1. Introduction
Zinc oxide (ZnO) has been employed for a variety of applications, for example, in antibacterial agents [
1], photo-catalysts [
2], nanogenerators [
3] and gas sensors [
4,
5]. Because of the key properties of ZnO nanoparticles, i.e., high electron mobility, good chemical stability, low toxicity and biological compatibility [
6], ZnO biosensors are also widely used in applications for detection of small molecules such as glucose [
7], cholesterol [
8] and DNA [
9].
Enhancements of material properties have been demonstrated by incorporating other materials or elements within a ZnO matrix. For example, the implantation of iron oxide (Fe
O
) in ZnO nanosheets decreases the charge transfer resistance and also ensures that the nanosheets are photostable and reusable, and thus, suitable for application as photocatalysts [
2]. In order to enhance the electrocatalytic properties of a modified electrode, carbon xerogel-zinc oxide composites were synthesized by the sol-gel method, contributing to good material conductivity [
10].
The addition of copper oxide (CuO) to ZnO has been shown to improve sensitivity by enhancing the redox property and electron transfer. ZnO surfaces have been created by an electro-spinning method followed by hydrothermal treatment, with CuO subsequently being added by a wet method [
4]. These “CuO-decorated ZnO hierarchical nanostructures” were employed to enhance the performance of a Hydrogen Sulfide (H
S) gas-sensor. Furthermore, a novel free-standing ZnO–CuO composite film, which was fabricated by a modified hydrothermal method, in order to remove the need for a substrate [
5], formed the basis for a gas sensor which showed a good improvement in response for sensing carbon monoxide (CO) gas compared with pure ZnO nanowires [
5]. Finally, a ZnO–CuO composite matrix was fabricated on an ITO (Indium tin oxide) coated corning glass substrate by pulsed laser deposition [
11]. The inclusion of CuO resulted in excellent redox properties, and consequently, in the absence of any external mediator, good oxidation and reduction peaks were visible on a cyclic voltammogram. The improvement in properties in these composite nanoparticles has been related to the decrease in the band gap energy. In fact, it has been shown that the higher the concentration of CuO in a ZnO–CuO composite, the smaller the band gap [
1].
However, for all of these examples plus others in the literature, the formation of ZnO/CuO composite nanoparticles/nano-surfaces requires complex processes, typically involving high temperature and long time periods. For example, ZnO nanowires have been grown by thermal evaporation and CuO films have been synthesized by thermal oxidation (at 400
C for 12 h in 80% oxygen/20% argon atmosphere); the Cu films initially being produced by a radio frequency (RF) sputtering technique [
12]. Methods described for fabricating CuO–ZnO nanocomposites are also complex. For example, Widiarti et al. [
1] added copper(II) sulphate pentahydrate (CuSO
·5H
O) to citric acid stirring until homogenous and the precursor solution made by ethylene glycol mixed with zinc(II) acetate dihydrate (Zn(CH
COO)
·2H
O), deionized water and citric acid by vigorous stirring for an hour. The solution was added by sodium hydroxide (NaOH) drop by drop stirring for one hour and kept for 48 h for aging. The gel was washed and dried in oven to constant weight and then calcined at 500
C for 4 h [
1].
The study presented here investigated mixtures of ZnO and CuO nanoparticles to fabricate nanocrystal surfaces for the immobilisation of anti-CRP antibodies. An enzyme labelled antibody was used to assess protein uptake on the nano-surfaces. The fabrication of the biosensor nano-surfaces used a simple and inexpensive colloidal dispersion technique. ZnO nanocrystals were deposited on PET, coated with anti-CRP antibodies and then sandwiched between two glass coverslips for the detection of CRP. The binding of CRP to the antibody on the biosensor surface was detected and quantified using impedance spectroscopy. Fabrication of the ZnO–CuO nano-surfaces was easy to fabricate, with a process that is compatible with large scale manufacture.
C-reactive protein (CRP) was chosen as the model target for the biosensor as it is an important marker of inflammation and can also predict myocardial infarction and sudden cardiac death [
13]. A rapid test which can be used in a doctors surgery of clinic will allow the assessment of patients during their consultation without the need to send a blood sample to a centralise laboratory for testing. The use of paper based lateral flow or paper based microfluidic assays have been described [
14] highlighting a point-of-care application for the measurement of CRP.
