Sum-Frequency Generation Spectroscopy of Plasmonic Nanomaterials: A Review
Abstract
:1. Introduction
2. Sum-Frequency Generation Spectroscopy of Metallic Nanoparticles
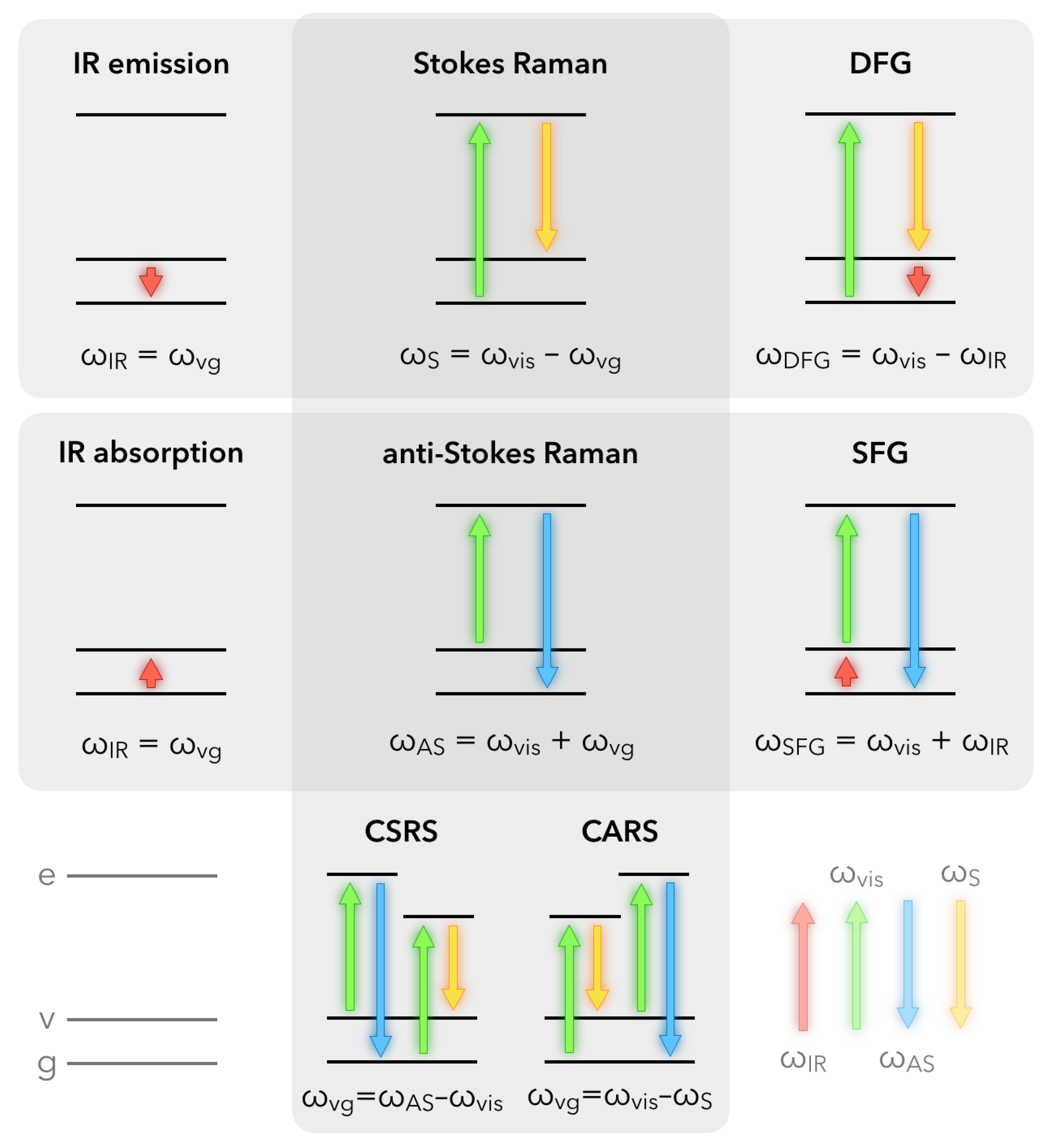
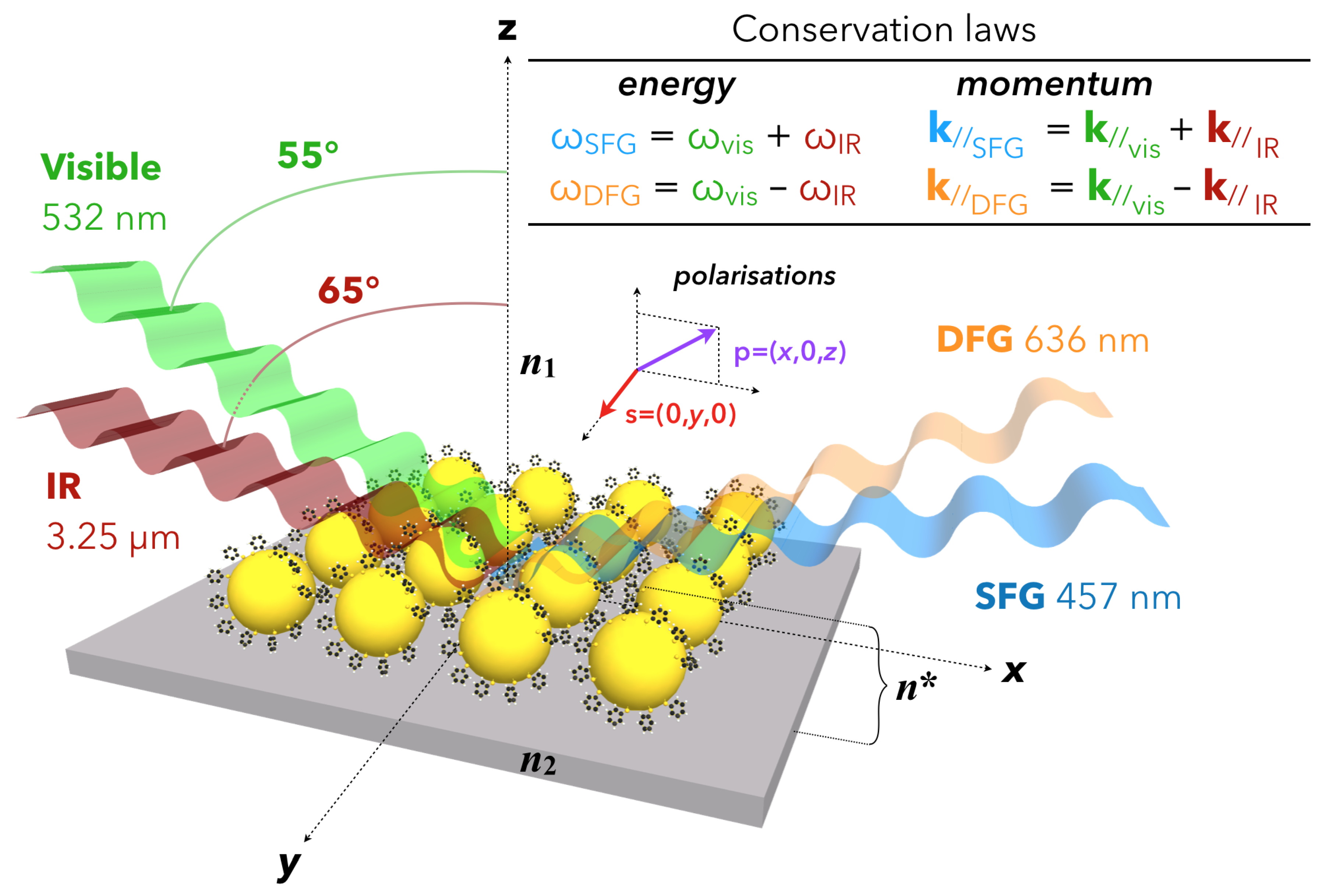
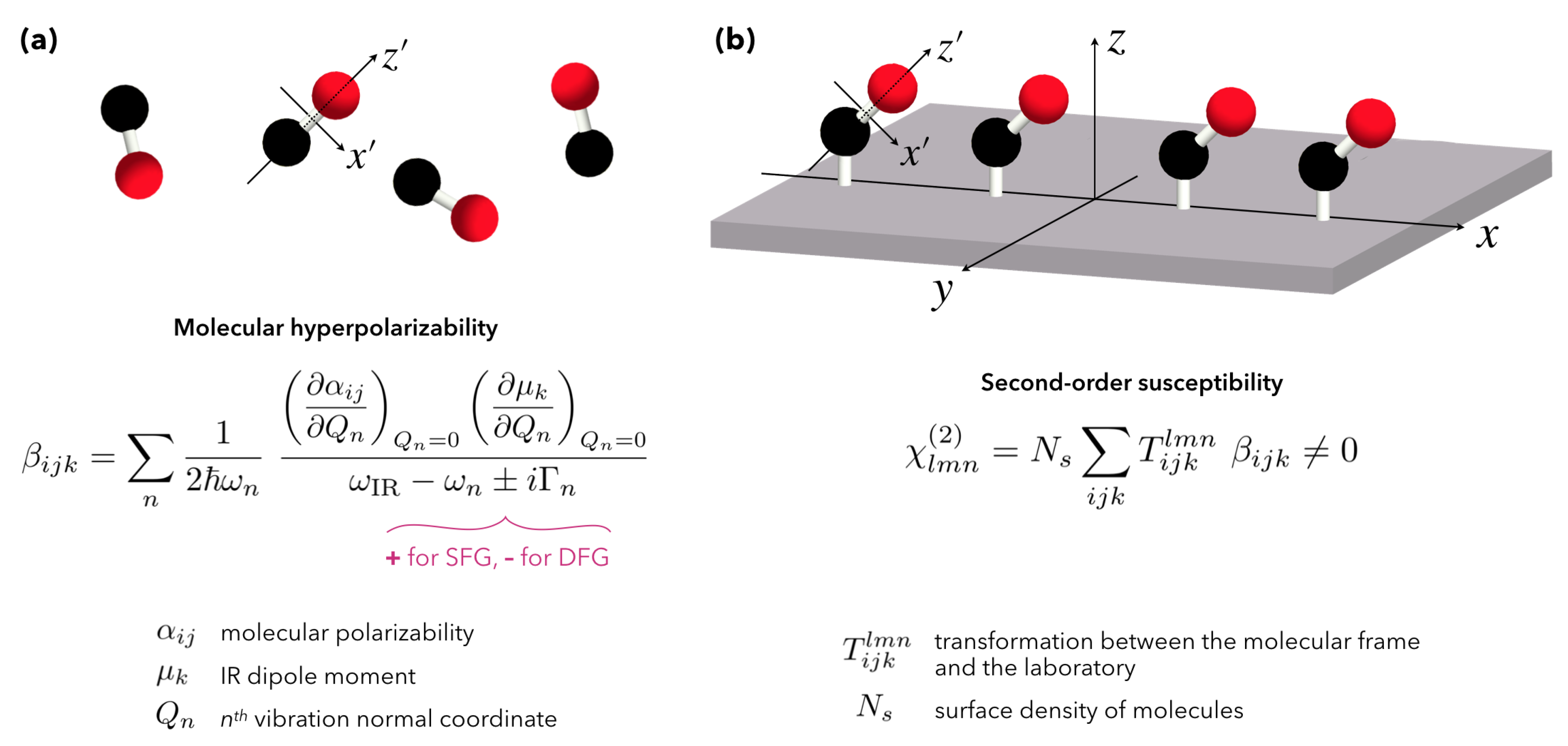
2.1. Fundamentals of Sum-Frequency Generation Spectroscopy
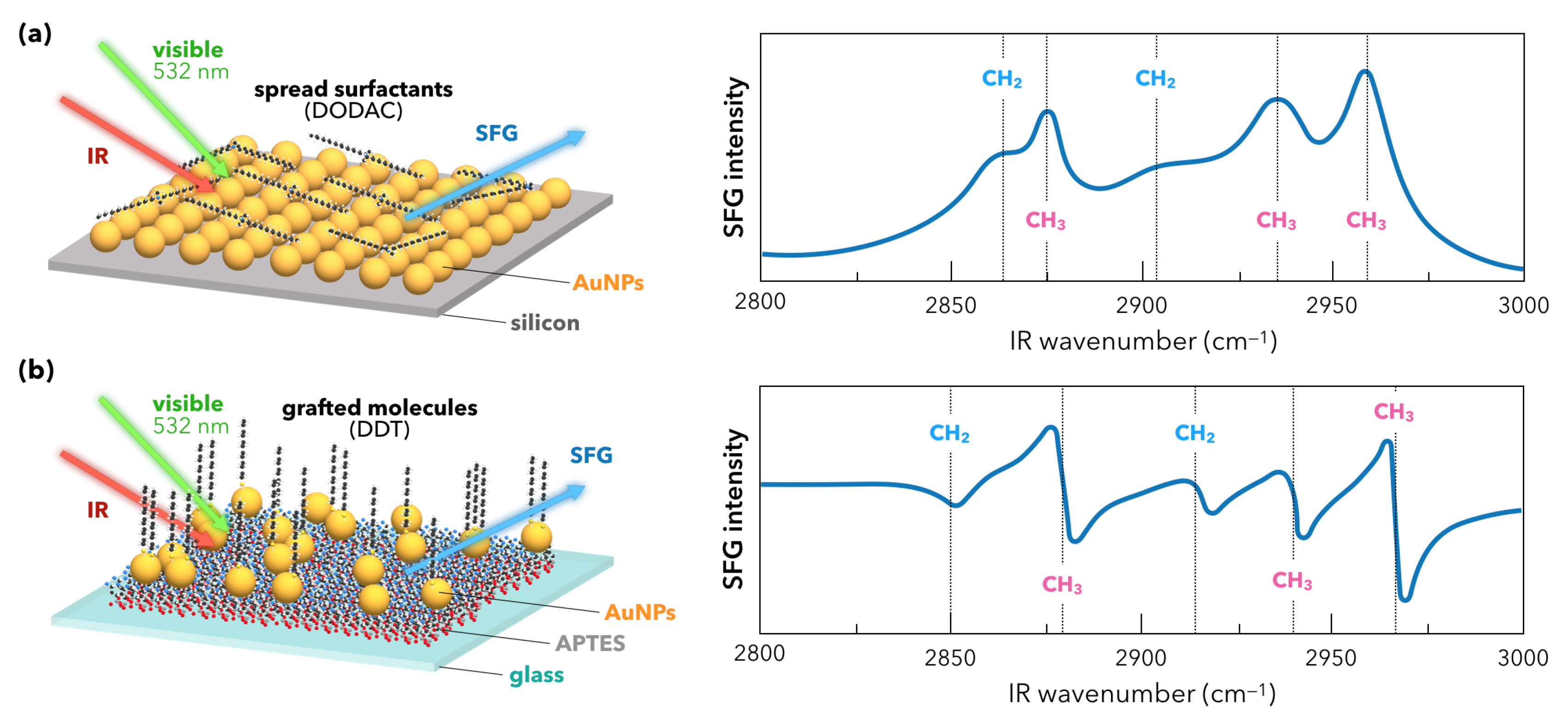
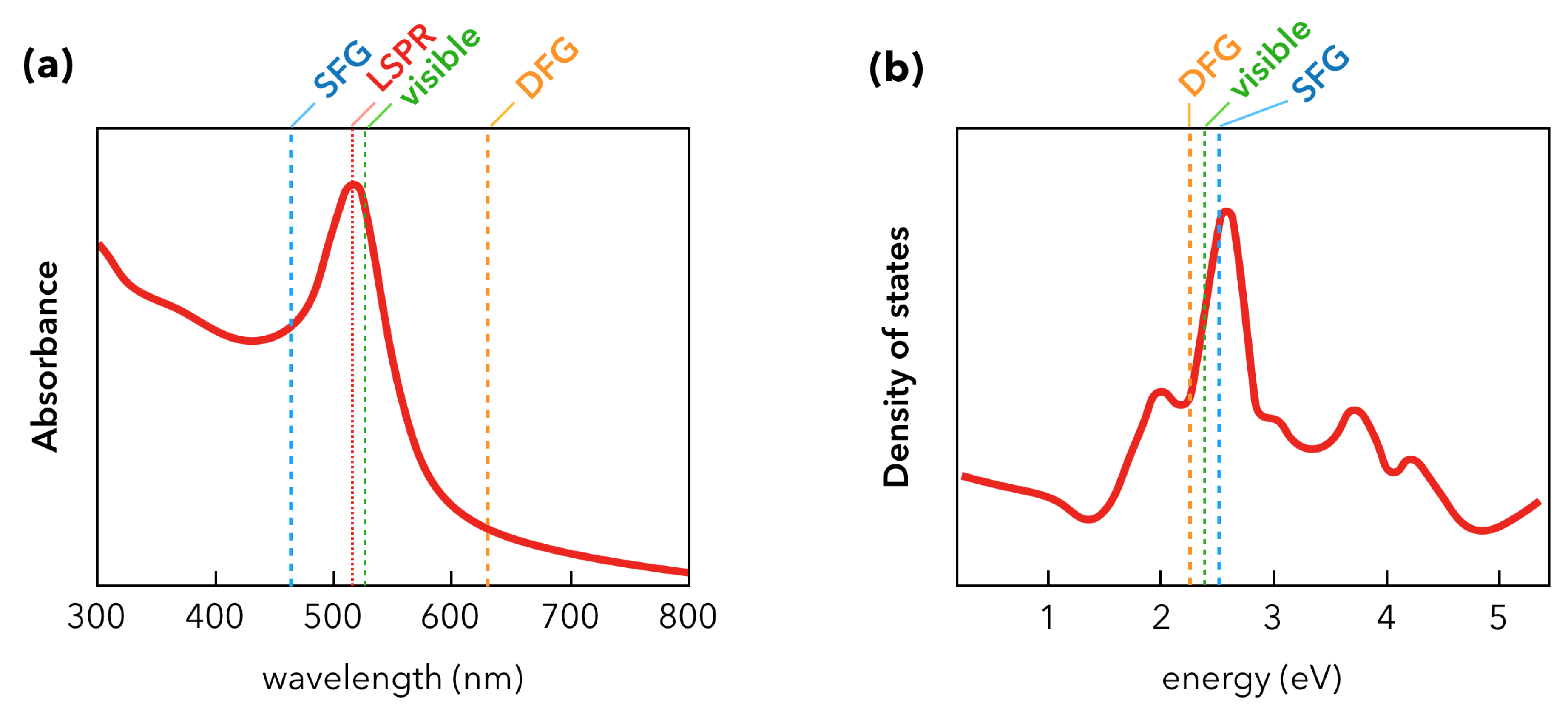
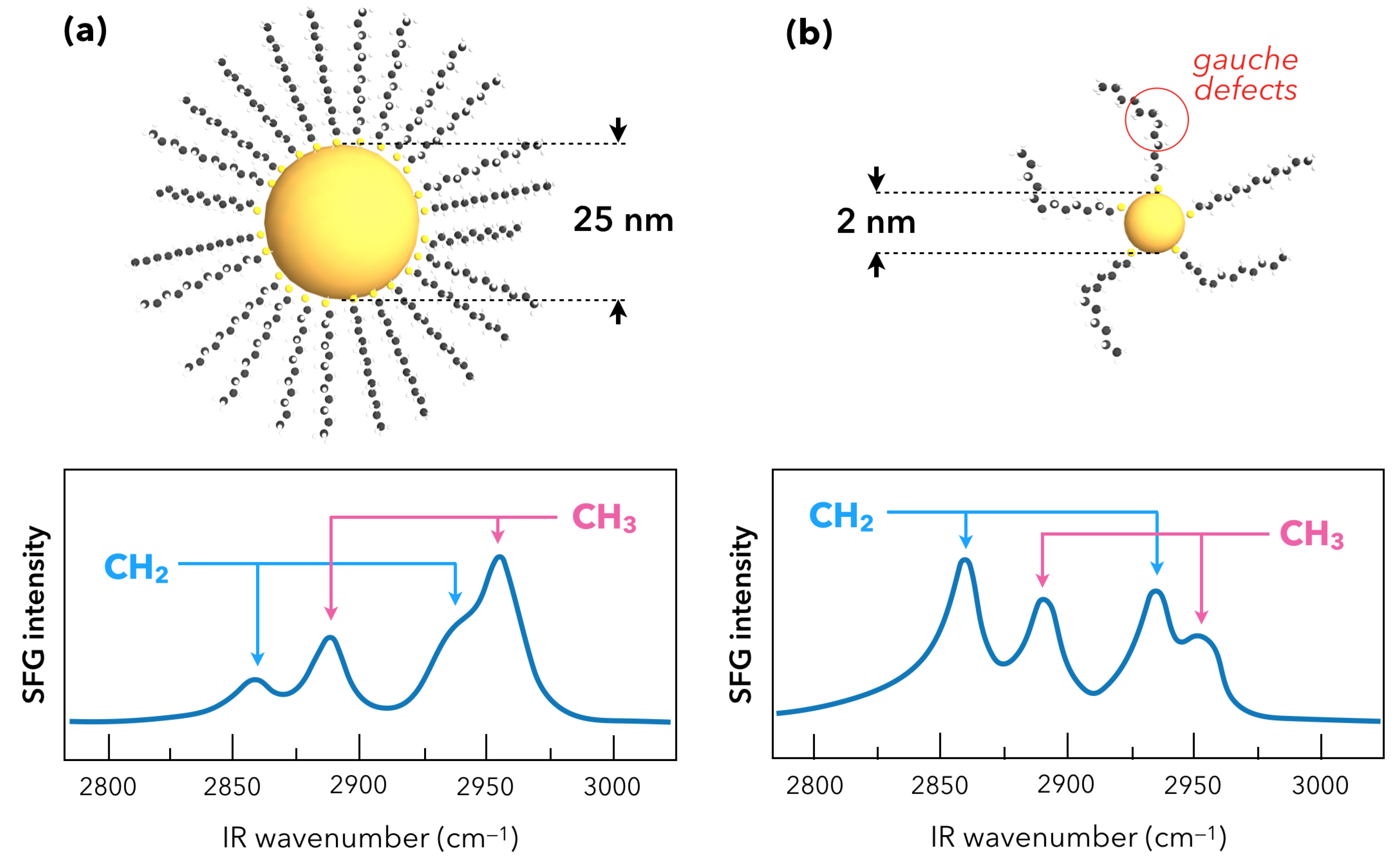
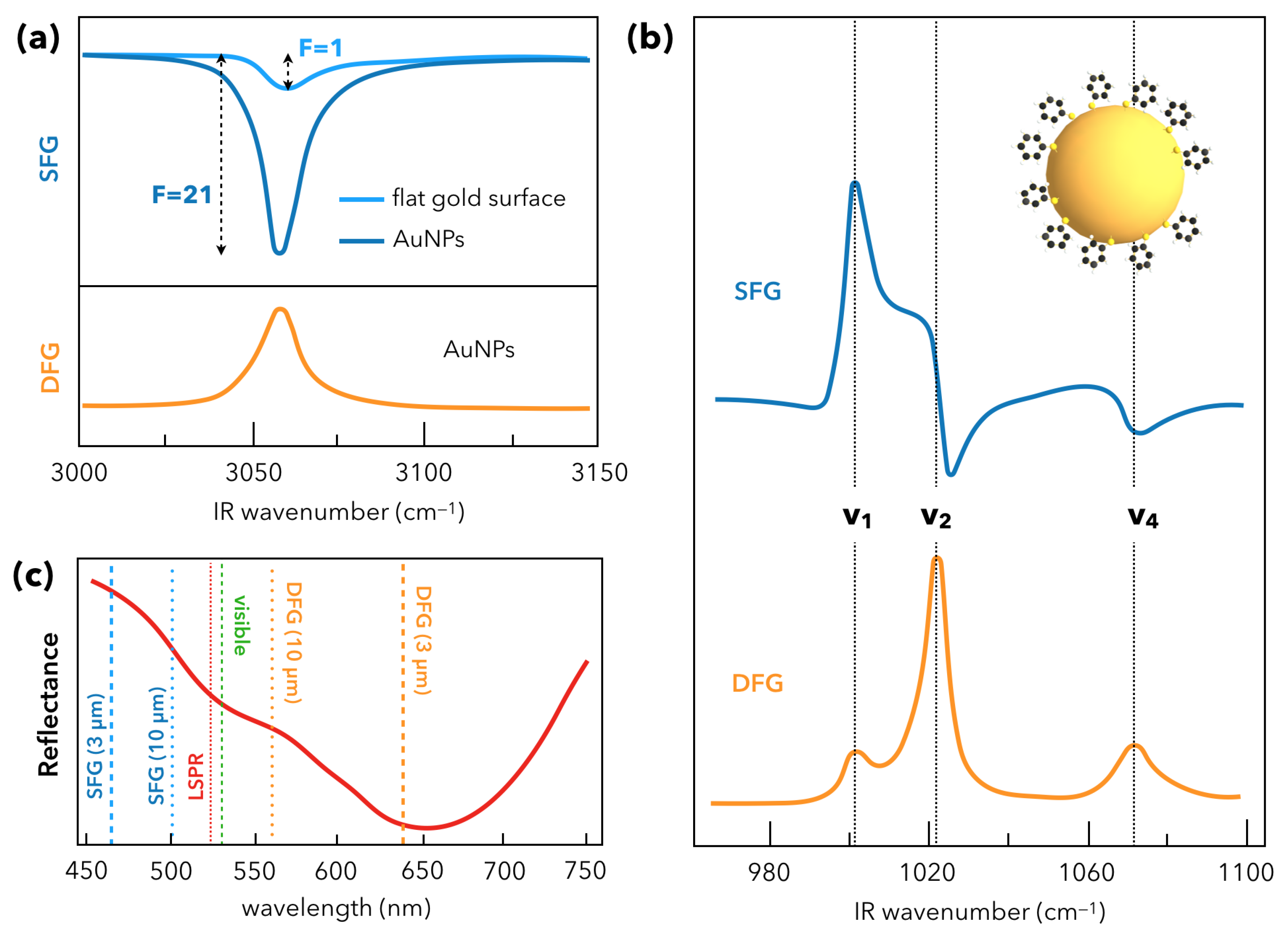
2.2. Nanoparticles and Sum-Frequency Generation: The Gold Case
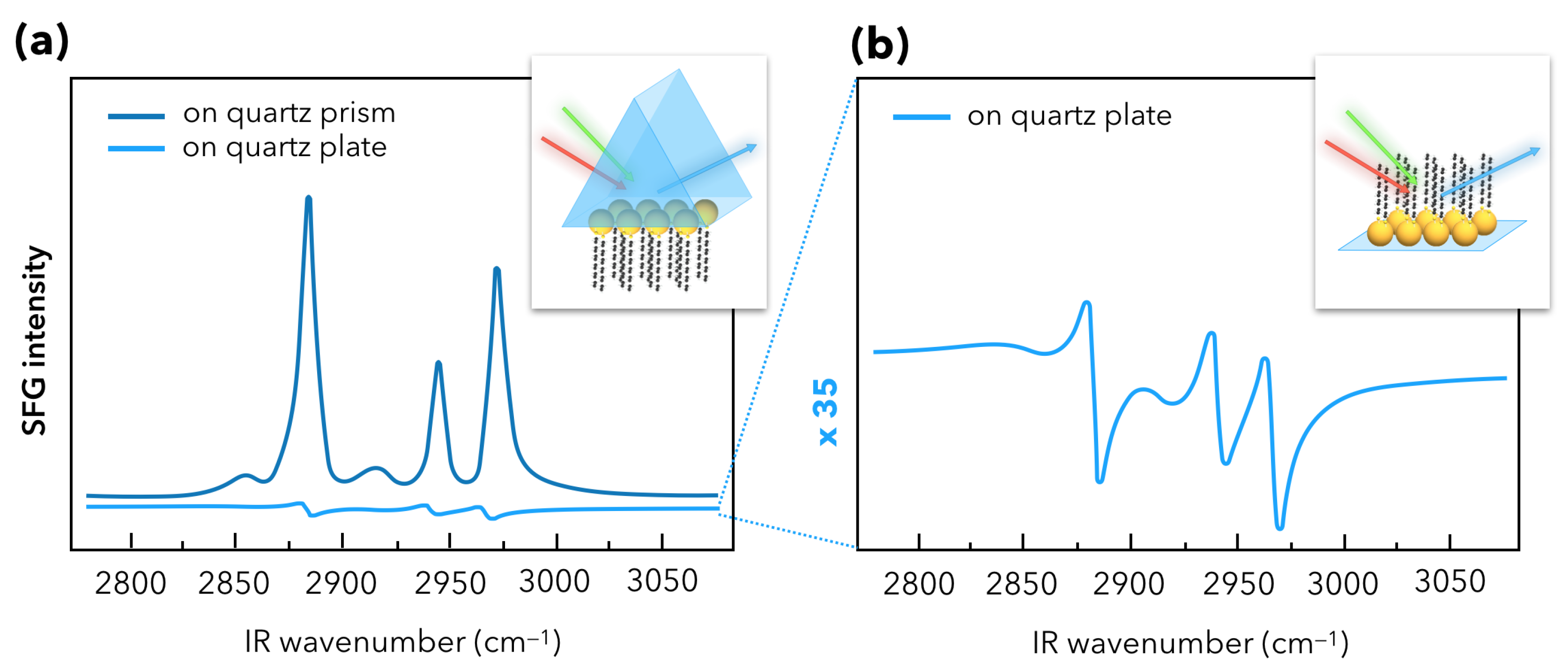
3. Recent Developments in SFG Spectroscopy of Plasmonic Materials
3.1. Towards Applications of Gold Nanoparticles
3.2. Complex Plasmonic Nanostructures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Miller, J.T.; Kropf, A.J.; Zha, Y.; Regalbuto, J.R.; Delannoy, L.; Louis, C.; Bus, E.; van Bokhoven, J.A. The effect of gold particle size on Au-Au bond length and reactivity toward oxygen in supported catalysts. J. Catal. 2006, 240, 222–234. [Google Scholar] [CrossRef]
- Morel, A.-L.; Boujday, S.; Méthivier, C.; Kraff, J.-M.; Pradier, C.-M. Biosensors elaborated on gold nanoparticles, a PM-IRRAS characterisation of the IgG binding efficiency. Talanta 2011, 85, 35–42. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Thompson, D. Michael Faraday’s recognition of ruby gold: The birth of modern nanotechnology. Gold Bull. 2007, 40, 267–269. [Google Scholar] [CrossRef]
- Barbillon, G.; Faure, A.-C.; El Kork, N.; Moretti, P.; Roux, S.; Tillement, O.; Ou, M.G.; Descamps, A.; Perriat, P.; Vial, A.; et al. How nanoparticles encapsulating fluorophores allow a double detection of biomolecules by localized surface plasmon resonance and luminescence. Nanotechnology 2008, 19, 035705. [Google Scholar] [CrossRef]
- Barbillon, G.; Bijeon, J.-L.; Lérondel, G.; Plain, J.; Royer, P. Detection of chemical molecules with integrated plasmonic glass nanotips. Surf. Sci. 2008, 602, L119–L122. [Google Scholar] [CrossRef]
- Faure, A.-C.; Barbillon, G.; Ou, M.; Ledoux, G.; Tillement, O.; Roux, S.; Fabregue, D.; Descamps, A.; Bijeon, J.-L.; Marquette, C.A.; et al. Core/shell nanoparticles for multiple biological detection with enhanced sensitivity and kinetics. Nanotechnology 2008, 19, 485103. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: the role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Ong, Q.K.; Zhao, S.; Reguera, J.; Biscarini, F.; Stellacci, F. Comparative STM studies of mixed ligand monolayers on gold nanoparticles in air and in 1-phenyloctane. Chem. Commun. 2014, 50, 10456–10459. [Google Scholar] [CrossRef]
