A Novel Phototransistor Device with Dual Active Layers Composited of CsPbBr3 and ZnO Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
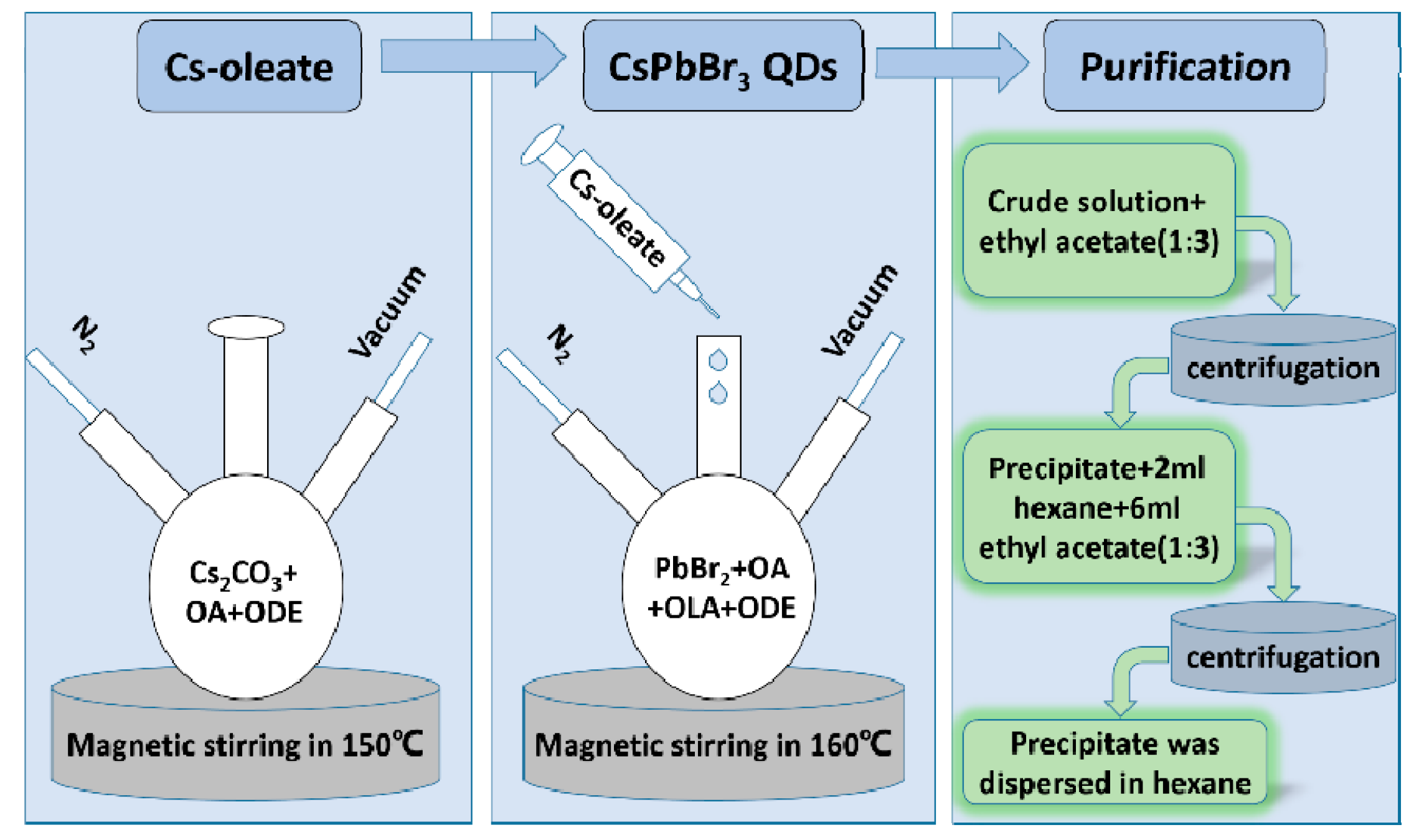
2.1. Preparation of CsPbBr3 QDs
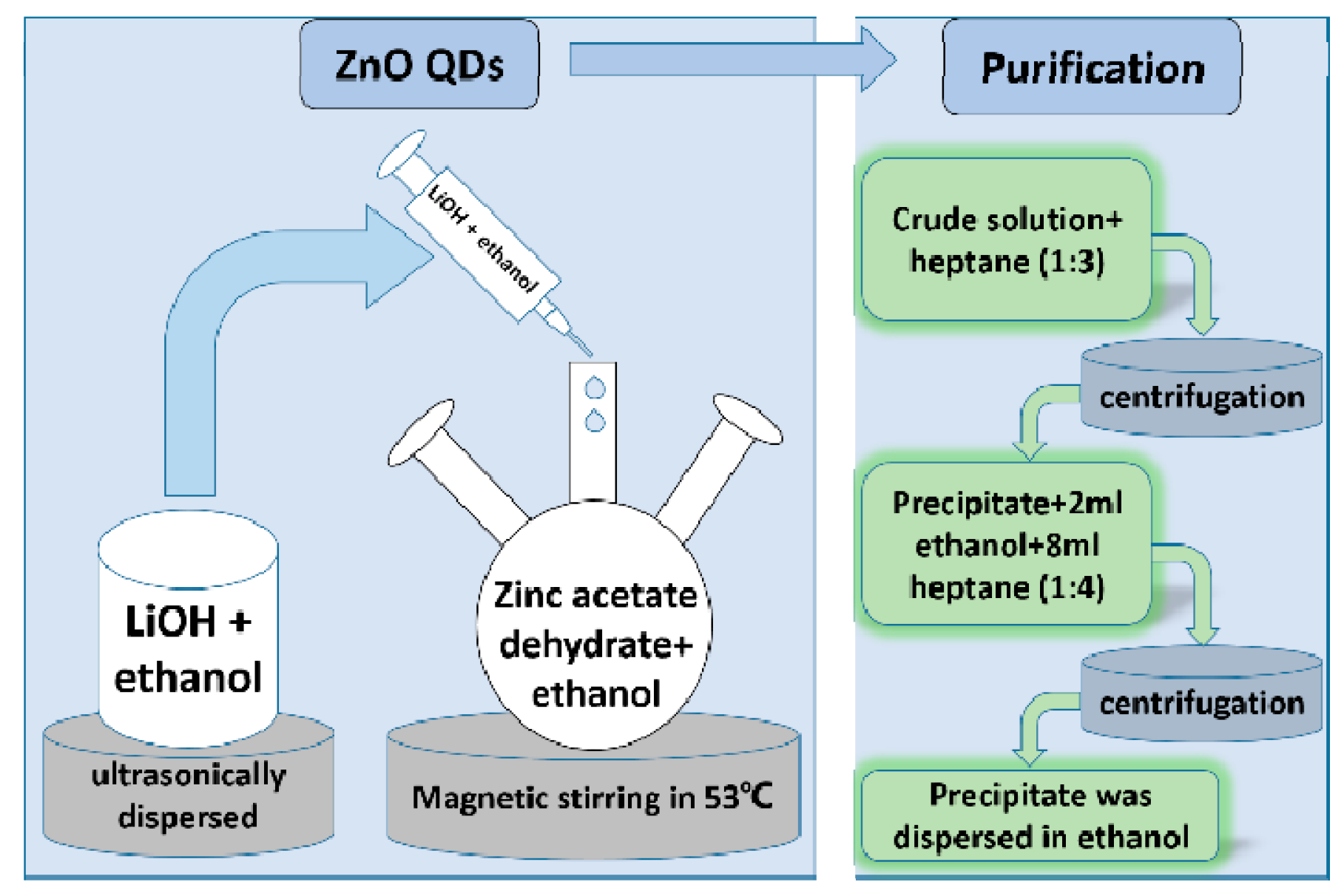
2.2. Preparation of ZnO QDs
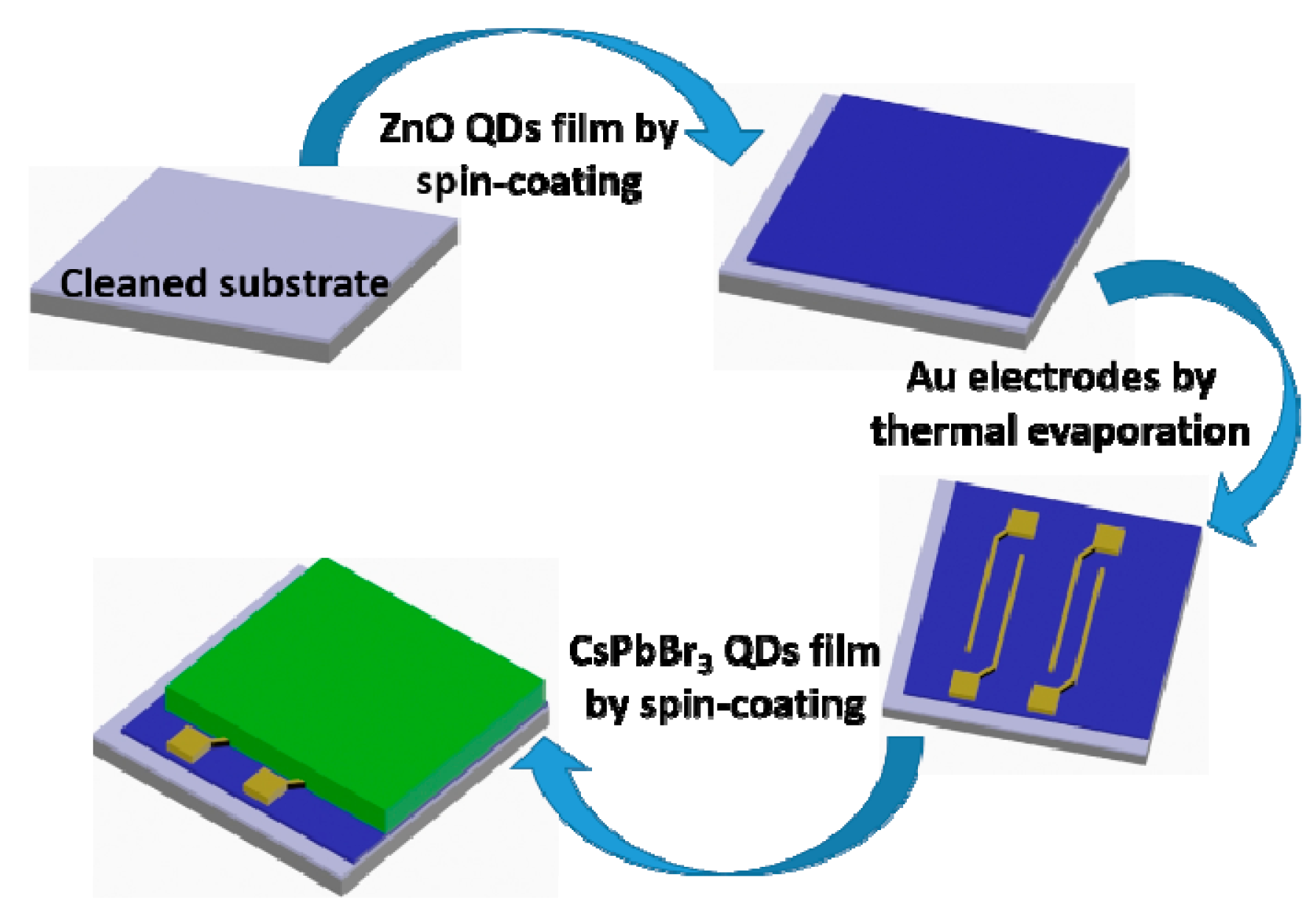
2.3. Fabrication of the Phototransistor
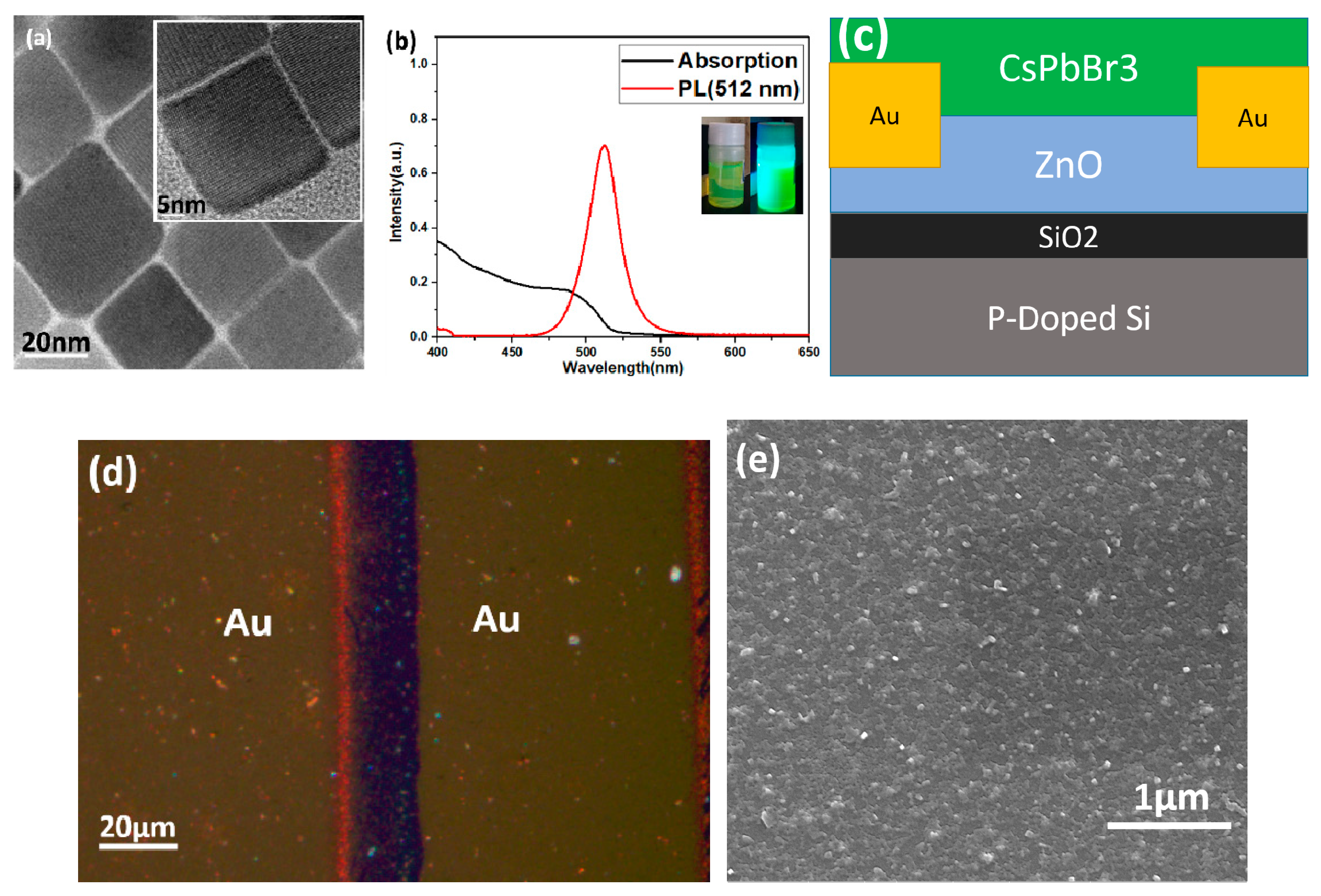
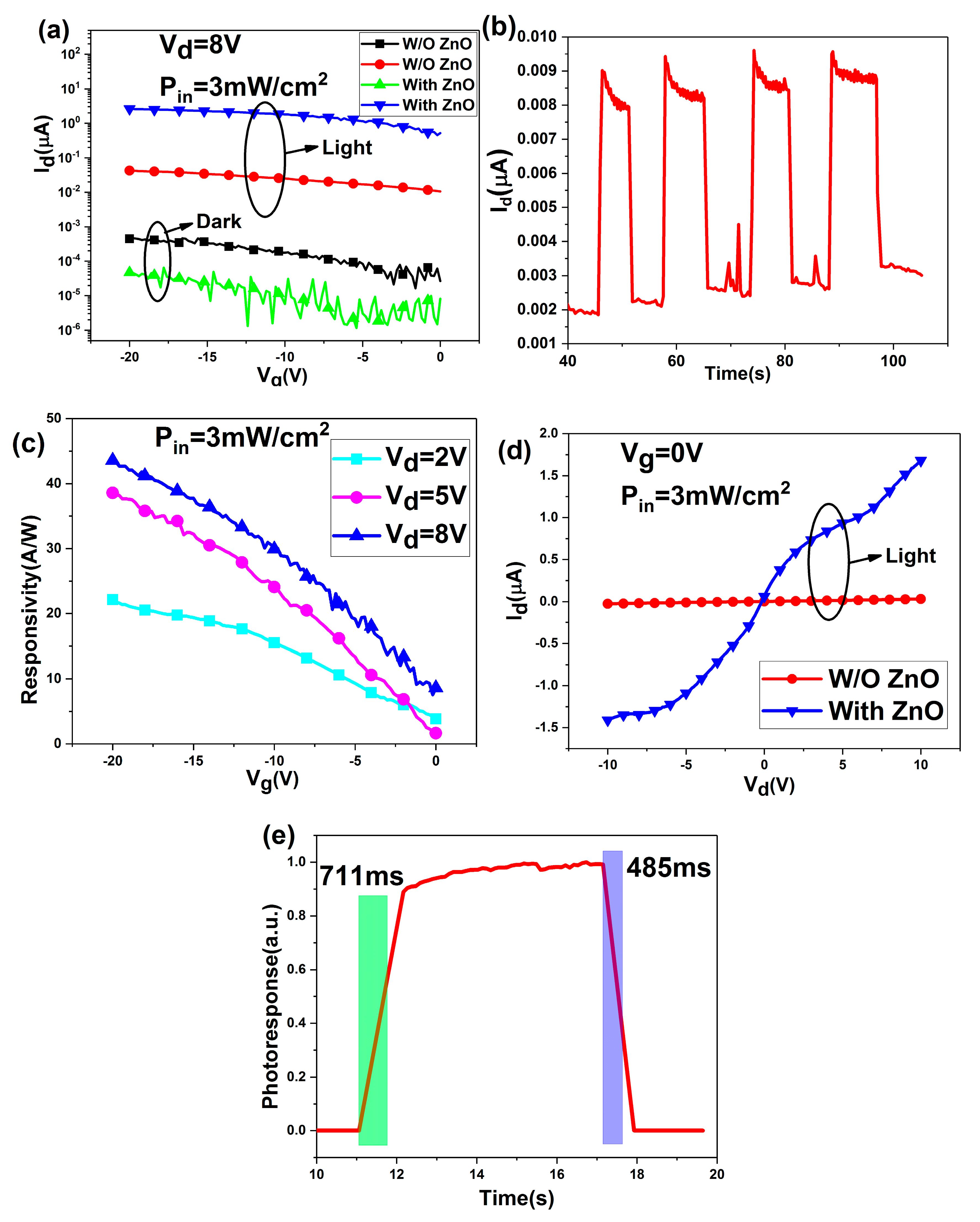
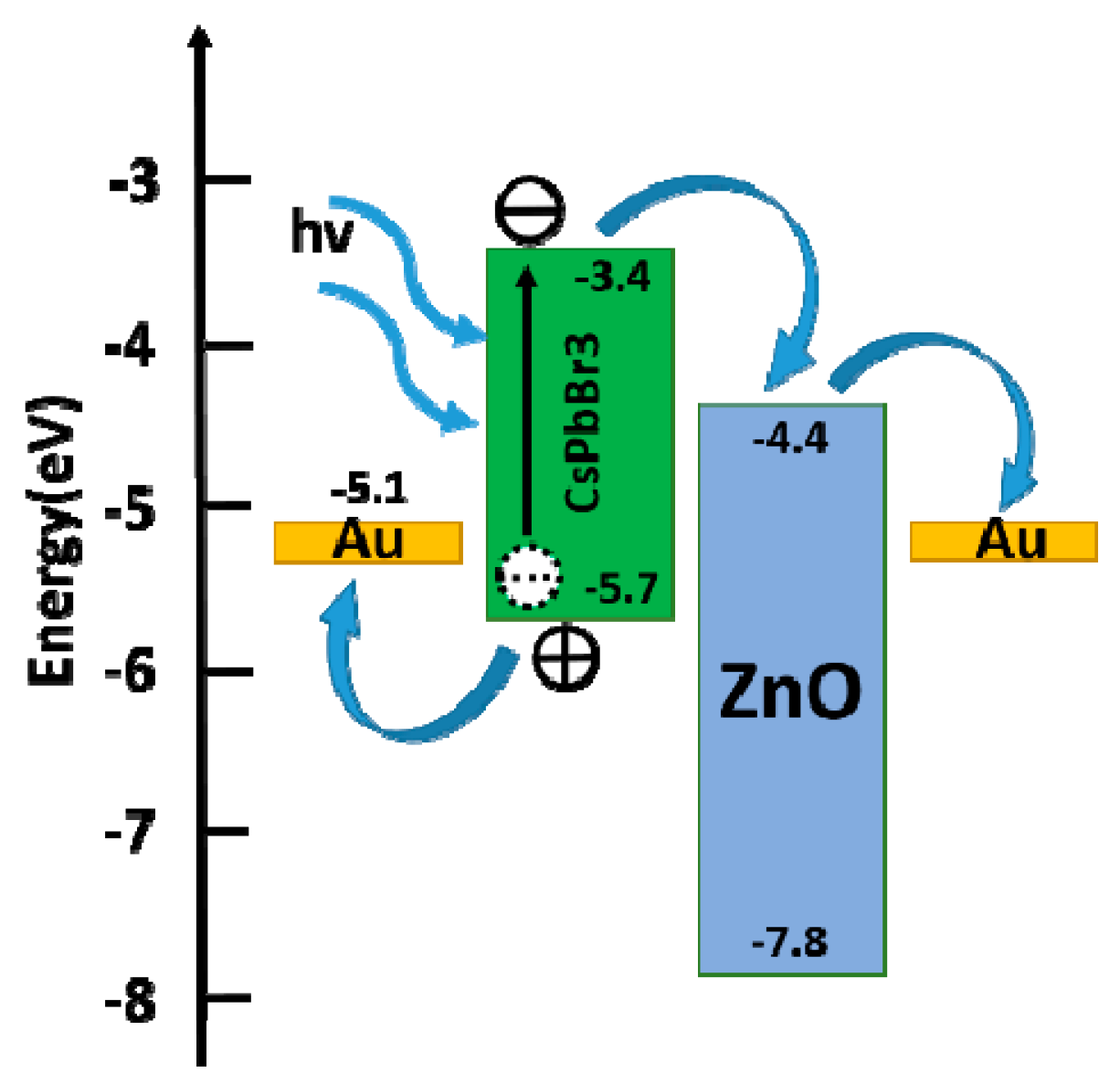
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.S.; Lee, C.R.; Im, J.-H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Leijtens, T.; Stranks, S.D.; Eperon, G.E.; Lindblad, R.; Johansson, E.M.J.; McPherson, I.J.; Rensmo, H.; Ball, J.M.; Lee, M.M.; Snaith, H.J. Electronic Properties of Meso-Superstructured and Planar Organometal Halide Perovskite Films: Charge Trapping, Photodoping, and Carrier Mobility. ACS Nano 2014, 8, 7147–7155. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, J.; Su, D.; Chen, X.; Liang, Z. Efficient and Balanced Charge Transport Revealed in Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 4471–4475. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Graetzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic–inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Fu, P.; Shan, Q.; Shang, Y.; Song, J.; Zeng, H.; Ning, Z.; Gong, J. Perovskite nanocrystals: Synthesis, properties and applications. Sci. Bull. 2017, 62, 369–380. [Google Scholar] [CrossRef]
- Nakazaki, J.; Segawa, H. Evolution of organometal halide solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 74–107. [Google Scholar] [CrossRef]
- Sutherland, B.R.; Sargent, E.H. Perovskite photonic sources. Nat. Photonics 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Veldhuis, S.A.; Boix, P.P.; Yantara, N.; Li, M.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. Perovskite Materials for Light-Emitting Diodes and Lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar] [CrossRef]
- Choi, M.K.; Yang, J.; Kang, K.; Kim, D.C.; Choi, C.; Park, C.; Kim, S.J.; Chae, S.I.; Kim, T.-H.; Kim, J.H.; et al. Wearable red-green-blue quantum dot light-emitting diode array using high-resolution intaglio transfer printing. Nat. Commun. 2015, 6, 7149. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; et al. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detection. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yang, X.; Dong, D.; Li, B.; Yang, D.; Yuan, S.; Qiao, K.; Cheng, Y.-B.; Tang, J.; Song, H. Flexible and Semitransparent Organolead Triiodide Perovskite Network Photodetector Arrays with High Stability. Nano Lett. 2015, 15, 7963–7969. [Google Scholar] [CrossRef]
- Yu, J.; Chen, X.; Wang, Y.; Zhou, H.; Xue, M.; Xu, Y.; Li, Z.; Ye, C.; Zhang, J.; van Aken, P.A.; et al. A high-performance self-powered broadband photodetector based on a CH3NH3PbI3 perovskite/ZnO nanorod array heterostructure. J. Mater. Chem. C 2016, 4, 7302–7308. [Google Scholar] [CrossRef]
- Dong, Y.; Gu, Y.; Zou, Y.; Song, J.; Xu, L.; Li, J.; Xue, J.; Li, X.; Zeng, H. Improving All-Inorganic Perovskite Photodetectors by Preferred Orientation and Plasmonic Effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef]
- Kulbak, M.; Cahen, D.; Hodes, G. How Important Is the Organic Part of Lead Halide Perovskite Photovoltaic Cells? Efficient CsPbBr3 Cells. J. Phys. Chem. Lett. 2015, 6, 2452–2456. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, L.; Yin, Z.; Wang, D.; Meng, J.; Jiang, Q.; Wang, Y.; You, J. A high-performance photodetector based on an inorganic perovskite-ZnO heterostructure. J. Mater. Chem. C 2017, 5, 6115–6122. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.-F.; Li, S.; Lei, L.Z.; Ji, H.-F.; Wu, D.; Xu, T.-T.; Tian, Y.T.; Li, X.J. High-performance perovskite photodetectors based on solution-processed all-inorganic CsPbBr3 thin films. J. Mater. Chem. C 2017, 5, 8355–8360. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, J.; Hwang, E.; Ra, C.-H.; Yoo, W.J.; Ahn, J.-H.; Park, J.H.; Cho, J.H. High-Performance Perovskite-Graphene Hybrid Photodetector. Adv. Mater. 2015, 27, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shi, Y.; Hu, W.; Chiu, M.-H.; Liu, Z.; Bera, A.; Li, F.; Wang, H.; Li, L.-J.; Wu, T. Heterostructured WS2/CH3NH3PbI3 Photoconductors with Suppressed Dark Current and Enhanced Photodetection. Adv. Mater. 2016, 28, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Pae, S.R.; Shim, J.; Yoo, G.; Jeon, J.; Leem, J.W.; Yu, J.S.; Lee, S.; Shin, B.; Park, J.-H. An Ultrahigh-Performance Photodetector based on a Perovskite-Transition-Metal-Dichalcogenide Hybrid Structure. Adv. Mater. 2016, 28, 7799–7806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fullon, R.; Acerce, M.; Petoukhoff, C.E.; Yang, J.; Chen, C.; Du, S.; Lai, S.K.; Lau, S.P.; Voiry, D.; et al. Solution-Processed MoS2/Organolead Trihalide Perovskite Photodetectors. Adv. Mater. 