Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review
Abstract
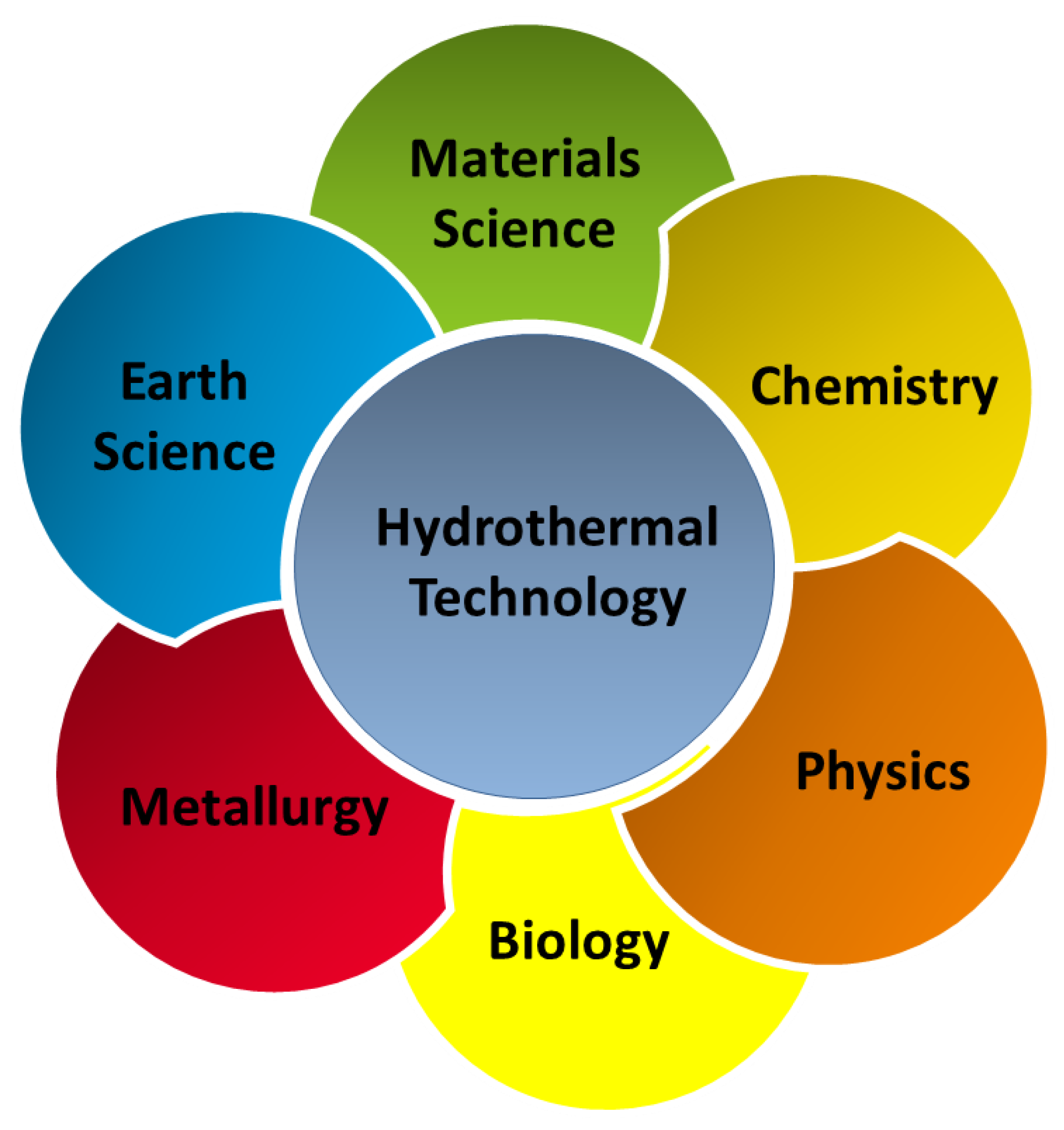
1. Introduction
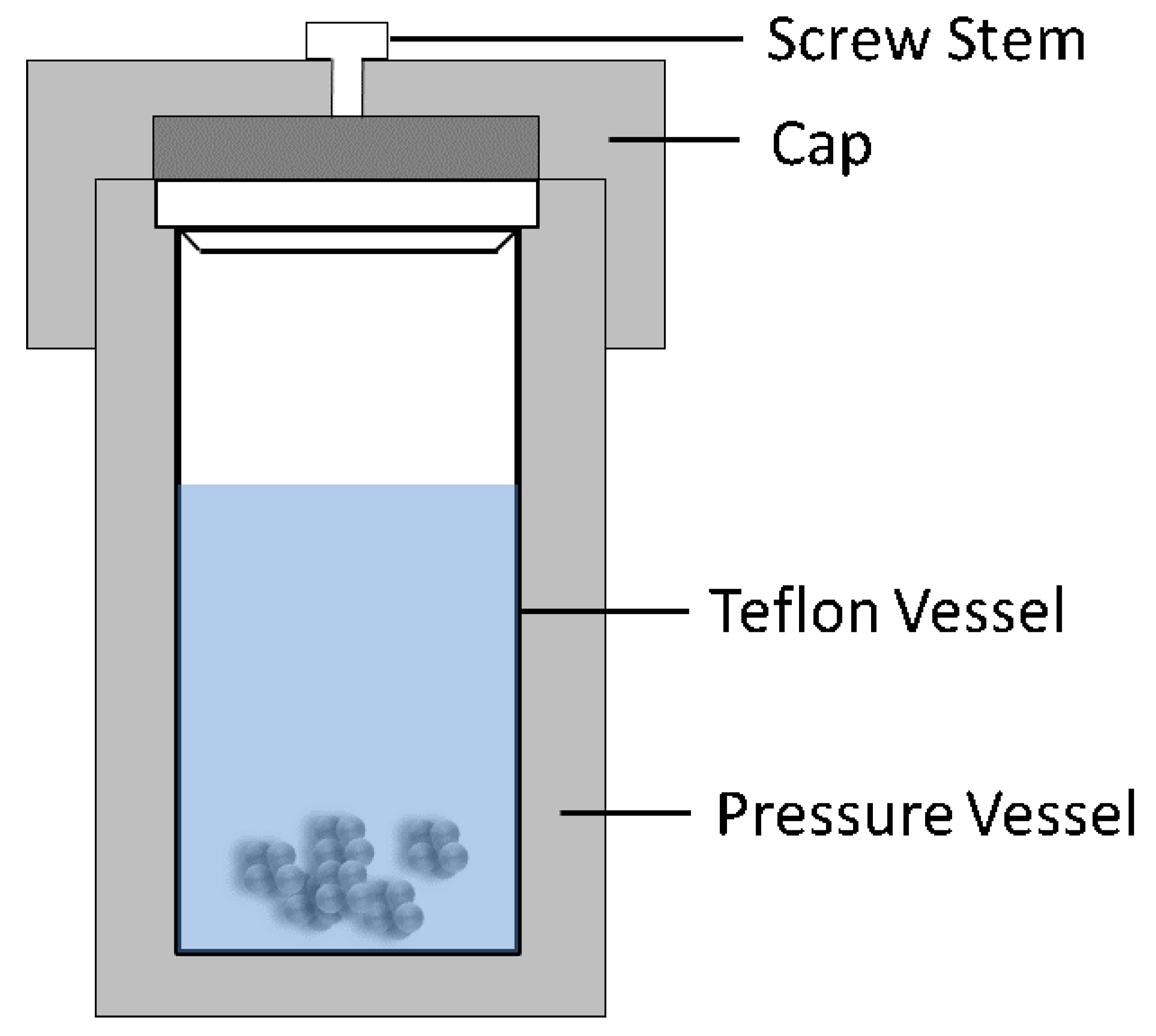
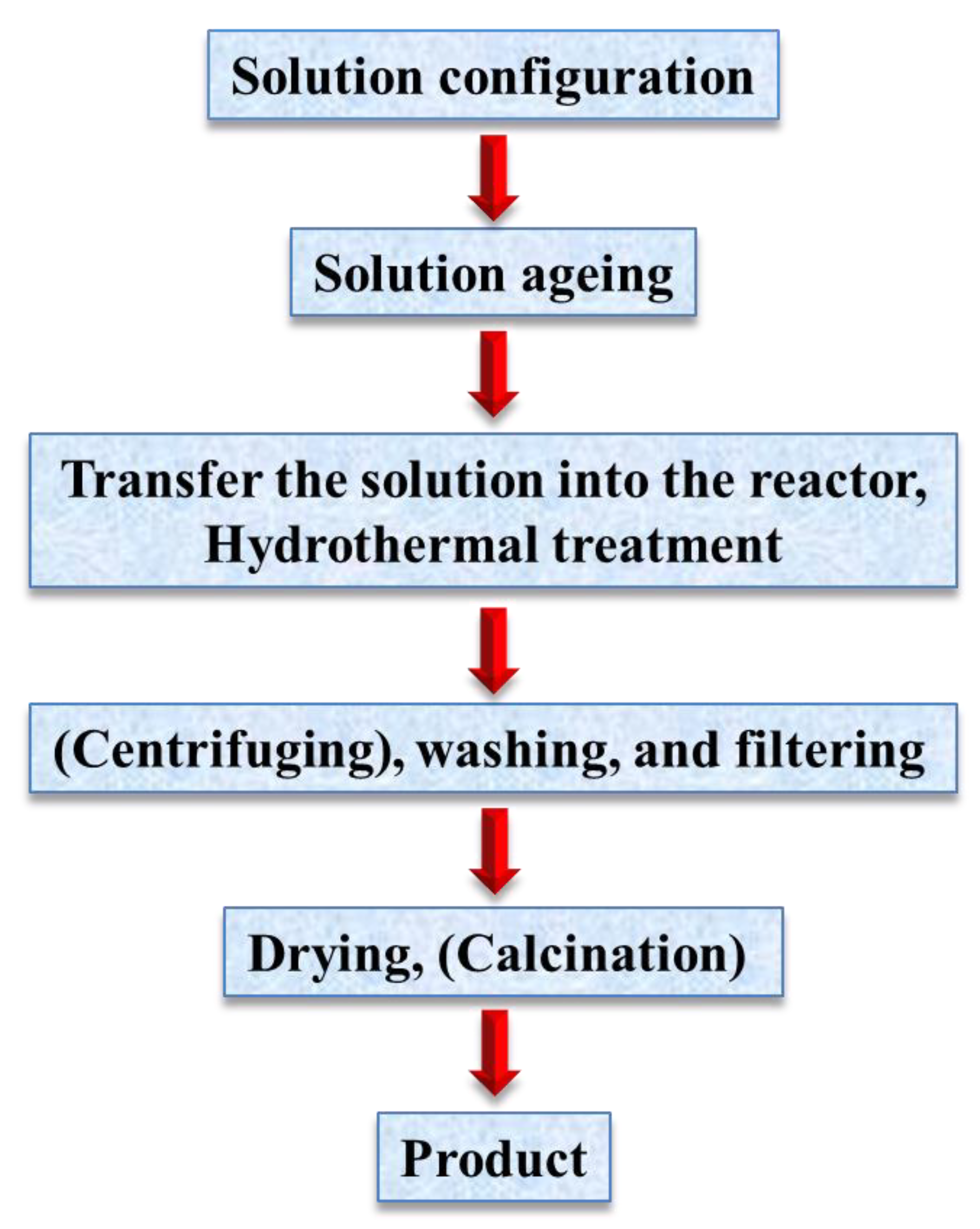
2. Hydrothermal Method
2.1. Reaction Kinetics and the Crystal Growth Mechanism of the Hydrothermal Method
2.2. The Role of Water in the Hydrothermal Method
2.3. Role of Mineralizer
2.4. Basic Classification of Hydrothermal Reactions
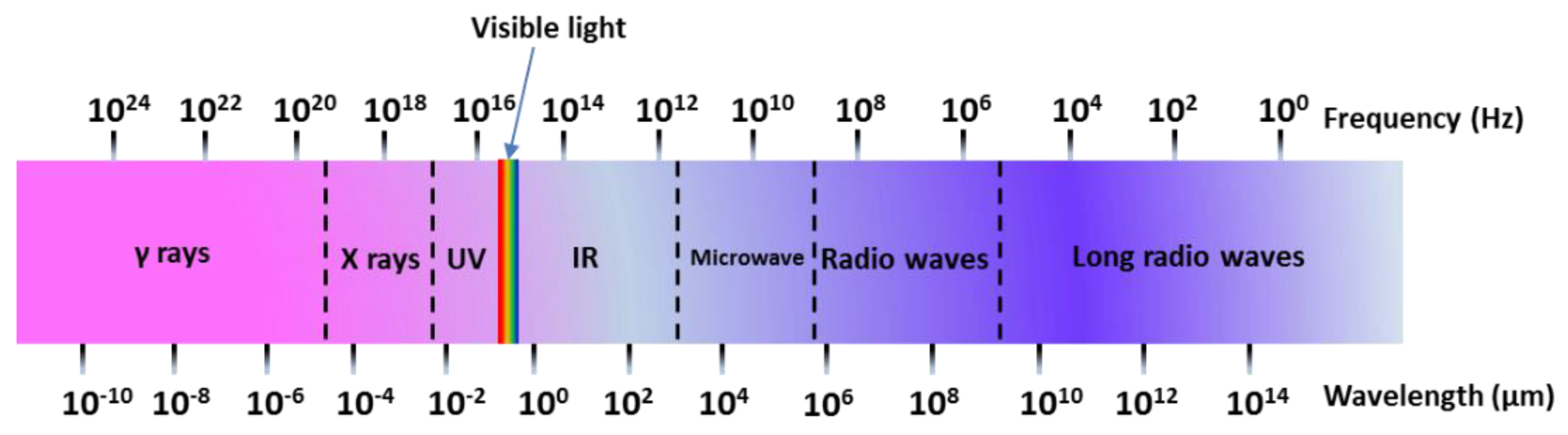
3. Microwave Hydrothermal Method
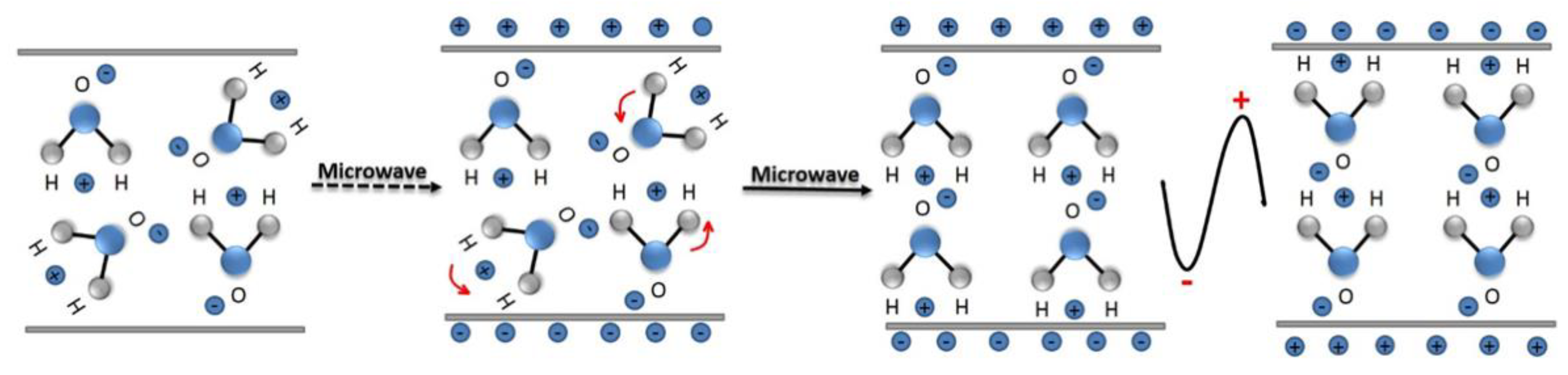
3.1. Reaction Mechanism of Microwave Heating
3.2. Characteristics of the Microwave Hydrothermal Method
4. Application
4.1. Simple Oxides
4.2. Mixed Oxides—Perovskite
4.3. Bioceramics
4.4. Thin Films
4.5. Vanadates
4.6. Garnets
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anyalebechi, P. Essentials of Materials Science and Engineering; Padnos College of Engineering and Computing: Grand Rapids, MI, USA, 2006. [Google Scholar]
- Cahn, R.W. The Coming of Materials Science; Pergamon Press: Oxford, UK, 2001. [Google Scholar]
- Callister, W.D. Fundamentals of Materials Science and Engineering; Wiley: London, UK, 2000; Volume 471660817. [Google Scholar]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef]
- Rajput, N. Methods of preparation of nanoparticles—A review. Int. J. Adv. Eng. Technol. 2015, 7, 1806–1811. [Google Scholar]
- Yang, G.; Yang, J.; Zhang, L. Formation mechanism of spinel LiTi2O4 prepared by carbon thermal reduction reaction. RSC Adv. 2015, 5, 97720–97723. [Google Scholar] [CrossRef]
- Ye, X.R.; Jia, D.Z.; Yu, J.Q.; Xin, X.Q.; Xue, Z. One-Step Solid-State Reactions at Ambient Temperatures—A Novel Approach to Nanocrystal Synthesis. Adv. Mater. 1999, 11, 941–942. [Google Scholar] [CrossRef]
- Li, J.; Jin, Y.-L.; Zhang, X.-G.; Yang, H. Microwave solid-state synthesis of spinel Li4Ti5O12 nanocrystallites as anode material for lithium-ion batteries. Solid State Ion. 2007, 178, 1590–1594. [Google Scholar] [CrossRef]
- Izquierdo, M.; Turan, A.; Garcia, S.; Maroto-Valer, M. Optimization of Li4SiO4 synthesis conditions by a solid state method for maximum CO2 capture at high temperature. J. Mater. Chem. A 2018, 6, 3249–3257. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Wang, Y.; Liu, P.; Zhong, Y.L.; Yin, H.; Su, X.; Zhang, H.; Yang, H.; Wang, D.; Tang, Z. One-step solid phase synthesis of a highly efficient and robust cobalt pentlandite electrocatalyst for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 18314–18321. [Google Scholar] [CrossRef]
- Ghorbani, H.R.; Mehr, F.P.; Pazoki, H.; Rahmani, B.M. Synthesis of ZnO nanoparticles by precipitation method. Orient. J. Chem. 2015, 31, 1219–1221. [Google Scholar] [CrossRef]
- Ramezani, M.; Hosseinpour-Mashkani, S.M.; Sobhani-Nasab, A.; Estarki, H.G. Synthesis, characterization, and morphological control of ZnMoO4 nanostructures through precipitation method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2015, 26, 7588–7594. [Google Scholar] [CrossRef]
- Nejati-Moghadam, L.; Esmaeili Bafghi-Karimabad, A.; Salavati-Niasari, M.; Safardoust, H. Synthesis and characterization of SnO2 nanostructures prepared by a facile precipitation method. J. Nanostruct. 2015, 5, 47–53. [Google Scholar]
- Yang, G.; Park, S.-J. Facile hydrothermal synthesis of NiCo2O4-decorated filter carbon as electrodes for high performance asymmetric supercapacitors. Electrochim. Acta 2018, 285, 405–414. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloys Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Kappis, K.; Papadopoulos, C.; Papavasiliou, J.; Vakros, J.; Georgiou, Y.; Deligiannakis, Y.; Avgouropoulos, G. Tuning the Catalytic Properties of Copper-Promoted Nanoceria via a Hydrothermal Method. Catalysts 2019, 9, 138. [Google Scholar] [CrossRef]