- Tian, C.S.; Shen, Y.R. Recent progress on sum-frequency spectroscopy. Surf. Sci. Rep. 2014, 69, 105–131. [Google Scholar] [CrossRef]
- Blaudez, D.; Castano, S.; Desbat, B. PM-IRRAS at liquid interfaces. In Biointerface Characterization by Advanced IR Spectroscopy, 1st ed.; Pradier, C.M., Chabal, Y.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 27–55. [Google Scholar]
- Stiles, P.L.; Dieringer, J.A.; Shahand, N.C.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Bryche, J.-F.; Gillibert, R.; Barbillon, G.; Sarkar, M.; Coutrot, A.-L.; Hamouda, F.; Aassime, A.; Moreau, J.; Lamy de la Chapelle, M.; Bartenlian, B.; et al. Density effect of gold nanodisks on the SERS intensity for a highly sensitive detection of chemical molecules. J. Mater. Sci. 2015, 50, 6601–6607. [Google Scholar] [CrossRef]
- Bryche, J.-F.; Gillibert, R.; Barbillon, G.; Gogol, P.; Moreau, J.; Lamy de la Chapelle, M.; Bartenlian, B.; Canva, M. Plasmonic enhancement by a continuous gold underlayer: Application to SERS sensing. Plasmonics 2016, 11, 601–608. [Google Scholar] [CrossRef]
- Bryche, J.-F.; Tsigara, A.; Bélier, B.; Lamy de la Chapelle, M.; Canva, M.; Bartenlian, B.; Barbillon, G. Surface enhanced Raman scattering improvement of gold triangular nanoprisms by a gold reflective underlayer for chemical sensing. Sens. Actuators B 2016, 228, 31–35. [Google Scholar] [CrossRef]
- Arnolds, H.; Bonn, M. Ultrafast surface vibrational dynamics. Surf. Sci. Rep. 2010, 65, 45–66. [Google Scholar] [CrossRef]
- Walter, S.R.; Geiger, F.M. DNA on Stage: Showcasing Oligonucleotides at Surfaces and Interfaces with Second Harmonic and Vibrational Sum Frequency Generation. J. Phys. Chem. Lett. 2010, 1, 9–15. [Google Scholar] [CrossRef]
- Jena, K.C.; Covert, P.A.; Hore, D.K. The Effect of Salt on the Water Structure at a Charged Solid Surface: Differentiating Second- and Third-order Nonlinear Contributions. J. Phys. Chem. Lett. 2011, 2, 1056–1061. [Google Scholar] [CrossRef]
