Quality Control of High Carbon Steel for Steel Wires
Abstract
1. Introduction
2. Control of Centerline Segregation
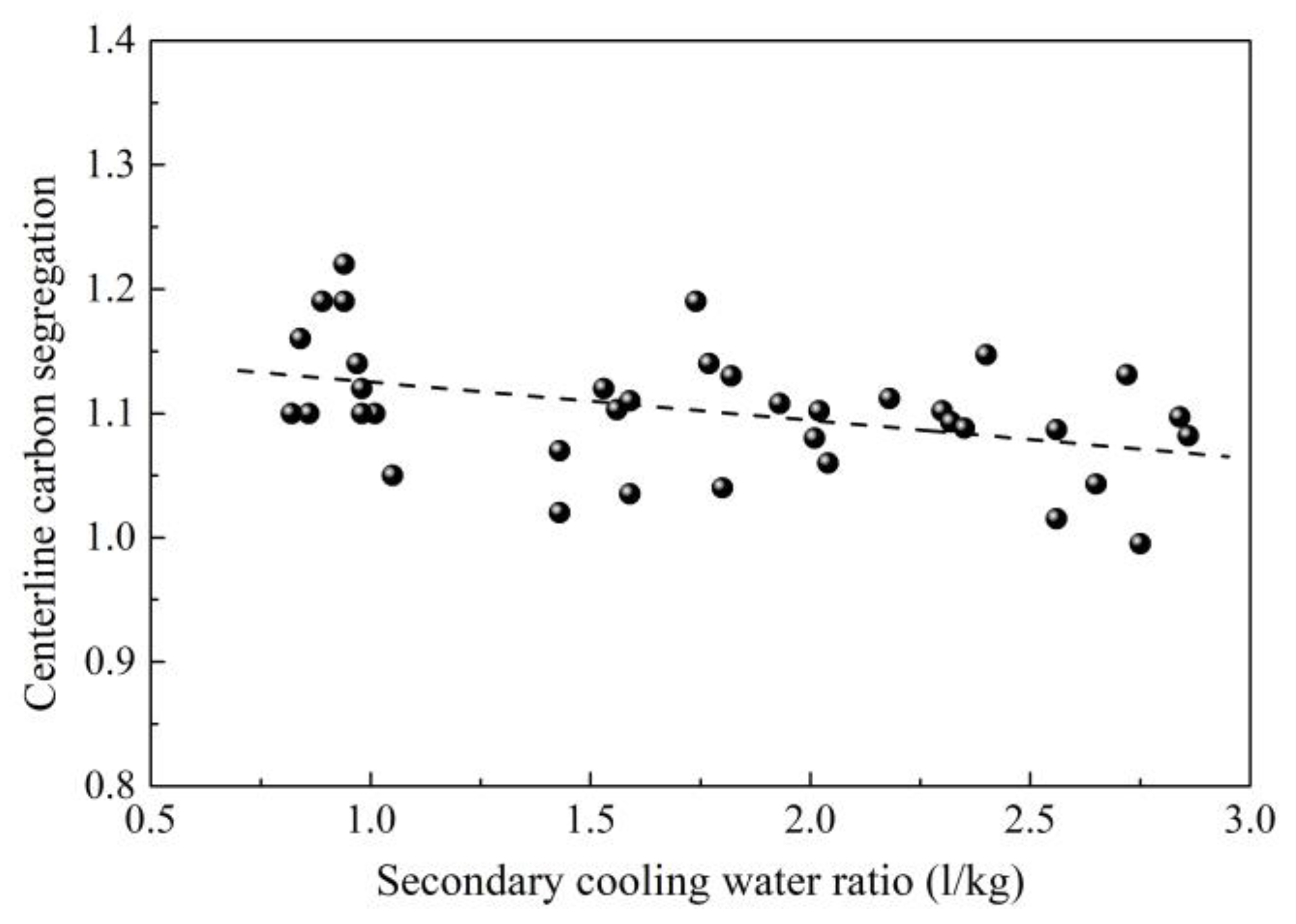
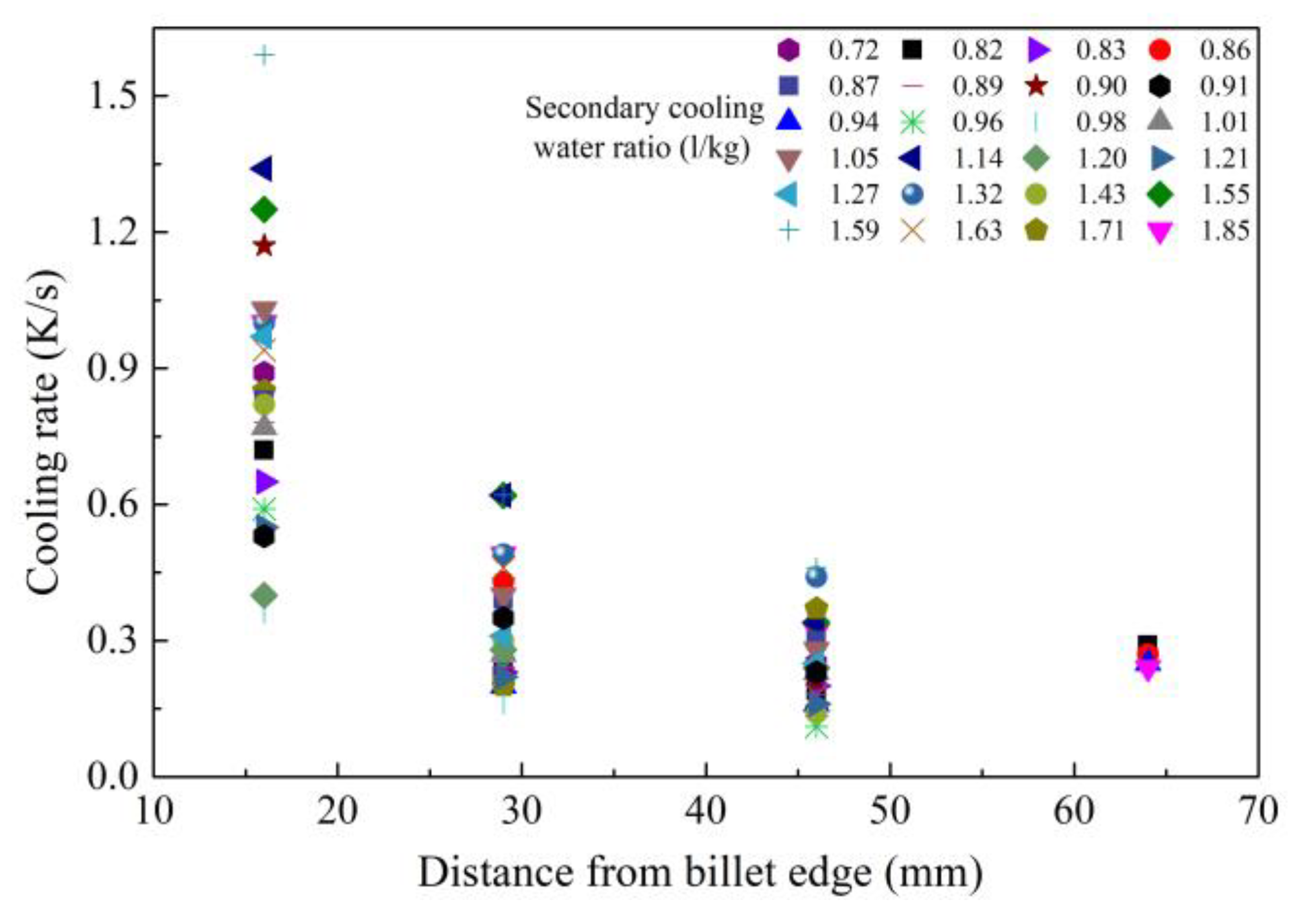
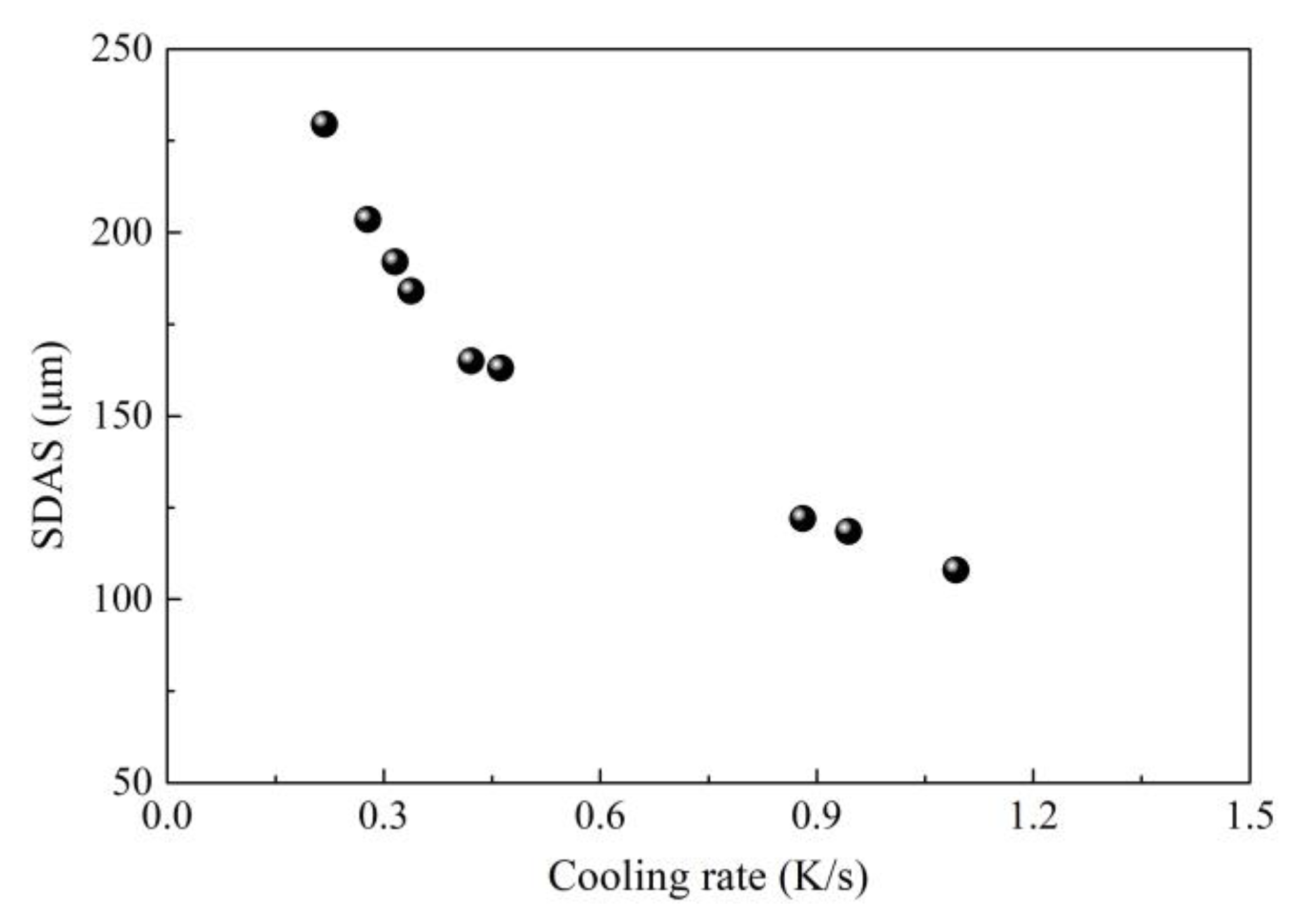
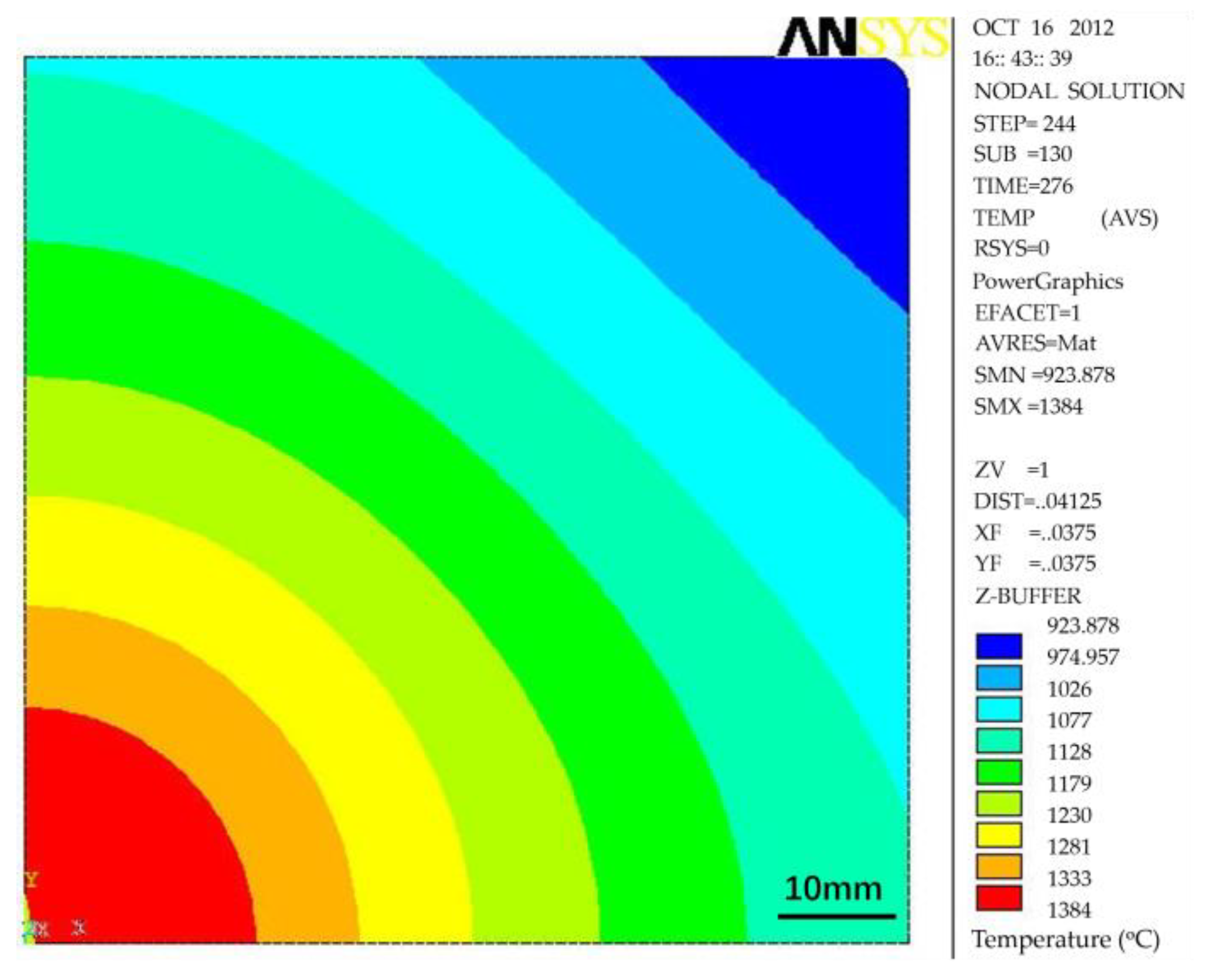
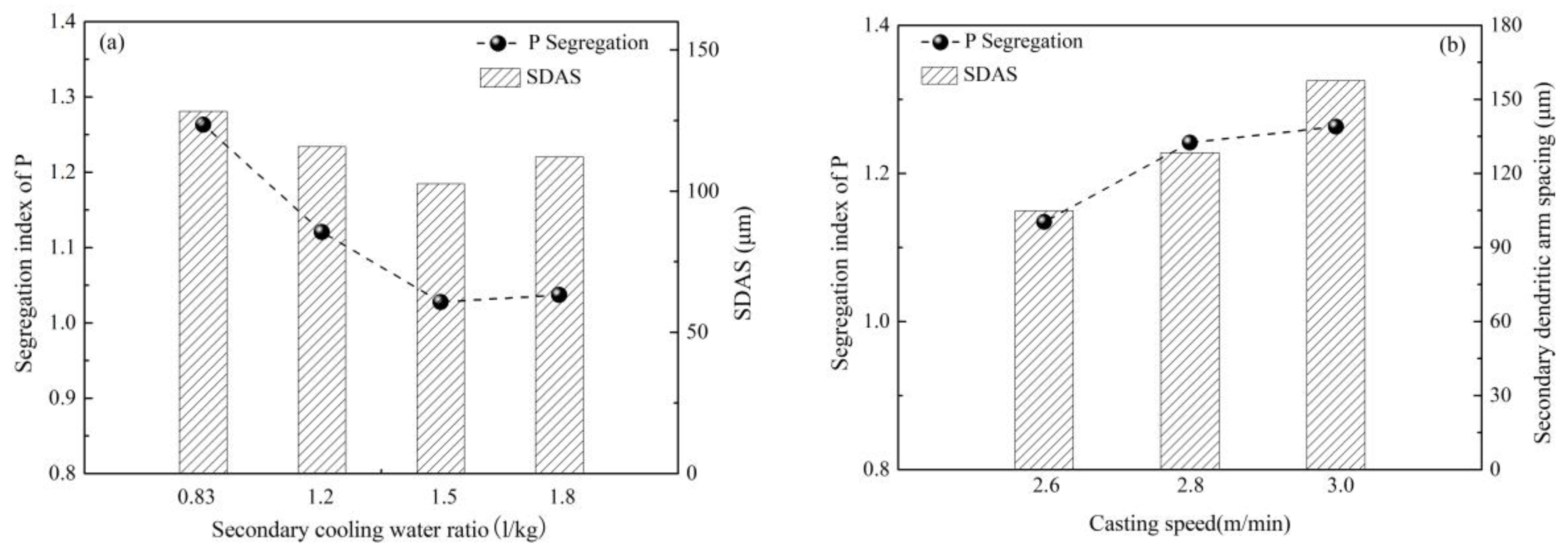
2.1. Control of Centerline Segregation Using Intensive Secondary Cooling
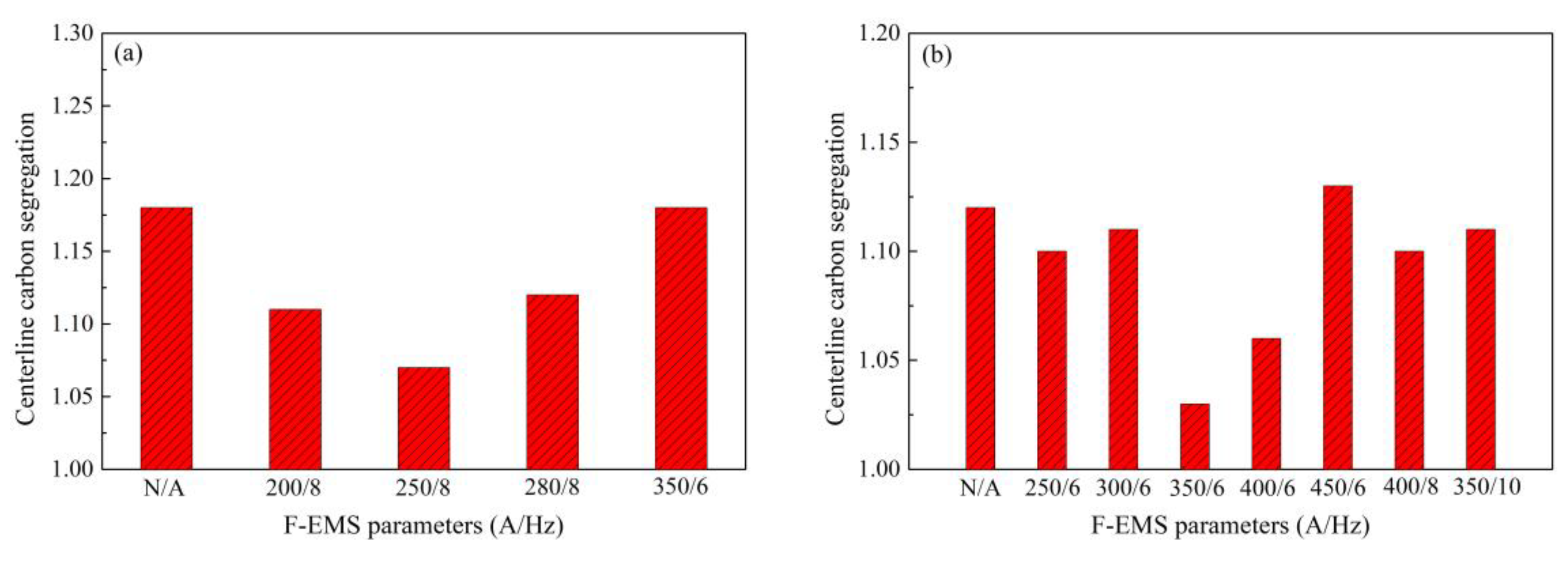
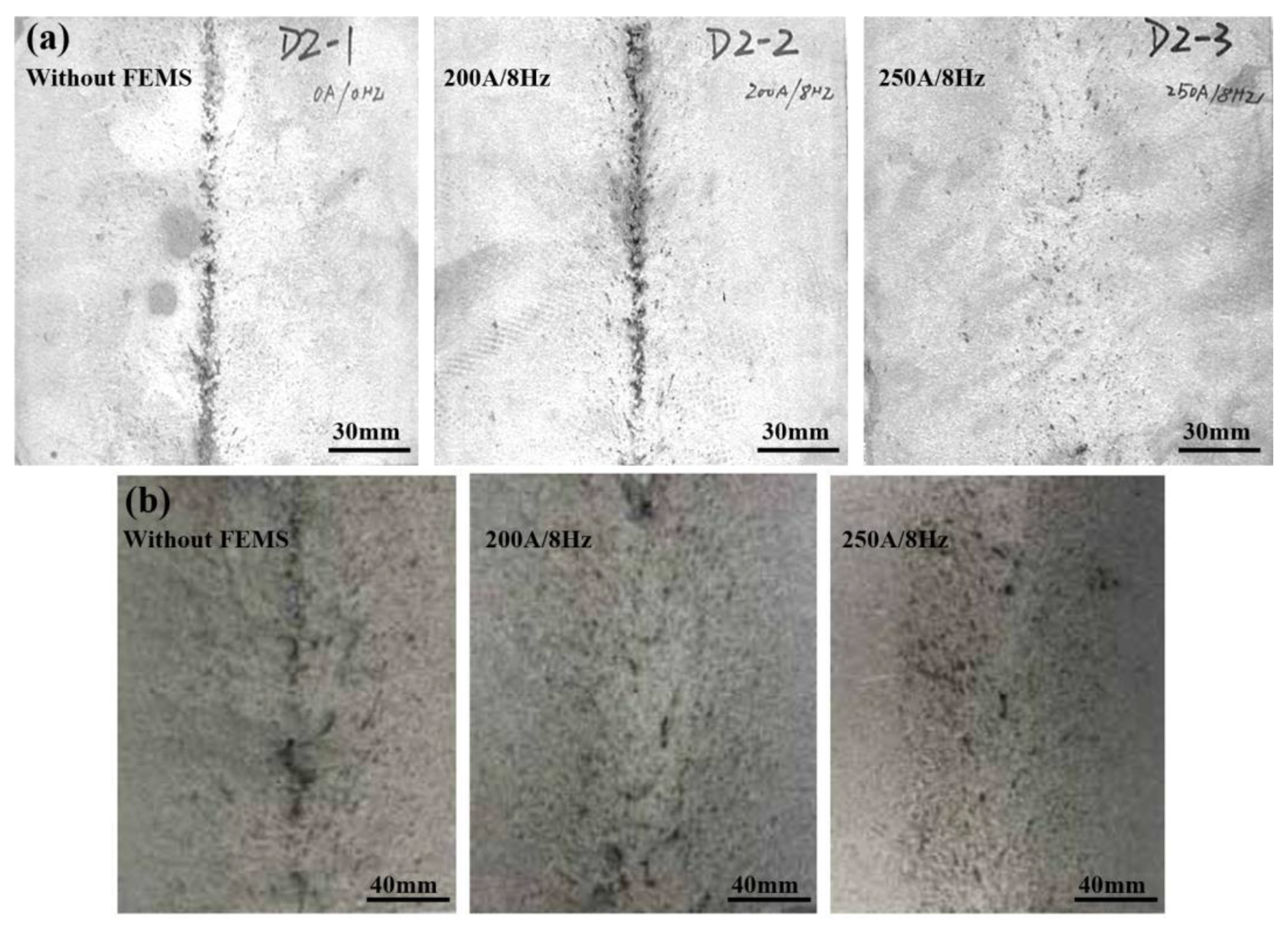
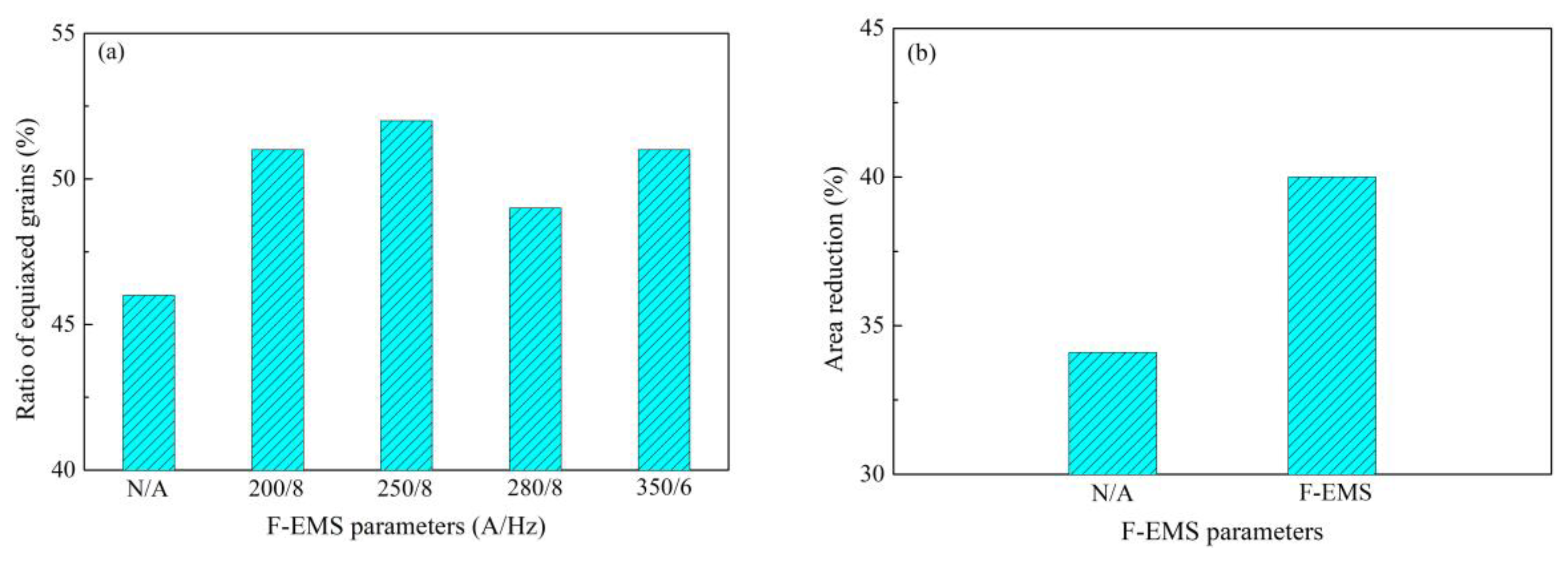
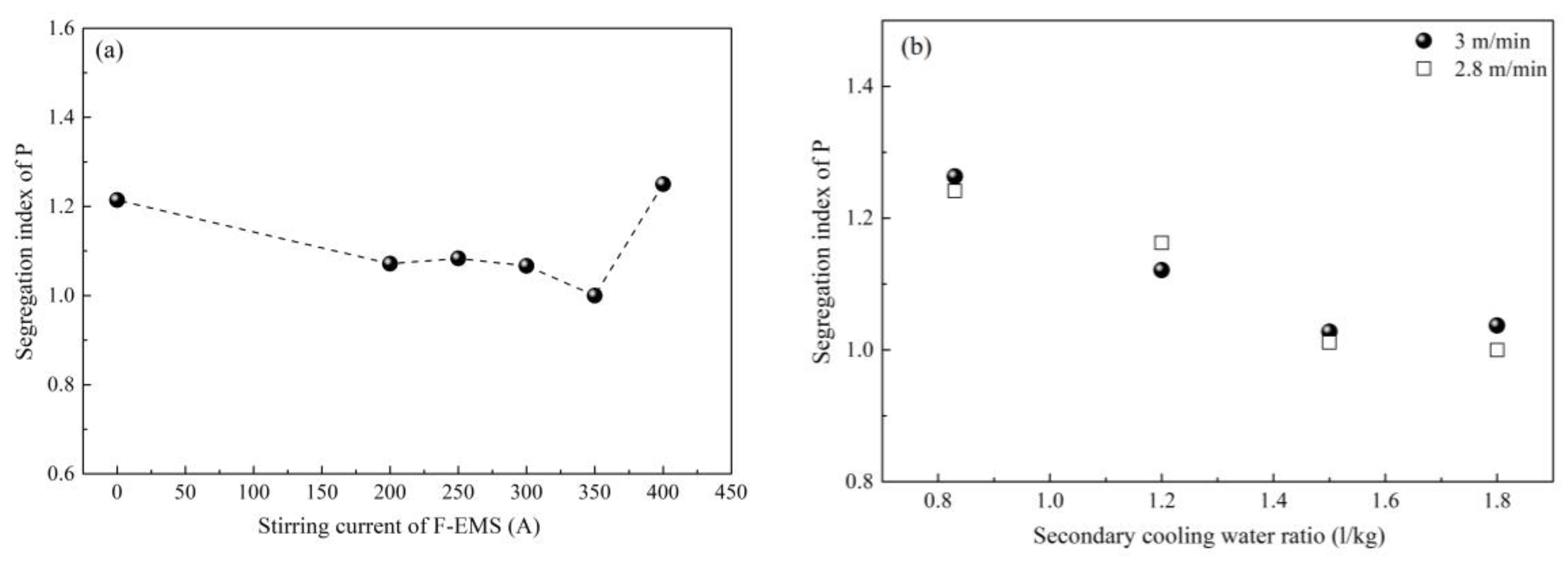
2.2. Control of Centerline Segregation Using F-EMS
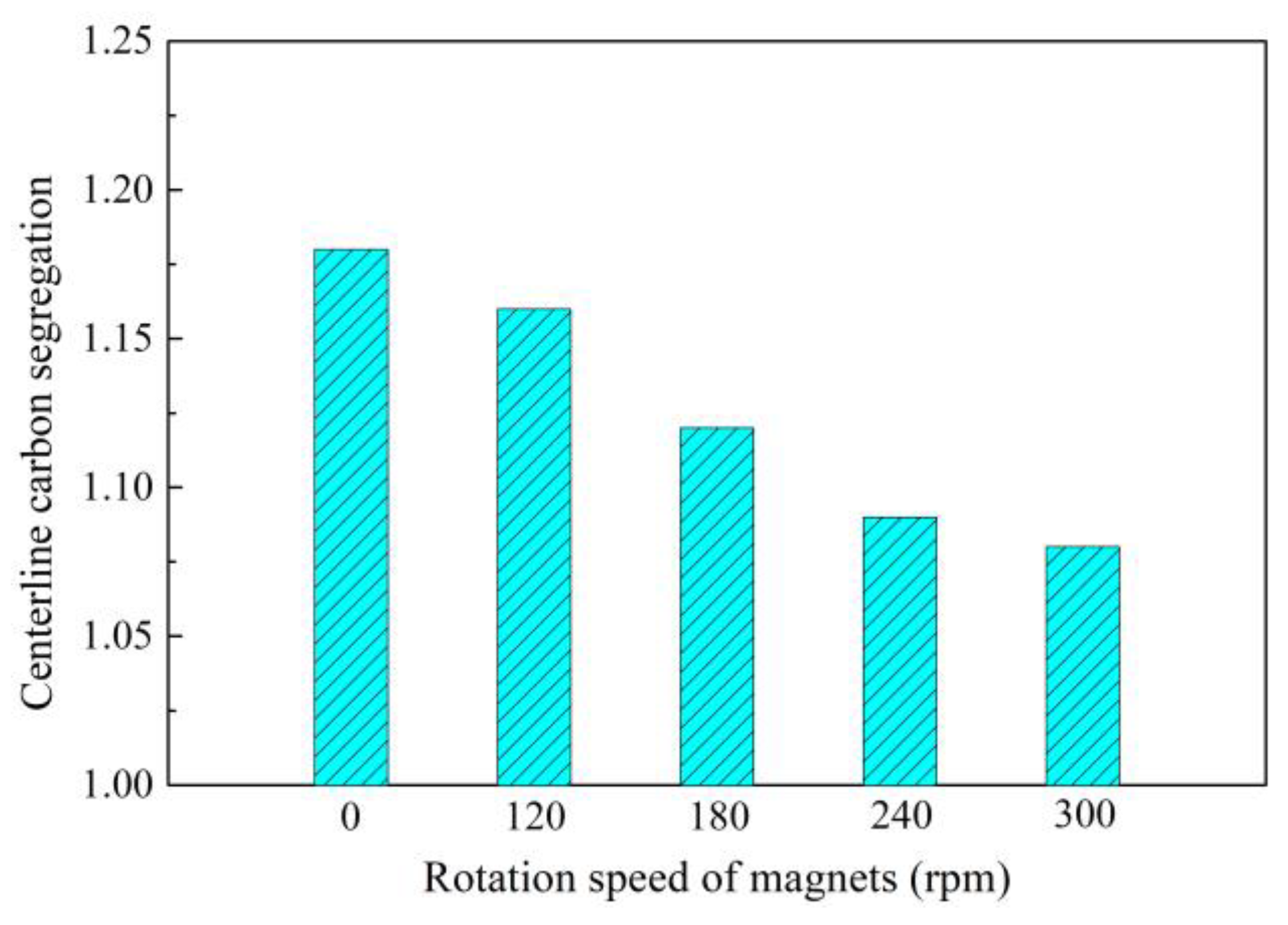
2.3. Control of Central Segregation Using F-PMS
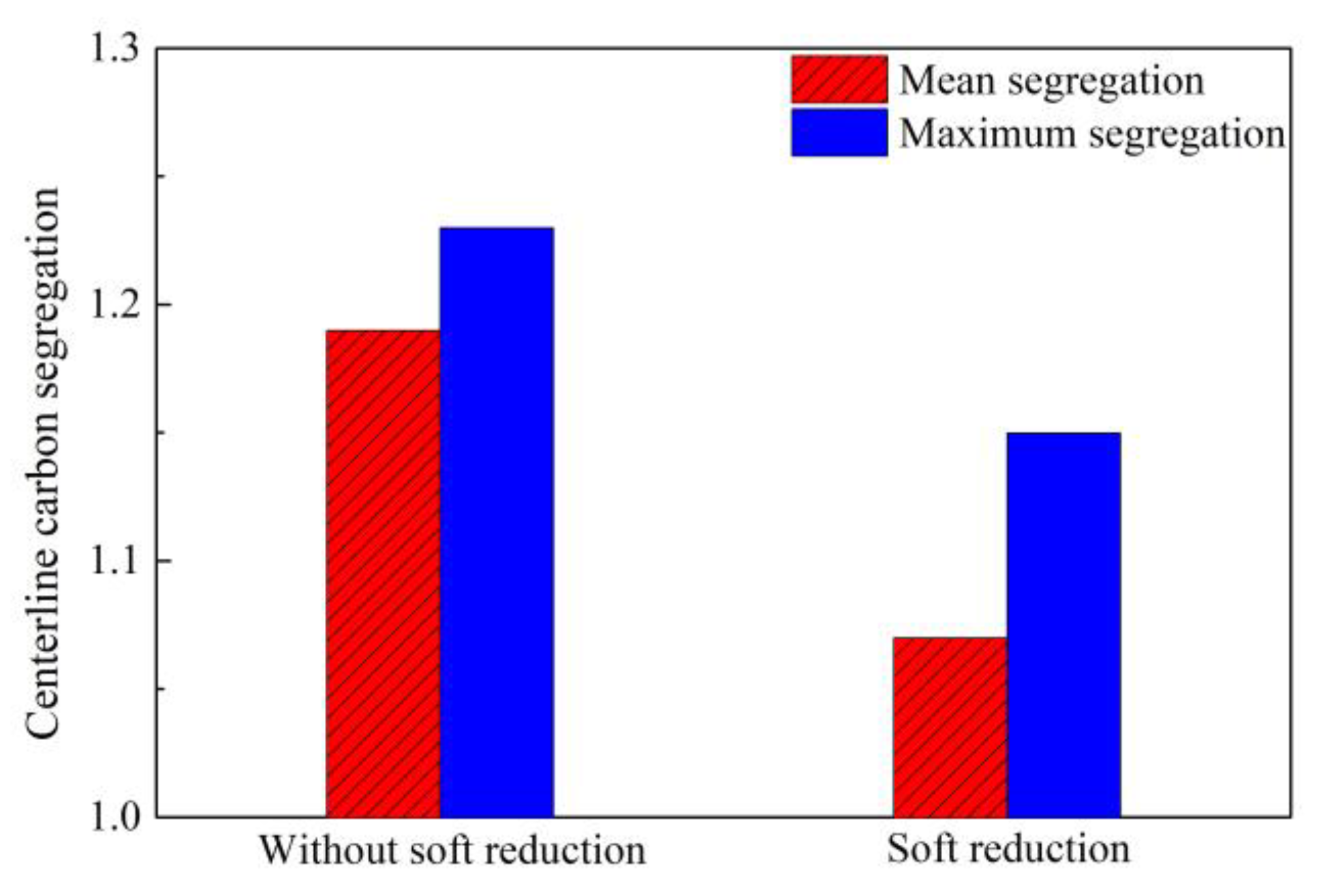
2.4. Control of Central Segregation Using Soft Reduction
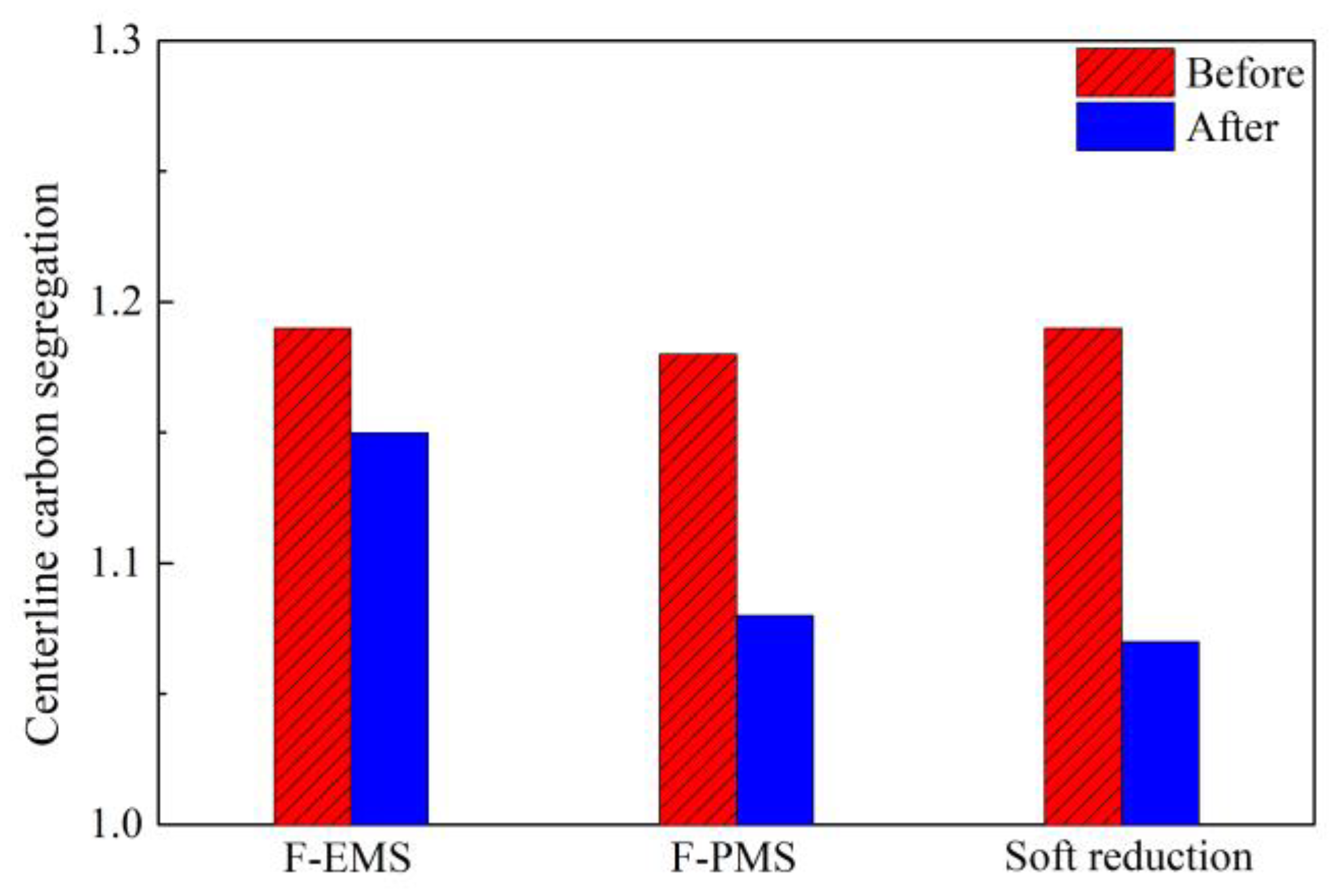
2.5. Comparison in Control of Segregation
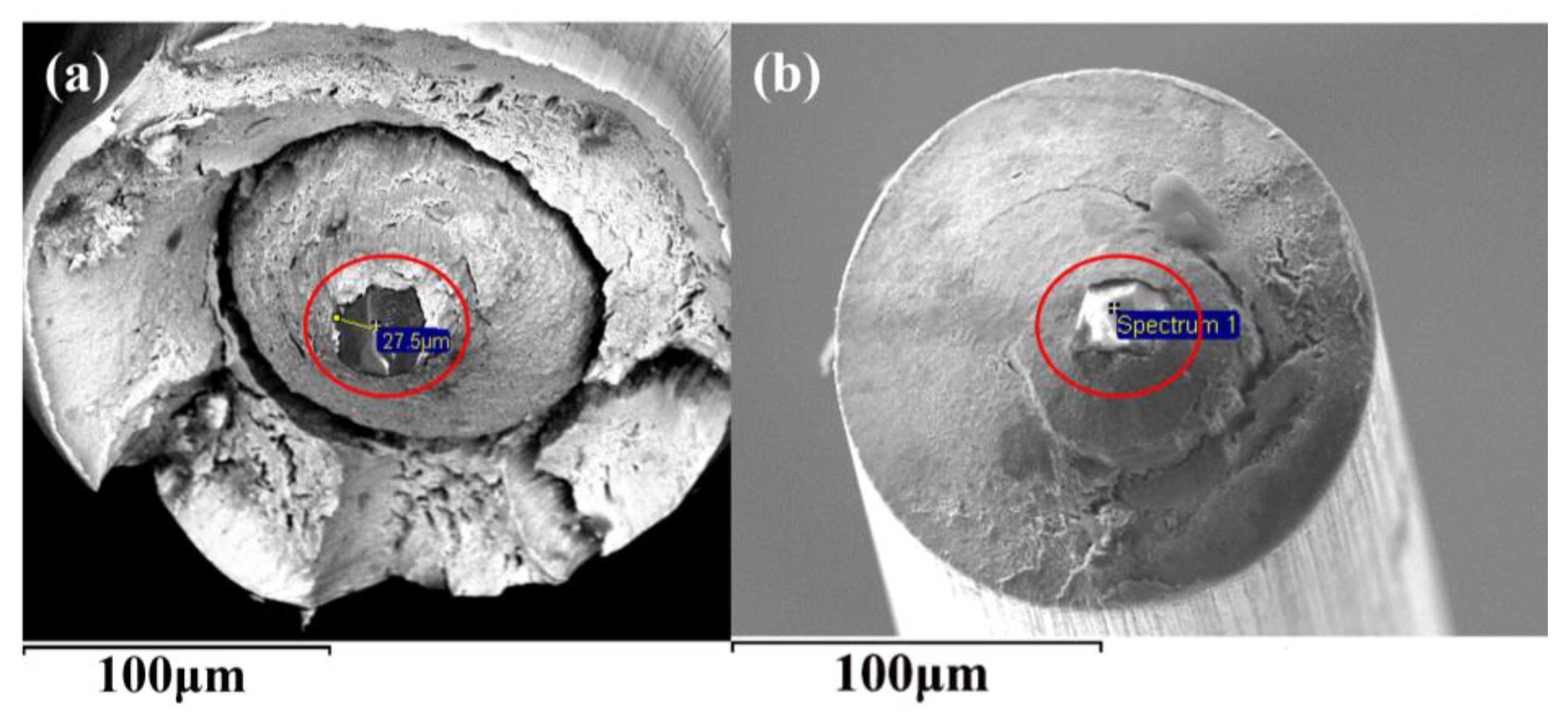
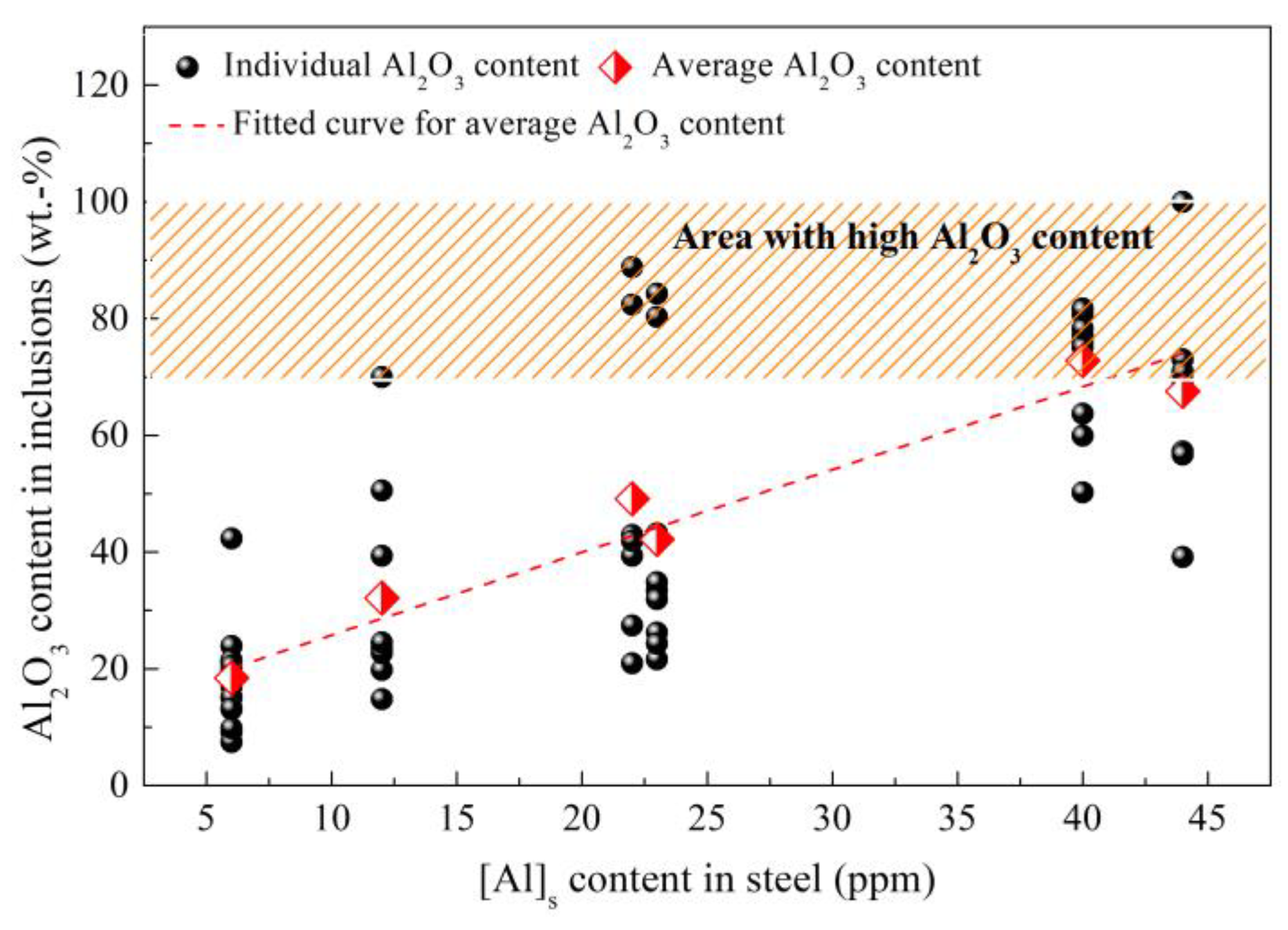
3. Control of Inclusions
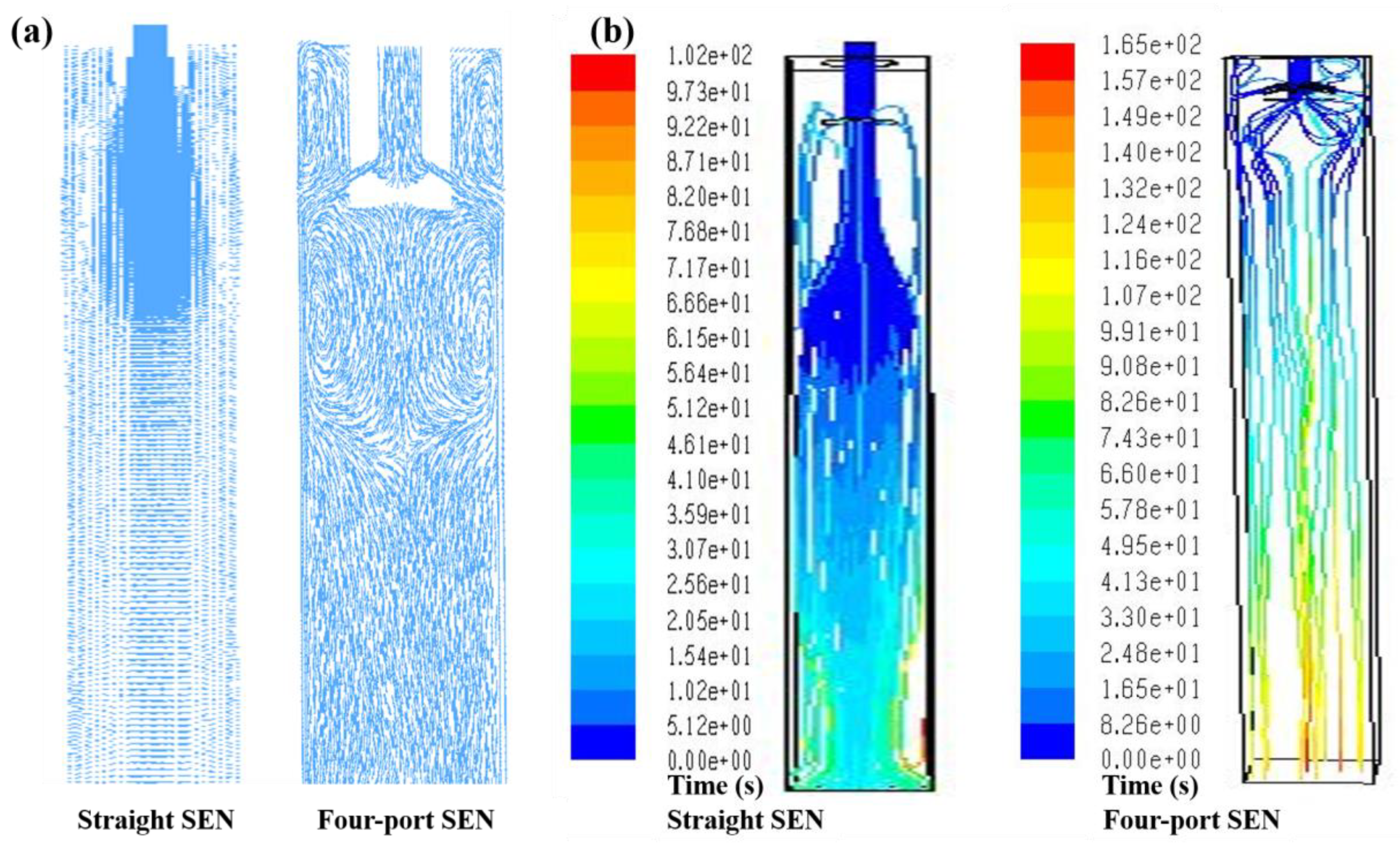
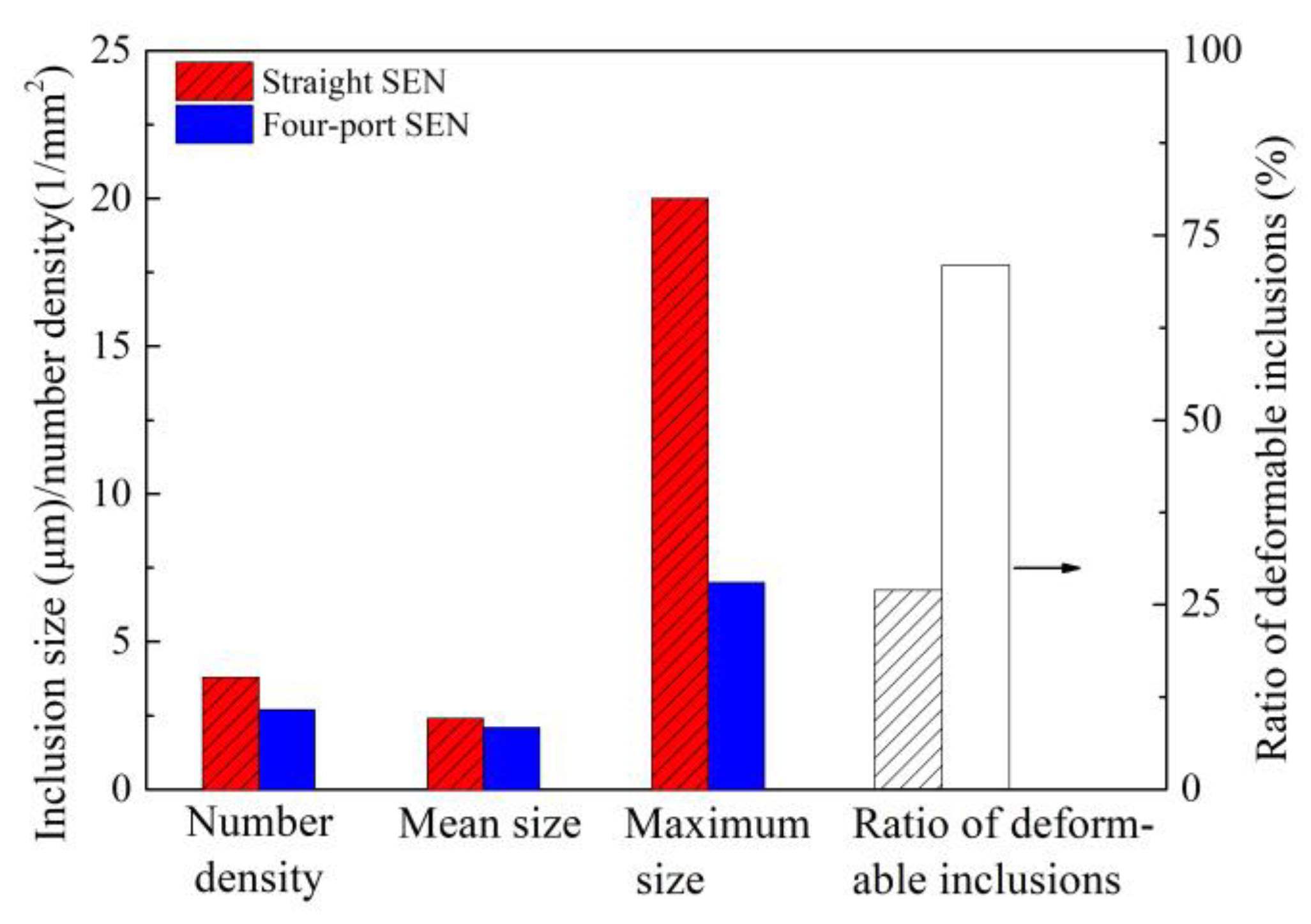
3.1. Control of Inclusions through Redesigning SEN
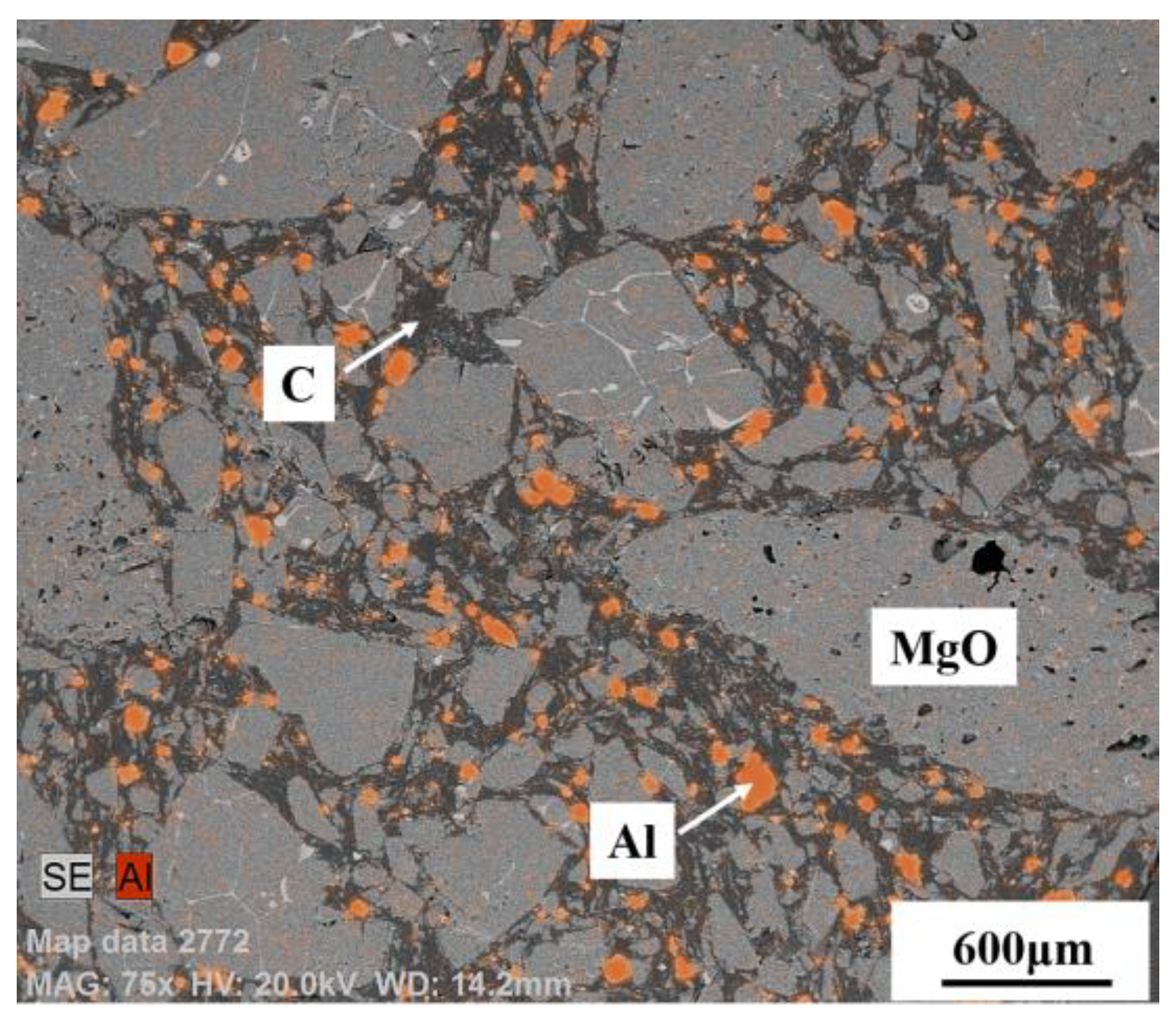
3.2. Control of Inclusions through Refractory
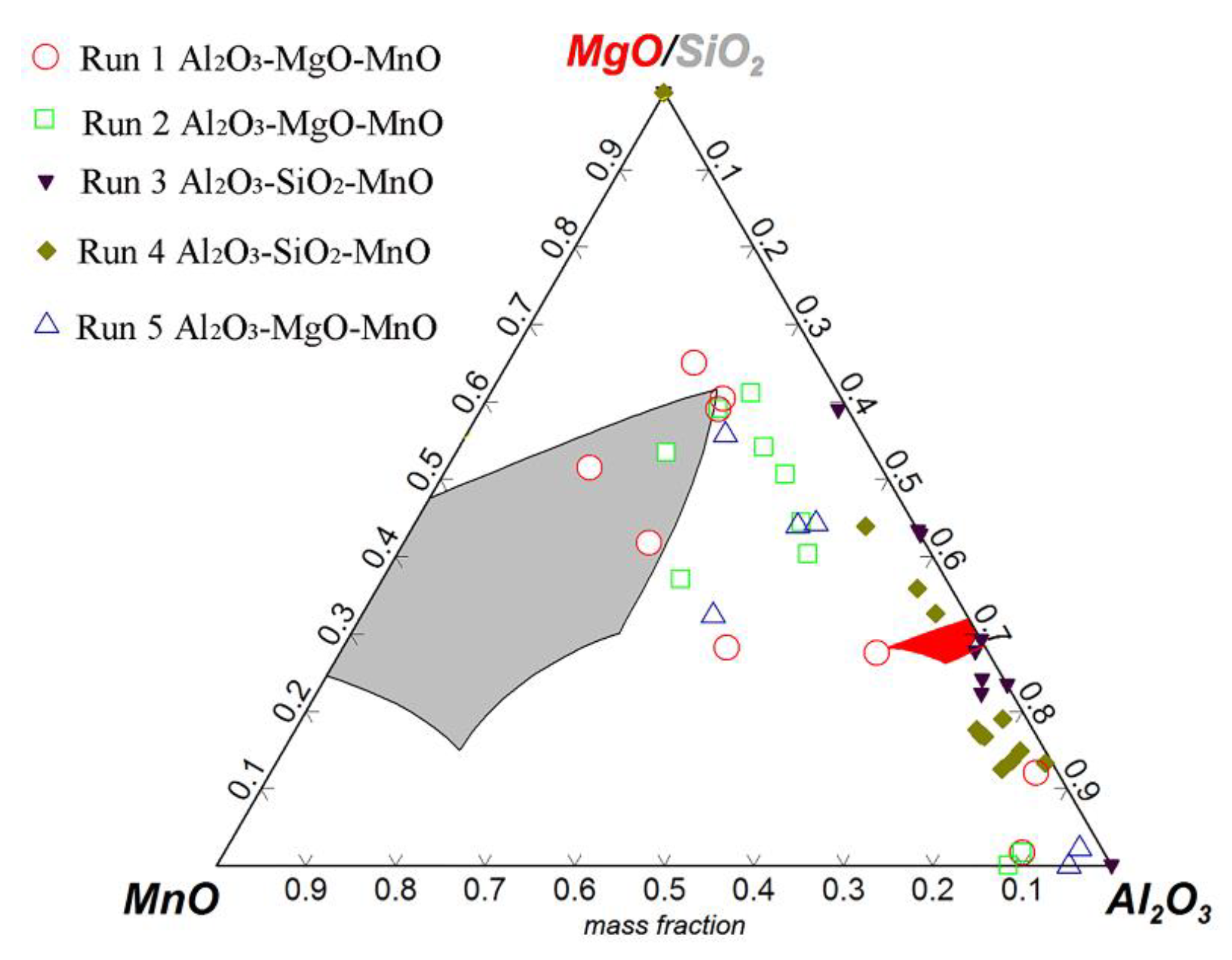
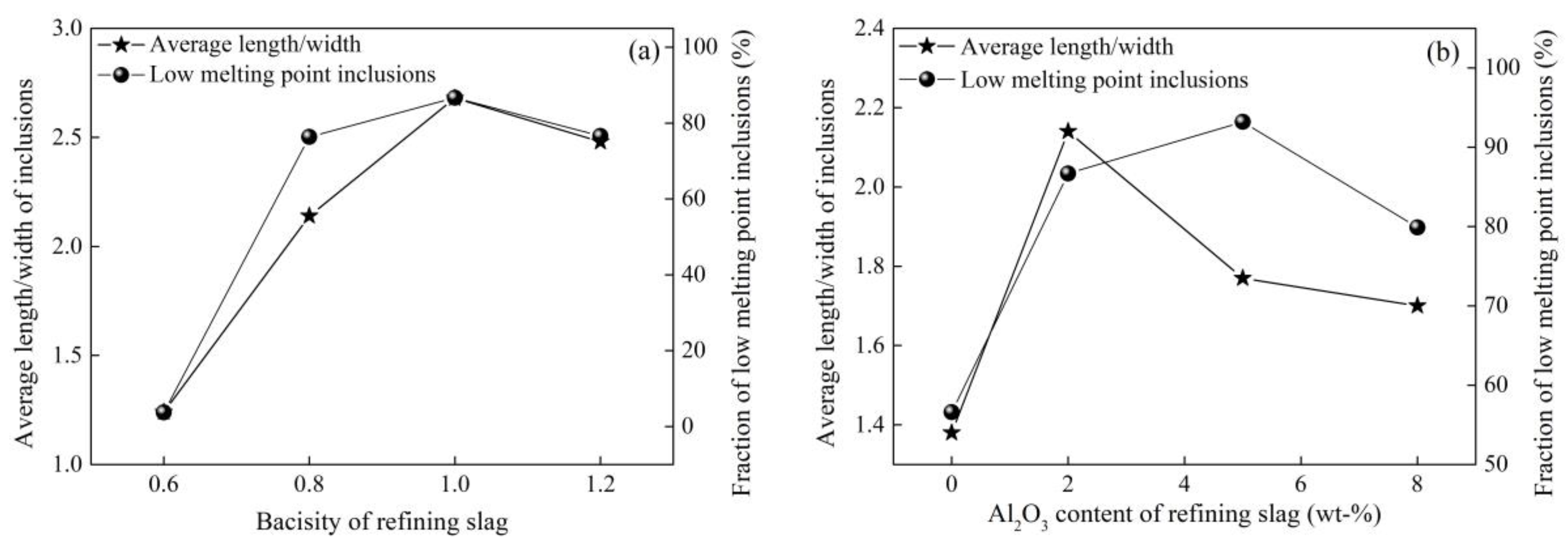
3.3. Control of Inclusions through Redesigning Refining Slag
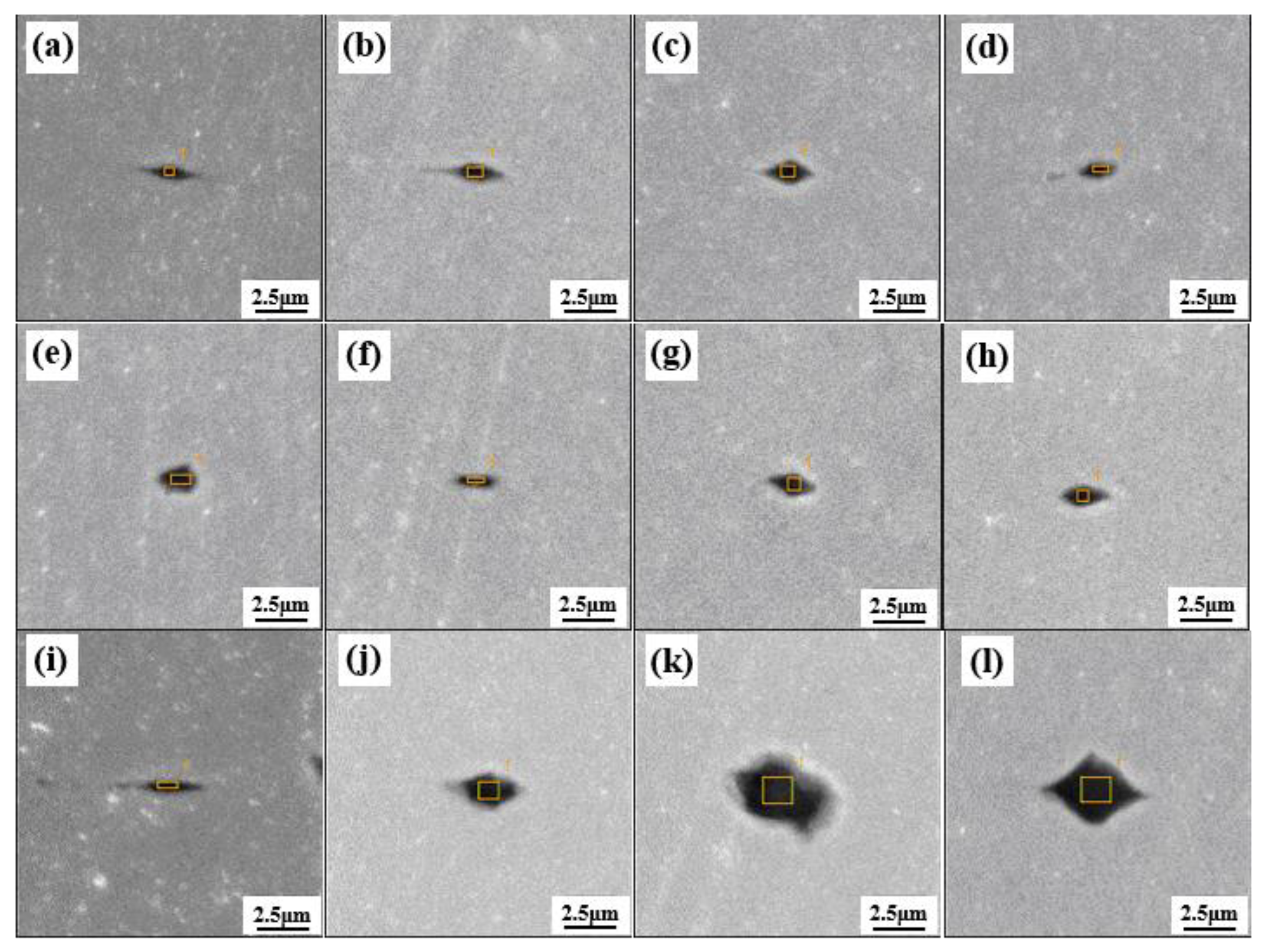
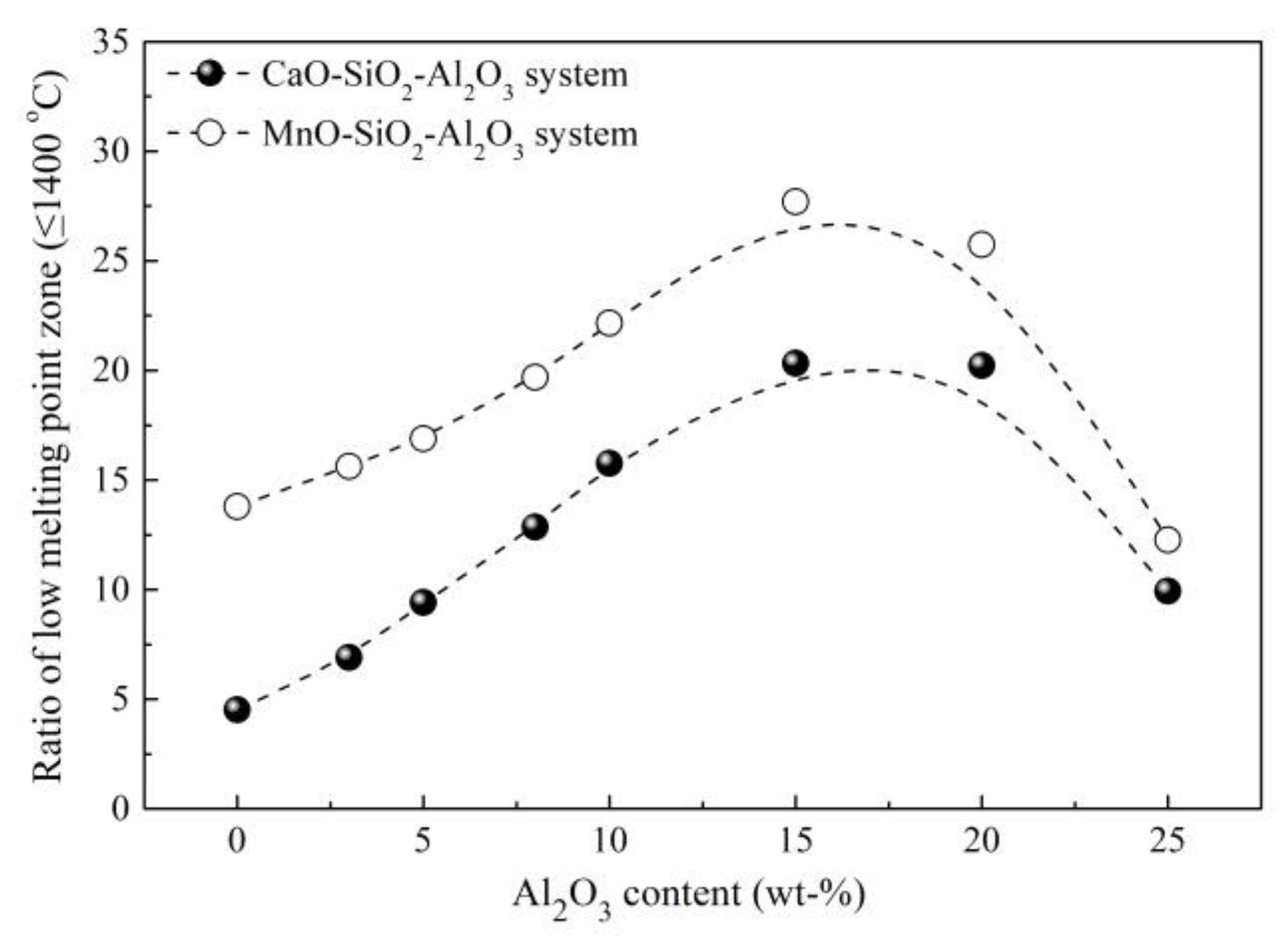
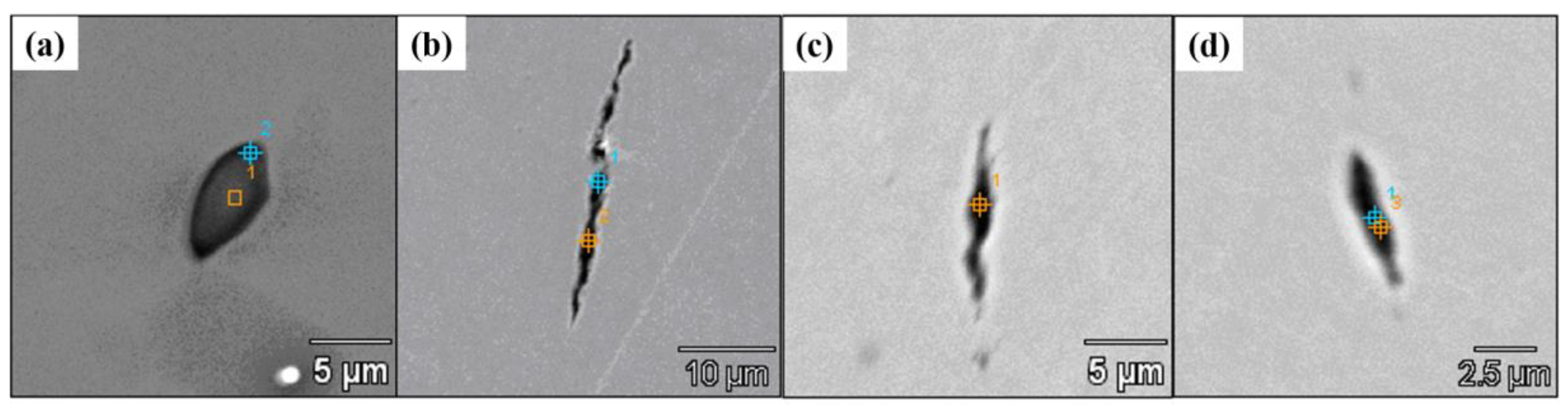
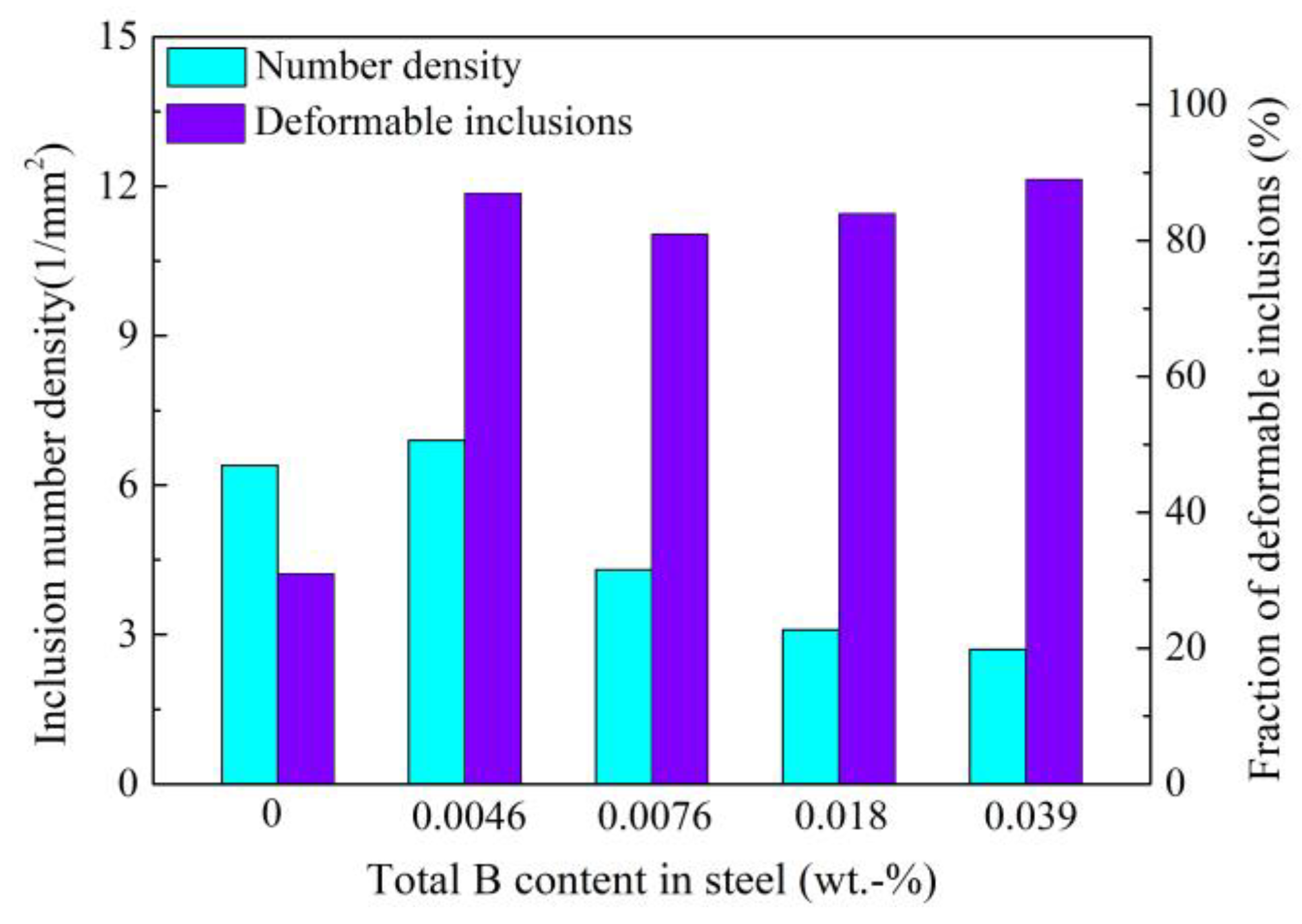
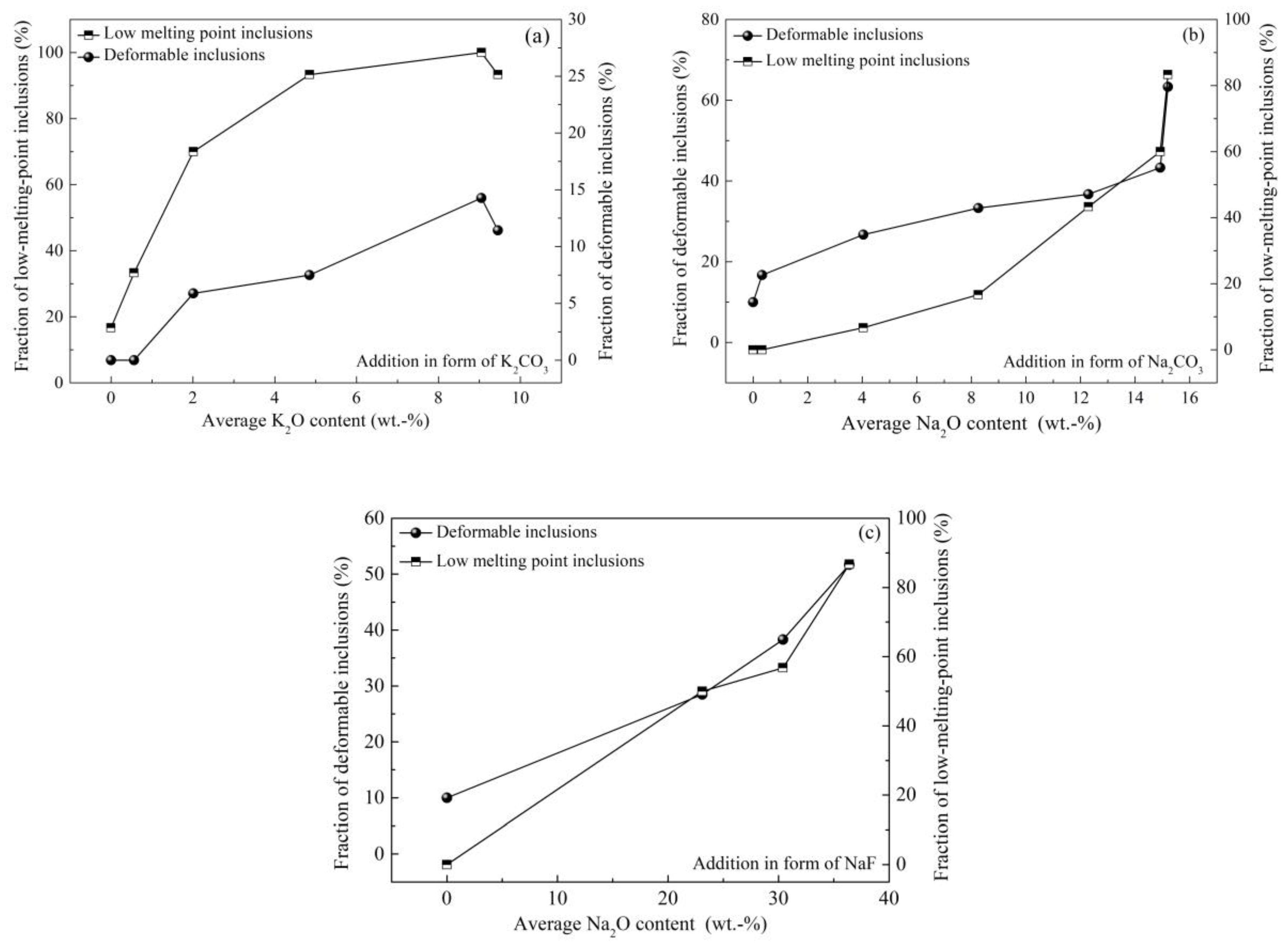
3.4. Control of Inclusions through Using Low-Melting-Point Compounds
4. Control of Microstructure
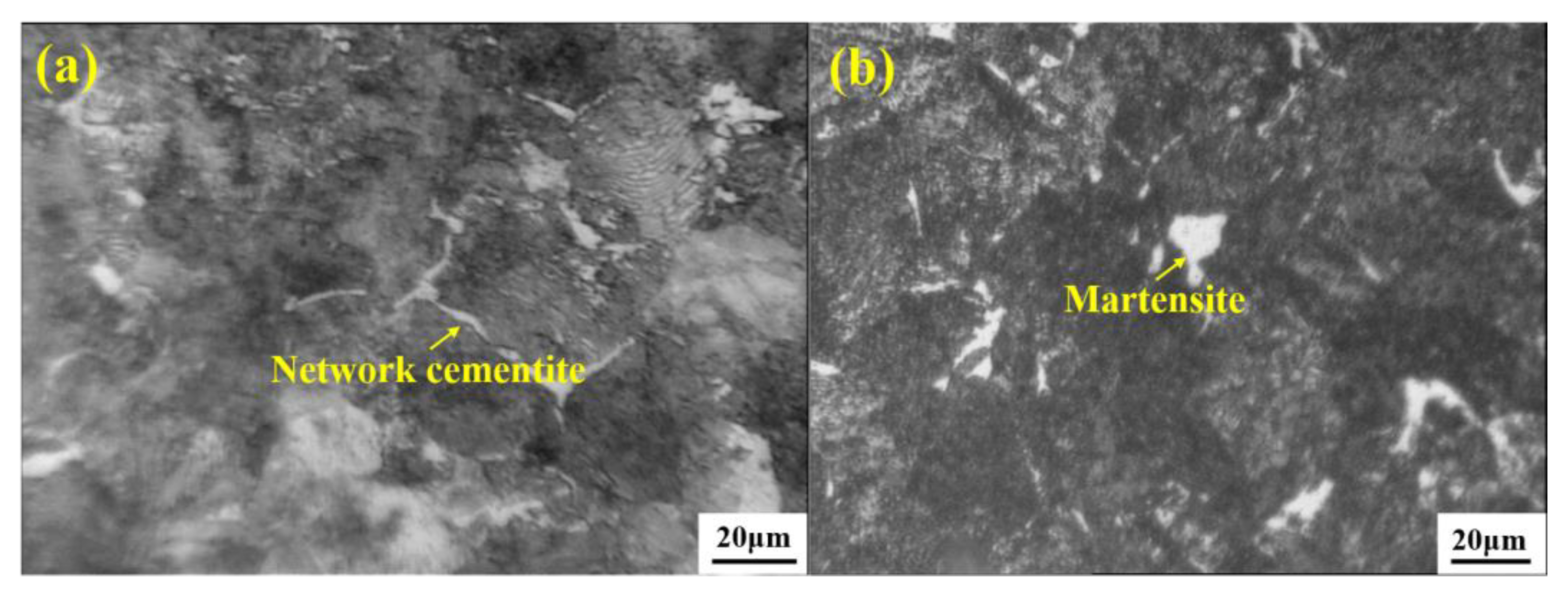
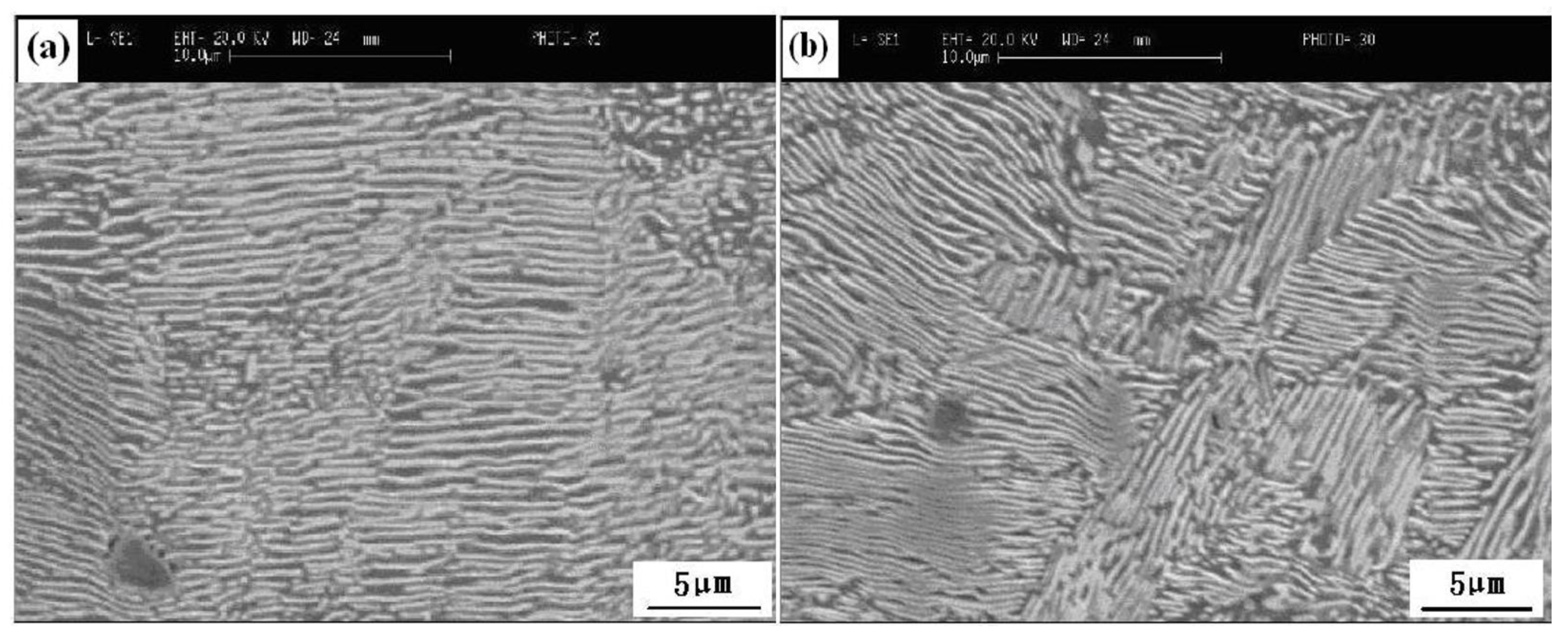
4.1. Control of Pearlite, Network Cementite, and Martensite
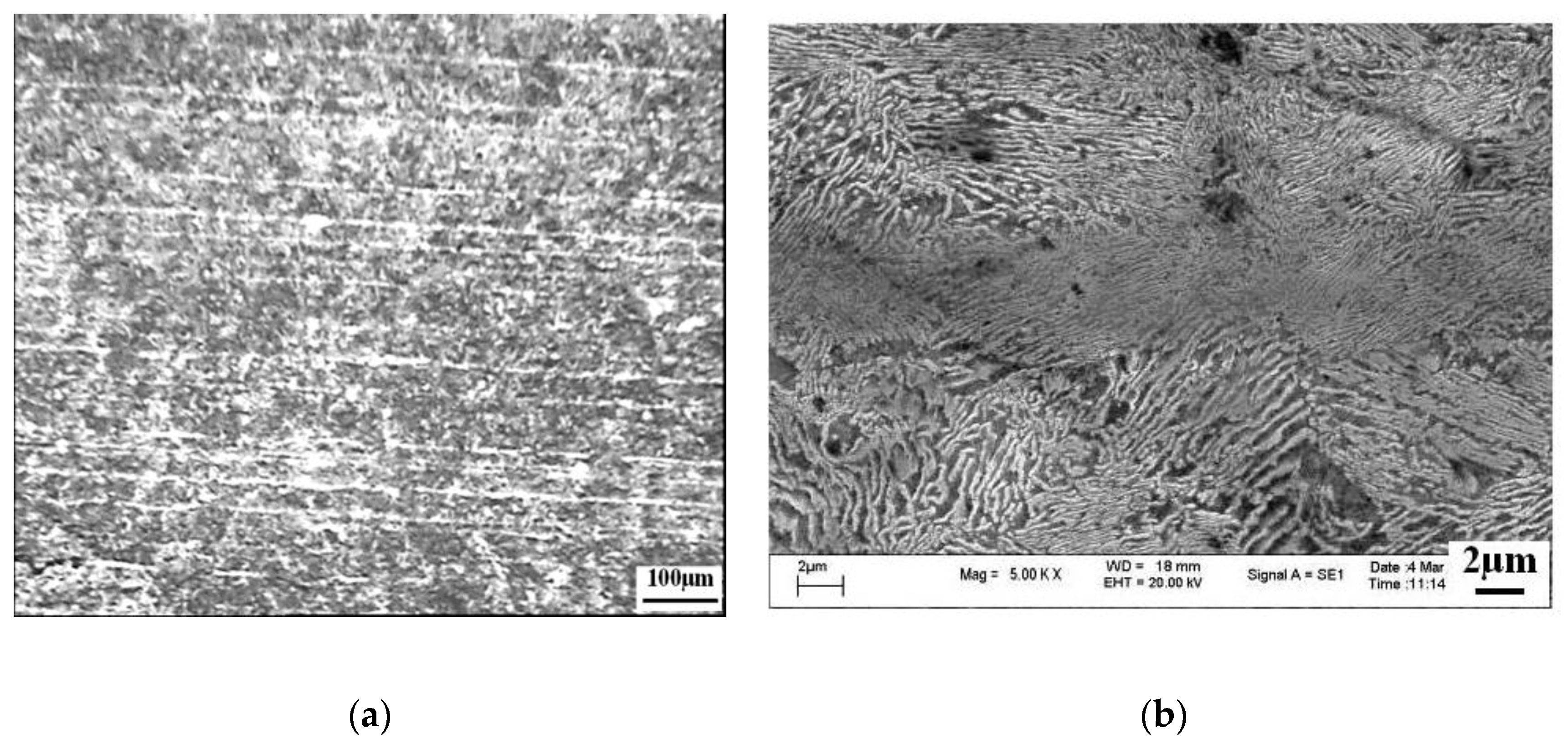
4.2. Control of Banded Structure
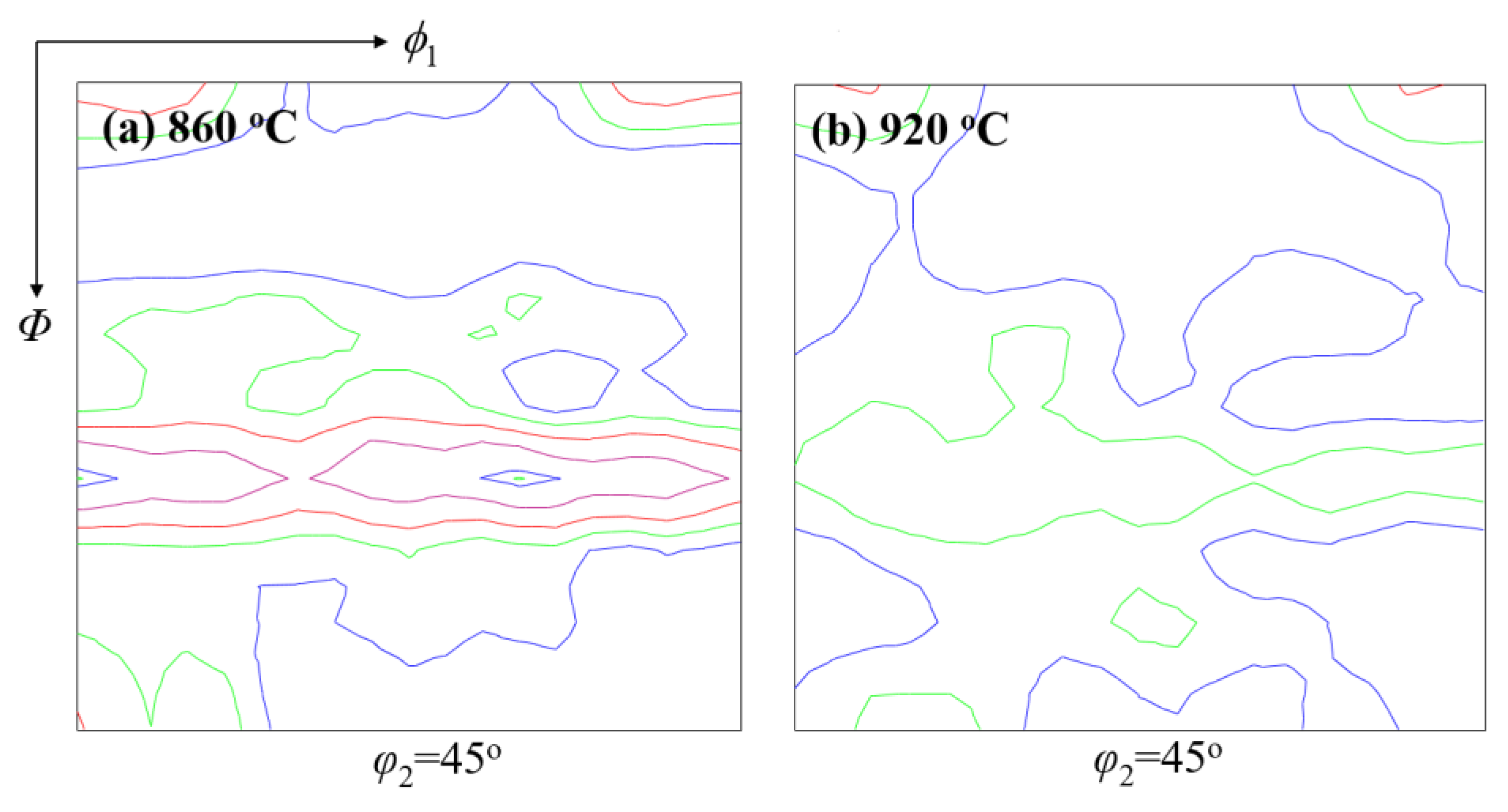
4.3. Control of Texture
5. Summary
- 1)
- Intensive secondary cooling, F-EMS, F-PMS and soft reduction in control of segregation have been investigated and applied, and their application scopes have been defined. In particular, F-PMS has been developed and applied innovatively in the industrial production of high-carbon steel and shows advantages in terms of reducing the cost and improving the quality of products.