2. Materials and Methods
2.1. Surface Preparation
The sum of 0.15 g ZnO nanoparticles (99.9+%, 80–200 nm from US Research Nanomaterials Inc., Houston, TX, USA) was added to 15 mL double deionized water and 0.1 g CuO nanoparticles (99.5+%, width 10–30 nm, Length: 200–800 nm, Long fibrous shape from US Research Nanomaterials Inc.) was added to 10 mL double deionized water. The suspensions of both 1% ZnO and 1% CuO were stirred at room temperature for 1 h. Five different nano-surfaces were produced as follows: 0% CuO (ZnO only), 33% CuO (2:1 ZnO:CuO via volume ratio), 50% CuO (1:1 ZnO:CuO), 67% (1:2 ZnO:CuO) and 100% CuO (CuO only). 1.5 mL aliquots of each preparation (prepared by volume ratios) were ultra-sonicated for 7 periods of 20 s, at 4 min intervals using an exponential microprobe (Soniprep 150) at 30 watts. 200
L of each suspension were dropped on to clean polyethylene terephthalate (PET) substrates (20 mm × 20 mm) separately to make ZnO/CuO nano-surfaces. Finally, they were dried in an oven at 65
C for 80 min and cooled down to room temperature, then stored in a dry atmosphere with silica gel for up to 2 days [
15].
2.2. CRP Sensor Fabrication and Test