- Kutz, R.B.; Braunschweig, B.; Mukherjee, P.; Dlott, D.D.; Wieckowski, A. Study of Ethanol Electrooxidation in Alkaline Electrolytes with Isotope Labels and Sum-Frequency Generation. J. Phys. Chem. Lett. 2011, 2, 2236–2240. [Google Scholar] [CrossRef]
- Penalber, C.Y.; Baldelli, S. Observation of Charge Inversion of an Ionic Liquid at the Solid Salt–Liquid Interface by Sum Frequency Generation Spectroscopy. J. Phys. Chem. Lett. 2012, 3, 844–847. [Google Scholar] [CrossRef]
- Dalstein, L.; Potapova, E.; Tyrode, E. The elusive silica/water interface: Isolated silanols under water as revealed by vibrational sum frequency spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 10343–10349. [Google Scholar] [CrossRef]
- Dellwig, T.; Rupprechter, G.; Unterhalt, H.; Freund, H.-J. Bridging the pressure and materials gaps: High pressure sum frequency generation study on supported Pd nanoparticles. Phys. Rev. Lett. 2000, 85, 776–779. [Google Scholar] [CrossRef]
- Rupprechter, G.; Freund, H.-J. Adsorbate-induced restructuring and pressure-dependent adsorption on metal nanoparticles studied by electron microscopy and sum frequency generation spectroscopy. Top. Catal. 2000, 14, 3–14. [Google Scholar] [CrossRef]
- Olson, J.Z.; Johansson, P.K.; Castner, D.G.; Schlenker, C.W. Operando Sum-Frequency Generation Detection of Electrolyte Redox Products at Active Si Nanoparticle Li-Ion Battery Interfaces. Chem. Mater. 2018, 30, 1239–1248. [Google Scholar] [CrossRef]
- Tatsumi, H.; Liu, F.; Han, H.-L.; Carl, L.M.; Sapi, A.; Somorjai, G.A. Alcohol Oxidation at Platinum-Gas and Platinum-Liquid Interfaces: The Effect of Platinum Nanoparticle Size, Water Coadsorption, and Alcohol Concentration. J. Phys. Chem. C 2017, 121, 7365–7371. [Google Scholar] [CrossRef]
- Ouvrard, A.; Ghalgaoui, A.; Michel, C.; Barth, C.; Wang, J.; Carrez, S.; Zheng, W.; Henry, C.R.; Bourguignon, B. CO Chemisorption on Ultrathin MgO-Supported Palladium Nanoparticles. J. Phys. Chem. C 2017, 121, 5551–5564. [Google Scholar] [CrossRef]
- Kawai, T.; Neivandt, D.J.; Davies, P.B. Sum frequency generation on surfactant-coated gold nanoparticles. J. Am. Chem. Soc. 2000, 122, 12031–12032. [Google Scholar] [CrossRef]
- Lis, D.; Cecchet, F. Localized surface plasmon resonances in nanostructures to enhance nonlinear vibrational spectroscopies: Towards an astonishing molecular sensitivity. Beilstein J. Nanotechnol. 2014, 5, 2275–2292. [Google Scholar] [CrossRef]
