Monodisperse Fe3O4/SiO2 and Fe3O4/SiO2/PPy Core-Shell Composite Nanospheres for IBU Loading and Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fe3O4 Nanoparticles
2.2. Preparation of Fe3O4/SiO2 Composite Nanospheres
2.3. Preparation of Fe3O4/SiO2/PPy Composite Nanospheres
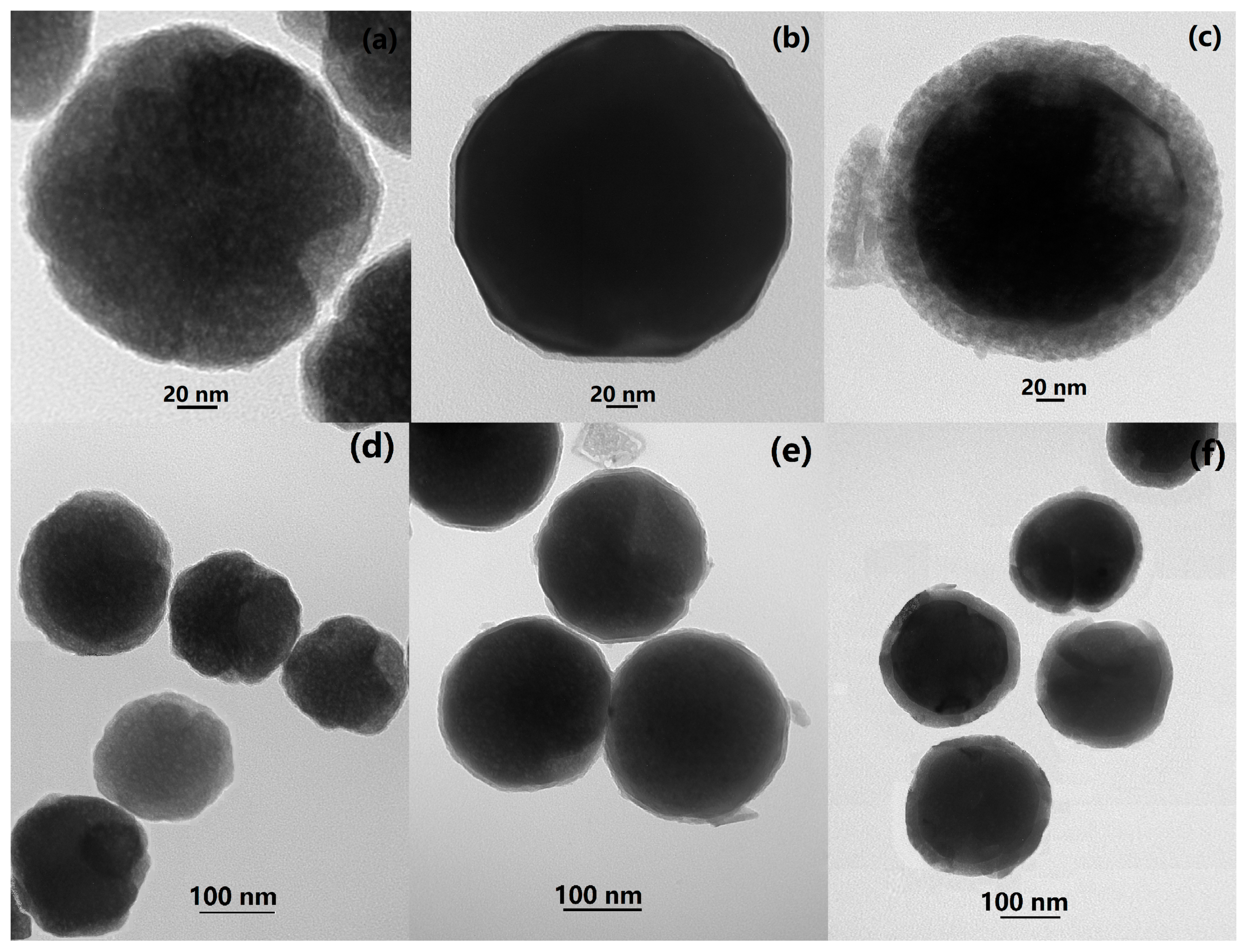
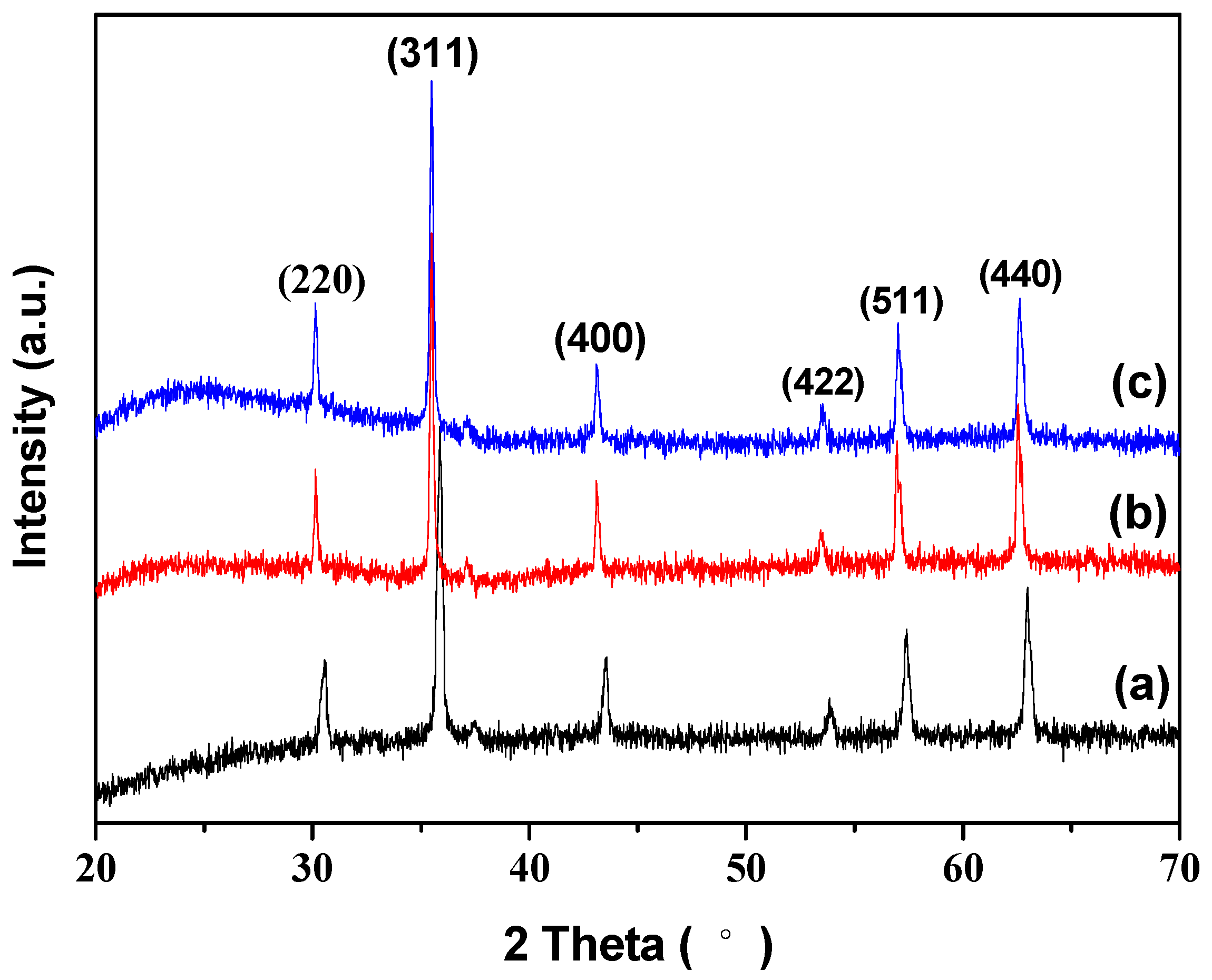
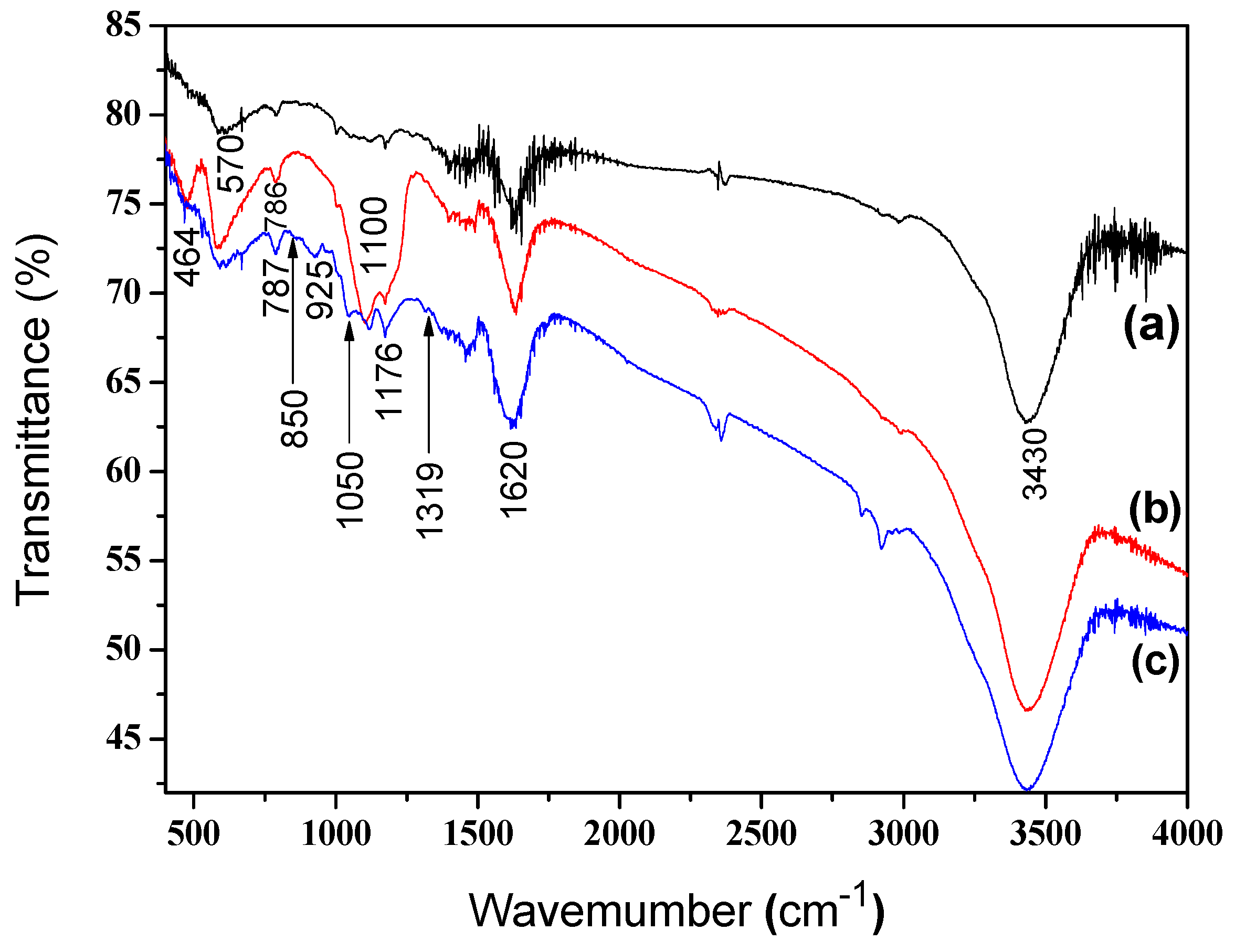
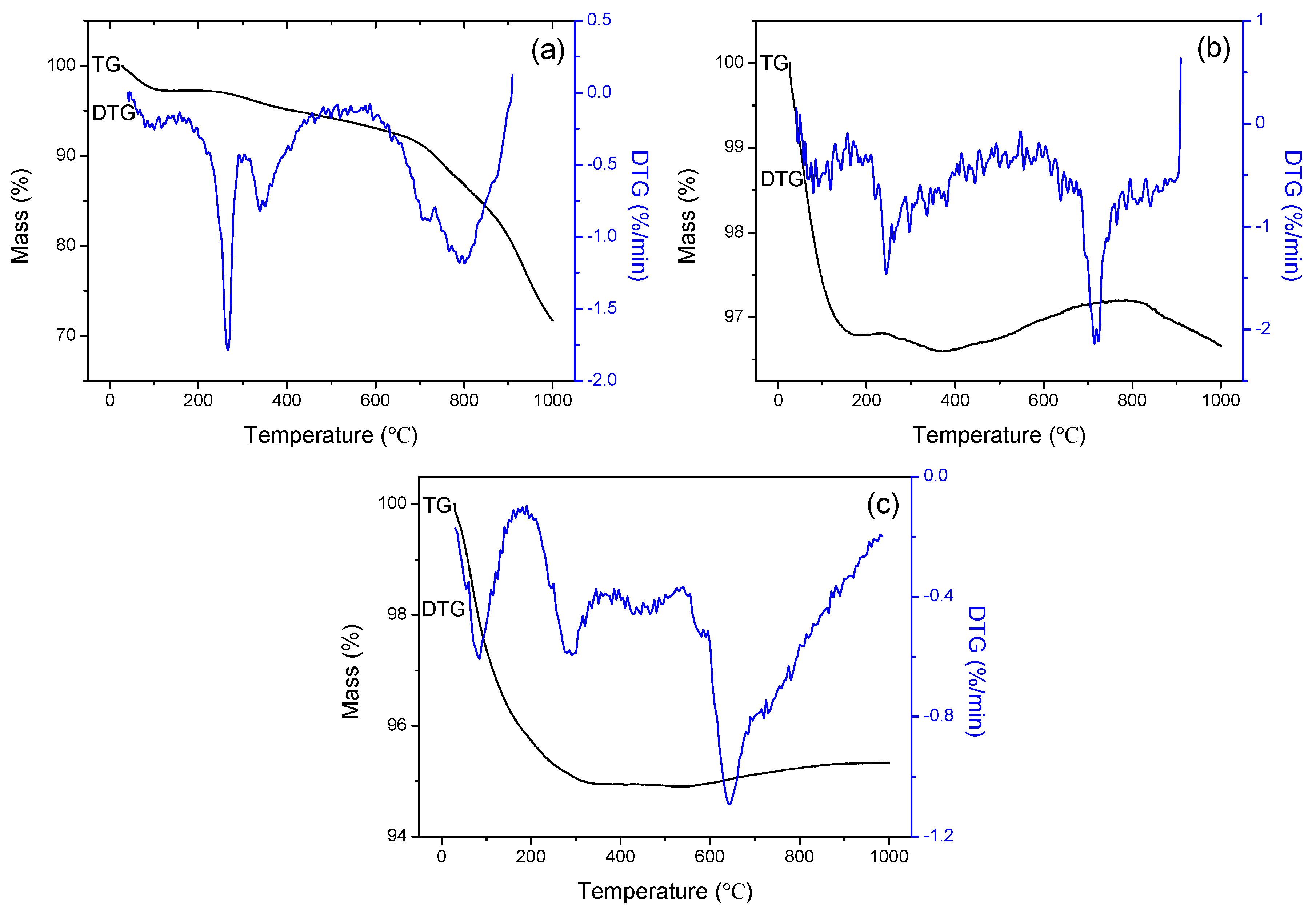
2.4. Characterizations
2.5. Drug Loading and Drug Release
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kamari, Y.; Ghiaci, M. Preparation and characterization of ibuprofen/modified chitosan/TiO2 hybrid composite as a controlled drug-delivery system. Micropor. Mesopor. Mat. 2016, 234, 361–369. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Wang, X.; He, S.; Zhai, G. Development and evaluation of ibuprofen loaded mixed micelles preparations for topical delivery. J. Drug Deliv. Sci. Technol. 2018, 48, 363–371. [Google Scholar] [CrossRef]
