Electroless-Deposited Platinum Antennas for Wireless Surface Acoustic Wave Sensors
Abstract
1. Introduction
2. Materials and Methods
3. Results
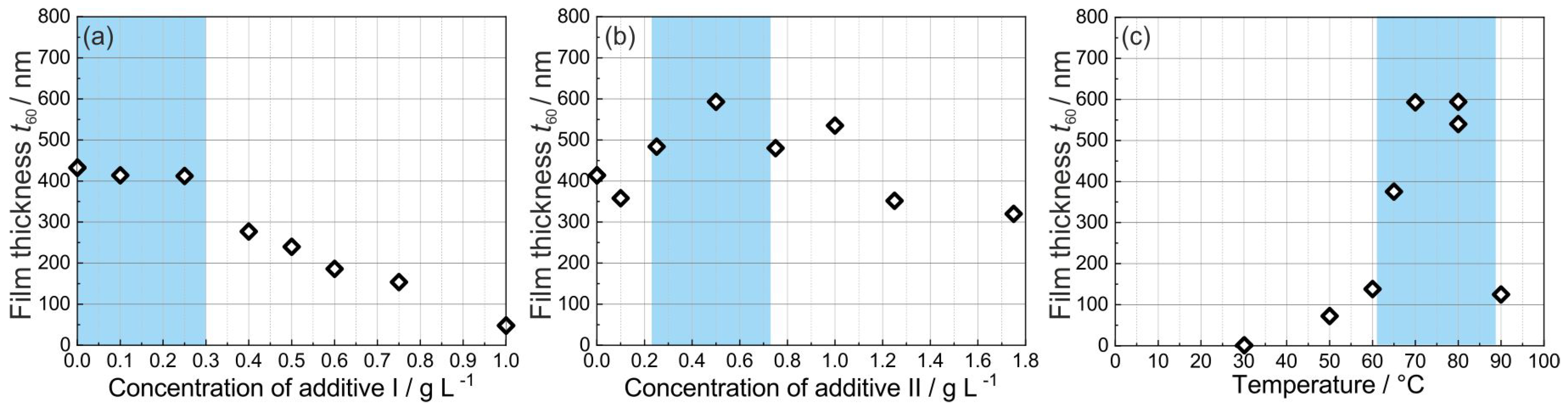
3.1. Optimization of the Platinum Electrolyte and the Deposition Conditions
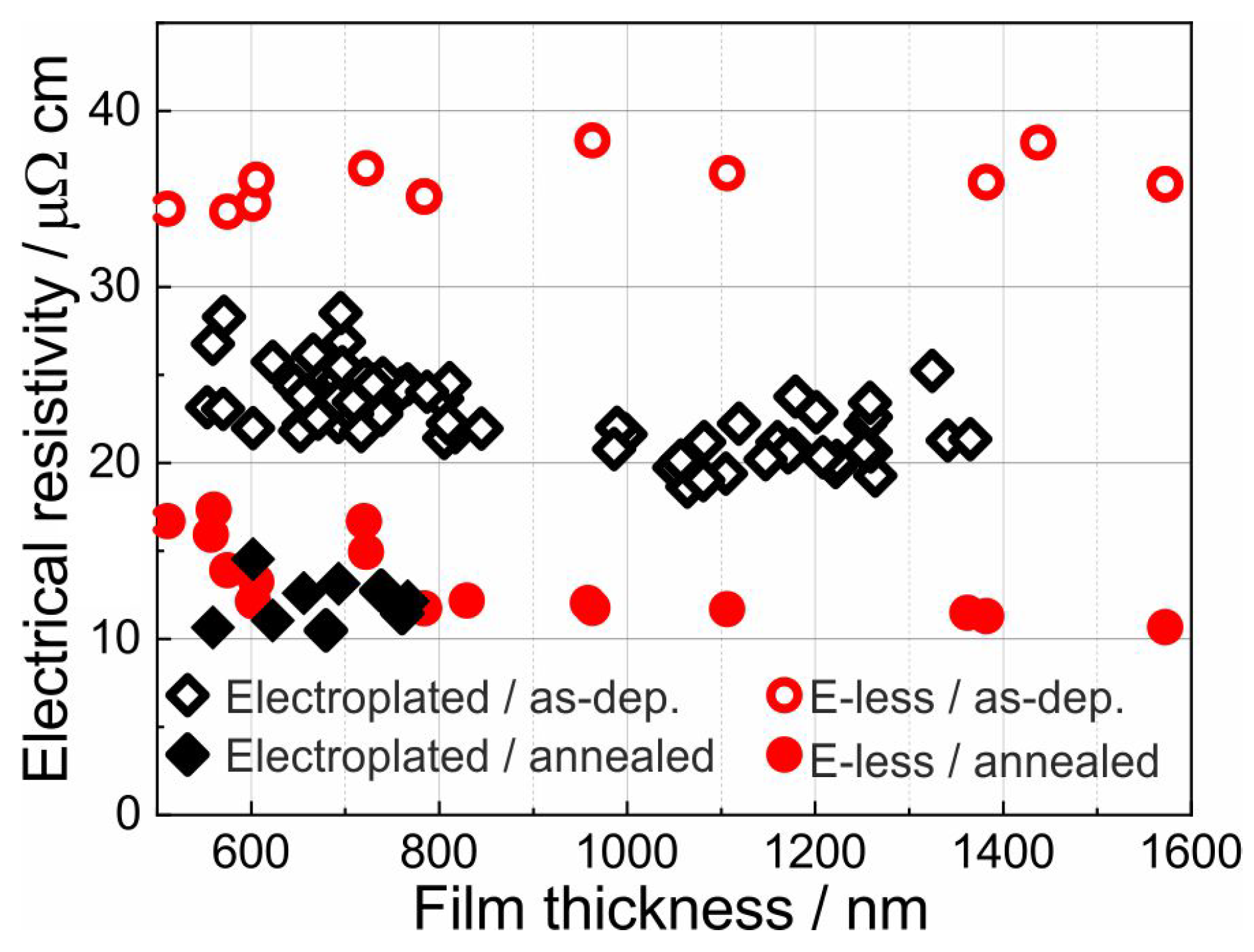
3.2. Electrical Resistance Measurements
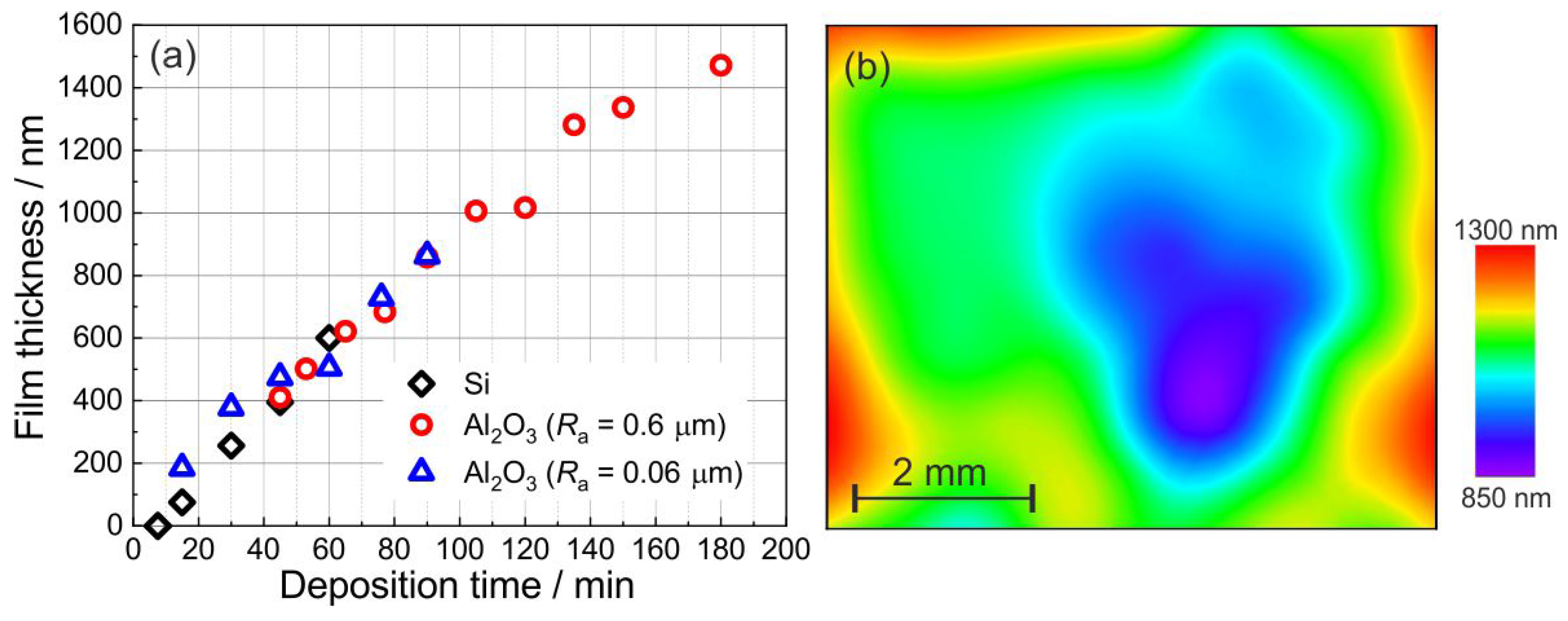
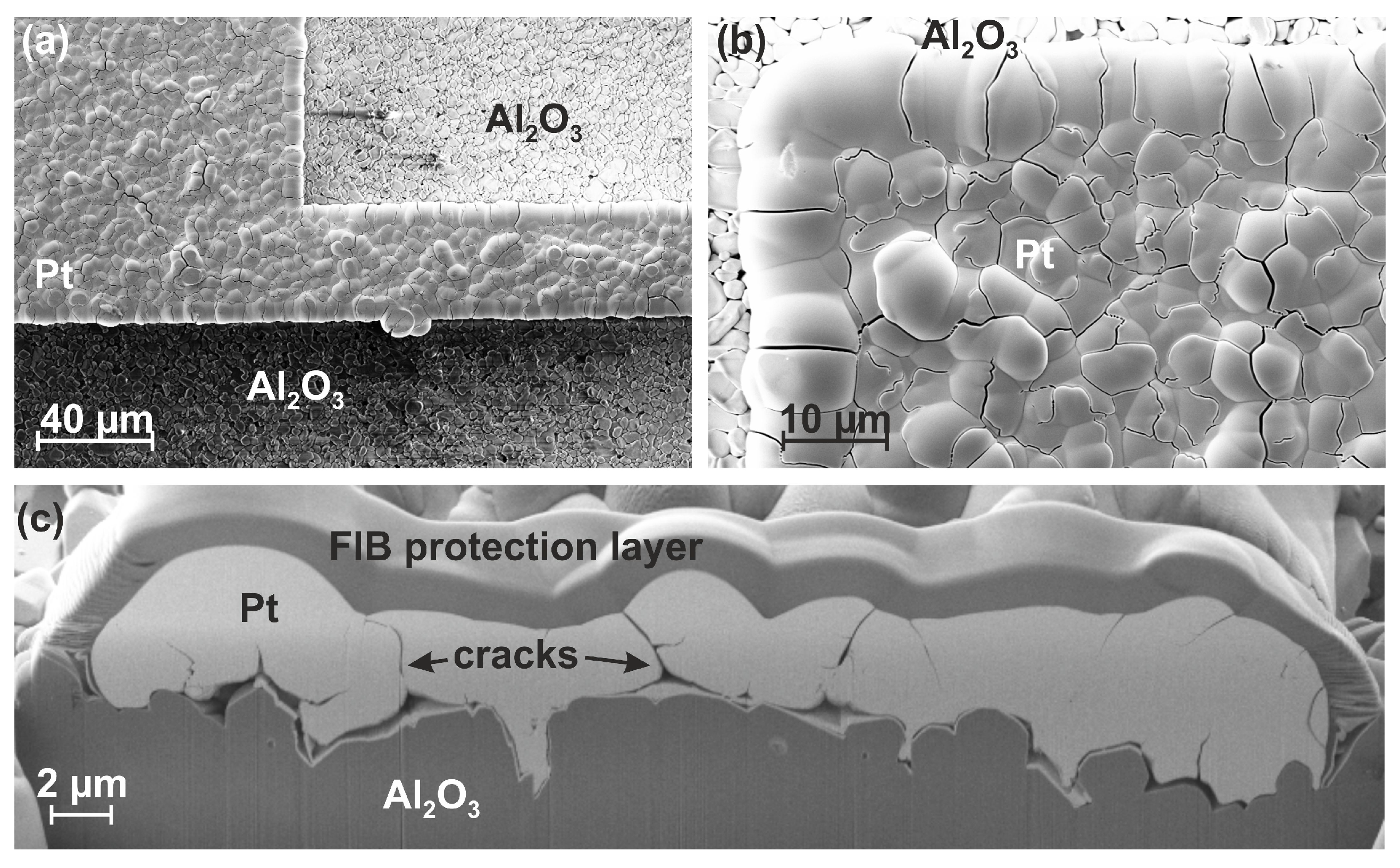
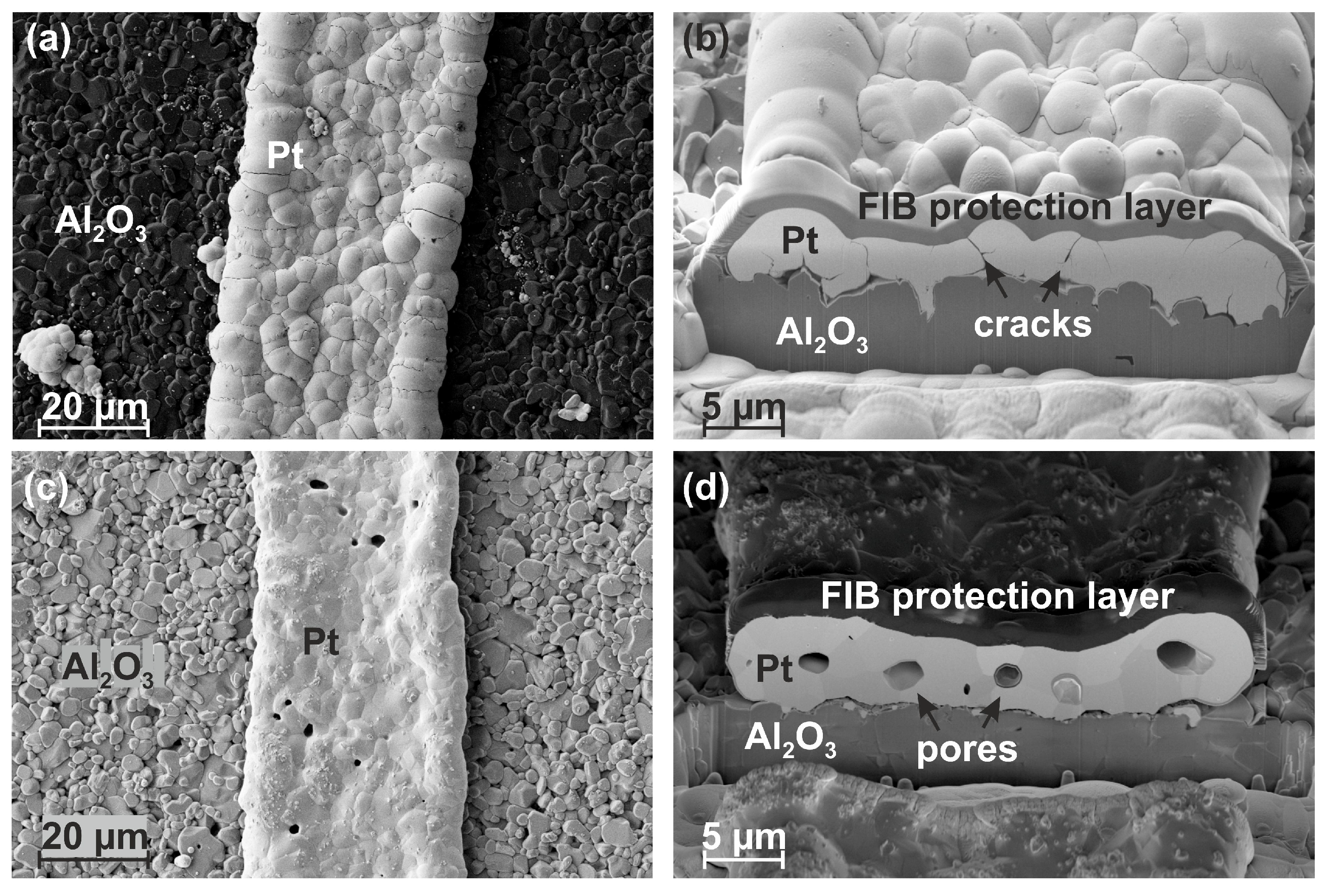
3.3. Film Morphology
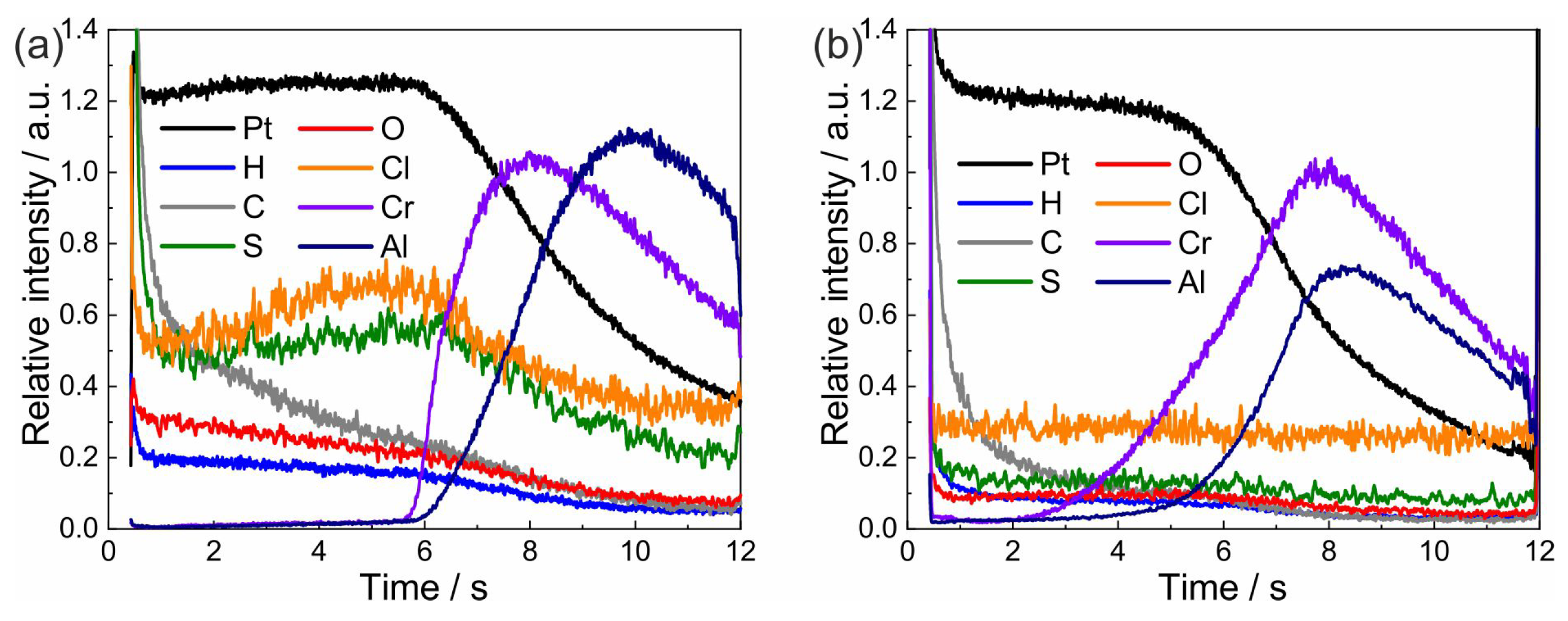
3.4. Chemical Composition Analysis
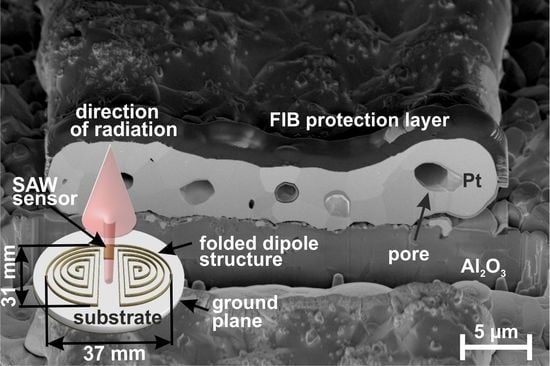
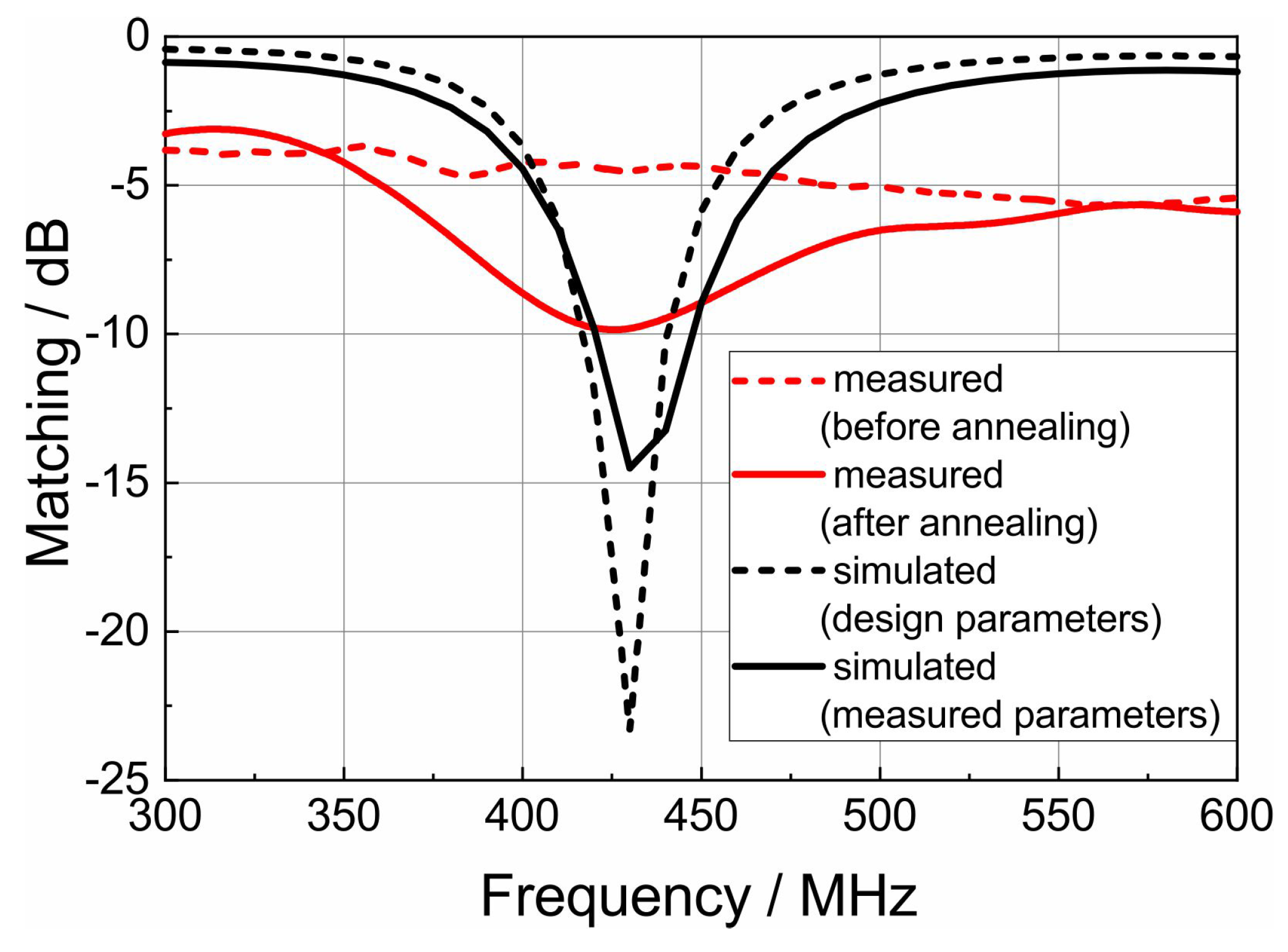
3.5. Antenna Simulation and Fabrication
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FIB | focused ion beam |
| GDOES | glow discharge optical emission spectrometry |
| PVD | physical vapor deposition |