- Morán-Lázaro, J.; Guillen-López, E.; López-Urias, F.; Muñoz-Sandoval, E.; Blanco-Alonso, O.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.; Sanchez-Tizapa, M.; Olvera-Amador, M. Synthesis of ZnMn2O4 nanoparticles by a microwave-assisted colloidal method and their evaluation as a gas sensor of propane and carbon monoxide. Sensors 2018, 18, 701. [Google Scholar] [CrossRef]
- Murase, N.; Jagannathan, R.; Kanematsu, Y.; Watanabe, M.; Kurita, A.; Hirata, K.; Yazawa, T.; Kushida, T. Fluorescence and EPR characteristics of Mn2+-doped ZnS nanocrystals prepared by aqueous colloidal method. J. Phys. Chem. B 1999, 103, 754–760. [Google Scholar] [CrossRef]
- Kim, H.; Popov, B.N. Characterization of hydrous ruthenium oxide/carbon nanocomposite supercapacitors prepared by a colloidal method. J. Power Sources 2002, 104, 52–61. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Pylypchuk, I.V.; Tertykh, V.A.; Yanovska, E.S.; Kolodynska, D. Synthesis and adsorption properties of chitosan-silica nanocomposite prepared by sol-gel method. Nanoscale Res. Lett. 2015, 10, 87. [Google Scholar] [CrossRef]
- Ismail, I.; Jani, M.; Mutalib, A.; Osman, N. Preparation of Nano-Structured Cathode for Proton-Conducting Fuel Cell by Dispersing Agent-Assisted Sol-Gel Method. Mater. Sci. Forum 2018, 917, 78–82. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Xue, Y.; Yao, G.; Zheng, S. A facile synthesis of g-C3N4/TiO2 hybrid photocatalysts by sol–gel method and its enhanced photodegradation towards methylene blue under visible light. Adv. Powder Technol. 2016, 27, 330–337. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Gao, T.; Yao, T.; Han, J.; Han, Z.; Zhang, Z.; Wu, Q.; Song, B. One-pot evaporation–condensation strategy for green synthesis of carbon nitride quantum dots: An efficient fluorescent probe for ion detection and bioimaging. Mater. Chem. Phys. 2017, 194, 293–301. [Google Scholar] [CrossRef]
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef]
- Sasaki, Y.; Hyakkai, M.; Kita, E.; Tasaki, A.; Tanimoto, H.; Iwamoto, Y. Magnetic properties and Mössbauer study of Fe nanocrystals prepared by the gas-deposition method. J. Appl. Phys. 1997, 81, 4736–4738. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2008, 9, 30–35. [Google Scholar] [CrossRef]
- Lee, Y.H.; Zhang, X.Q.; Zhang, W.; Chang, M.T.; Lin, C.T.; Chang, K.D.; Yu, Y.C.; Wang, J.T.W.; Chang, C.S.; Li, L.J. Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Melo, R.; Silva, F.; Moura, K.; De Menezes, A.; Sinfrônio, F. Magnetic ferrites synthesised using the microwave-hydrothermal method. J. Magn. Magn. Mater. 2015, 381, 109–115. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Li, J.-F.; Chen, H.; Wang, L.; Zheng, S.; Lu, G. Systhesizing SnTe nanocrystals leading to thermoelectric performance enhancement via an ultra-fast microwave hydrothermal method. Nano Energy 2016, 28, 78–86. [Google Scholar] [CrossRef]
- Athayde, D.D.; Souza, D.F.; Silva, A.M.; Vasconcelos, D.; Nunes, E.H.; da Costa, J.C.D.; Vasconcelos, W.L. Review of perovskite ceramic synthesis and membrane preparation methods. Ceram. Int. 2016, 42, 6555–6571. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, P.; Liu, D.; Yu, W.; Laipan, M.; Deng, L.; Chen, F. In situ hydrothermal synthesis of a novel hierarchically porous TS-1/modified-diatomite composite for methylene blue (MB) removal by the synergistic effect of adsorption and photocatalysis. J. Colloid Interface Sci. 2016, 462, 191–199. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Soleimani, S.; Ganji, D. Effect of electric field on hydrothermal behavior of nanofluid in a complex geometry. J. Mol. Liq. 2016, 213, 153–161. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ellahi, R. Electrohydrodynamic nanofluid hydrothermal treatment in an enclosure with sinusoidal upper wall. Appl. Sci. 2015, 5, 294–306. [Google Scholar] [CrossRef]