- 2)
- A combination of the redesign of SEN, the redesign of refining slag, the application of Al-free refractory during the vacuum process, and the improvement of the deformability of inclusions through the addition of B2O3 and alkali metal compounds has been investigated and applied in the industrial production of high-carbon steel, promoting the inclusion removal and greatly improving the deformability of inclusions.
- 3)
- Final stirring and using an appropriate cooling rate are beneficial for effective control of network cementite and martensite. Formation of a hard banded structure is close to P segregation, which can be controlled through effective secondary cooling, casting speed, and final stirring. Undesired {111} texture can be controlled through improvements of heating time, heating temperature, and especially, the laying temperature.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirhara, K. Production technology of wire rod for high tensile strength steel cord. Kobelco Technol. Rev. 2011, 30, 62–65. [Google Scholar]
- Chen, L.J. Inclusion Control in High-Carbon Steel for Saw Wire. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 21 December 2018. [Google Scholar]
- Jie, W.Q. Solute redistribution and segregation in solidification processes. Sci. Technol. Adv. Mat. 2001, 2, 29–35. [Google Scholar] [CrossRef]
- Irving, W.R.; Perkins, A.; Broooks, M.G. Effect of chemical, operational, and engineering factors on segregation in continuously cast slabs. Ironmak. Steelmak. 1984, 11, 152–161. [Google Scholar]
- Reger, M.; Vero, B.; Felde, I.; Kardos, I. The effect of heat treatment on the stability of centerline segregation. J. Mech. Eng. 2010, 56, 143–149. [Google Scholar]
- Cai, K.K. Quality Control of Casting Slabs; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Yilmaz, M.; Ertunc, H.M. The prediction of mechanical behavior for steel wires and cord materials using neural networks. Mater. Des. 2007, 28, 599–608. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R. Thermodynamics on control of inclusions composition in ultra-clean steels. ISIJ Int. 1996, 36, 528–536. [Google Scholar] [CrossRef]
