Fast Removal of Propranolol from Water by Attapulgite/Graphene Oxide Magnetic Ternary Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Preparation
2.3. Batch Experiment
2.4. Characterization Techniques
3. Results
3.1. Material Characterization
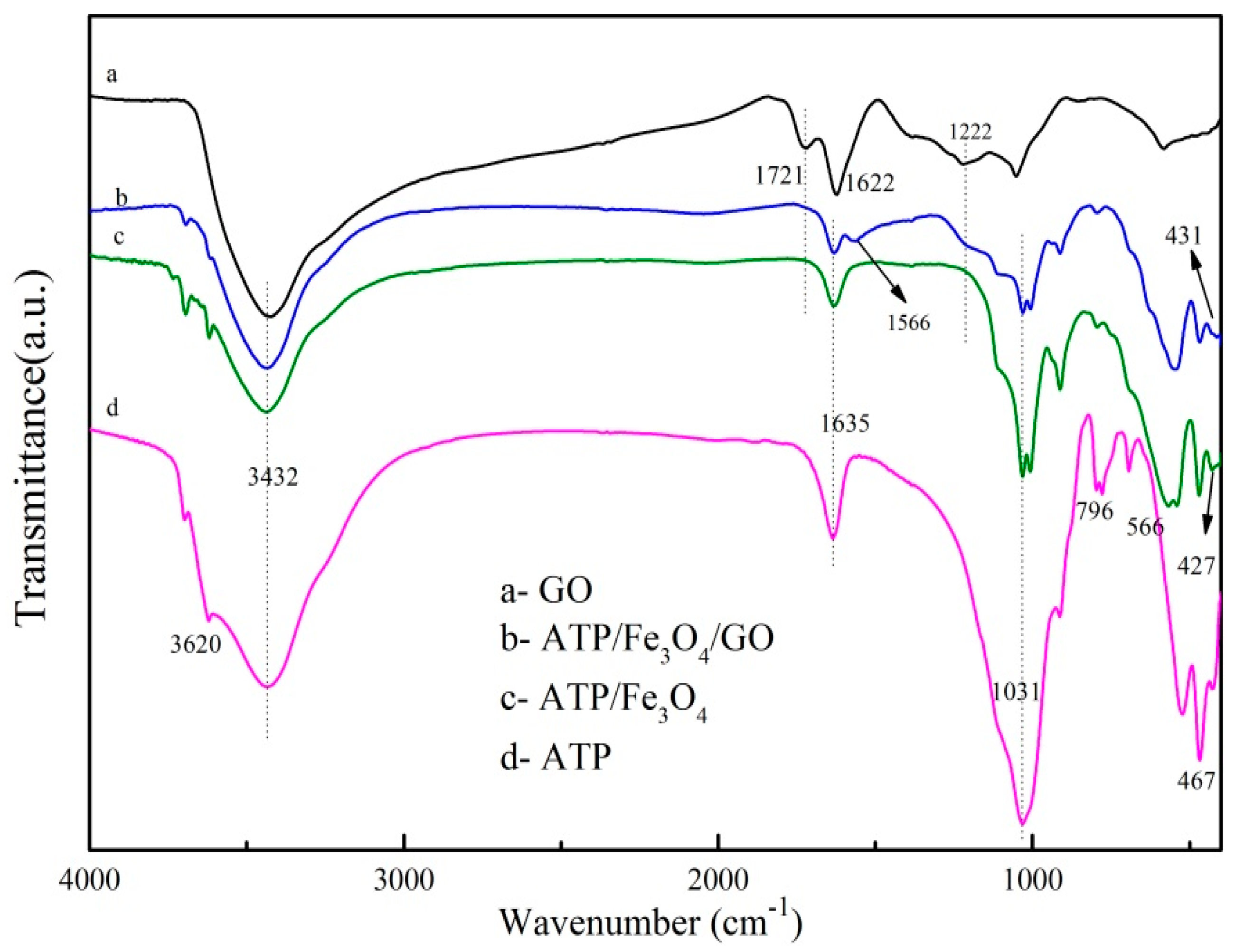
3.1.1. FTIR Spectra Analysis
3.1.2. Zeta Potential Analysis
3.1.3. XRD Analysis
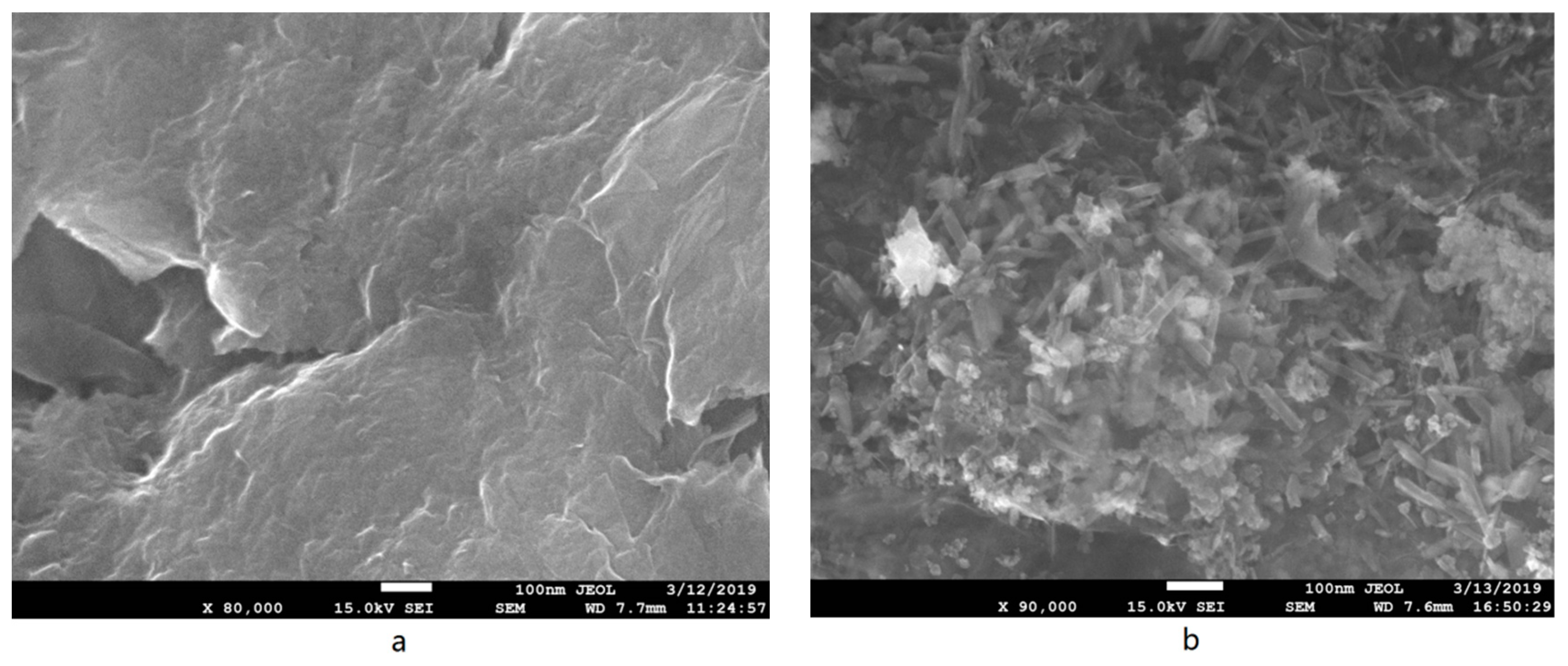
3.1.4. SEM Micrographs
3.2. Comparison of the Adsorption Capacities of PRO onto All Adsorbent
3.3. Effect Factors
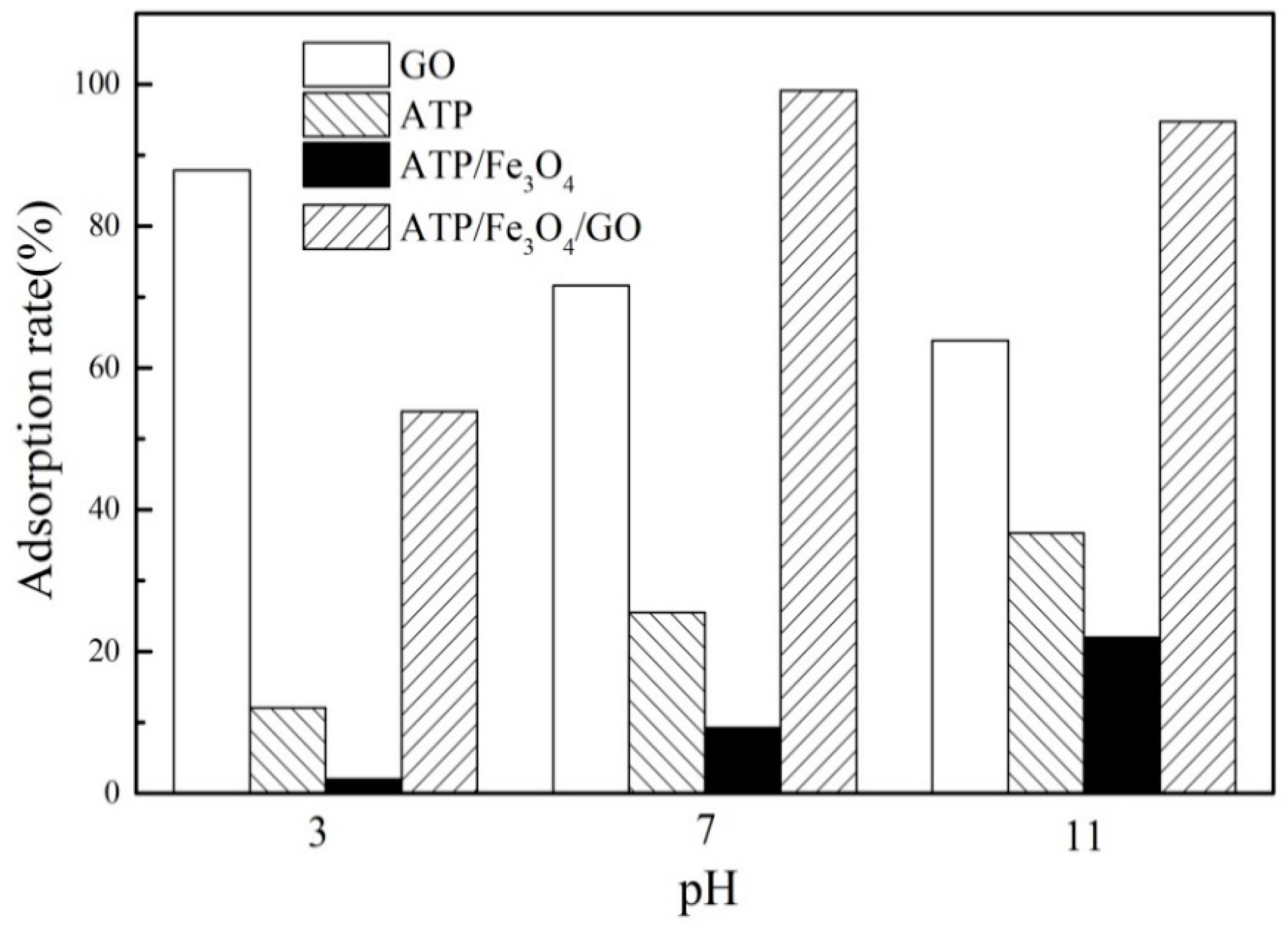
3.3.1. Effect of pH
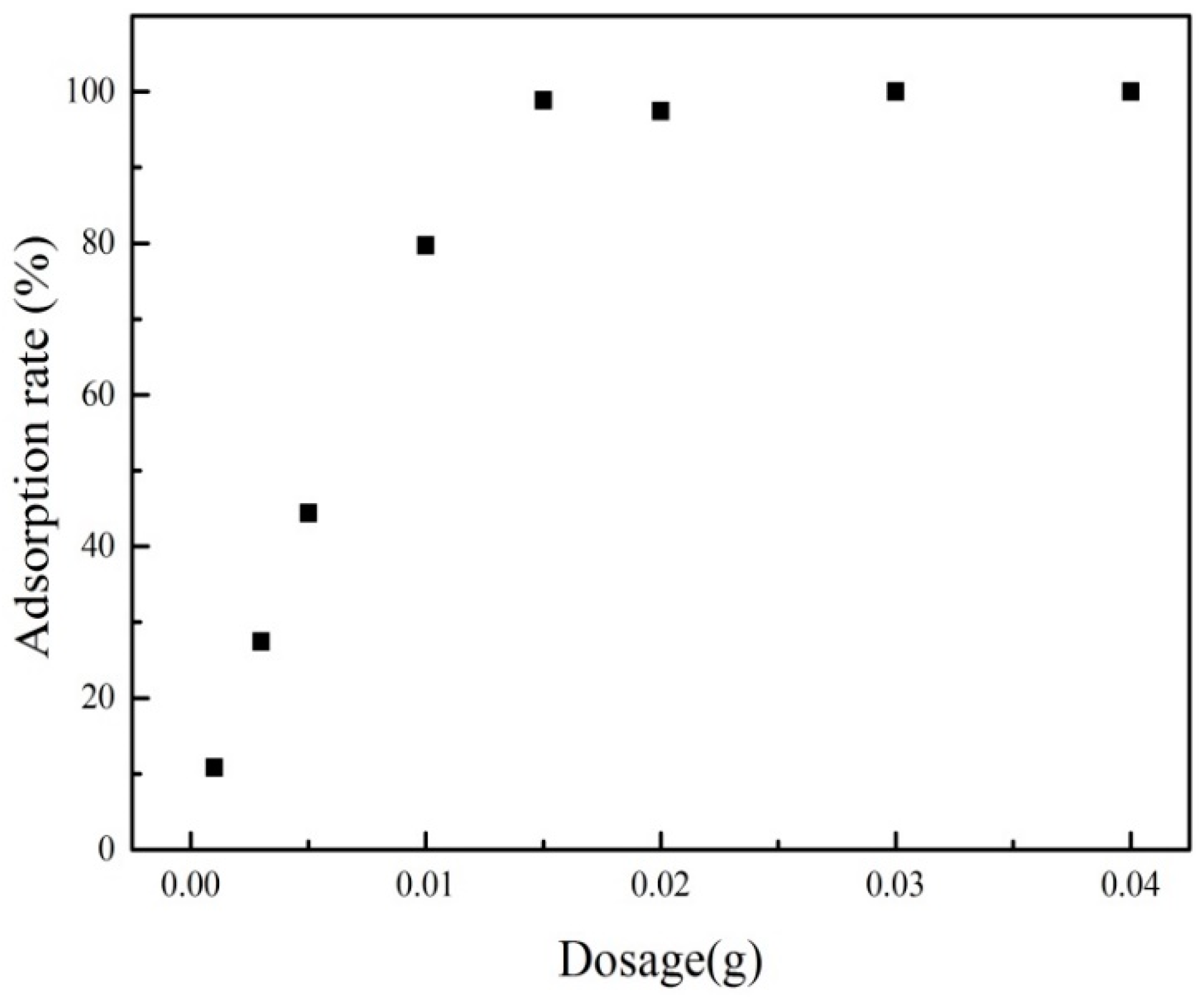
3.3.2. Effect of Adsorbent Dosage
3.3.3. Effect of Ionic Strength
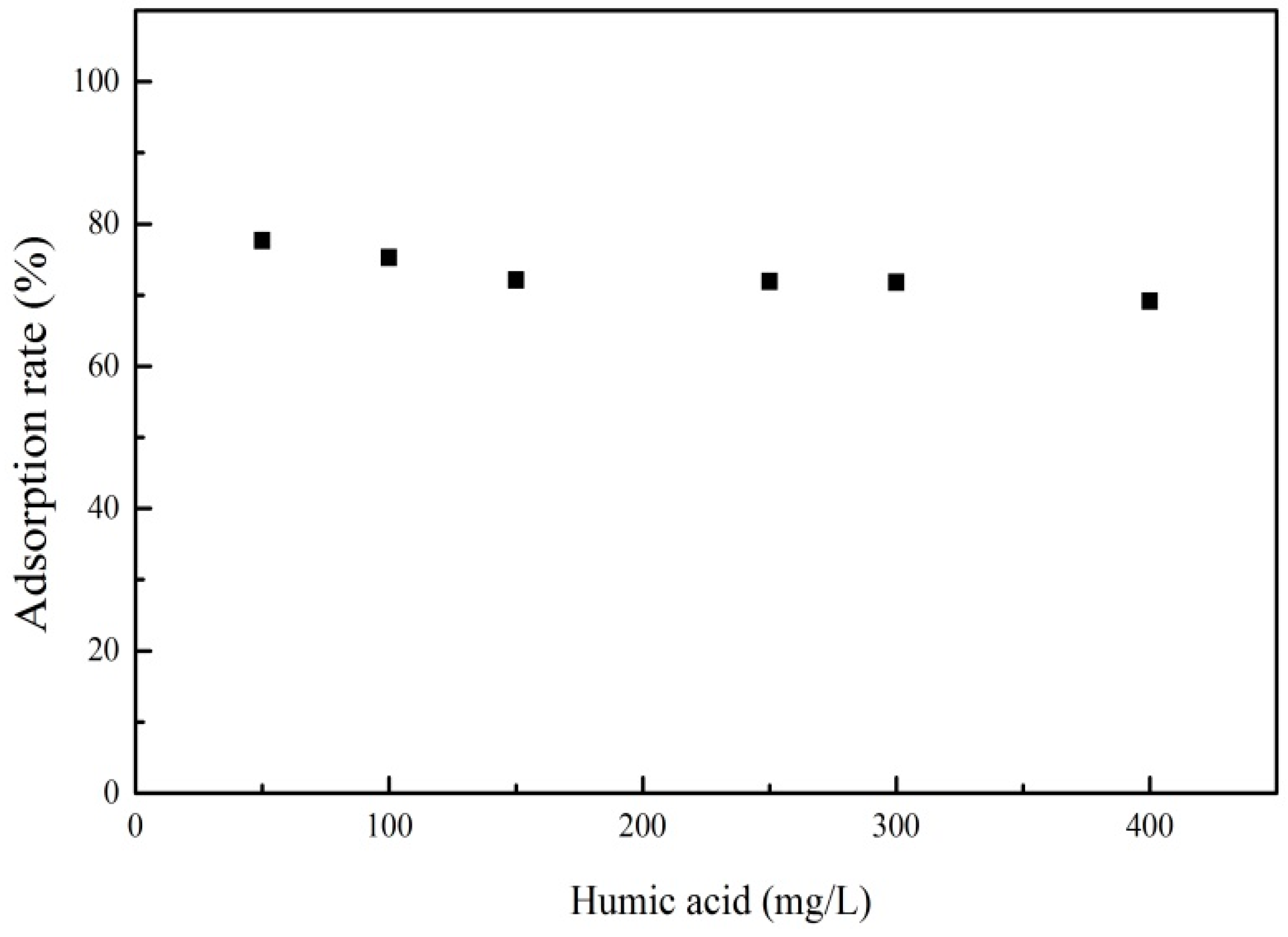
3.3.4. Effect of Humic Acid
3.4. Adsorption Kinetics
3.5. Adsorption Isotherm
3.6. Adsorption Thermodynamics
3.7. Comparison of ATP/Fe3O4/GO with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Botero-Coy, M.A.; Martínez-Pachón, D.; Boix, C.; Rincón, J.R.; Castillo, N.; Arias-Marín, P.L.; Manrique-Losada, L.; Torres-Palma, R.; Moncayo-Lasso, A.; Hernández, F. An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater. Sci. Total Environ. 