- Morey, G.W.; Niggli, P. The hydrothermal formation of silicates, a review. J. Am. Chem. Soc. 1913, 35, 1086–1130. [Google Scholar] [CrossRef]
- Yoshimura, M.; Suda, H. Hydrothermal processing of hydroxyapatite: Past, present, and future. In Hydroxyapatite and Related Compounds; CRC Press Inc.: Boca Raton, FL, USA, 1994; pp. 45–72. [Google Scholar]
- Penn, R.L.; Banfield, J.F. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from Titania. Geochim. Cosmochim. Acta 1999, 63, 1549–1557. [Google Scholar] [CrossRef]
- Polsongkram, D.; Chamninok, P.; Pukird, S.; Chow, L.; Lupan, O.; Chai, G.; Khallaf, H.; Park, S.; Schulte, A. Effect of synthesis conditions on the growth of ZnO nanorods via hydrothermal method. Physica B 2008, 403, 3713–3717. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Miyashita, S.; Obara, K.; Shishido, T.; Sakagami, N. Hydrothermal growth of ZnO single crystals and their optical characterization. J. Cryst. Growth 2000, 214, 72–76. [Google Scholar] [CrossRef]
- Donnay, J.H.; Harker, D. A new law of crystal morphology extending the Law of Bravais. Am. Mineral. J. Earth Planet. Mater. 1937, 22, 457–477. [Google Scholar]
- Hartman, P.; Perdok, W.G. On the relations between structure and morphology of crystals: I. Acta Crystallogr. 2010, 8, 521–524. [Google Scholar] [CrossRef]
- Davey, R.J.; Milisavljevic, B.; Bourne, J.R. Solvent interactions at crystal surfaces: The kinetic story of .alpha.-resorcinol. J. Phys. Chem. 1988, 92, 2032–2036. [Google Scholar] [CrossRef]
- Andonov, P.; Chieux, P.; Kimura, S. A local order study of molten LiNbO3 by neutron diffraction. J. Phys. Condens. Matter 1993, 5, 4865. [Google Scholar] [CrossRef]
- Tuttle, O.F.; Bowen, N.L. Origin of Granite in the Light of Experimental Studies in the System NaAlSi3O8–KAlSi3O8–SiO2–H2O; Geological Society of America: Boulder, CO, USA, 1958. [Google Scholar]
- Luth, W.C.; Jahns, R.H.; Frank, T.O. The granite system at pressures of 4 to 10 kilobars. J. Geophys. Res. 1964, 69, 759–773. [Google Scholar] [CrossRef]
- Fenn, P.M. Nucleation and growth of alkali feldspars from hydrous melts. Can. Miner. 1977, 15, 135–161. [Google Scholar]
- Dell’Agli, G.; Colantuono, A.; Mascolo, G. The effect of mineralizers on the crystallization of zirconia gel under hydrothermal conditions. Solid State Ion. 1999, 123, 87–94. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, J.; Zhao, Z.; Qi, L. Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem. Mater. 1995, 7, 663–671. [Google Scholar] [CrossRef]
- Roble, M.; Rojas, S.; Wheatley, R.; Wallentowitz, S.; Cabrera, A.; Diaz-Droguett, D. Hydrothermal Improvement For 3R-CuFeO2 Delafossite Growth by Control of Mineralizer and Reaction Atmosphere. J. Solid State Chem. 2019, 271, 314–325. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, K.; Yuan, L.; Feng, S. Mineralizer effect on facet-controllable hydrothermal crystallization of perovskite structure YbFeO3 crystals. CrystEngComm 2018, 20, 470–476. [Google Scholar] [CrossRef]
- Lestari, W.; Hasanah, D.; Putra, R.; Mukti, R.; Nugrahaningtyas, K. Transformation of Indonesian Natural Zeolite into Analcime Phase under Hydrothermal Condition; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 012068. [Google Scholar]
- Kang, X.; Floyd, R.; Lowum, S.; Cabral, M.; Dickey, E.; Maria, J.P. Mechanism studies of hydrothermal cold sintering of zinc oxide at near room temperature. J. Am. Ceram. Soc. 2018. [Google Scholar] [CrossRef]