2017, 29, 1603995. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhao, S.L.; Xu, Z.; Yang, Y.F.; Liu, Z.-M.; Xie, X.Y. Heterojunction of ZnO nanoparticle/PMMA and its ultraviolet electroluminescence. Opt. Lett. 2014, 39, 2633–2636. [Google Scholar] [CrossRef] [PubMed]
- Konenkamp, R.; Word, R.C.; Godinez, M. Ultraviolet electroluminescence from ZnO/polymer heterojunction light-emitting diodes. Nano Lett. 2005, 5, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.J.; Li, Q.; Pan, J.Y.; Liu, X.; Li, D.P.; Chen, J.; Tolner, H. Optical Properties of Ultraviolet Quantum Dot Light-Emitting Devices Using ZnO-Cores with a MgO-Shell. J. Disp. Technol. 2015, 11, 461–465. [Google Scholar] [CrossRef]
- Kuang, W.J.; Li, Q.; Sun, Y.; Chen, J.; Tolner, H. Near-band-edge emission characteristics of ZnO-MgO core-shell quantum-dots. Mater. Lett. 2016, 178, 27–30. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Y.; Zhang, X.; Zhang, Z.; Khan, Q.; Chen, J.; Lei, W.; Xia, J. Research on ZnO-MgO QDs and its Application in QLED. Presented at the SID, Los Angeles, CA, USA, 30 May 2018. [Google Scholar]