- Hayashi, M.; Lin, S.H.; Raschke, M.B.; Shen, Y.R. A molecular theory for doubly resonant IR-UV-Vis sum-frequency generation. J. Phys. Chem. A 2002, 106, 2271–2282. [Google Scholar] [CrossRef]
- Dalstein, L.; Ben Haddada, M.; Barbillon, G.; Humbert, C.; Tadjeddine, A.; Boujday, S.; Busson, B. Revealing the interplay between adsorbed molecular layers and gold nanoparticles by linear and nonlinear optical properties. J. Phys. Chem. C 2015, 119, 17146–17155. [Google Scholar] [CrossRef]
- Noblet, T.; Dreesen, L.; Boujday, S.; Méthivier, C.; Busson, B.; Tadjeddine, A.; Humbert, C. Semiconductor quantum dots reveal dipolar coupling from exciton to ligand vibration. Commun. Chem. 2018, 1, 76. [Google Scholar] [CrossRef]
- Frederick, M.T.; Achtyl, J.L.; Knowles, K.E.; Weiss, E.A.; Geiger, F.M. Surface-amplified ligand disorder in CdSe quantum dots determined by electron and coherent vibrational spectroscopies. J. Am. Chem. Soc. 2011, 133, 7476–7481. [Google Scholar] [CrossRef]
- Humbert, C.; Dahi, A.; Dalstein, L.; Busson, B.; Lismont, M.; Colson, P.; Dreesen, L. Linear and nonlinear optical properties of functionalized CdSe quantum dots prepared by plasma sputtering and wet chemistry. J. Colloid Interface Sci. 2015, 445, 69–75. [Google Scholar] [CrossRef]
- Sengupta, S.; Bromley, L.; Velarde, L. Aggregated states of chalcogenorhodamine dyes on nanocrystalline titania revealed by doubly resonant sum frequency spectroscopy. J. Phys. Chem. C 2017, 121, 3424–3436. [Google Scholar] [CrossRef]
- Franken, P.A.; Ward, J.F. Optical harmonics and nonlinear phenomena. Rev. Mod. Phys. 1963, 35, 23. [Google Scholar] [CrossRef]
- Ward, J.F. Calculation of nonlinear optical susceptibilities using diagrammatic perturbation theory. Rev. Mod. Phys. 1965, 37, 1. [Google Scholar] [CrossRef]
- Hirose, C.; Akamatsu, N.; Domen, K. Formulas for the analysis of the surface SFG spectrum and transformation coefficients of cartesian SFG tensor components. Appl. Spectrosc. 1992, 46, 1051–1072. [Google Scholar] [CrossRef]
- Li, X.; Roiaz, M.; Pramhaas, V.; Rameshan, C.; Rupprechter, G. Polarization-Dependent SFG Spectroscopy of Near Ambient Pressure CO Adsorption on Pt(111) and Pd(111) Revisited. Top. Catal. 2018, 61, 751–762. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Boughton, A.P.; Kristalyn, C.B.; Chen, Z. Multiple Orientation of Melittin inside a Single Lipid Bilayer Determined by Combined Vibrational Spectroscopic Studies. J. Am. Chem. Soc. 2007, 129, 1420–1427. [Google Scholar] [CrossRef]