- Tani, E.; Yoshimura, M.; Sōmiya, S. Formation of ultrafine tetragonal ZrO2 powder under hydrothermal conditions. J. Am. Ceram. Soc. 1983, 66, 11–14. [Google Scholar] [CrossRef]
- Sutton, W.H. Microwave processing of ceramic materials. Am. Ceram. Soc. Bull. 1989, 68, 376–386. [Google Scholar]
- Metaxas, A.; Meredith, R. Industrial Microwave Heating; Peter Peregrinus Ltd. (IEE): London, UK, 1983. [Google Scholar]
- Crane, C.; Pantoya, M.; Weeks, B. Spatial observation and quantification of microwave heating in materials. Rev. Sci. Instrum. 2013, 84, 084705. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef]
- Cheng, J.; Roy, R.; Agrawal, D. Experimental proof of major role of magnetic field losses in microwave heating of metal and metallic composites. J. Mater. Sci. Lett. 2001, 20, 1561–1563. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Casagrande, A.; Leonelli, C. Microwave ignition of the combustion synthesis of aluminides and field-related effects. J. Alloys Compd. 2016, 657, 59–67. [Google Scholar] [CrossRef]
- Thostenson, E.; Chou, T.-W. Microwave processing: Fundamentals and applications. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Bugrov, A.N.; Rodionov, I.A.; Zvereva, I.A.; Smyslov, R.Y.; Almjasheva, O.V. Photocatalytic activity and luminescent properties of Y, Eu, Tb, Sm and Er-doped ZrO2 nanoparticles obtained by hydrothermal method. Int. J. Nanotechnol. 2016, 13, 147–157. [Google Scholar] [CrossRef]
- Xue, G.; Huang, X.; Zhao, N.; Xiao, F.; Wei, W. Hollow Al2O3 spheres prepared by a simple and tunable hydrothermal method. RSC Adv. 2015, 5, 13385–13391. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, Z.J.; Li, X.L.; Lee, S.W. Controlled hydrothermal synthesis of colloidal boehmite (γ-AlOOH) nanorods and nanoflakes and their conversion into γ-Al2O3 nanocrystals. Solid State Commun. 2008, 145, 368–373. [Google Scholar] [CrossRef]
- Wu, H.; Wu, G.; Wang, L. Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: Facile synthesis and electromagnetic properties. Powder Technol. 2015, 269, 443–451. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z.; Wang, N.; Huang, Y.; Wang, J.; Liu, W.; Fu, Y.; Wang, Z. Facile synthesis of α-Fe2O3 micro-ellipsoids by surfactant-free hydrothermal method for sub-ppm level H2S detection. Mater. Des. 2016, 110, 532–539. [Google Scholar] [CrossRef]
- Li, N.; Zhu, X.; Zhang, C.; Lai, L.; Jiang, R.; Zhu, J. Controllable synthesis of different microstructured MnO2 by a facile hydrothermal method for supercapacitors. J. Alloys Compd. 2017, 692, 26–33. [Google Scholar] [CrossRef]
- Chu, J.; Lu, D.; Ma, J.; Wang, M.; Wang, X.; Xiong, S. Controlled growth of MnO2 via a facile one-step hydrothermal method and their application in supercapacitors. Mater. Lett. 2017, 193, 263–265. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Nagajyothi, P.; Anil Kumar Reddy, P.; Kotesh Kumar, M.; Shim, J.; Byon, C. Tiny MoO3 nanocrystals self-assembled on folded molybdenum disulfide nanosheets via a hydrothermal method for supercapacitor. Mater. Res. Lett. 2018, 6, 432–441. [Google Scholar] [CrossRef]
- Gong, J.; Zeng, W.; Zhang, H. Hydrothermal synthesis of controlled morphologies of MoO3 nanobelts and hierarchical structures. Mater. Lett. 2015, 154, 170–172. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Deng, D.; Liu, X.; Xing, X.; Xiao, X.; Wang, Y. Acetone sensing performances based on nanoporous TiO2 synthesized by a facile hydrothermal method. Sens. Actuators B Chem. 2017, 238, 491–500. [Google Scholar] [CrossRef]
- Iraj, M.; Nayeri, F.D.; Asl-Soleimani, E.; Narimani, K. Controlled growth of vertically aligned TiO2 nanorod arrays using the improved hydrothermal method and their application to dye-sensitized solar cells. J. Alloys Compd. 2016, 659, 44–50. [Google Scholar] [CrossRef]