2018, 642, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.T.; Cabrera-Codony, A.; Barcel, D.; Rodriguez-Mozaz, S.; Pinheiro, A.; Gonzalez-Olmos, R. Influencing factors on the removal of pharmaceuticals from water with micro-grain activated carbon. Water Res. 2018, 144, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shen, X.; Han, Y.; Liu, Z.; Dai, W.; Dutta, A.; Kumar, A.; Liu, J. Selective adsorption and removal of drug contaminants by using an extremely stable Cu(II)-based 3D metal-organic framework. Chemosphere 2019, 215, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Bensaadi, Z.; Yeddou-Mezenner, N.; Trari, M.; Medjene, F. Kinetic studies of β-blocker photodegradation on TiO2. J. Environ. Chem. Eng. 2014, 2, 1371–1377. [Google Scholar] [CrossRef]
- Marques, C.R.S.; Mestre, S.A.; Machuqueiro, M.; Gotvajnb, Ž.A.; Marinšek, M.; Carvalho, P.A. Apple tree branches derived activated carbons for the removal of β-blocker atenolol. Chem. Eng. J. 2018, 345, 669–678. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Mohamad, A.M.; Jaafar, J.; Ismail, F.A.; Harun, Z. Hybrid membrane filtration-advanced oxidation processes for removal of pharmaceutical residue. J. Colloid Interf. Sci. 2018, 532, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Escher, I.B.; Richle, P.; Schaffner, C.; Alder, C.A. Elimination of β-blockers in sewage treatment plants. Water Res. 2007, 41, 1614–1622. [Google Scholar] [CrossRef]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y.; Hayashi, T. Removal of pharmaceuticals in water by introduction of ozonated microbubbles. Sep. Purif. Technol. 2019, 212, 483–489. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, D.B.; Oelgemoller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manage. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Wilt, D.A.; Gijn, V.K.; Verhoek, T.; Vergnes, A.; Hoek, M.; Rijnaarts, H.; Langenhoff, A. Enhanced pharmaceutical removal from water in a three step bio-ozone-bio process. Water Res. 2018, 138, 97–105. [Google Scholar] [CrossRef]
- Barbieri, M.; Licha, T.; Ndler, K.; Carrera, J.; Ayora, C.; Sanchez-Vila, X. Fate of β-blockers in aquifer material under nitrate reducing conditions: Batch experiments. Chemosphere 2012, 89, 1272–1277. [Google Scholar] [CrossRef]