- Feng, J.; Chen, W.Q. Study on refining process for improvement of cleanliness of high carbon steel. Steelmaking 2005, 21, 41–52. (In Chinese) [Google Scholar]
- Chen, L.J.; Chen, W.Q.; Hu, Y.; Chen, Z.P.; Xu, Y.T.; Yan, W. Investigation on the origin of Al2O3-rich inclusions in valve spring steel under vacuum condition. Steel Res. Int. 2017, 88, 1600376. [Google Scholar] [CrossRef]
- Costa e Silva, A. Non-metallic inclusions in steels–origin and control. J. Mater. Res. Technol. 2018, 3, 283–299. [Google Scholar] [CrossRef]
- Thomas, B.G.; Yuan, Q.; Zhang, L.; Vanka, S.P. Flow dynamics and inclusion transport in continuous casting of steel. NSF Design, Service. In Proceedings of the Manufacturing and Industrial Innovation Research Conference, Birmingham, UK, 6–9 January 2003; pp. 2328–2362. [Google Scholar]
- Li, F.S.; Chen, W.Q. Effect of SEN type on inclusions in tire cord steel. Int. Tech. Rep. 2015, 19, 22–27. [Google Scholar]
- Han, Y.S.; Chen, W.Q. Effect of weir and dam in 6-strand asymmetric 40 t tundish on inclusions in coil of tire cord steel. Spec. Steel 2016, 37, 25–27. (In Chinese) [Google Scholar]
- Zhao, H.Q.; Chen, W.Q. Effect of crucible material and top slag composition on the inclusion composition of tire cord steel. J. Iron Steel Res. 2012, 24, 12–16. (In Chinese) [Google Scholar]
- Chen, S.H.; Jiang, M.; He, X.F.; Wang, X.H. Top slag refining for inclusion composition transform control in tire cord steel. Int. J. Min. Met. Mater. 2012, 19, 490–498. [Google Scholar] [CrossRef]
- Liu, H.J.; Fu, J.; Wang, G.J.; He, H.Y.; Liu, H.T.; Chen, W.Q. Effect of carburetant in BOF tapping and RH refining on cleanliness of high carbon steel SWRH82B. Spec. Steel 2011, 32, 25–27. (In Chinese) [Google Scholar]
- Gobinath, R.; Naveen Kumar, N.; Balachandran, G. Metallurgical characterization of commercial high carbon steel wire rods. Int. J. Adv. Res. Sci. Eng. 2018, 7, 229–242. [Google Scholar]
- Takahashi, T.; Ochiai, I.; Tashiro, H.; Ohashi, S.; Nishida, S.; Tarui, T. Strengthening of steel wire for tire cord. Nippon Steel Tech. Rep. 1995, 64, 45–49. [Google Scholar]