The sensing area (10 mm × 4 mm) of each ZnO–CuO nano-surface on PET was defined by tape. Subsequently, 40 L (100 ng or 200 ng) of monoclonal mouse anti-human C-reactive protein from HyTest Ltd. (Turku, Finland) was added to the surface. Phosphate Buffered Saline (PBS, pH 7.3 ± 0.2 at 25 C) was purchased from OXOID Microbiology products (Fisher Scientific, Loughborough, Leicestershire, UK). Anti-CRP antibodies were stored and diluted in 0.025 M PBS with pH 7.40. The biosensor was then dried in a desiccator with silica gel at 4 C overnight for 18 h. The nano-surface biosensors were then aligned above a pair of D-shape electrodes to perform impedance measurements.
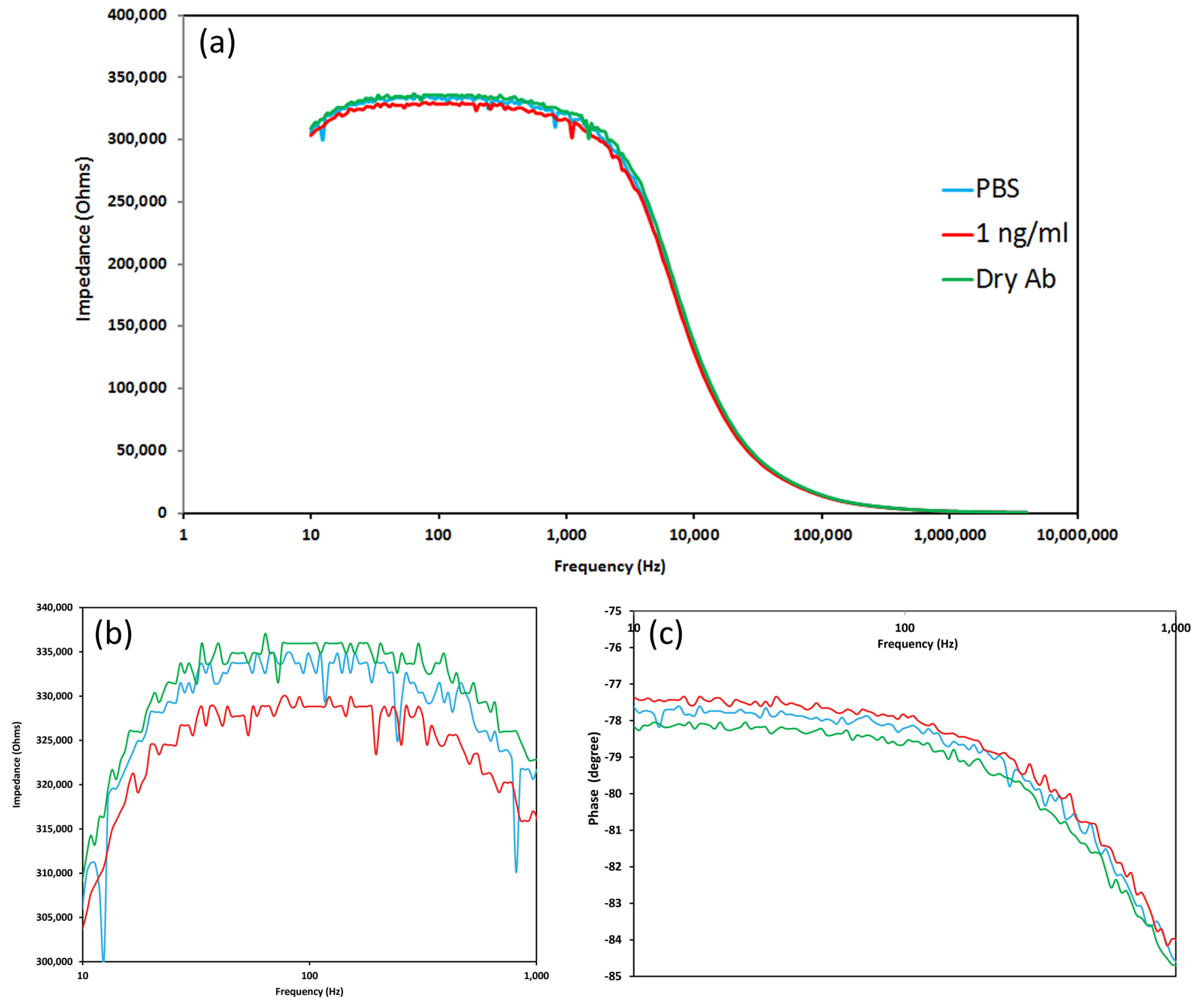
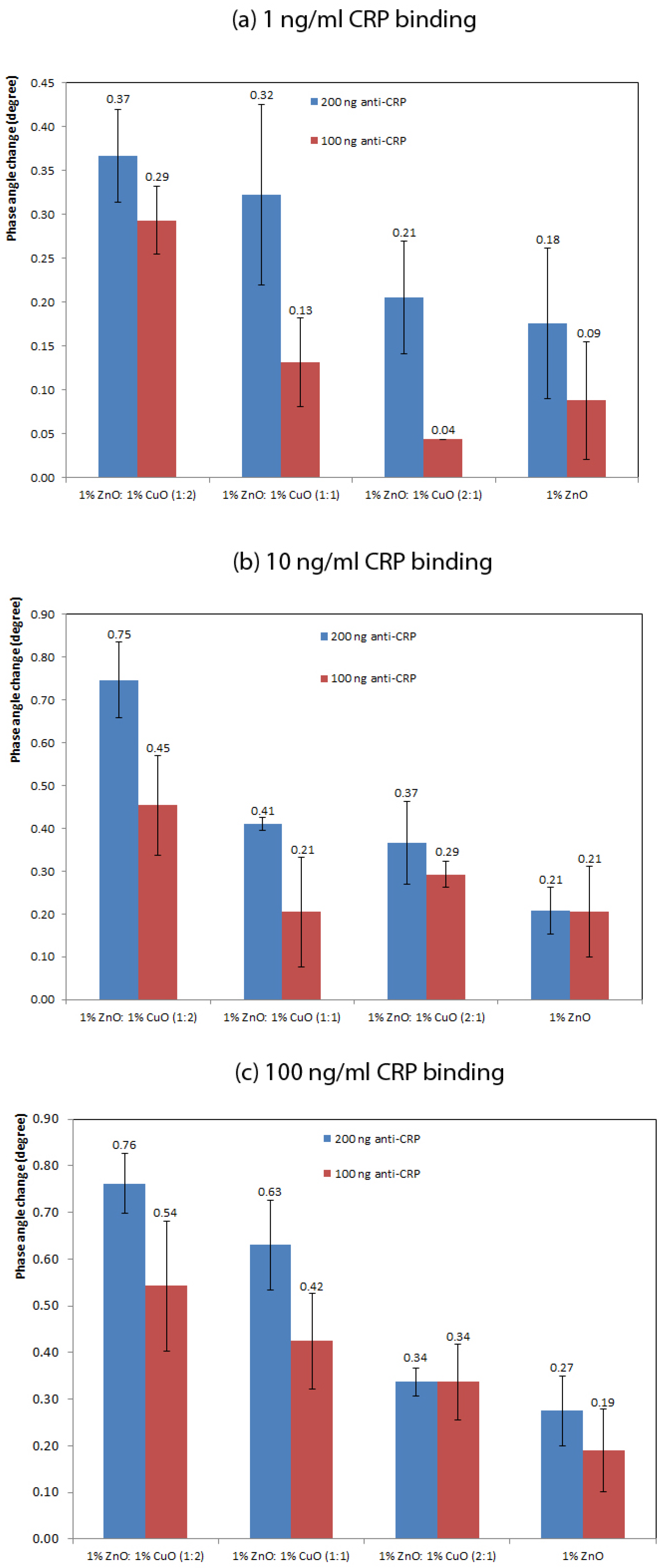
A Cypher Instruments C60 Impedance-Amplitude-Phase Analyser (Cypher Instruments, UK) was used to measure the impedance of the nano-crystal surfaces. The frequency was scanned from 10 Hz to 4 MHz at a voltage of 2 Vpp with a DC offset of 0.9 mV, with 300 test points. The impedance plots were analysed by Cypher Graph V1.21.0, Impedance Amplitude and Phase Analyser graphing application software. Impedance spectra of the ZnO–CuO nano-surfaces were acquired in triplicate for each of the following types of surface: 1% ZnO and 1:1, 1:2 and 2:1 volume ratios of 1% ZnO and 1% CuO suspensions.