- Lambert, A.G.; Davies, P.B.; Neivandt, D.J. Implementing the Theory of Sum Frequency Generation Vibrational Spectroscopy: A Tutorial Review. Appl. Spec. Rev. 2005, 40, 103–145. [Google Scholar] [CrossRef]
- Caudano, Y.; Silien, C.; Humbert, C.; Dreesen, L.; Mani, A.A.; Peremans, A.; Thiry, P.A. Electron-phonon couplings at C60 interfaces: A case study by two-color, infrared-visible sum-frequency generation spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 2003, 129, 139–147. [Google Scholar] [CrossRef]
- Kakudji, E.; Silien, C.; Lis, D.; Cecchet, F.; Nouri, A.; Thiry, P.A.; Peremans, A.; Caudano, Y. In situ nonlinear optical spectroscopy of electron–phonon couplings at alkali-doped C60/Ag(111) interfaces. Phys. Status Solidi B 2010, 8, 1992–1996. [Google Scholar] [CrossRef]
- Elsenbeck, D.; Dasa, S.K.; Velarde, L. Substrate influence on the interlayer electron-phonon couplings in fullerene films probed with doubly-resonant SFG spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 18519–18528. [Google Scholar] [CrossRef]
- Dreesen, L.; Humbert, C.; Sartenaer, Y.; Caudano, Y.; Volcke, C.; Mani, A.A.; Peremans, A.; Thiry, P.A.; Hanique, S.; Frere, J.-M. Electronic and molecular properties of an adsorbed protein monolayer probed by two-colour sum-frequency generation spectroscopy. Langmuir 2004, 20, 7201–7207. [Google Scholar] [CrossRef]
- Covert, P.A.; Hore, D.K. Assessing the Gold Standard: The Complex Vibrational Nonlinear Susceptibility of Metals. J. Phys. Chem. C 2015, 119, 271–276. [Google Scholar] [CrossRef]
- Dalstein, L.; Revel, A.; Humbert, C.; Busson, B. Nonlinear optical response of a gold surface in the visible range: A study by Two-Color Sum-Frequency Generation spectroscopy. I. Experimental determination. J. Chem. Phys. 2018, 148, 134701. [Google Scholar] [CrossRef]
- Mendoza, B.S.; Mochan, W.L.; Maytorena, J.A. Visible-infrared sum and difference frequency generation at adsorbed-covered Au. Phys. Rev. B 1999, 60, 14334–14340. [Google Scholar] [CrossRef]
- Busson, B.; Dalstein, L. Nonlinear optical response of a gold surface in the visible range: A study by two-color sum-frequency generation spectroscopy. III. Simulations of the experimental SFG intensities. J. Chem. Phys. 2018, 149, 154701. [Google Scholar] [CrossRef]