- Hassanpour, A.; Bogdan, N.; Capobianco, J.A.; Bianucci, P. Hydrothermal selective growth of low aspect ratio isolated ZnO nanorods. Mater. Des. 2017, 119, 464–469. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Kong, J.; Yu, M.; Jin, H. Synthesis of Mg-doped hierarchical ZnO nanostructures via hydrothermal method and their optical properties. J. Alloys Compd. 2016, 657, 261–267. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-temperature high-performance H2S sensor based on porous CuO nanosheets prepared by hydrothermal method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Gopalakrishnan, N. Hydrothermally synthesized Copper Oxide (CuO) superstructures for ammonia sensing. J. Colloid Interface Sci. 2016, 480, 76–84. [Google Scholar] [CrossRef]
- Zdravković, J.; Simović, B.; Golubović, A.; Poleti, D.; Veljković, I.; Šćepanović, M.; Branković, G. Comparative study of CeO2 nanopowders obtained by the hydrothermal method from various precursors. Ceram. Int. 2015, 41, 1970–1979. [Google Scholar] [CrossRef]
- Paparazzo, E.; Moretti, G. Comment on: “Structural, morphological and optical properties of shuttle-like CeO2 synthesized by a facile hydrothermal method,” by Li et al., J. Alloys Comp., 722 (2017) 489. J. Alloys Compd. 2019, 770, 942–944. [Google Scholar] [CrossRef]
- Lu, H.; Xiang, K.; Bai, N.; Zhou, W.; Wang, S.; Chen, H. Urchin-shaped Nb2O5 microspheres synthesized by the facile hydrothermal method and their lithium storage performance. Mater. Lett. 2016, 167, 106–108. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Zhang, X.; Liu, B.; Xu, L.; Zhang, Z.; Zhang, Y. 2D ultrathin nanosheet-assembled Nb2O5 microflowers for lithium ion batteries. Mater. Lett. 2018, 227, 112–115. [Google Scholar] [CrossRef]
- Roy, N.; Park, Y.; Sohn, Y.; Leung, K.T.; Pradhan, D. Green synthesis of anatase TiO2 nanocrystals with diverse shapes and their exposed facets-dependent photoredox activity. ACS Appl. Mater. Interfaces 2014, 6, 16498–16507. [Google Scholar] [CrossRef]
- Zavala, M.Á.L.; Morales, S.A.L.; Ávila-Santos, M. Synthesis of stable TiO2 nanotubes: Effect of hydrothermal treatment, acid washing and annealing temperature. Heliyon 2017, 3, e00456. [Google Scholar] [CrossRef]
- Komarneni, S.; Roy, R.; Li, Q. Microwave-hydrothermal synthesis of ceramic powders. Mater. Res. Bull. 1992, 27, 1393–1405. [Google Scholar] [CrossRef]
- Wilson, G.J.; Will, G.D.; Frost, R.L.; Montgomery, S.A. Efficient microwave hydrothermal preparation of nanocrystalline anatase TiO2 colloids. J. Mater. Chem. 2002, 12, 1787–1791. [Google Scholar] [CrossRef]
- Yao, K.; Liu, S.; Dong, Y.-Y.; Wang, B.; Bian, J.; Ma, M.-G. Comparative study of CuO crystals on the cellulose substrate by the microwave-assisted hydrothermal method and hydrothermal method. Mater. Des. 2016, 90, 129–136. [Google Scholar] [CrossRef]
- Noh, H.-J.; Seo, D.-S.; Kim, H.; Lee, J.-K. Synthesis and crystallization of anisotropic shaped ZrO2 nanocrystalline powders by hydrothermal process. Mater. Lett. 2003, 57, 2425–2431. [Google Scholar] [CrossRef]
- Bondioli, F.; Ferrari, A.M.; Leonelli, C.; Siligardi, C.; Pellacani, G.C. Microwave-hydrothermal synthesis of nanocrystalline zirconia powders. J. Am. Ceram. Soc. 2001, 84, 2728–2730. [Google Scholar] [CrossRef]
- Li, C.; Li, K.; Li, H.; Zhang, Y.; Ouyang, H.; Liu, L.; Sun, C. Effect of reaction temperature on crystallization of nanocrystalline zirconia synthesized by microwave-hydrothermal process. J. Alloys Compd. 2013, 561, 23–27. [Google Scholar] [CrossRef]