- Montesin, T.; Heizmann, J.; Abdellaoui, A. Relation between texture and drawability of steel cord. Wire J. Int. 1993, 26, 86. [Google Scholar]
- Kanetsuki, Y.; Ibaraki, N.; Ashida, S. Effect of cobalt addition on transformation behavior and drawability of hypereutectoid steel wire. ISIJ Int. 1991, 31, 304–311. [Google Scholar] [CrossRef]
- Khalid, F.A.; Farooque, M.; Haq, A.; Khan, A.Q. Role of ferrite/pearlite banded structure and segregation on mechanical properties of microalloyed hot rolled steel. Mater. Sci. Technol. 1999, 15, 1209–1215. [Google Scholar] [CrossRef]
- Cui, H.Z.; Chen, W.Q.; Cao, C.F. Analysis of banded structure in wire rod for tire cord steel. Heat Treat. Met.-CH 2011, 36, 41–45. (In Chinese) [Google Scholar]
- Cui, H.Z.; Chen, W.Q.; Mao, W.M.; Cao, C.F.; Zhang, Q.Z. Effects of rolling processes on textures of wires for tire cord steel. J. Univ. Sci. Technol. B 2011, 33, 947–951. (In Chinese) [Google Scholar]
- Zhang, S.L.; Feng, J.; Chen, W.Q.; Chen, S.H. Study on F-EMS of billet continues casting. In Proceedings of the Third International Conference on Continuous Casting of Steel in Developing Countries, Beijing, China, 14–17 September 2004. [Google Scholar]
- Zhang, S.L.; Feng, J.; Chen, W.Q. Experimental study on F-EMS during continuous casting of billet. Iron Steel 2004, 39, 135–138. (In Chinese) [Google Scholar]
- Feng, J.; Chen, W.Q. Effect of concasting technology parameters on center carbon segregation of high carbon steel billet. Spec. Steel 2005, 26, 33–35. (In Chinese) [Google Scholar]
- Feng, J.; Chen, W.Q. Effect of high secondary cooling intensity on solidification structure and center carbon segregation of high carbon steel billet. Spec. Steel 2006, 27, 42–44. (In Chinese) [Google Scholar]
- Feng, J. The Study on Inner Quality and Cleanliness of High Carbon Steel Billets. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 15 March 2006. [Google Scholar]
- Feng, J.; Han, J.; Chen, W.Q.; Duan, G.S. Effect of CC process parameters on secondary dendrite arm spacing of high carbon steel billets. Iron Steel 2006, 41, 37–43. (In Chinese) [Google Scholar]
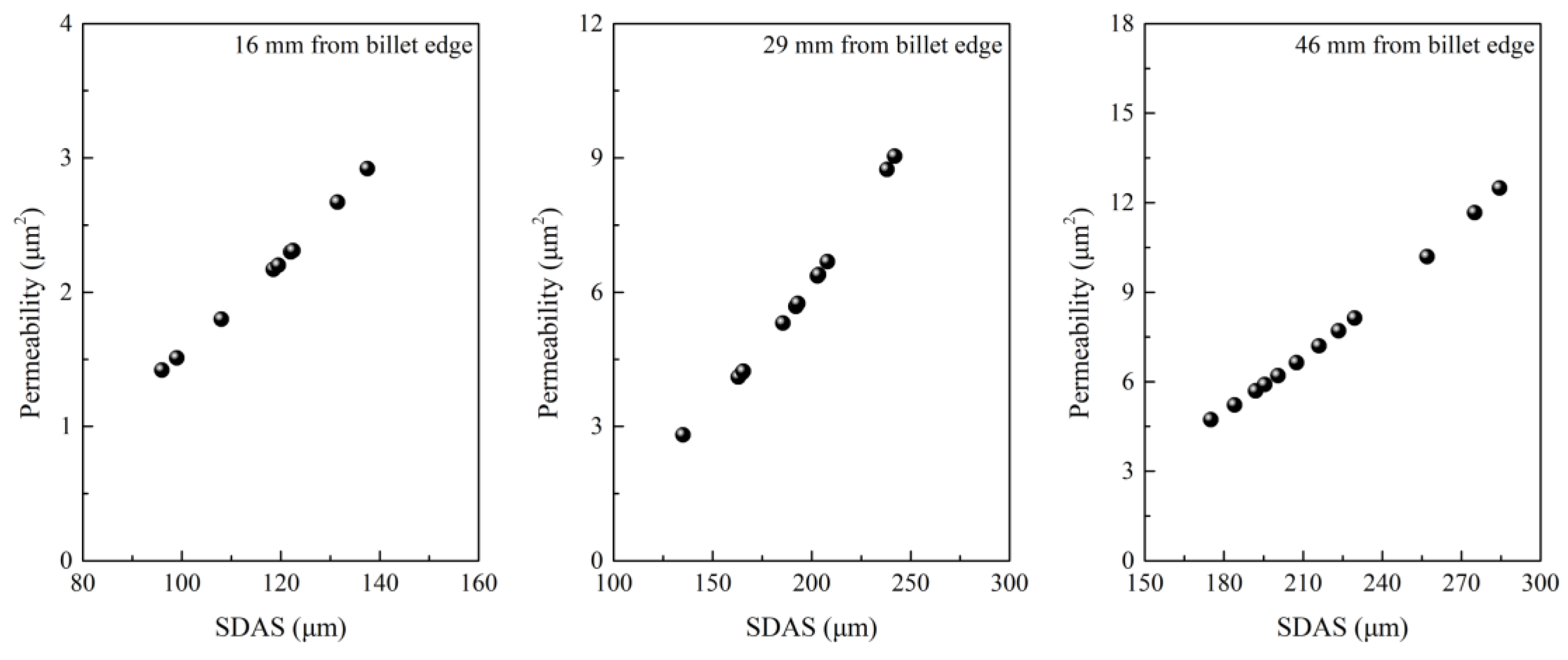
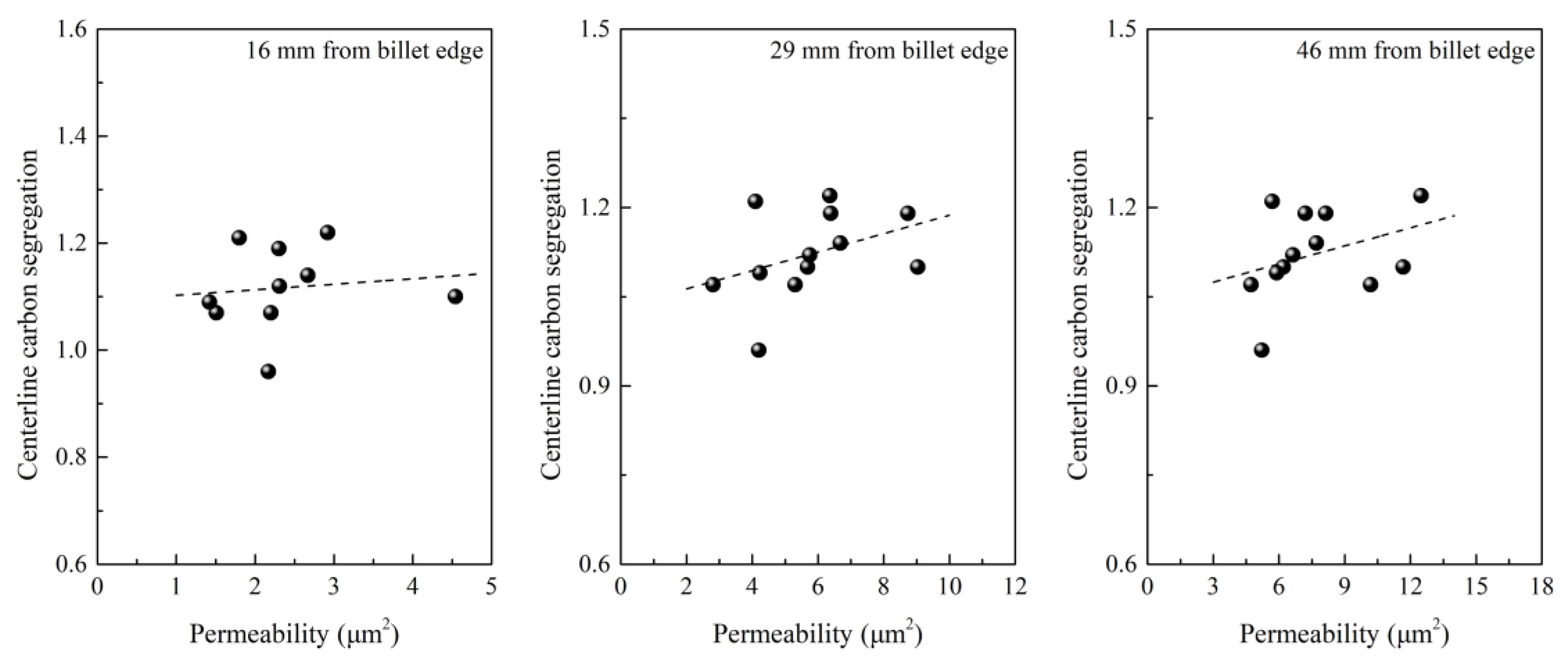