| SAW | surface acoustic wave |
| SEM | scanning electron microscope |
| XRF | X-ray fluorescence analysis |
References
- Pohl, A. A review of wireless SAW sensors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Drafts, B. Acoustic wave technology sensors. IEEE Trans. Microw. Theory 2001, 49, 795–802. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Y.; Liu, X.; Liang, Y. Surface acoustic wave acceleration sensor with high sensitivity incorporating ST-X quartz cantilever beam. Smart Mater. Struct. 2015, 24, 015015. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chen, Y.Y.; Wu, T.T. A passive wireless hydrogen surface acoustic wave sensor based on Pt-coated ZnO nanorods. Nanotechnology 2010, 21, 095503. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.P.; Moonlight, T.; Lad, R.; Frankel, D.; Bernhardt, G. High Temperature Sensing Technology for Applications Up To 1000 °C. In Proceedings of the 2008 IEEE SENSORS, Lecce, Italy, 26–29 October 2008; pp. 752–755. [Google Scholar]
- Shu, L.; Peng, B.; Yang, Z.; Wang, R.; Deng, S.; Liu, X. High-Temperature SAW Wireless Strain Sensor with Langasite. Sensors 2015, 15, 28531–28542. [Google Scholar] [CrossRef] [PubMed]
- Aubert, T.; Bardong, J.; Elmazria, O.; Bruckner, G.; Assouar, B. Iridium Interdigital Transducers for High-Temperature Surface Acoustic Wave Applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 194. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wang, W.; Kim, T.; Yang, S. A novel 440 MHz wireless SAW microsensor integrated with pressure–temperature sensors and ID tag. J. Micromech. Microeng. 2007, 17, 515–523. [Google Scholar] [CrossRef]