The impedance amplitude and phase were measured after 10 min incubation time following the addition of 40 L of different concentrations of human C-reactive protein (CRP) from HyTest Ltd. and sealing the sensing region to form a chamber using cover slip. The specific concentrations of CRP were as follows: 0, 1, 5, 10 and 100 ng/mL diluted in 0.025 M PBS with pH 7.40. To demonstrate specificity of the anti-CRP antibody, a negative control consisting of 125 ng/mL cortisol (Co-pharma) was added to the sensor surface composed of 1% ZnO (n = 2) with 200 ng anti-CRP antibody.
The cross section of one of the biosensor (ZnO only) is illustrated in
Figure 1. This shows the sensing area with ZnO nanocrystals, on PET and dry anti-CRP antibodies for the detection of CRP sandwiched between two coverslips.
The sensor response was plotted using readings taken at a frequency of 138 Hz. This frequency was selected as in previous work it demonstrated maximum changes in response to addition of a target analyte [
15]. This observation concurred with Jacobs et al., who found that the electrical double layer is greater at frequencies below 1000 Hz and the most significant changes occurred around 100 Hz in their experiment to measure the protein troponin-T on a ZnO surface [
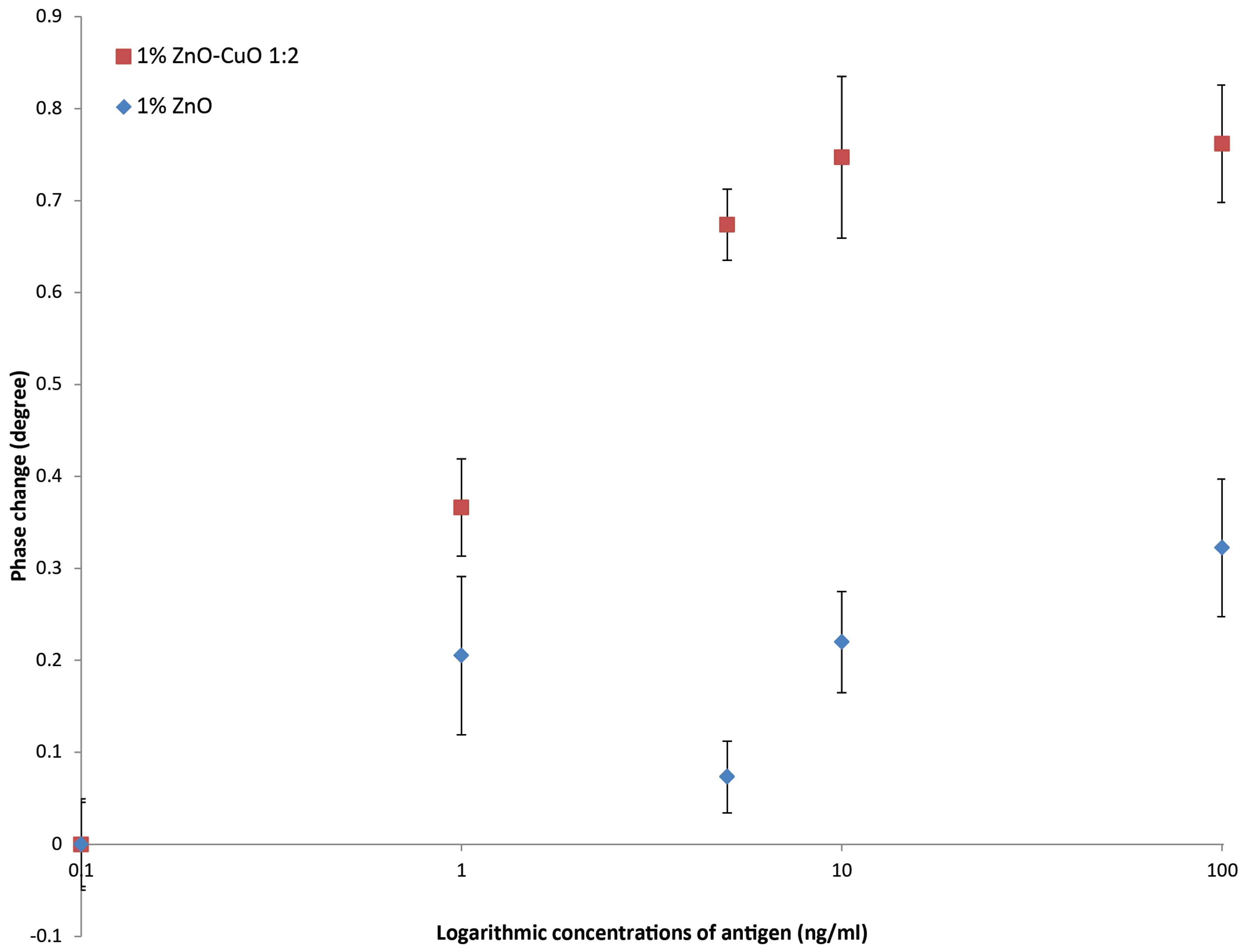
16]. Impedance values were compared with both 100 ng and 200 ng anti-CRP capture antibody. The phase change was defined as the difference of the phase value after adding CRP, after 10 min incubation, and the control. In order to plot logarithmic concentrations of CRP, the measurement of PBS buffer only with no CRP (the blank), was defined as 0.1 ng/mL CRP (rather than 0 ng/mL).
2.3. Characterization
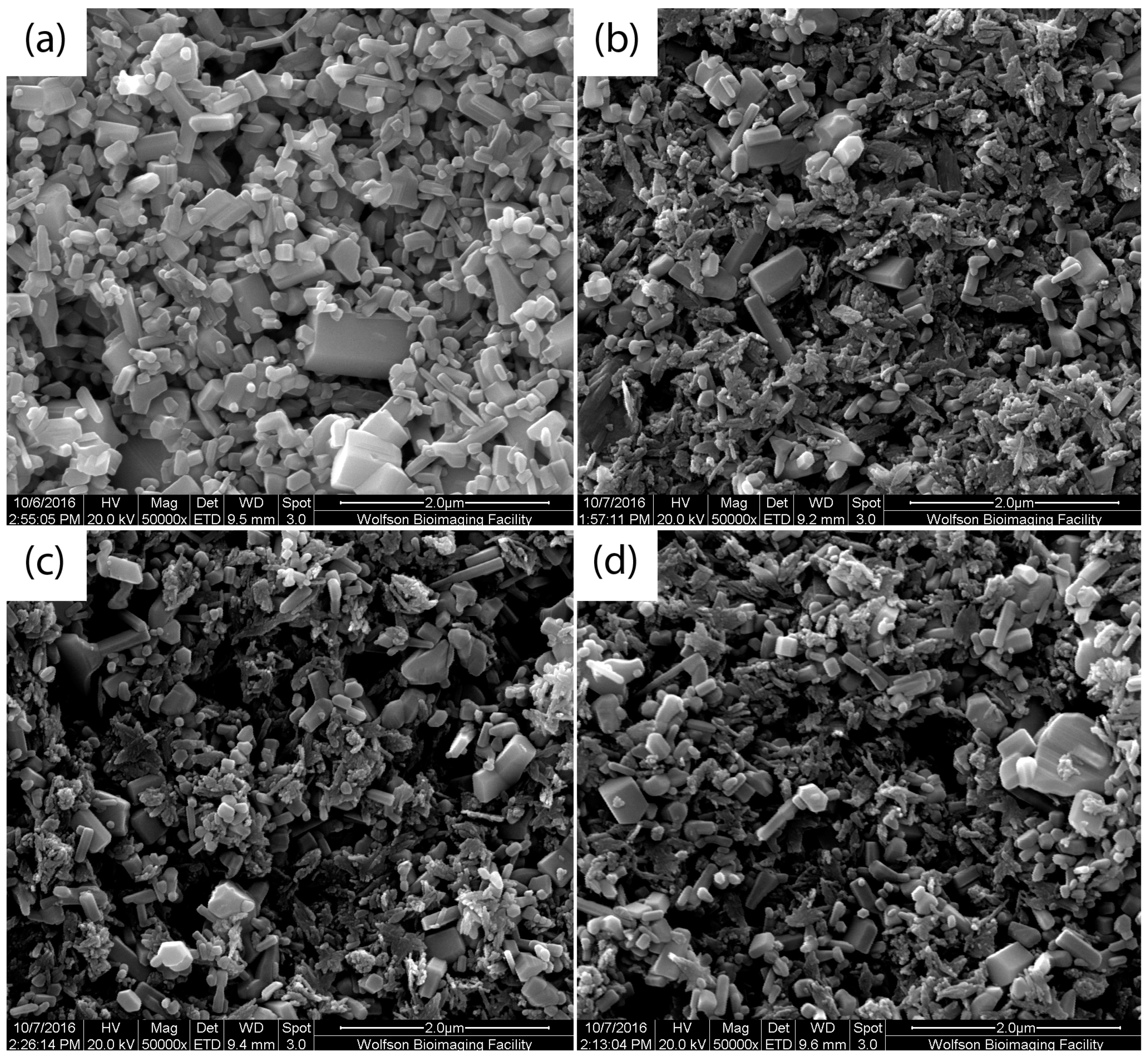
The morphology of pure ZnO and mixed ZnO–CuO composite nano-surfaces were analysed by the Quanta 200 Scanning Electron Microscope (Thermo Fisher Scientific, Hillsboro, OR, USA). The samples were coated with a thin layer of Au prior to analysis.
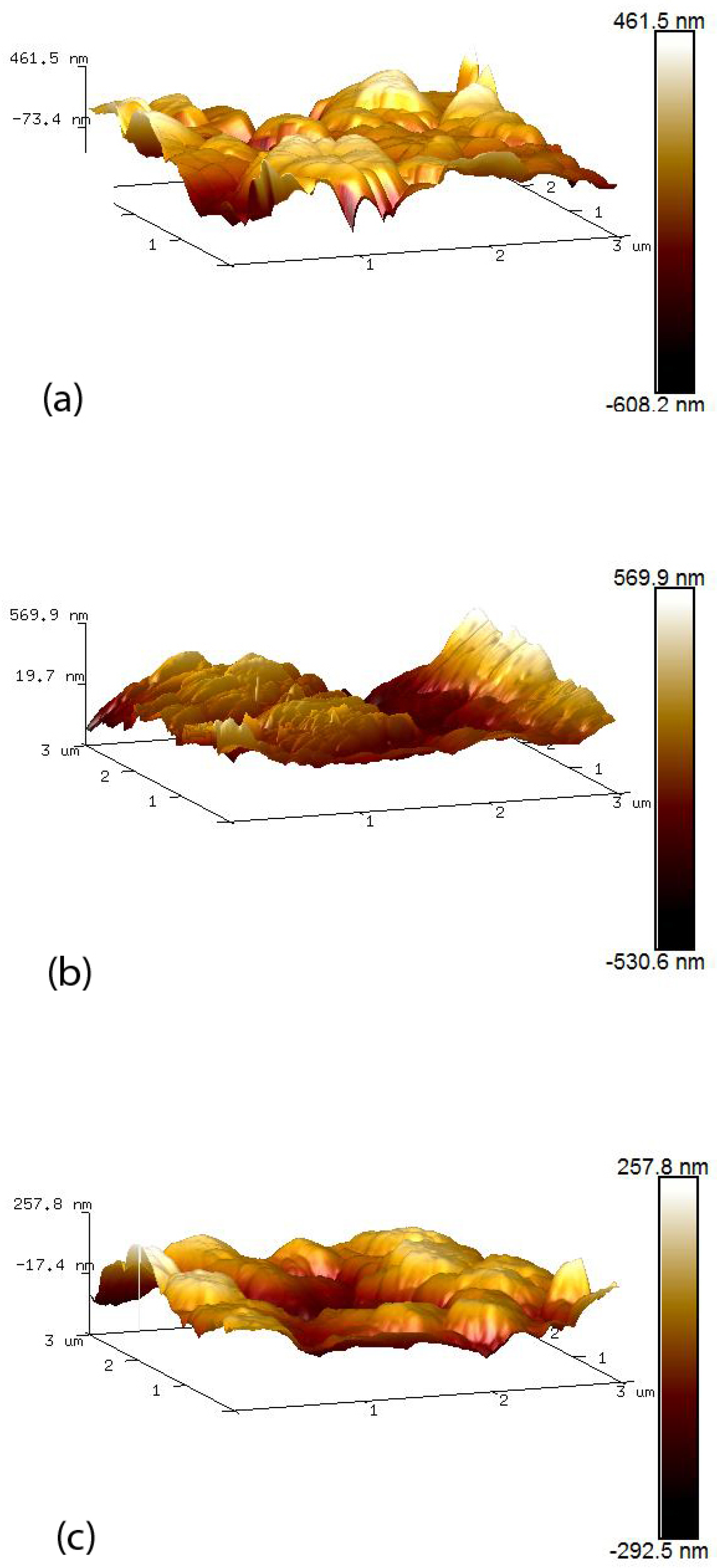
AFM was used to examine the topology of the different nano-surfaces using Bruker Innova Atomic Force Microscope (Bruker UK limited, Conventry, UK) with an antimony (n) doped silicon tip. Each AFM image was analysed using the NanoScope Analysis software (version 1.8). Image surface