- Lai, C.B.; Xin, B.; Chen, W.Q.; Lv, R.G.; Wu, S.J. Effect of secondary dendrite arm spacing on center carbon segregation of slab. Steelmaking 2009, 25, 42–45. (In Chinese) [Google Scholar]
- Yoo, H.; Viskanta, R. Effect of anisotropic permeability on the transport process during solidification of a binary mixture. Int. J. Heat Mass Trans. 1992, 35, 2335–2346. [Google Scholar] [CrossRef]
- Lin, S.C. Magnetic field numerical simulation of F-EMS in billet continuous casting. J. Iron Steel Res. 2008, 20, 18–21. (In Chinese) [Google Scholar]
- Feng, J.; Chen, W.Q.; Wang, X.F.; Wang, Q.X.; Su, W. Effect of white band on quality of billet cast with F-EMS. Iron Steel 2006, 41, 26–28. (In Chinese) [Google Scholar]
- He, H.Y.; Liu, H.T.; Chen, W.Q.; Wang, G.J.; Fu, J.; Chen, H.J. Optimization of Continuous Casting Parameters for High Carbon Steel Billet and Control of Central Segregation of Billets. Iron Steel Vanadium Titan. 2011, 32, 84–88. (In Chinese) [Google Scholar]
- Wang, T.; Chen, W.Q.; Wang, H.B.; Wang, J.Z.; Zhang, Z.Q. Effect of concasting parameter and FEMS on center carbon segregation of 82B steel billet. Spec. Steel 2013, 34, 49–51. (In Chinese) [Google Scholar]
- Zeng, J.; Chen, W.Q.; Wang, Q.X.; Wang, G.S. Improving inner quality in continuous casting rectangular billets comparison between mechanical soft reduction and final electromagnetic stirring. Trans. Indian Inst. Met. 2016, 69, 1623–1632. [Google Scholar] [CrossRef]
- Wang, T. Study on Center Carbon Segregation of 82B Billet and Wire Rod Quality. Master’s Thesis, University of Science and Technology Beijing, Beijing, China, 10 December 2012. [Google Scholar]
- Bucenieks, I.E. High pressure and high flowrate induction pumps with permanent magnets. Magnetohydrodynamics 2003, 39, 411–418. [Google Scholar]
- Plevachuk, Y.; Sklyarchuk, V.; Eckert, S.; Gerbeth, G.; Novakovic, R. Thermophysical properties of the liquid Ga-In-Sn eutectic alloy. J. Chem. Eng. Data 2014, 59, 757–763. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, W.Q.; Yan, W.; Yang, Y.D.; McLean, A. Effect of permanent magnet stirring on solidification of Sn-Pb alloy. Mater. Des. 2016, 108, 364–373. [Google Scholar] [CrossRef]
- Kobayashi, S. Development of the rotating magnet stirrer: Development of an in-the-mold EMS method I. Tetsu-to-Hagane 1981, 67, S834. [Google Scholar]