- Yuan, X.; Capomacchia, A.C. Influence of physicochemical properties on the in vitro skin permeation of the enantiomers, racemate, and eutectics of ibuprofen for enhanced transdermal drug delivery. J. Pharmaceut. Sci. 2013, 102, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–222. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Pawlik, A.; Szuwarzyński, M.; Jaskuła, M.; Sulka, G.D. Nanoporous anodic titanium dioxide layers as potential drug delivery systems: Drug release kinetics and mechanism. Colloid Surf. B 2016, 143, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.M.; Fitzpatrick, R.; Volans, G.N. Ibuprofen toxicity. A review of adverse reactions and overdose. Advers. Drug React. Toxicol. Rev. 1993, 12, 107–128. [Google Scholar] [CrossRef]
- Tang, C.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Preparation of ibuprofen-loaded chitosan films for oral mucosal drug delivery using supercritical solution impregnation. Int. J. Pharmaceut. 2014, 473, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Ahmed, K.; Rahim, A.; Muhammad, N.; Tariq, S.; Azhar, U.; Khan, A.J.; Sama, Z.; Volpe, P.L.O.; Airoldi, C. Organo-bridged silsesquioxane incorporated mesoporous silica as a carrier for the controlled delivery of ibuprofen and fluorouracil. J. Mol. Liq. 2018, 258, 319–326. [Google Scholar] [CrossRef]
- Javanbakht, S.; Nezhad-Mokhtari, P.; Shaabani, A.; Arsalani, N.; Ghorbani, M. Incorporating Cu-based metal-organic framework/drug nanohybrids into gelatin microsphere for ibuprofen oral delivery. Mater. Sci. Eng. C 2019, 96, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, L.; Zhang, X.; Liu, H.; Yan, X.; Qiu, J.; Yeung, K.L. Synthesis and characterization of ZIF-8@SiO2@Fe3O4 core@double-shell microspheres with noble metal nanoparticles sandwiched between two shell layers. Mater. Lett. 2015, 148, 17–21. [Google Scholar] [CrossRef]