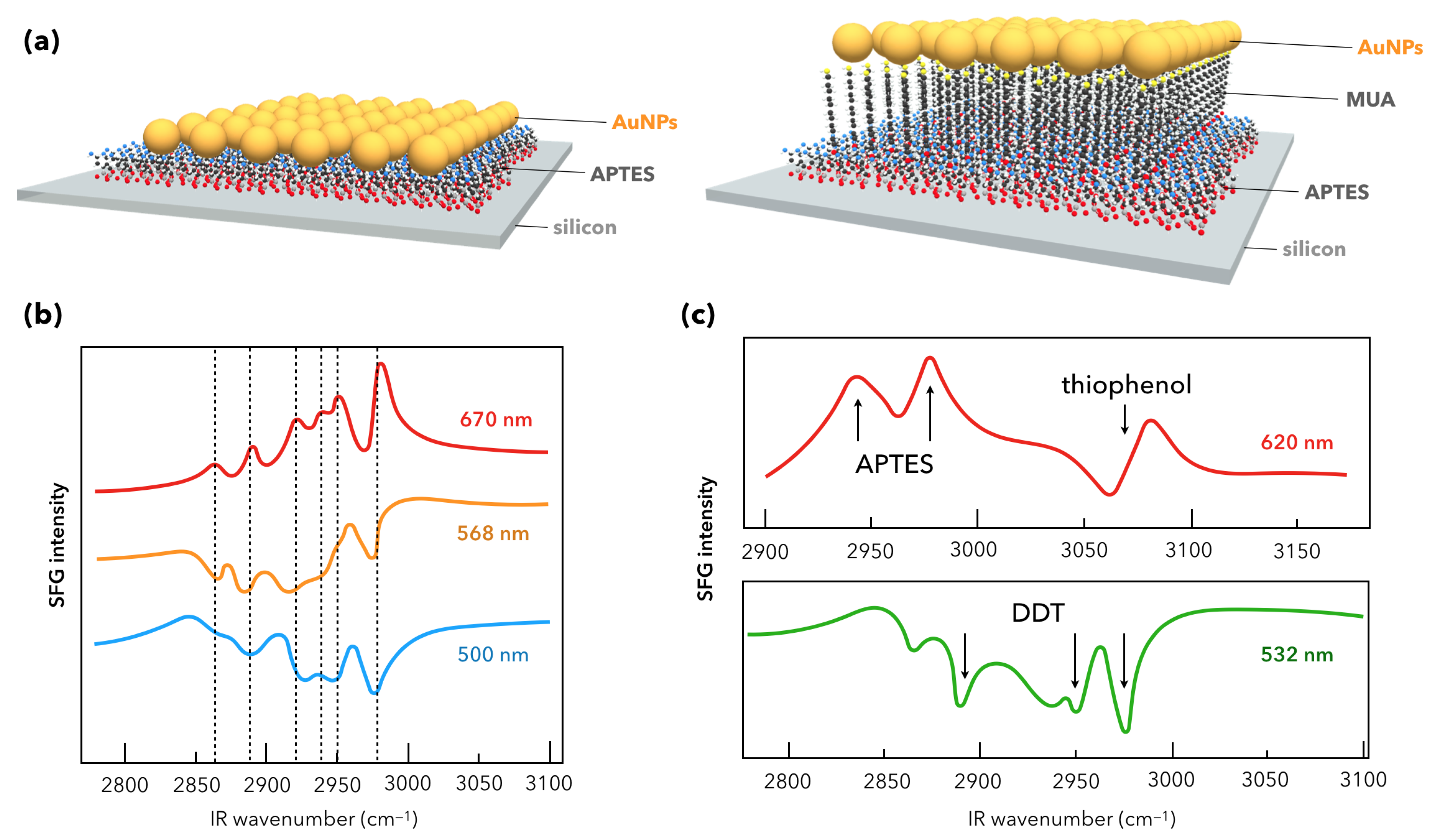
- Humbert, C.; Busson, B.; Abid, J.-P.; Six, C.; Girault, H.H.; Tadjeddine, A. Self-assembled organic monolayers on gold nanoparticles: A study by sum-frequency generation combined with UV-Vis spectroscopy. Electrochim. Acta 2005, 50, 3101–3110. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Boyd, R.W. Nonlinear Optics, 2nd ed.; Academic Press–Elsevier: San Diego, CA, USA, 2003; pp. 1–578. [Google Scholar]
- Weeraman, C.; Yatawara, A.K.; Bordenyuk, A.N.; Benderskii, A.V. Effect of Nanoscale Geometry on Molecular Conformation: Vibrational Sum-Frequency Generation of Alkanethiols on Gold Nanoparticles. J. Am. Chem. Soc. 2006, 128, 14244–14245. [Google Scholar] [CrossRef]
- Bordenyuk, A.N.; Weeraman, C.; Yatawara, A.K.; Jayathilake, H.D.; Stiopkin, Y.; Liu, Y.; Benderskii, A.V. Vibrational Sum Frequency Generation Spectroscopy of Dodecanethiol on Metal Nanoparticles. J. Phys. Chem. C 2006, 111, 8925–8933. [Google Scholar] [CrossRef]
- Traverse, A.; Humbert, C.; Six, C.; Gayral, A.; Busson, B. Nonlinear optical properties of Ag nanoparticles embedded in Si3N4. EPL 2008, 83, 64004. [Google Scholar] [CrossRef]
- Tourillon, G.; Dreesen, L.; Volcke, C.; Sartenaer, Y.; Thiry, P.A.; Peremans, A. Total internal reflection sum-frequency generation spectroscopy and dense gold nanoparticles monolayer: A route for probing adsorbed molecules. Nanotechnology 2007, 18, 415301–415308. [Google Scholar] [CrossRef]
- Tourillon, G.; Dreesen, L.; Volcke, C.; Sartenaer, Y.; Thiry, P.A.; Peremans, A. Close-packed array of gold nanoparticles and Sum Frequency Generation spectroscopy in total internal reflection: A platform for studying biomolecules and biosensors. J. Mater. Sci. 2009, 44, 6805–6810. [Google Scholar] [CrossRef]
- Eliel, E.R.; van der Ham, E.W.M.; Vrehen, Q.H.F. Enhancing the yield in surface sum-frequency generation by the use of surface polaritons. Appl. Phys. B 1999, 68, 349–353. [Google Scholar] [CrossRef]
- Williams, C.T.; Yan, Y.; Bain, C.D. Total internal reflection sum-frequency spectroscopy: A strategy for studying molecular adsorption on metal surfaces. Langmuir 2000, 16, 2343–2350. [Google Scholar] [CrossRef]