- Zhanga, B.; Hua, H.; Yeb, A.; Zhanga, P. A surface acoustic wave ICP sensor with good temperature stability. Technol. Health Care 2017, 25, S435–S441. [Google Scholar] [CrossRef] [PubMed]
- François, B.; Sakharov, S.; Droit, C.; Davis, Z.; Richter, D.; Fritze, H.; Martin, G.; Friedt, J.M.; Plessky, V.P.; Brückner, G.; et al. Wireless Temperature Measurements above 500 °C using Surface Acoustic Wave Sensors. Procedia Eng. 2012, 47, 1227–1230. [Google Scholar] [CrossRef]
- Hamidon, M.N.; Skarda, V.; White, N.M.; Krispel, F.; Krempl, P.; Binhack, M.; Buff, W. Fabrication of high temperature surface acoustic wave devices for sensor applications. Sens. Actuators A-Phys. 2005, 123, 403–407. [Google Scholar] [CrossRef]
- Schmid, U.; Seidel, H. Effect of high temperature annealing on the electrical performance of titanium/platinum thin films. Thin Solid Films 2008, 516, 898–906. [Google Scholar] [CrossRef]
- Richter, D.; Sakharov, S.; Forsén, E.; Mayer, E.; Reindl, L.; Fritze, H. Thin Film Electrodes for High Temperature Surface Acoustic Wave Devices. Procedia Eng. 2011, 25, 168–171. [Google Scholar] [CrossRef]
- Aubert, T.; Elmazria, O.; Assouar, B.; Bouvot, L.; Hehn, M.; Weber, S.; Oudich, M.; Genève, D. Behavior of platinum/tantalum as interdigital transducers for SAW devices in high-temperature environments. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 603–610. [Google Scholar] [CrossRef]
- Thiele, J.A.; da Cunha, M.P. Platinum and palladium high-temperature transducers on langasite. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Moulzolf, S.C.; Frankel, D.J.; Pereira da Cunha, M.; Lad, R.J. Electrically Conductive Pt-Rh/ZrO2 and Pt-Rh/HfO2 Nanocomposite Electrodes for High Temperature Harsh Environment Sensors. Microsyst. Technol. 2014, 20, 523–531. [Google Scholar] [CrossRef]