- Wick, A.; Fink, G.; Joss, A.; Siegrist, H.; Ternes, A.T. Fate of beta blockers and psycho-active drugs in conventional wastewater treatment. Water Res. 2009, 43, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, H.; Zhang, Y.; Alder, C.A. Occurrence and enantiomer profiles of β-blockers in wastewater and a receiving water body and adjacent soil in Tianjin, China. Sci. Total Environ. 2019, 650, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-blockers in the environment: Part II. Ecotoxicity study. Sci. Total Environ. 2014, 493, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, Z.G.; Koltsakidou, A.; Nanaki, G.S.; Bikiaris, N.D.; Lambropoulou, A.D. Removal of beta-blockers from aqueous media by adsorption onto graphene oxide. Sci. Total Environ. 2015, 537, 411–420. [Google Scholar] [CrossRef]
- Ding, J.; Lu, G.; Li, S.; Nie, Y.; Liu, J. Biological fate and effects of propranolol in an experimental aquatic food chain. Sci. Total Environ. 2015, 532, 31–39. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, N.; Wang, W.; Kang, S.; Xu, J.; Xiang, H.; Yin, D. Ultrasound-assisted heterogeneous activation of persulfate by nano zerovalent iron (nZVI) for the propranolol degradation in water. Ultrason. Sonochem. 