- Dreesen, L.; Sartenaer, Y.; Humbert, C.; Mani, A.A.; Méthivier, C.; Pradier, C.-M.; Thiry, P.A.; Peremans, A. Probing Ligand-Protein Recognition with Sum-Frequency Generation Spectroscopy: The Avidin-Biocytin Case. ChemPhysChem 2004, 5, 1719–1725. [Google Scholar] [CrossRef]
- Pluchery, O.; Humbert, C.; Valamanesh, M.; Lacaze, E.; Busson, B. Enhanced detection of thiophenol adsorbed on Gold Nanoparticles by SFG and DFG nonlinear optical spectroscopy. Phys. Chem. Chem. Phys. 2009, 11, 7729–7737. [Google Scholar] [CrossRef]
- Humbert, C.; Pluchery, O.; Lacaze, E.; Tadjeddine, A.; Busson, B. A Multiscale description of molecular adsorption on gold nanoparticles by nonlinear optical spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 280–289. [Google Scholar] [CrossRef]
- Humbert, C.; Pluchery, O.; Lacaze, E.; Tadjeddine, A.; Busson, B. Optical spectroscopy of functionalized gold nanoparticles assemblies as a function of the surface coverage. Gold Bull. 2013, 46, 299–309. [Google Scholar] [CrossRef]
- Feugmo, C.G.T.; Liegeois, V. Analyzing the vibrational signatures of thiophenol adsorbed on small gold clusters by DFT calculations. ChemPhysChem 2013, 14, 1633–1645. [Google Scholar] [CrossRef]
- De Aguiar, H.B.; Scheu, R.; Jena, K.C.; de Beer, A.G.F.; Roke, S. Comparison of scattering and reflection SFG: A question of phase matching. Phys. Chem. Chem. Phys. 2012, 14, 6826–6832. [Google Scholar] [CrossRef]
- Roke, S.; Gonella, G. Nonlinear light scattering and spectroscopy of particles and droplets in liquids. Annu. Rev. Phys. Chem. 2012, 63, 353–378. [Google Scholar] [CrossRef]
- Humbert, C.; Busson, B. Sum frequency generation spectroscopy of biointerfaces. In Biointerface Characterization by Advanced IR Spectroscopy, 1st ed.; Pradier, C.M., Chabal, Y.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 279–321. [Google Scholar]
- Chen, Z. Sum frequency generation vibrational spectroscopy studies on molecular conformation and orientation of biological molecules at interfaces. Int. J. Mod. Phys. B 2005, 19, 691–713. [Google Scholar] [CrossRef]
- Uehara, T.M.; Spolon Marongoni, V.; Pasquale, N.; Miranda, P.B.; Lee, K.-B.; Zucolotto, V.A. Detailed investigation on the interactions between magnetic nanoparticles and cell membrane models. ACS Appl. Mater. Interfaces 2013, 5, 13063–13068. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, X.; Zhang, C.; Chen, Z. Molecular interactions between gold nanoparticles and model cell membranes. Phys. Chem. Chem. Phys. 2015, 17, 9873–9884. [Google Scholar] [CrossRef]