- Zhang, X.; Li, Q.; Zhang, Z.; Yan, S. P-9.13: Research on the Regulating of Luminescence Region of ZnO-MgO QLED. SID Symp. Dig. Tech. Pap. 2018, 49, 700–703. [Google Scholar] [CrossRef]
- Yang, L.; Liu, K.; Xu, H.; Liu, W.; Ma, J.; Zhang, C.; Liu, C.; Wang, Z.; Yang, G.; Liu, Y. Enhanced Electroluminescence from ZnO Quantum Dot Light-Emitting Diodes via Introducing Al2O3 Retarding Layer and Ag@ZnO Hybrid Nanodots. Adv. Opt. Mater. 2017, 5, 1700493. [Google Scholar] [CrossRef]
- Kumar, M.H.; Yantara, N.; Dharani, S.; Graetzel, M.; Mhaisalkar, S.; Boix, P.P.; Mathews, N. Flexible, low-temperature, solution processed ZnO-based perovskite solid state solar cells. Chem. Commun. 2013, 49, 11089–11091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Barboux, P.; Pauporte, T. Electrochemical Design of Nanostructured ZnO Charge Carrier Layers for Efficient Solid-State Perovskite-Sensitized Solar Cells. Adv. Energy Mater. 2014, 4, 1400932. [Google Scholar] [CrossRef]
- Pan, J.; Chen, J.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W.; Nathan, A. Size Tunable ZnO Nanoparticles To Enhance Electron Injection in Solution Processed QLEDs. ACS Photonics 2016, 3, 215–222. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.-H.; Song, W.-S.; Ko, H.; Lee, C.; Lee, J.-H.; Yang, H. Highly Efficient, Color-Pure, Color-Stable Blue Quantum Dot Light-Emitting Devices. ACS Nano 2013, 7, 7295–7302. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tavakoli, R.; Nourbakhsh, Z.; Waleed, A.; Virk, U.S.; Fan, Z. High Efficiency and Stable Perovskite Solar Cell Using ZnO/rGO QDs as an Electron Transfer Layer. Adv. Mater. Interfaces 2016, 3, 1500790. [Google Scholar] [CrossRef]
- Tan, W.C.; Huang, L.; Ng, R.J.; Wang, L.; Hasan, D.M.N.; Duffin, T.J.; Kumar, K.S.; Nijhuis, C.A.; Lee, C.; Ang, K.-W. A Black Phosphorus Carbide Infrared Phototransistor. Adv. Mater. 2018, 30, 1705039. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, X.; Zeng, H. ZrO2 quantum dots/graphene phototransistors for deep UV detection. Mater. Res. Bull. 2017, 96, 458–462. [Google Scholar] [CrossRef]
- Li, X.; Yu, D.; Cao, F.; Gu, Y.; Wei, Y.; Wu, Y.; Song, J.; Zeng, H. Healing All-Inorganic Perovskite Films via Recyclable Dissolution-Recyrstallization for Compact and Smooth Carrier Channels of Optoelectronic Devices with High Stability. Adv. Funct. Mater. 2016, 26, 5903–5912. [Google Scholar] [CrossRef]