- Nie, L.; Deng, K.; Yuan, S.; Zhang, W.; Tan, Q. Microwave-assisted hydrothermal synthesis of hierarchically porous γ-Al2O3 hollow microspheres with enhanced Cu2+ adsorption performance. Mater. Lett. 2014, 132, 369–372. [Google Scholar] [CrossRef]
- Ren, T.-Z.; Yuan, Z.-Y.; Su, B.-L. Microwave-assisted preparation of hierarchical mesoporous– macroporous boehmite AlOOH and γ-Al2O3. Langmuir 2004, 20, 1531–1534. [Google Scholar] [CrossRef]
- Ma, R.; Bando, Y.; Zhang, L.; Sasaki, T. Layered MnO2 nanobelts: Hydrothermal synthesis and electrochemical measurements. Adv. Mater. 2004, 16, 918–922. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, T.; Shi, D.; Zhang, Y.; Zeng, W.; Li, T.; Miao, B. Hydrothermal synthesis of urchin-like MnO2 nanostructures and its electrochemical character for supercapacitor. Appl. Surf. Sci. 2015, 351, 862–868. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, Y.; Banis, M.N.; Liu, J.; Geng, D.; Li, R.; Sun, X. Facile controlled synthesis and growth mechanisms of flower-like and tubular MnO2 nanostructures by microwave-assisted hydrothermal method. J. Colloid Interface Sci. 2012, 369, 123–128. [Google Scholar] [CrossRef]
- Ming, B.; Li, J.; Kang, F.; Pang, G.; Zhang, Y.; Chen, L.; Xu, J.; Wang, X. Microwave–hydrothermal synthesis of birnessite-type MnO2 nanospheres as supercapacitor electrode materials. J. Power Sources 2012, 198, 428–431. [Google Scholar] [CrossRef]
- Chung, C.-C.; Chung, T.-W.; Yang, T.C.-K. Rapid synthesis of titania nanowires by microwave-assisted hydrothermal treatments. Ind. Eng. Chem. Res. 2008, 47, 2301–2307. [Google Scholar] [CrossRef]
- Corradi, A.B.; Bondioli, F.; Focher, B.; Ferrari, A.M.; Grippo, C.; Mariani, E.; Villa, C. Conventional and microwave-hydrothermal synthesis of TiO2 nanopowders. J. Am. Ceram. Soc. 2005, 88, 2639–2641. [Google Scholar] [CrossRef]
- Lozano-Sánchez, L.; Lee, S.-W.; Sekino, T.; Rodríguez-González, V. Practical microwave-induced hydrothermal synthesis of rectangular prism-like CaTiO3. CrystEngComm 2013, 15, 2359–2362. [Google Scholar] [CrossRef]
- Han, C.; Liu, J.; Yang, W.; Wu, Q.; Yang, H.; Xue, X. Photocatalytic activity of CaTiO3 synthesized by solid state, sol–gel and hydrothermal methods. J. Sol-Gel Sci. Technol. 2017, 81, 806–813. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lima, A.; Godinho, M.; Moura, A.; Espinosa, J.; Longo, E.; Marques, A. Synthesis of Pr3+-doped CaTiO3 using polymeric precursor and microwave-assisted hydrothermal methods: A comparative study. Ceram. Int. 2015, 41, 12841–12848. [Google Scholar] [CrossRef]
- Chybczyńska, K.; Markiewicz, E.; Błaszyk, M.; Hilczer, B.; Andrzejewski, B. Dielectric response and electric conductivity of ceramics obtained from BiFeO3 synthesized by microwave hydrothermal method. J. Alloys Compd. 2016, 671, 493–501. [Google Scholar] [CrossRef]
- Liu, S.-F.; Abothu, I.R.; Komarneni, S. Barium titanate ceramics prepared from conventional and microwave hydrothermal powders. Mater. Lett. 1999, 38, 344–350. [Google Scholar] [CrossRef]
- Suchanek, K.; Bartkowiak, A.; Perzanowski, M.; Marszałek, M.; Sowa, M.; Simka, W. Electrochemical properties and bioactivity of hydroxyapatite coatings prepared by MEA/EDTA double-regulated hydrothermal synthesis. Electrochim. Acta 2019, 298, 685–693. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Takahashi, H.; Ioku, K. Tubular hydroxyapatite formation through a hydrothermal process from α-tricalcium phosphate with anatase. J. Mater. Sci. 2012, 47, 4194–4199. [Google Scholar] [CrossRef]
- Jokić, B.; Mitrić, M.; Radmilović, V.; Drmanić, S.; Petrović, R.; Janaćković, D. Synthesis and characterization of monetite and hydroxyapatite whiskers obtained by a hydrothermal method. Ceram. Int. 2011, 37, 167–173. [Google Scholar] [CrossRef]