2018, 49, 33–40. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, N.; Yin, D.; Tian, F.; Zheng, Q. Oxidation of the β-blocker propranolol by UV/persulfate: Effect, mechanism and toxicity investigation. Chemosphere 2018, 201, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Al, I.; Alothman, A.Z.; Alwarthan, A. Uptake of propranolol on ionic liquid iron nanocomposite adsorbent: Kinetic, thermodynamics and mechanism of adsorption. J. Mol. Liq. 2017, 236, 205–213. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Zhang, X.; Zhang, X.; Jiang, J.; Yao, J. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals. J. Colloid Interf. Sci. 2018, 514, 190–198. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Tan, X.; Jiang, L.; Zeng, G.; Liu, S.; Tian, S.; Liu, S.; Liu, N.; Li, M. Adsorption of 17 β-estradiol by a novel attapulgite/biochar nanocomposite: Characteristics and influencing factors. Process Saf. Environ. 2019, 121, 155–164. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, F.; Liu, B.; Hu, X.; Sun, C. Sorptive removal of β-blocker propranolol from aqueous solution by modified attapulgite: Effect factors and sorption mechanisms. Chem. Eng. J. 2011, 174, 571–578. [Google Scholar] [CrossRef]
- Lu, Z.; Hao, Z.; Wang, J.; Chen, L. Efficient removal of europium from aqueous solutions using attapulgite-iron oxide magnetic composites. J. Ind. Eng. Chem. 2016, 34, 374–381. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Su, Z.; Li, F.; Wen, F. Attapulgite-Fe3O4 magnetic nanoparticles via co-precipitation technique. Appl. Surf. Sci. 2008, 255, 2020–2025. [Google Scholar] [CrossRef]
- Pan, J.; Xu, L.; Dai, J.; Li, X.; Hang, H.; Huo, P.; Li, C.; Yan, Y. Magnetic molecularly imprinted polymers based on attapulgite/Fe3O4 particles for the selective recognition of 2,4-dichlorophenol. Chem. Eng. J. 2011, 174, 68–75. [Google Scholar] [CrossRef]
- Qin, W.; Qian, G.; Tao, H.; Wang, J.; Sun, J.; Cui, X.; Zhang, Y.; Zhang, X. Adsorption of Hg(II) ions by PAMAM dendrimers modified attapulgite composites. React. Funct. Polym. 2019, 136, 75–85. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Molla, A.; Li, Y.; Mandal, B.; Kang, G.S.; Hur, H.S.; Chung, S.J. Selective adsorption of organic dyes on graphene oxide: Theoretical and experimental analysis. Appl. Surf. Sci. 2019, 464, 170–177. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Bakar, N.F.A.; Him, N.R.N.; Lau, W.J. Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide. J. Environ. Chem. Eng. 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Sun, J.; Alsaedi, A.; Hayat, T.; Li, J.; Wang, X. Insight into the impact of interaction between attapulgite and graphene oxide on the adsorption of U(VI). Chem. Eng. J. 2018, 343, 217–224. [Google Scholar] [CrossRef]
- Hummers, S.W.; Offeman, E.R. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Du, L.; Wang, P.; Li, X.; Tan, Z. Effect of attapulgite colloids on uranium migration in quartz column. Appl. Geochem. 2019, 100, 363–370. [Google Scholar] [CrossRef]




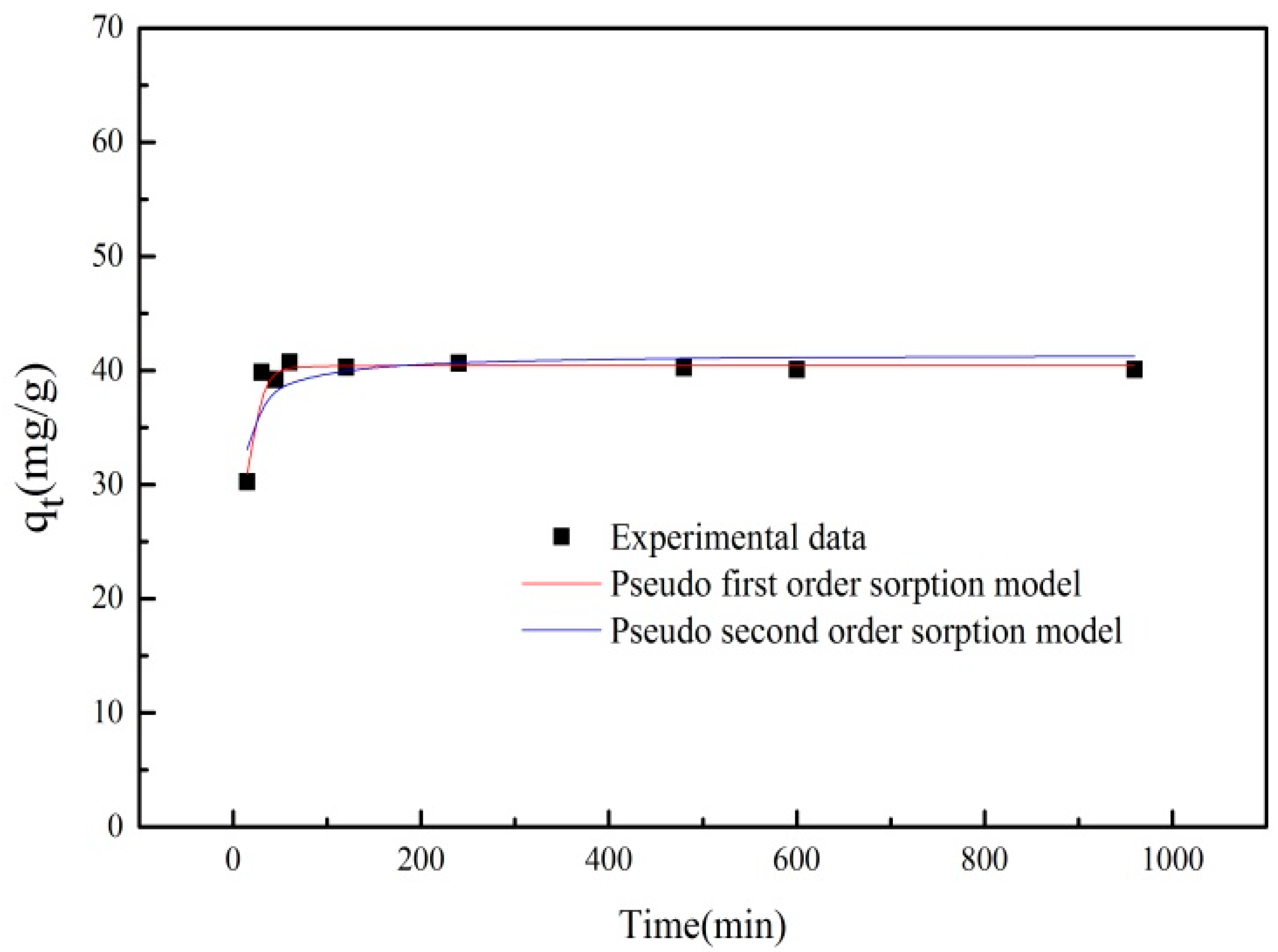

| qexp (mg/g) | Pseudo-First-Order Equation | Pseudo-Second-Order Equation | ||||
|---|---|---|---|---|---|---|
| K1 (1/min) | qcal (mg/g) | R2 | K2 (g/(mg·min)) | qcal (mg/g) | R2 | |
| 42.07 | 0.097 | 40.432 | 0.947 | 0.0064 | 41.415 | 0.682 |
| Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g) | n | R2 |
| 54.763 | 21.747 | 0.841 | 32.361 | 7.683 | 0.945 |
