Simulation-driven Selection of Electrode Materials Based on Mechanical Performance for Lithium-Ion Battery
Abstract
1. Introduction
2. Mathematical and Parametric Analysis
2.1. Mathematical Model
2.2. Material Characterization for Lithium Electrodes
Constraints and Free Variables:
3. Results and Discussion
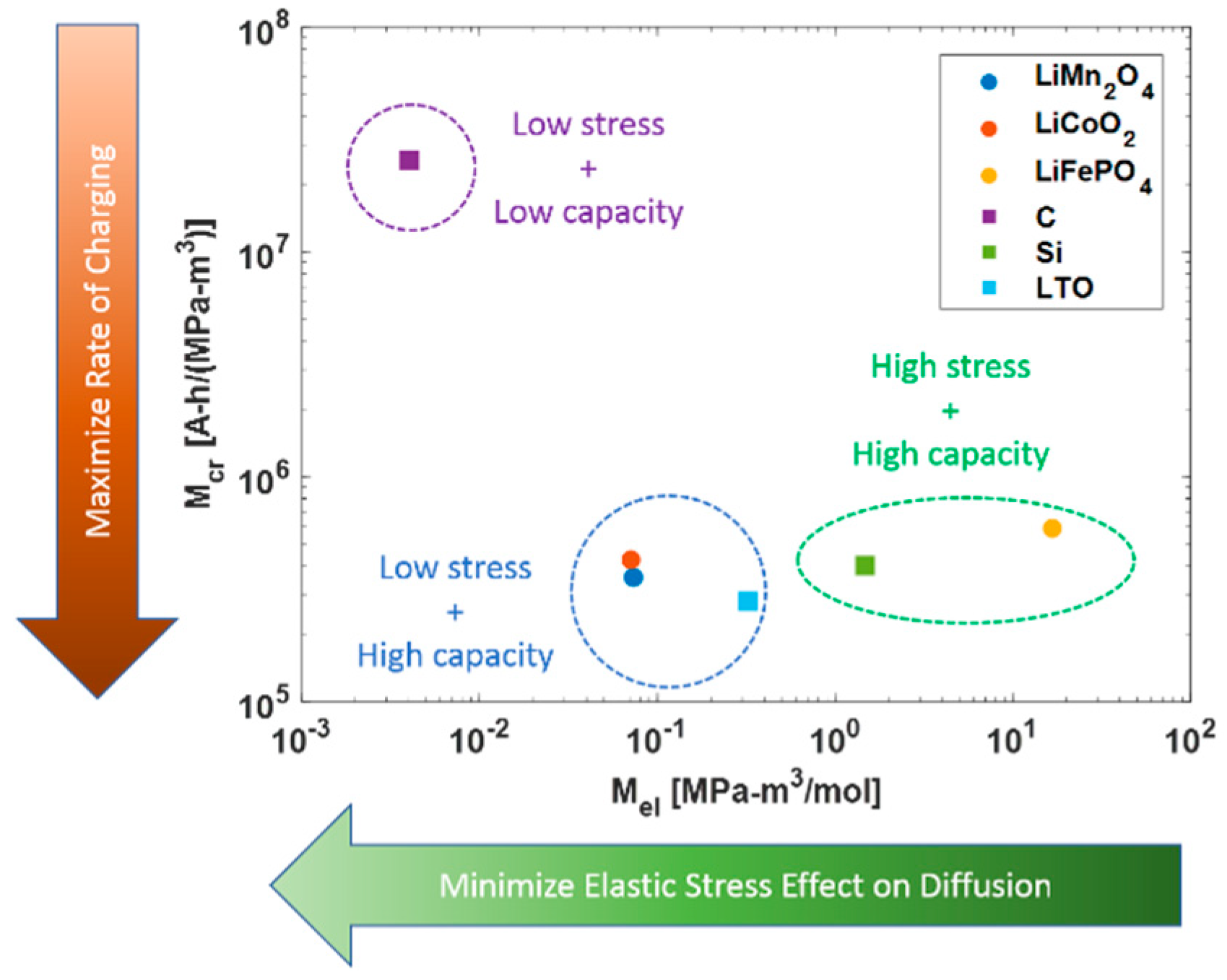
3.1. Charging Index
3.2. Elastic and Plastic Indices
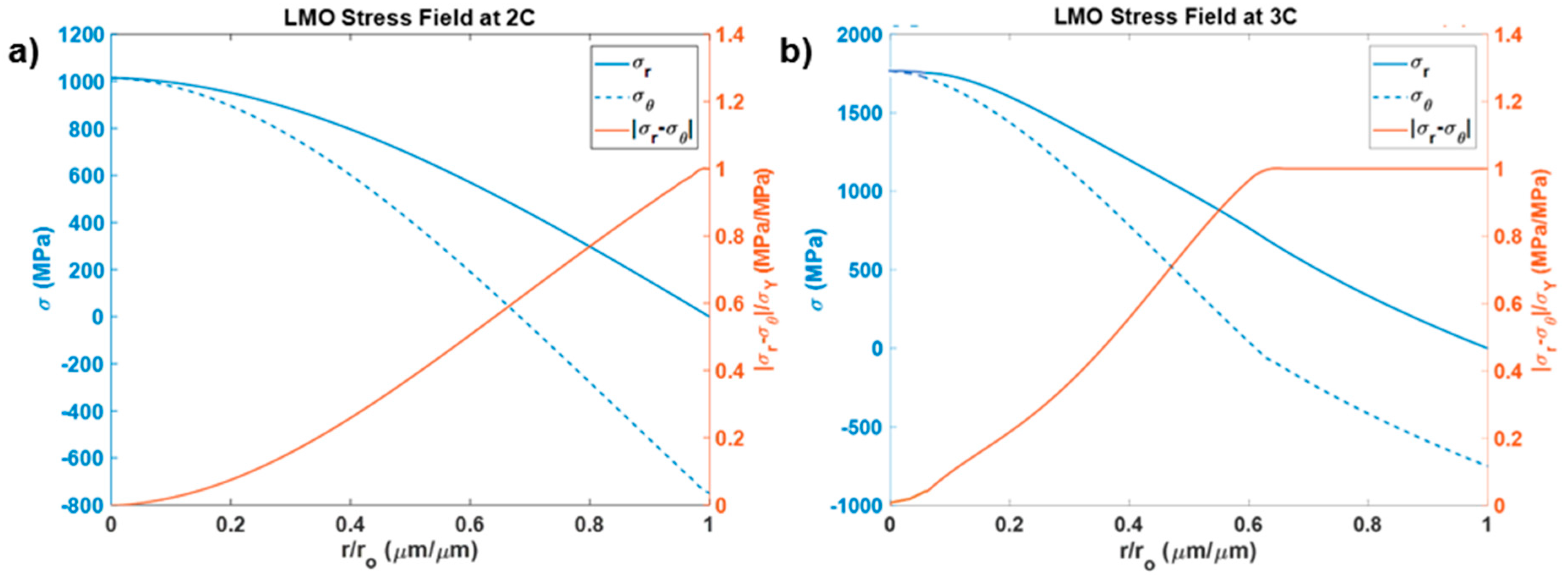
3.3. Stress Index
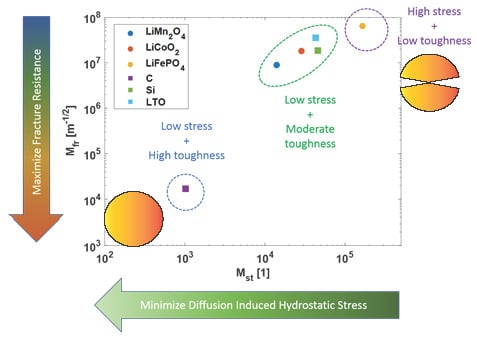
3.4. Fracture Index
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.L.; Lee, K.T.; Nazar, L.F. Positive electrode materials for li-ion and li-batteries. Chem. Mater. 2010, 22, 691–714. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Ramadesigan, V.; Northrop, P.W.C.; De, S.; Santhanagopalan, S.; Braatz, R.D.; Subramanian, V.R. Modeling and simulation of lithium-ion batteries from a systems engineering perspective. J. Electrochem. Soc. 2012, 159, R31–R45. [Google Scholar] [CrossRef]
- Kermani, G.; Sahraei, E. Review: Characterization and modeling of the mechanical properties of lithium-ion batteries. Energies 2017, 10, 1730. [Google Scholar] [CrossRef]
- Xu, J.J.; Kinser, A.J.; Owens, B.B.; Smyrl, W.H. Amorphous manganese dioxide: A high capacity lithium intercalation host. Electrochem. Solid State Lett. 1998, 1, 1–3. [Google Scholar] [CrossRef]
- Paulsen, J.M.; Dahn, J.R. Phase diagram of Li-Mn-O spinel in air. Chem. Mater. 1999, 11, 3065–3079. [Google Scholar] [CrossRef]
- Gabrisch, H.; Yazami, R.; Fultz, B. Hexagonal to cubic spinel transformation in lithiated cobalt oxide TEM investigation. J. Electrochem. Soc. 2004, 151, A891–A897. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Chang, J.; Huang, X.; Zhou, G.; Cui, S.; Hallac, P.B.; Jiang, J.; Hurley, P.T.; Chen, J. Lithium-ion batteries: Multilayered si nanoparticle/reduced graphene oxide hybrid as a high-performance lithium-ion battery anode. Adv. Mater. 2014, 26, 665. [Google Scholar] [CrossRef]