- Kawami, A.; Maruta, Y.; Kameko, S. Improvement of surface quality by rotating magnet stirrer in the mold on C.C. bloom: Development of an in-the-mold EMS method II. Tetsu-to-Hagane 1981, 67, S835. [Google Scholar]
- Hagiwara, T.; Taki, M.; Kimura, K. Improvement of internal quality by rotating magnet stirrer in the mold on C.C. bloom: Development of an in-the-mold EMS method III. Tetsu-to-Hagane 1981, 67, S836. [Google Scholar]
- Zeng, J.; Chen, W.Q.; Zhang, S.L.; Li, Y.; Wang, Q.L. Development and application of final permanent magnet stirring during continuous casting of high carbon rectangular billet. ISIJ Int. 2015, 55, 2142–2149. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, W.Q.; Yang, Y.D.; McLean, A. A Review of permanent magnet stirring during metal solidification. Metall. Mater. Trans. B 2017, 48, 3083–3100. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, W.Q.; Zhang, S.L. Experimental study of molten metal flow and numerical simulation of magnetic field during permanent magnet stirring and its application in continuous casting. Metall. Res. Technol. 2016, 113, 609. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, W.Q.; Yang, Y.D.; McLean, A. Effect of permanent magnet stirring on internal quality of steel. Ironmak. Steelmak. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, S.L.; Liu, C.; Chen, W.Q.; Li, B. Final permanent magnet stirring in high carbon rectangular billet continuous casting. Iron Steel 2016, 51, 22–27. (In Chinese) [Google Scholar]
- Zeng, J.; Zhang, S.L.; Cao, C.F.; Wang, G.S.; Chen, W.Q. Comparison between permanent magnet stirring and final electromagnetic stirring during continuous casting bloom. Steelmaking 2016, 32, 36–40. (In Chinese) [Google Scholar]
- Zeng, J. Effect of Mechanical Soft Reduction and Permanent Magnet Stirring on Internal Quality of Continuous Casting Steel. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 21 December 2017. [Google Scholar]