- Frankel, D.J.; Bernhardt, G.P.; Sturtevant, B.T.; Moonlight, T.; Pereira da Cunha, M.; Lad, R.J. Stable electrodes and ultrathin passivation coatings for high temperature sensors in harsh environments. In Proceedings of the IEEE International Sensor 2008 Conference Proceedings, Lecce, Italy, 26–29 October 2008; pp. 82–85. [Google Scholar]
- Mantese, J.V.; Alcini, W.V. Platinum wire wedge bonding: A new IC and microsensor interconnect. J. Electron. Mater. 1988, 17, 285–289. [Google Scholar] [CrossRef]
- Brachmann, E.; Seifert, M.; Ernst, D.; Menzel, S.B.; Gemming, T. Pt-wire bonding optimization for electroplated Pt films on γ-Al2O3 for high temperature and harsh environment applications. Sens. Actuators A-Phys. 2018, 284, 129–134. [Google Scholar] [CrossRef]
- Malocha, D.C.; Gallagher, M.; Fischer, B.; Humphries, J.; Gallagher, D.; Kozlovski, N. A passive wireless multi-sensor SAW technology device and system perspectives. Sensors 2013, 13, 5897–5922. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.W.; Santos, B.C.; Malocha, D.C. Wireless wideband SAW sensor-antenna design. In Proceedings of the 2010 IEEE International Frequency Control Symposium, Newport Beach, CA, USA, 1–4 June 2010; pp. 291–296. [Google Scholar]
- Rao, C.; Trivedi, D. Chemical and electrochemical depositions of platinum group metals and their applications. Coord. Chem. Rev. 2005, 249, 613–631. [Google Scholar] [CrossRef]
- Walter, C.W.; Leaman, F.H. Method and Composition for Platinum Plating and Articles Plated Therewith; Dentsply International Inc.: York, PA, USA, 1968; Available online: https://patents.google.com/patent/US3562911A/en (accessed on 5 October 2013).