- Wang, F.; Wu, L.; Key, B.; Yang, X.-Q.; Grey, C.P.; Zhu, Y.; Graetz, J. Electrochemical reaction of lithium with nanostructured silicon anodes: A study by in-situ synchrotron X-Ray diffraction and electron energy-loss spectroscopy. Adv. Energy Mater. 2013, 3, 1324–1331. [Google Scholar] [CrossRef]
- Zhao, K.; Pharr, M.; Wan, Q.; Wang, W.L.; Kaxiras, E.; Vlassak, J.J.; Suo, Z. Concurrent reaction and plasticity during initial lithiation of crystalline silicon in lithium-ion batteries. J. Electrochem. Soc. 2012, 159, A238–A243. [Google Scholar] [CrossRef]
- Pharr, M.; Zhao, K.; Wang, X.; Suo, Z.; Vlassak, J.J. Kinetics of initial lithiation of crystalline silicon electrodes of lithium-ion batteries. Nano Lett. 2012, 12, 5039–5047. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Newman, J. A mathematical model of stress generation and fracture in lithium manganese oxide. J. Electrochem. Soc. 2006, 153, A1019–A1030. [Google Scholar] [CrossRef]
- Zhang, X.; Shyy, W.; Sastry, A.M. Numerical simulation of intercalation-induced stress in li-ion battery electrode particles. J. Electrochem. Soc. 2007, 154, A910–A916. [Google Scholar] [CrossRef]
- Zhang, X.; Sastry, A.M.; Shyy, W. Intercalation-induced stress and heat generation within single lithium-ion battery cathode particles. J. Electrochem. Soc. 2008, 155, A542–A552. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Nguyen, A.; Chon, M.J.; Nadimpalli, S.P.V.; Wang, H.; Abraham, D.P.; Bower, A.F.; Shenoy, V.B.; Guduru, P.R. Stress evolution in composite silicon electrodes during lithiation/delithiation. J. Electrochem. Soc. 2013, 160, A739–A746. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Xiang, X.; Knight, J.C.; Li, W.; Manthiram, A. Understanding the effect of Co3+ substitution on the electrochemical properties of lithium-rich layered oxide cathodes for lithium-ion batteries. J. Phys. Chem. C 2014, 118, 21826–21833. [Google Scholar] [CrossRef]