- Zeng, J.; Chen, W.Q.; Wang, G.S.; Cao, C.F.; Gao, Y.B. Development and application of an off-line soft reduction model during continuous casting of high-carbon rectangular billet. Metall. Res. Technol. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, W.Q. Formation mechanism of center negative segregation in continuous casting of high carbon rectangular billet with soft reduction. J. Iron Steel Res. 2016, 28, 51–55. (In Chinese) [Google Scholar]
- Zeng, J.; Chen, W.Q.; Cao, C.F.; Gao, Y.B.; Wang, G.S. Effect of soft reduction on inner quality of 82B steel rectangular bloom. Steelmaking 2015, 31, 63–67. (In Chinese) [Google Scholar]
- Yan, W.; Xu, H.C.; Chen, W.Q. Study on inclusions in wire rod of tire cord steel by means of electrolysis of wire rod. Steel Res. Int. 2014, 85, 53–59. [Google Scholar] [CrossRef]
- Chen, L.J.; Chen, W.Q.; Hu, Y.; Chen, Z.P.; Xu, Y.T.; Yan, W. Effect of Al antioxidant in MgO-C refractory on the formation of Al2O3-rich inclusions in high-carbon steel for saw wire under vacuum conditions. Ironmak. Steelmak. 2018, 45, 272–279. [Google Scholar] [CrossRef]
- Chen, L.J.; Yan, W.; Chen, W.Q.; Chen, Z.P.; Yang, Y.D.; McLean, A. Effect of the interaction between Al-containing MgO-C refractory and steel under vacuum on inclusions in Si-Mn killed steel. In Proceedings of the AISTech, Philadelphia, PA, USA, 7–10 May 2018. [Google Scholar]
- Maeda, S.; Soejima, T.; Saito, T. Shape control of inclusions in wire rods for high tensile tire cord by refining with synthetic slag. In Proceedings of the Steelmaking Conference, Warrendale, PA, USA, 25–28 March 1989. [Google Scholar]
- McPherson, N.A.; McLean, A. Continuous Casting: Non-Metallic Inclusions in Continuously Cast Steel; Iron & Steel Society: Warrendale, PA, USA, 1995. [Google Scholar]
- Faulring, G.M. Inclusion Modification in Semi-Killed Steels. Iron Steelmak. 1998, 26, 29–36. [Google Scholar]
- Zhao, H.Q. Study on the Inclusion Plasticity of Tire Cord Steel. Master’s Thesis, University of Science and Technology Beijing, Beijing, China, 15 December 2011. [Google Scholar]
- Cui, H.Z.; Chen, W.Q. Effect of boron on inclusion in tire cord steel. J. Iron Steel Res. Int. 2012, 19, 22–27. [Google Scholar] [CrossRef]
- Cui, H.Z. Study on the Quality of Wire Rod for Tire Cord Steel. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 15 May 2011. [Google Scholar]