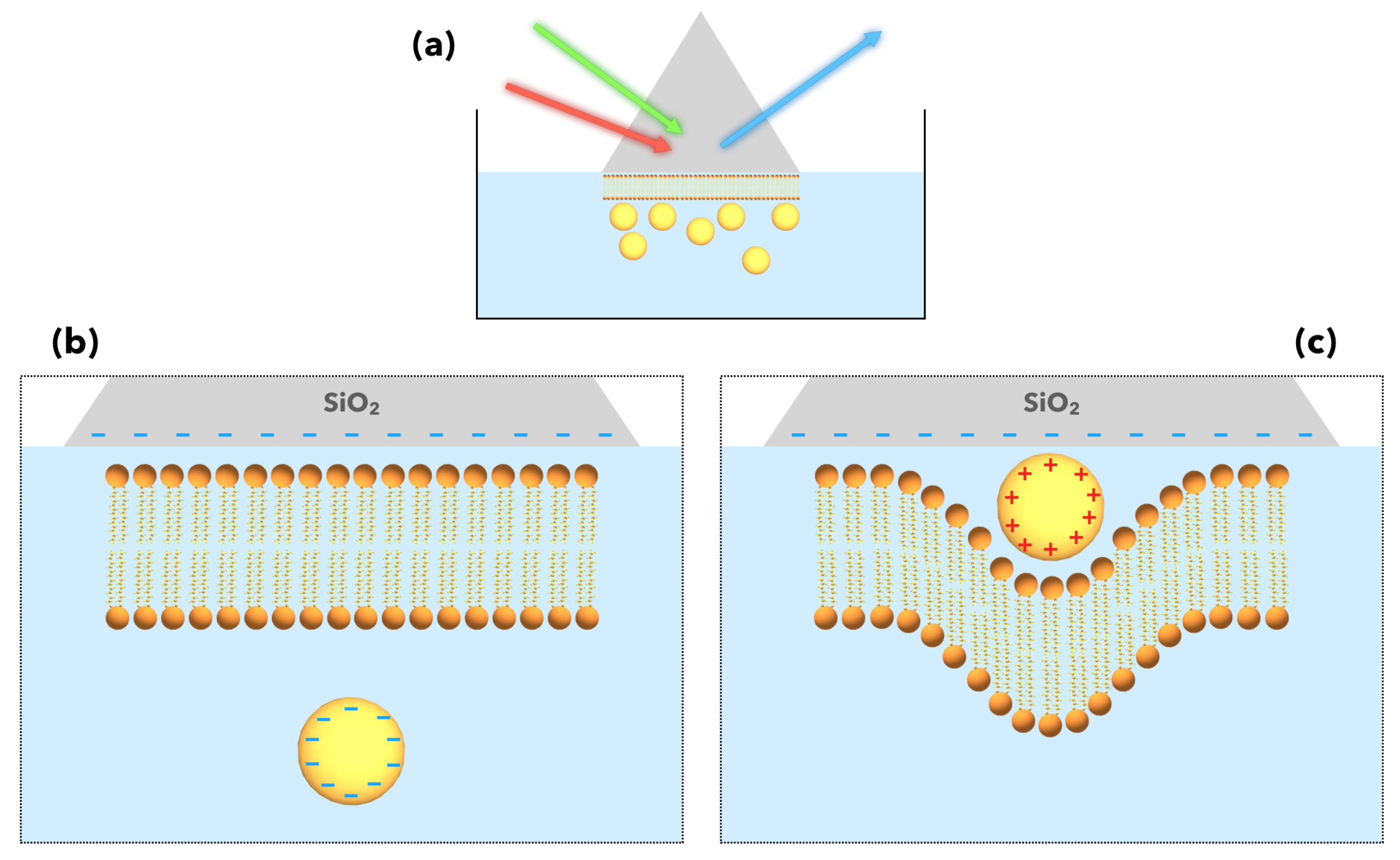
- Toledo-Fuentes, X.; Lis, D.; Cecchet, F. Structural Changes to Lipid Bilayers and Their Surrounding Water upon Interaction with Functionalized Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 21399–21409. [Google Scholar] [CrossRef]
- Toledo-Fuentes, X.; Molinaro, C.; Cecchet, F. Interfacial charges drive the organization of supported lipid membranes and their interaction with nanoparticles. Colloids Surf. B 2018, 172, 254–261. [Google Scholar] [CrossRef]
- Barbillon, G.; Sandana, V.E.; Humbert, C.; Bélier, B.; Rogers, D.J.; Teherani, F.H.; Bove, P.; McClintock, R.; Razeghi, M. Study of Au coated ZnO nanoarrays for surface enhanced Raman scattering chemical sensing. J. Mater. Chem. C 2017, 5, 3528–3535. [Google Scholar] [CrossRef]
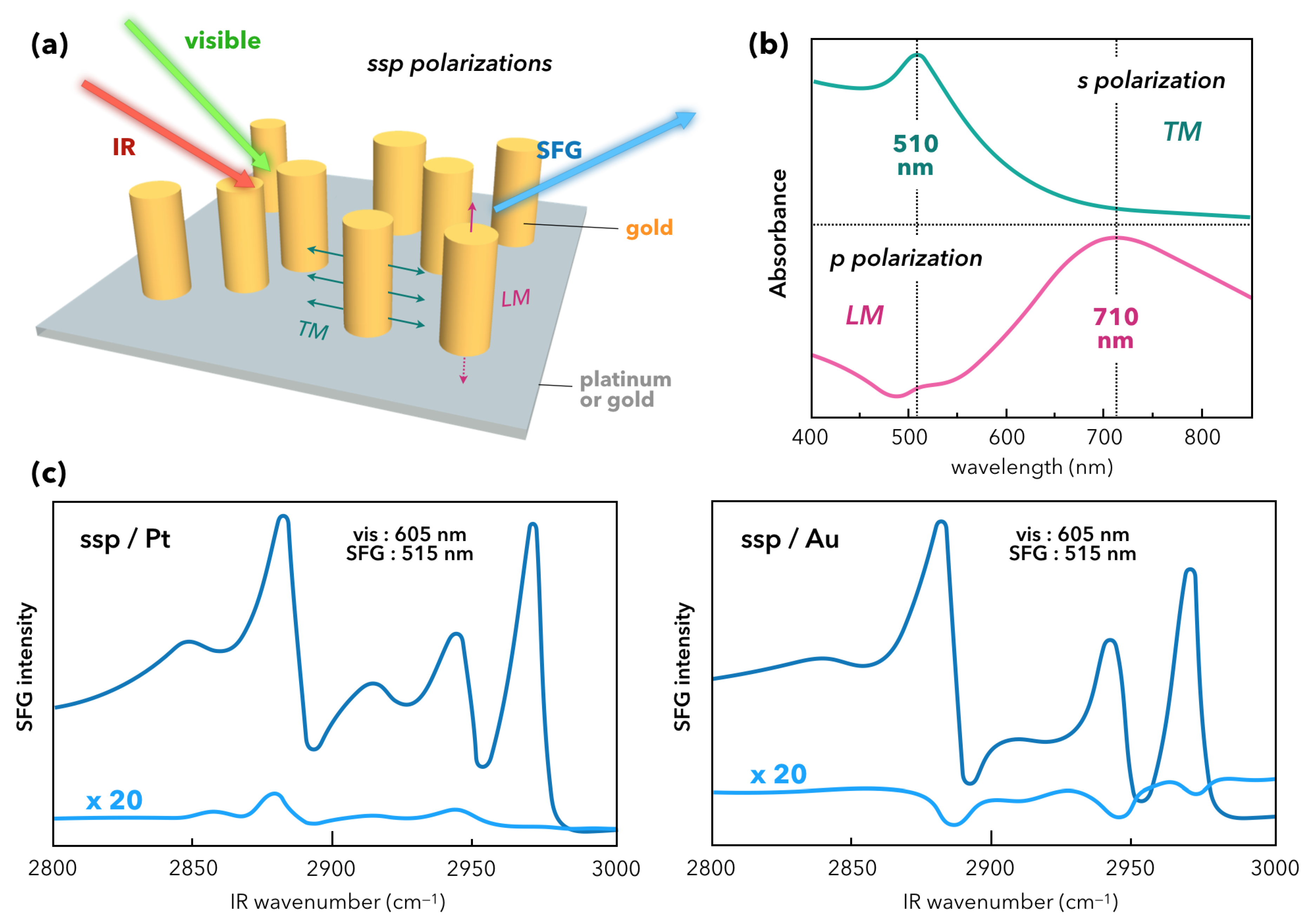
- Lis, D.; Caudano, Y.; Henry, M.; Demoustier-Champagne, S.; Ferain, E.; Cecchet, F. Selective Plasmonic Platforms Based on Nanopillars to Enhance Vibrational Sum-Frequency Generation Spectroscopy. Adv. Opt. Mater. 2013, 1, 244–255. [Google Scholar] [CrossRef]
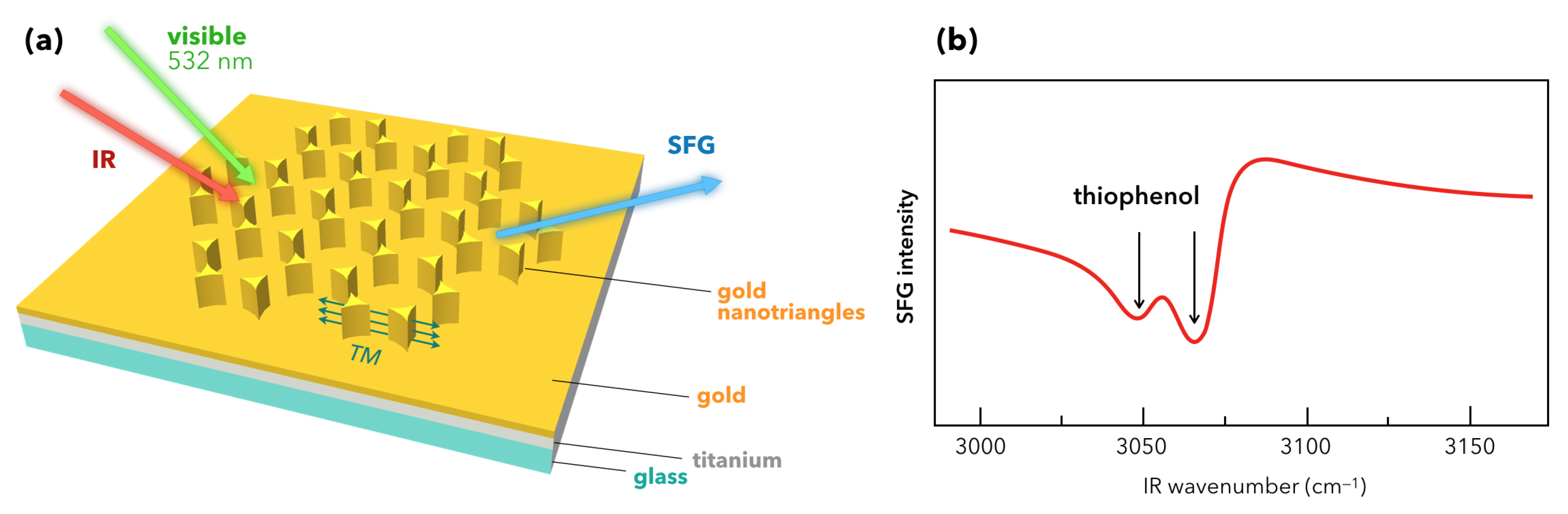
- Barbillon, G.; Noblet, T.; Busson, B.; Tadjeddine, A.; Humbert, C. Localised detection of thiophenol with gold nanotriangles highly structured as honeycombs by nonlinear sum frequency generation spectroscopy. J. Mater. Sci. 2018, 53, 4554–4562. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humbert, C.; Noblet, T.; Dalstein, L.; Busson, B.; Barbillon, G. Sum-Frequency Generation Spectroscopy of Plasmonic Nanomaterials: A Review. Materials 2019, 12, 836. https://doi.org/10.3390/ma12050836
Humbert C, Noblet T, Dalstein L, Busson B, Barbillon G. Sum-Frequency Generation Spectroscopy of Plasmonic Nanomaterials: A Review. Materials. 2019; 12(5):836. https://doi.org/10.3390/ma12050836
Chicago/Turabian StyleHumbert, Christophe, Thomas Noblet, Laetitia Dalstein, Bertrand Busson, and Grégory Barbillon. 2019. "Sum-Frequency Generation Spectroscopy of Plasmonic Nanomaterials: A Review" Materials 12, no. 5: 836. https://doi.org/10.3390/ma12050836
APA StyleHumbert, C., Noblet, T., Dalstein, L., Busson, B., & Barbillon, G. (2019). Sum-Frequency Generation Spectroscopy of Plasmonic Nanomaterials: A Review. Materials, 12(5), 836. https://doi.org/10.3390/ma12050836