areas were compared within a 3 m by 3 m scan area. Calculation of the total surface area and mean roughness (Ra) within the scanned region provided a method of comparing the roughness of the various nano-surfaces.
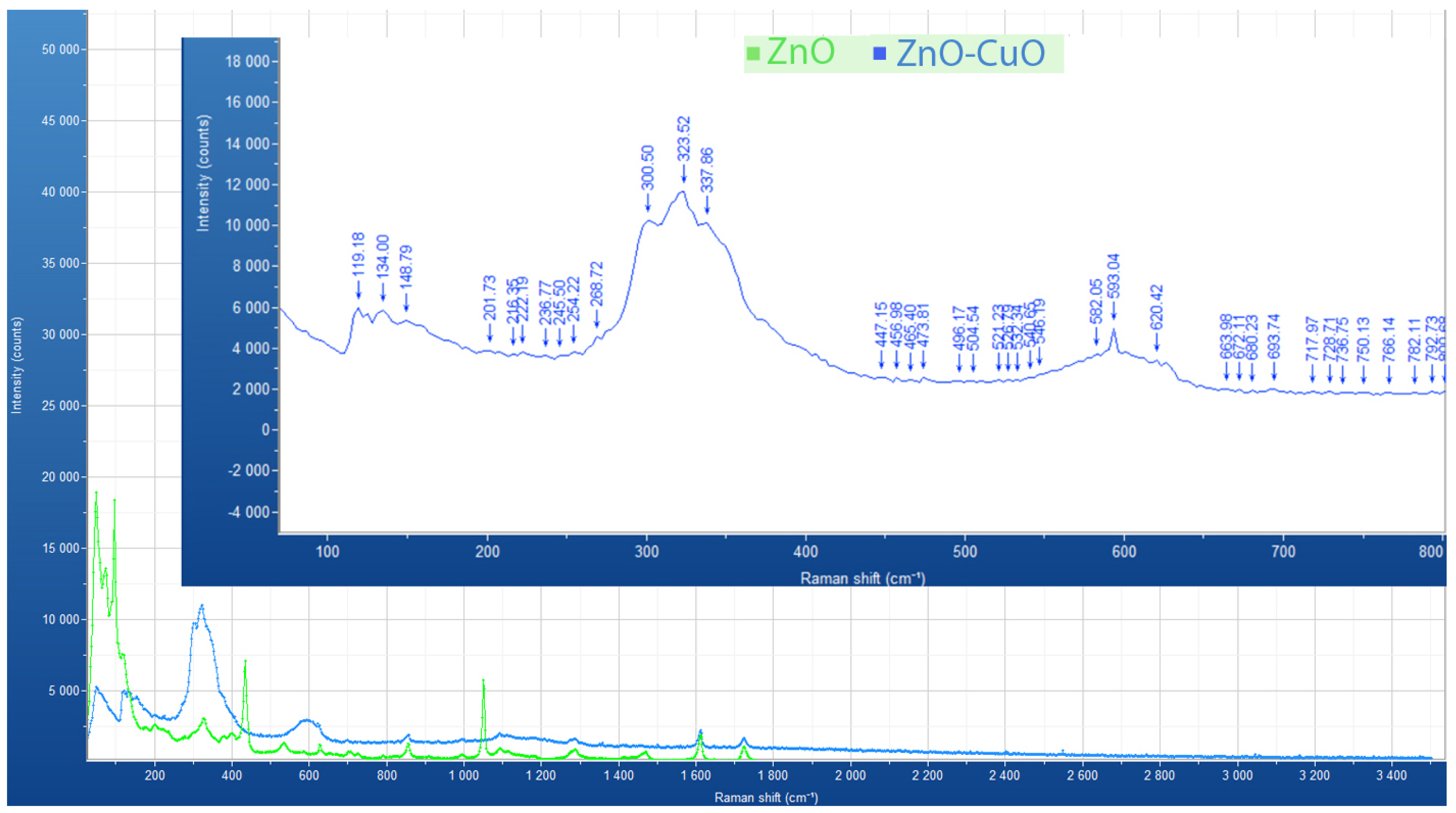
Raman spectroscopy was used to analyse the chemical compositions of the pure 1% ZnO and mixed 1% ZnO–CuO (1:2) nano-surfaces. An XploRA Raman spectrometer from Horiba (HORIBA UK Limited, Northampton, UK), equipped with a confocal microscope, was used. The Raman signals were collected in a range of 0–3500 cm using a 785 nm red laser excitation. The laser beam was focused on the sample using objective magnification of 50×.
A Kruskal-Wallis test was used to analyse the data and level of significance was defined as p ≤ 0.05.
2.4. Surface Uptake of Antibody
To understand how ZnO and CuO captured protein on their surfaces an antibody-enzyme conjugate was used in place of the anti-CRP. Here, 75 L anti-mouse Ig-HRP (2.5 ng/L) from Bio-Rad Antibodies Inc. (Kidlington, UK) was dropped on each surface within the sensing area and dried in a desiccator with silica gel at 4 C overnight for 18 h. Following a thorough