- Yu, H.-P.; Zhu, Y.-J.; Lu, B.-Q. Highly efficient and environmentally friendly microwave-assisted hydrothermal rapid synthesis of ultralong hydroxyapatite nanowires. Ceram. Int. 2018, 44, 12352–12356. [Google Scholar] [CrossRef]
- Kumar, G.S.; Karunakaran, G.; Girija, E.K.; Kolesnikov, E.; Van Minh, N.; Gorshenkov, M.V.; Kuznetsov, D. Size and morphology-controlled synthesis of mesoporous hydroxyapatite nanocrystals by microwave-assisted hydrothermal method. Ceram. Int. 2018, 44, 11257–11264. [Google Scholar] [CrossRef]
- Zawawi, C.Z.N.C.M.; Salleh, S.; Jew, L.O.; Chaudhary, K.T.; Helmi, M.; Aziz, M.S.; Haider, Z.; Ali, J. In Two steps hydrothermal growth and characterisations of BaTiO3 films composed of nanowires. J. Phys. Conf. Ser. 2018, 1027, 012014. [Google Scholar] [CrossRef]
- Velasco-Davalos, I.; Ambriz-Vargas, F.; Gómez-Yáñez, C.; Thomas, R.; Ruediger, A. Polarization reversal in BaTiO3 nanostructures synthesized by microwave-assisted hydrothermal method. J. Alloys Compd. 2016, 667, 268–274. [Google Scholar] [CrossRef]
- Lancaster, M.; Powell, J.; Porch, A. Thin-film ferroelectric microwave devices. Supercond. Sci. Technol. 1998, 11, 1323. [Google Scholar] [CrossRef]
- Ishizawa, N.; Banno, H.; Hayashi, M.; Yoo, S.E.; Yoshimura, M. Preparation of BaTiO3 and SrTiO3 polycrystalline thin films on flexible polymer film substrate by hydrothermal method. Jpn. J. Appl. Phys. 1990, 29, 2467. [Google Scholar] [CrossRef]
- Sun, Y.; Fuge, G.M.; Fox, N.A.; Riley, D.J.; Ashfold, M.N. Synthesis of aligned arrays of ultrathin ZnO nanotubes on a Si wafer coated with a thin ZnO film. Adv. Mater. 2005, 17, 2477–2481. [Google Scholar] [CrossRef]
- Liew, L.L.; Hong, Q.L.; Goh, G.K.L. Microwave-assisted hydrothermally grown epitaxial ZnO films on <1 1 1> MgAl2O4 substrate. J. Solid State Chem. 2012, 189, 90–95. [Google Scholar] [CrossRef]
- Doh, J.G.; Hong, J.S.; Vittal, R.; Kang, M.G.; Park, N.G.; Kim, K.J. Enhancement of Photocurrent and Photovoltage of Dye-Sensitized Solar Cells with TiO2 Film Deposited on Indium Zinc Oxide Substrate. Chem. Mater. 2014, 16, 493–497. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, M.; Choung, S.J.; Ogino, K.; Miyata, S.; Kim, M.S.; Park, J.Y.; Kim, J.B. The preparation of TiO2 nanometer photocatalyst film by a hydrothermal method and its sterilization performance for Giardia lamblia. Water Res. 2004, 38, 713–719. [Google Scholar] [CrossRef]
- Wu, H.; Hu, Z.; Li, B.; Wang, H.; Peng, Y.; Zhou, D.; Zhang, X. High-quality ZnO thin film grown on sapphire by hydrothermal method. Mater. Lett. 2015, 161, 565–567. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, W.; Jiang, D.; Zhao, H.; John, R.; Wilson, G.J.; Will, G.D. Photoelectrochemical characterisation of TiO2 thin films derived from microwave hydrothermally processed nanocrystalline colloids. J. Photochem. Photobiol. A 2006, 179, 305–313. [Google Scholar] [CrossRef]
- Zhou, Z.; Bowland, C.C.; Patterson, B.A.; Malakooti, M.H.; Sodano, H.A. Conformal BaTiO3 films with high piezoelectric coupling through an optimized hydrothermal synthesis. ACS Appl. Mater. Interfaces 2016, 8, 21446–21453. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Molero-Sánchez, B.; Ávila-Brande, D.; Morán, E.; Pérez-Flores, J.C.; Kuhn, A.; García-Alvarado, F. The intercalation chemistry of H2V3O8 nanobelts synthesised by a green, fast and cost-effective procedure. J. Power Sources 2013, 232, 173–180. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Xie, M.; Jing, L.; Xu, H.; She, X.; Li, H.; Xie, J. Construction of novel CNT/LaVO4 nanostructures for efficient antibiotic photodegradation. Chem. Eng. J. 2019, 357, 487–497. [Google Scholar] [CrossRef]