- Yang, F. Interaction between diffusion and chemical stresses. Mater. Sci. Eng. A 2005, 409, 153–159. [Google Scholar] [CrossRef]
- Bower, A.F. Applied Mechanics of Solids; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Ashby, M.F. Materials selection in mechanical design. MRS Bull. 2005, 30, 995. [Google Scholar] [CrossRef]
- Renganathan, S.; Sikha, G.; Santhanagopalan, S.; White, R.E. Theoretical analysis of stresses in a lithium ion cell. J. Electrochem. Soc. 2010, 157, A155–A163. [Google Scholar] [CrossRef]
- Satyavani, T.; Kiran, B.R.; Kumar, V.R.; Kumar, A.S.; Naidu, S.V. Effect of particle size on dc conductivity, activation energy and diffusion coefficient of lithium iron phosphate in li-ion cells. Eng. Sci. Technol. Int. J. 2016, 19, 40–44. [Google Scholar] [CrossRef]
- Ma, Z.S.; Xie, Z.C.; Wang, Y.; Zhang, P.P.; Pan, Y.; Zhou, Y.C.; Lu, C. Failure modes of hollow core—Shell structural active materials during the lithiation—delithiation process. J. Power Sources 2015, 290, 114–122. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Q.; Li, Y.; Wu, K.; Liu, B.; Peng, P.; Wang, Q. A thermal runaway simulation on a lithium titanate battery and the battery module. Energies 2015, 8, 490–500. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C. Galvanostatic intermittent titration technique for phase-transformation electrodes. J. Phys. Chem. C 2010, 114, 2830–2841. [Google Scholar] [CrossRef]
- Dash, R.; Pannala, S. Theoretical limits of energy density in silicon-carbon composite anode based lithium ion batteries. Sci. Rep. 2016, 6, 27449. [Google Scholar] [CrossRef]
- Lithium Titanate. Available online: https://en.wikipedia.org/wiki/Lithium_titanate (accessed on 5 December 2017).