wash, to remove unbound protein, with 0.05 M PBS, 40 L 3,3’,5,5’-Tetramethylbenzidine (TMB) was added on the surface for 10 min after which 50 L stop TMB solution was added. The colour intensity of each test was read at 450 nm using a microplate reader (EZ Read 400, Biochrom Ltd., Cambridge, UK). The relative amounts of surface bound antibody were defined by the optical density of the tests of the different sensor surfaces.
Data were presented with one blank sample on each nano-surface, without anti-mouse Ig-HRP, as a control and two test samples (with antibody) on each nano-surface.
4. Conclusions
ZnO–CuO composite nano-surfaces were fabricated using a colloidal dispersion technique, incorporating sonication. SEM images and AFM 3D surfaces indicated that the nano-surfaces were modified when increasing amounts of CuO were added to form ZnO–CuO nano-surfaces. The results illustrated that the smaller CuO nanoparticle flakes filled the large voids in the ZnO surface and also align themselves flat against the ZnO surface area due to electrostatic attraction, thereby reducing the overall surface area.
Although there was not a significance difference in the antibody loading on the 1:2 ZnO–CuO compared with the ZnO only nano-surface, there was a significantly higher signal produced from the biosensor fabricated with 1:2 ZnO–CuO compared with the biosensor fabricated with ZnO only. This suggests that the electrical interaction between ZnO and CuO nano-structures plays an important part in the generation of the enhanced signal. This supposition is supported by the observations that increasing the amount of CuO in the nano-surface increases the sensor output.
We believe that this is the first time that the enhancing effect of CuO as a component of a nano-structured surface of a biosensor has been described emphasising the role of nano-material play in the development of future biosensor technologies. In addition to enhance sensitivity the biosensor is easily fabricated and would be well suited to development of point-of-care devices for the health care industry. The ability to monitor health conditions at home or to rapidly diagnose inflammatory responses or infections is driven by the development of technologies such as described in this paper, leading to cost reductions in state health care.