| C0 (mg/L) | ΔH (kJ/mol) | ΔS (J/(mol·K)) | ΔG (kJ/mol) | ||
|---|---|---|---|---|---|
| 298 (K) | 308 (K) | 318 (K) | |||
| 50 | 12.22 | 45.72 | −1.41 | −1.87 | −2.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Li, Y.; Nie, W.; Gao, X.; Zhang, L.; Yang, P.; Tan, X. Fast Removal of Propranolol from Water by Attapulgite/Graphene Oxide Magnetic Ternary Composites. Materials 2019, 12, 924. https://doi.org/10.3390/ma12060924
Deng Y, Li Y, Nie W, Gao X, Zhang L, Yang P, Tan X. Fast Removal of Propranolol from Water by Attapulgite/Graphene Oxide Magnetic Ternary Composites. Materials. 2019; 12(6):924. https://doi.org/10.3390/ma12060924
Chicago/Turabian StyleDeng, Yuehua, Yani Li, Wenjie Nie, Xiang Gao, Lei Zhang, Pengli Yang, and Xiaochun Tan. 2019. "Fast Removal of Propranolol from Water by Attapulgite/Graphene Oxide Magnetic Ternary Composites" Materials 12, no. 6: 924. https://doi.org/10.3390/ma12060924
APA StyleDeng, Y., Li, Y., Nie, W., Gao, X., Zhang, L., Yang, P., & Tan, X. (2019). Fast Removal of Propranolol from Water by Attapulgite/Graphene Oxide Magnetic Ternary Composites. Materials, 12(6), 924. https://doi.org/10.3390/ma12060924