- Chen, L.J.; Chen, W.Q.; Hu, Y.; Chen, Z.P.; Xu, Y.T.; Yan, W. Effects of K2CO3 addition on inclusions in high-carbon steel for saw wire. High Temp. Mater. Process. 2018, 37, 701–709. [Google Scholar] [CrossRef]
- Chen, L.J.; Chen, W.Q.; Hu, Y.; Chen, Z.P.; Xu, Y.T.; Yan, W. Effect of Na2CO3 addition on inclusions in high-carbon steel for saw wire. Trans. Indian Inst. Met. 2018, 71, 383–391. [Google Scholar] [CrossRef]
- Chen, L.J.; Chen, W.Q.; Yan, W.; Peng, H.B.; Yang, Y.D.; McLean, A. Inclusion modification in Al-killed valve spring steel by Na2CO3 addition. Ironmak. Steelmak. 2018, 45, 397–405. [Google Scholar] [CrossRef]
- Taleff, E.M.; Lewandowski, J.J.; Pourladian, B. Microstructure-property relationships in pearlitic eutectoid and hypereutectoid carbon steels. JOM 2002, 54, 25–30. [Google Scholar] [CrossRef]
- Xiong, Y. Study on Quality Control of Tire Cord Steel 72A. Master’s Thesis, University of Science and Technology Beijing, Beijing, China, 5 November 2013. [Google Scholar]
- He, H.Y. Study on Prodution Technology of High Carbon Steel SWRH82B. Master’s Thesis, University of Science and Technology Beijing, Beijing, China, 18 December 2010. [Google Scholar]
- Cui, H.Z.; Chen, W.Q. Segregation behavior of phosphorus in casting billet of high carbon steel 82B. Spec. Steel 2011, 32, 25–27. [Google Scholar]
- Abdellaoui, A.; Montesin, T.; Heizmannj, J.J.; Pelletier, B. Study of the texture of steel cord during the wet drawing process—Influence of the patenting and the friction on the dies. Mater. Sci. Forum 1994, 157–162, 611–616. [Google Scholar]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Chen, W.; Li, J. Quality Control of High Carbon Steel for Steel Wires. Materials 2019, 12, 846. https://doi.org/10.3390/ma12060846
Yan W, Chen W, Li J. Quality Control of High Carbon Steel for Steel Wires. Materials. 2019; 12(6):846. https://doi.org/10.3390/ma12060846
Chicago/Turabian StyleYan, Wei, Weiqing Chen, and Jing Li. 2019. "Quality Control of High Carbon Steel for Steel Wires" Materials 12, no. 6: 846. https://doi.org/10.3390/ma12060846
APA StyleYan, W., Chen, W., & Li, J. (2019). Quality Control of High Carbon Steel for Steel Wires. Materials, 12(6), 846. https://doi.org/10.3390/ma12060846