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative issues of cathode materials for li-ion batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Kam, K.C.; Doeff, M.M. Electrode materials for lithium ion batteries. Mater. Matters 2012, 7, 56–62. [Google Scholar]
- Chen, L.; Fan, F.; Hong, L.; Chen, J.; Ji, Y.Z.; Zhang, S.L.; Zhu, T.; Chen, L.Q. A phase-field model coupled with large elasto-plastic deformation: Application to lithiated silicon electrodes. J. Electrochem. Soc. 2014, 161, F3164–F3172. [Google Scholar] [CrossRef]
- Lundgren, H. Thermal Aspects and Electrolyte Mass Transport in Lithium-ion Batteries. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 11 June 2015. [Google Scholar]
- Zhao, K.; Pharr, M.; Vlassak, J.J.; Suo, Z. Fracture of electrodes in lithium-ion batteries caused by fast charging. J. Appl. Phys. 2010, 108, 73517. [Google Scholar] [CrossRef]
- Kushima, A.; Huang, J.Y.; Li, J. Quantitative fracture strength and plasticity measurements of lithiated silicon nanowires by in situ TEM tensile experiments. ACS Nano 2012, 6, 9425–9432. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Hector, L.G.; James, C.; Kim, K.J. Lithium concentration dependent elastic properties of battery electrode materials from first principles calculations. J. Electrochem. Soc. 2014, 161, F3010–F3018. [Google Scholar] [CrossRef]
- Berla, L.A.; Lee, S.W.; Cui, Y.; Nix, W.D. Mechanical behavior of electrochemically lithiated silicon. J. Power Sources 2015, 273, 41–51. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Jo, H.; Cho, Y.-H.; David, I.N.; Askeland, P.; Case, E.D.; Kim, H.; Choe, H.; Sakamoto, J. A preliminary investigation of fracture toughness of Li7La3Zr2O12 and its comparison to other solid li-ionconductors. Mater. Lett. 2013, 96, 117–120. [Google Scholar] [CrossRef]
- Kosova, N.V.; Uvarov, N.F.; Devyatkina, E.T.; Avvakumov, E.G. Mechanochemical synthesis of LiMn2O4 cathode material for lithium batteries. Solid State Ionics 2000, 135, 107–114. [Google Scholar] [CrossRef]
- Schilcher, C.; Meyer, C.; Kwade, A. Structural and electrochemical properties of calendered lithium manganese oxide cathodes. Energy Technol. 2016, 4, 1604–1610. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sour. 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Fang, X.; Ge, M.; Chen, H.; Xu, J.; Zhou, C. Coaxial Si/anodic titanium oxide/Si nanotube arrays for lithium-ion battery anodes. Nano Res. 2013, 6, 182–190. [Google Scholar] [CrossRef]



| Properties | LiMn2O4 | LiCoO2 | LiFePO4 | LixC6 | LixSi15 | LixTiO2 |
|---|---|---|---|---|---|---|
| 7.08 × 10−15 [17] | 1.00 × 10−13 [26] | 7.96 × 10−16 [27] | 3.90 × 10−14 [26] | 1.00 × 10−16 [28] | 6.80 × 10−15 [29] | |
| 4100 [17] | 5030 [26] | 3600 [30] | 2100 [26] | 2328 [31] | 3510 [32] | |
| 148 [33] | 166 [33] | 170 [33] | 372 [33] | 4200 [33] | 175 [7] | |
| 2.29 × 104 [17] | 4.99 × 104 [26] | 2.12 × 104 | 3.05 × 104 [26] | 8.87 × 104 [34] | 5.00 × 104 [29] | |
| 3.50 × 10−6 [17] | 1.92 × 10−6 [26] | 67.32 × 10−6 [35] | 3.17 × 10−6 [26] | 32.25 × 10−6 [36] | 5.00 × 10−6 [29] | |
| 776 [37] | 1056 [38] | 500 [38] | 23 [33] | 720 [21] | 836 | |
| 194 [38] | 264 [38] | 125 [38] | 10 [38] | 12 [39] | 209 [38] | |
| 0.26 [38] | 0.32 [38] | 0.28 [38] | 0.24 [38] | 0.25 [38] | 0.19 [38] | |
| 1.50 [40] | 1.30 [40] | 1.50 [40] | 1.25 [40] | 1.00 [40] | 1.50 [40] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, A.; Shrotriya, P.; Chandra, A. Simulation-driven Selection of Electrode Materials Based on Mechanical Performance for Lithium-Ion Battery. Materials 2019, 12, 831. https://doi.org/10.3390/ma12050831
Sarkar A, Shrotriya P, Chandra A. Simulation-driven Selection of Electrode Materials Based on Mechanical Performance for Lithium-Ion Battery. Materials. 2019; 12(5):831. https://doi.org/10.3390/ma12050831
Chicago/Turabian StyleSarkar, Abhishek, Pranav Shrotriya, and Abhijit Chandra. 2019. "Simulation-driven Selection of Electrode Materials Based on Mechanical Performance for Lithium-Ion Battery" Materials 12, no. 5: 831. https://doi.org/10.3390/ma12050831
APA StyleSarkar, A., Shrotriya, P., & Chandra, A. (2019). Simulation-driven Selection of Electrode Materials Based on Mechanical Performance for Lithium-Ion Battery. Materials, 12(5), 831. https://doi.org/10.3390/ma12050831