778. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, J.R.; Park, S.-J. Thermal conductivity and thermo-physical properties of nanodiamond-attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. Part A Appl. Sci. Manuf. 2017, 101, 227–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Park, S.-J. Nanodiamond nanocluster-decorated graphene oxide/epoxy nanocomposites with enhanced mechanical behavior and thermal stability. Compos. Part B Eng. 2017, 114, 111–120. [Google Scholar] [CrossRef]
- Qina, W.Z.; Vautard, F.; Yu, J.R. Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber matrix interphase. Compos. Part B Eng. 2015, 69, 335–341. [Google Scholar] [CrossRef]
- Zhang, R.L.; Gao, B.; Du, W.T.; Zhang, J.; Cui, H.Z.; Liu, J.; Ma, Q.H.; Wang, C.G.; Li, F.H. Enhanced mechanical properties of multiscale carbon fiber/epoxy composites by fiber surface treatment with graphene oxide/polyhedral oligomeric silsesquioxane. Compos. Part A Appl. Sci. Manuf. 2016, 84, 455–463. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Qu, C.B.; Xiao, H.M.; Fu, S.Y. Enhanced mechanical properties of short carbon fiber reinforced polyethersulfone composites by graphene oxide coating. Polymer 2015, 59, 155–165. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, C.; Xu, H.; Liu, D.; Shi, P.; Zhu, Y.; Liu, J. Enhanced interfacial properties of carbon fiber reinforced polyamide 6 composites by grafting graphene oxide onto fiber surface. Appl. Surface Sci. 2018, 452, 286–298. [Google Scholar] [CrossRef]
- Park, M.; Park, J.H.; Yang, B.J.; Cho, J.; Kim, S.Y.; Jung, I. Enhanced interfacial, electrical, and flexural properties of polyphenylene sulfide composites filled with carbon fibers modified by electrophoretic surface deposition of multi-walled carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2018, 109, 124–130. [Google Scholar] [CrossRef]
- Kuang, T.R.; Mi, H.Y.; Peng, X.F. Fabrication of poly(lactic acid)/graphene oxide foams with highly oriented and elongated cell structure via unidirectional foaming using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2015, 54, 758–768. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhao, F.; Song, Y.J.; Li, J.; Hu, Z.; Huang, Y.D. Interfacial microstructure and properties of poly (phenylene benzobisoxazole) fiber grafted with grapheme oxide via solvothermal method. Appl. Surf. Sci. 2013, 266, 306–312. [Google Scholar] [CrossRef]
- Kurkjian, C.R. Intrinsic Strength, Damage and Failure. In Fractography of Glasses and Ceramics VI: Ceramic Transactions; Wiley-Blackwell: Hoboken, NJ, USA, 2012; Volume 230, pp. 213–223. [Google Scholar]
- Johson, D.J. Structural studies of PAN-based CFs. In Chemistry and Physics of Carbon Vol 20; Marcel Dekker: New York, NY, USA, 1987; pp. 1–58. [Google Scholar]
- Kang, S.K.; Lee, D.B.; Choi, N.S. Fiber/epoxy interfacial shear strength measured by the microdroplet test. Compos. Sci. Technol. 2009, 69, 45–51. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, T. An improved method to make the microdroplet single fiber composite specimen for determining the interfacial shear strength. J. Mater. Sci. 2012, 47, 4775–4778. [Google Scholar] [CrossRef]
- Livneh, T.; Haslett, T.L.; Moskovits, M. Distinguishing disorder-induced bands from allowed Raman bands in graphite. Phys. Rev. B 2002, 66, 195110. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, R.; He, M.; Sun, L.; Wang, C.; Liu, L.; Zhao, L.; Cui, H.; Cao, A. Effect of a multiscale reinforcement by carbon fiber surface treatment with graphene oxide/carbon nanotubes on the mechanical properties of reinforced carbon/carbon composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 433–440. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Kim, B.H.; Yang, K.S. Sturcture and electrochemical properties of electrospum carbon fiber composites containing graphene. J. Ind. Eng. Chem. 2014, 8, 36–41. [Google Scholar]
- Weihull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 290–293. [Google Scholar]
- Trustrum, K.; Jayatilaka, A.D.S. Estimating the Weibull modulus for a brittle material. J. Mater. Sci. 1979, 14, 1080–1084. [Google Scholar] [CrossRef]
- Matveev, M.Y.; Long, A.C.; Jones, I.A. Modelling of textile composites with fibre strength variability. Compos. Sci. Technol. 2014, 105, 44–50. [Google Scholar] [CrossRef]
- Osbeck, S.; Bradley, R.H.; Liu, C.; Idriss, H.; Ward, S. Effect of an ultraviolet/ozone treatment on the surface texture and functional groups on polyacrylonitrile carbon fibres. Carbon 2011, 49, 4322–4330. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Fan, X.Y. Interfacial microstructure and properties of carbon fiber composites modified with graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 1543–1552. [Google Scholar] [CrossRef]

| Fiber | L (mm) | Number | ||||
|---|---|---|---|---|---|---|
| Raw fiber | 7(±0.23) | 20 | 30 | 3.6556 | 4.922(±0.340) | 4.349 |
| Graphene grafted | 7(±0.27) | 20 | 30 | 5.5865 | 6.815(±0.47) | 6.177 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Liu, H.; Chen, B.; Niu, D.; Lei, B.; Ye, G.; Jiang, W.; Shi, Y.; Yin, L.; Lai, G. Amorphous Carbon-Induced Surface Defect Repair for Reinforcing the Mechanical Properties of Carbon Fiber. Materials 2019, 12, 1244. https://doi.org/10.3390/ma12081244
Li D, Liu H, Chen B, Niu D, Lei B, Ye G, Jiang W, Shi Y, Yin L, Lai G. Amorphous Carbon-Induced Surface Defect Repair for Reinforcing the Mechanical Properties of Carbon Fiber. Materials. 2019; 12(8):1244. https://doi.org/10.3390/ma12081244
Chicago/Turabian StyleLi, Dachao, Hongzhong Liu, Bangdao Chen, Dong Niu, Biao Lei, Guoyong Ye, Weitao Jiang, Yongsheng Shi, Lei Yin, and Guoquan Lai. 2019. "Amorphous Carbon-Induced Surface Defect Repair for Reinforcing the Mechanical Properties of Carbon Fiber" Materials 12, no. 8: 1244. https://doi.org/10.3390/ma12081244
APA StyleLi, D., Liu, H., Chen, B., Niu, D., Lei, B., Ye, G., Jiang, W., Shi, Y., Yin, L., & Lai, G. (2019). Amorphous Carbon-Induced Surface Defect Repair for Reinforcing the Mechanical Properties of Carbon Fiber. Materials, 12(8), 1244. https://doi.org/10.3390/ma12081244