- Mallory, G.O.; Hajdu, J.B. (Eds.) Electroless Plating: Fundamentals and Applications; American Electroplaters and Surface Finishers Society: Orlando, FL, USA, 1990. [Google Scholar]
- Seifert, M.; Brachmann, E.; Rane, G.K.; Menzel, S.B.; Gemming, T. Capability Study of Ti, Cr, W, Ta and Pt as Seed Layers for Electrodeposited Platinum Films on γ-Al2O3 for High Temperature and Harsh Environment Applications. Materials 2017, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.R.K.; Pushpavanam, M. Electroless deposition of platinum on titanium substrates. Mater. Chem. Phys. 2001, 68, 62–65. [Google Scholar] [CrossRef]
- Van der Pauw, L.J. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Res. Rep. 1958, 13, 1–9. [Google Scholar]
- Mizuhashi, S.; Cordonier, C.E.J.; Matsui, H.; Monma, H.; Taka, O. Comparative study on physical and electrochemical characteristics of thin films deposited from electroless platinum plating baths. Thin Solid Films 2016, 619, 328–335. [Google Scholar] [CrossRef]
- Van Den Meerakker, J.E.A.M. On the mechanism of electroless plating. II. One mechanism for different reductants. J. Appl. Electrochem. 1981, 11, 395. [Google Scholar] [CrossRef]
- James, J.R.; Hall, P.S.; Wood, C. Some Manufacturing and Operational Problems of Microstrip Antennas. In Microstrip Antenna: Theory and Design; No. 12; Peter Peregrinus Ltd.: London, UK, 1986; Chapter 8; pp. 225–232. [Google Scholar]
- Collier, R.J.; Skinner, A.D. IET Electrical Measurement Series: Microwave Measurements; Institution of Engineering and Technology: Stevenage, UK, 2007. [Google Scholar]
- Williams, D.F.; Wang, C.M.; Arz, U. An optimal multiline TRL calibration algorithm. In Proceedings of the IEEE MTT-S International Microwave Symposium Digest, Philadelphia, PA, USA, 8–13 June 2003; Volume 3, pp. 1819–1822. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brachmann, E.; Seifert, M.; Neumann, N.; Alshwawreh, N.; Uhlemann, M.; Menzel, S.B.; Acker, J.; Herold, S.; Hoffmann, V.; Gemming, T. Electroless-Deposited Platinum Antennas for Wireless Surface Acoustic Wave Sensors. Materials 2019, 12, 1002. https://doi.org/10.3390/ma12071002
Brachmann E, Seifert M, Neumann N, Alshwawreh N, Uhlemann M, Menzel SB, Acker J, Herold S, Hoffmann V, Gemming T. Electroless-Deposited Platinum Antennas for Wireless Surface Acoustic Wave Sensors. Materials. 2019; 12(7):1002. https://doi.org/10.3390/ma12071002
Chicago/Turabian StyleBrachmann, Erik, Marietta Seifert, Niels Neumann, Nidal Alshwawreh, Margitta Uhlemann, Siegfried Bernhard Menzel, Jörg Acker, Steven Herold, Volker Hoffmann, and Thomas Gemming. 2019. "Electroless-Deposited Platinum Antennas for Wireless Surface Acoustic Wave Sensors" Materials 12, no. 7: 1002. https://doi.org/10.3390/ma12071002
APA StyleBrachmann, E., Seifert, M., Neumann, N., Alshwawreh, N., Uhlemann, M., Menzel, S. B., Acker, J., Herold, S., Hoffmann, V., & Gemming, T. (2019). Electroless-Deposited Platinum Antennas for Wireless Surface Acoustic Wave Sensors. Materials, 12(7), 1002. https://doi.org/10.3390/ma12071002