- Wang, F.; Mei, J.; Wang, Y.; Zhang, L.; Zhao, H.; Zhao, D. Fast Photoconductive Responses in Organometal Halide Perovskite Photodetectors. ACS Appl. Mater. Interfaces 2016, 8, 2840–2846. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Q.; Yan, S.; Lei, W.; Chen, J.; Qasim, K. A Novel Phototransistor Device with Dual Active Layers Composited of CsPbBr3 and ZnO Quantum Dots. Materials 2019, 12, 1215. https://doi.org/10.3390/ma12081215
Zhang X, Li Q, Yan S, Lei W, Chen J, Qasim K. A Novel Phototransistor Device with Dual Active Layers Composited of CsPbBr3 and ZnO Quantum Dots. Materials. 2019; 12(8):1215. https://doi.org/10.3390/ma12081215
Chicago/Turabian StyleZhang, Xu, Qing Li, Shikai Yan, Wei Lei, Jing Chen, and Khan Qasim. 2019. "A Novel Phototransistor Device with Dual Active Layers Composited of CsPbBr3 and ZnO Quantum Dots" Materials 12, no. 8: 1215. https://doi.org/10.3390/ma12081215
APA StyleZhang, X., Li, Q., Yan, S., Lei, W., Chen, J., & Qasim, K. (2019). A Novel Phototransistor Device with Dual Active Layers Composited of CsPbBr3 and ZnO Quantum Dots. Materials, 12(8), 1215. https://doi.org/10.3390/ma12081215