- Jinhai, L.; Han, M.; Guo, Y.; Wang, F.; Meng, L.; Mao, D.; Ding, S.; Sun, C. Hydrothermal synthesis of novel flower-like BiVO4/Bi2Ti2O7 with superior photocatalytic activity toward tetracycline removal. Appl. Catal. A Gen. 2016, 524, 105–114. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, C.; Wei, C. Microstructure and photocatalytic performance of BiVO4 prepared by hydrothermal method. J. Alloys Compd. 2019, 781, 56–63. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Chen, X.; Bi, J.; Wu, L.; Li, Z.; Fu, X. Microwave hydrothermal synthesis and upconversion properties of NaYF4: Yb3+, Tm3+ with microtube morphology. Mater. Lett. 2009, 63, 1023–1026. [Google Scholar] [CrossRef]
- Laudise, R.; Kolb, E. Hydrothermal Crystallization of Yttrium-Iron Garnet on a Seed. J. Am. Ceram. Soc. 1962, 45, 51–53. [Google Scholar] [CrossRef]
- Cho, Y.S.; Burdick, V.L.; Amarakoon, V.R. Hydrothermal preparation and morphology characteristics of Y3Fe5O12. J. Am. Ceram. Soc. 1997, 80, 1605–1608. [Google Scholar] [CrossRef]
- Ramesh, T.; Shinde, R.; Murthy, S. Nanocrystalline gadolinium iron garnet for circulator applications. J. Magn. Magn. Mater. 2012, 324, 3668–3673. [Google Scholar] [CrossRef]
- Sadhana, K.; Murthy, S.R.; Praveena, K. Structural and magnetic properties of Dy3+ doped Y3Fe5O12 for microwave devices. Mater. Sci. Semicond. Proc. 2015, 34, 305–311. [Google Scholar] [CrossRef]
| Mineralizer | ZrO2 (300 °C, 24 h, 100 Mpa) | |
|---|---|---|
| Tetragonal | Monoclinic | |
| KF (8 wt.%) | No data | 16 nm |
| NaOH (30 wt.%) | No data | 40 nm |
| H2O | 15 nm | 17 nm |
| LiCl (15 wt.%) | 15 nm | 19 nm |
| KBr (15 wt.%) | 13 nm | 15 nm |
| Hydrothermal Method | Microwave Hydrothermal Method | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Morphology | Raw Materials | Conditions | Size | Ref. | Morphology | Raw Materials | Conditions | Size | Ref. | |
| ZrO2 | Spherical | ZrOCl2·8H2O, NH4OH, NaOH | 150 °C, 24 h | 20–30 nm | [87] | Monoclinic | ZrOCl2·8H2O, NaOH | 200 °C, 2 h, 2.45 GHz | 10–20 nm | [88] |
| Rod | ZrOCl2·8H2O, NH4OH, NaOH | 200 °C, 24 h 250 °C, 24 h | 50 nm × (200–400) nm 80 nm × (200–500) nm | [87] | Tetragonal-monoclinic | ZrOCl4, NaOH | 150–220 °C, 30 min | ~20 nm | [89] | |
| Al2O3 | Hollow | Al(NO3)3·9H2O, glucose | 160 °C, 3–8 h | 5.4–6.9 μm | [64] | Hollow | KAl(SO4)2·12H2O, CO(NH2)2 | 180 °C, 40 min, 300 W | 0.8–1.2 μm | [90] |
| Rod | Al(NO3)3·9H2O, N2H4⋅H2O | 200 °C, 12 h | 8 nm × (220–532) nm | [65] | Fiber | Surfactant Brij 56, H2SO4, Aluminum sec-butoxide | 80 °C, 30 min, 500 W | ~50 nm | [91] | |
| MnO2 | Belt | Mn2O3, NaOH | 170 °C, 12 h | 5–15 nm | [92] | Flower Nanosheet Fiber | KMnO4, HCl | 100 °C, 25 min 140 °C, 25 min 180 °C, 25 min | 200–400 nm 10 nm 2–6 μm | [94] |
| Urchin Urchin Nanowire | MnSO4, (NH4)2S2O8 | 80 °C, 4 h 110 °C, 4 h 140 °C, 4 h | 2–3 μm 30–40 μm ultrathin | [83] | Nanosphere | KMnO4, MnSO4·H2O | 75 °C, 30 min | 70–90 nm | [95] | |
| TiO2 | Nanotube | TiO2, NaOH | 150 °C, 48 h | 8.1–27.3 nm | [96] | Nanowire | TiO2, NaOH | 210 °C, 2 h, 350 W | 80–150 nm | [96] |
| Acicular | TiOCl2 | 195 °C, >8 h | 100 nm × 50 nm | [97] | Spherical | TiOCl2 | 195 °C, >30 min, 2.45 GHz | 10 nm | [97] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Park, S.-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. https://doi.org/10.3390/ma12071177
Yang G, Park S-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials. 2019; 12(7):1177. https://doi.org/10.3390/ma12071177
Chicago/Turabian StyleYang, Guijun, and Soo-Jin Park. 2019. "Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review" Materials 12, no. 7: 1177. https://doi.org/10.3390/ma12071177
APA StyleYang, G., & Park, S.-J. (2019). Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials, 12(7), 1177. https://doi.org/10.3390/ma12071177