- Pajchel, L.; Kolodziejski, W. Synthesis and characterization of MCM-48/hydroxyapatite composites for drug delivery: Ibuprofen incorporation, location and release studies. Mater. Sci. Eng. C 2018, 91, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Malfait, B.; Correia, N.T.; Mussi, A.; Paccou, L.; Guinet, Y.; Hédoux, A. Solid-state loading of organic molecular materials within mesoporous silica matrix: Application to ibuprofen. Micropor. Mesopor. Mat. 2019, 277, 203–207. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Nowak, I.; Marciniak, M.; Pietrzak, R. Ordered mesoporous silica modified with lanthanum for ibuprofen loading and release behavior. Eur. J. Pharm. Biopharm. 2015, 94, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, F.; Günther, G.; Nos, J.; Nonell, S.; Olea-Azar, C.; Morales, J. Antioxidant nanomaterial sased on core-shell silica nanospheres with surface-bound caffeic acid: A promising vehicle for oxidation-sensitive drugs. Nanomaterials 2019, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.M.P.; Carvalho, M.A.; Oliveira, C.S.; Profirio, D.M.; Ferreira, R.B.; Corbi, P.P.; Formiga, A.L.B. Enhanced performance of a metal-organic framework analogue to MIL-101(Cr) containing amine groups for ibuprofen and nimesulide controlled release. Inorg. Chem. Commun. 2016, 70, 47–50. [Google Scholar] [CrossRef]
- Pawlik, A.; Jarosz, M.; Syrek, K.; Sulka, G.D. Co-delivery of ibuprofen and gentamicin from nanoporous anodic titanium dioxide layers. Colloid Surf. B 2017, 152, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J.; Cegłowski, M.; Messyasz, B.; Schroeder, G. Dendrimer-functionalized halloysite nanotubes for effective drug delivery. Appl. Clay Sci. 2018, 153, 134–143. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A.; Brunella, V.; Scalarone, D.; Berlier, G. Poly(NIPAM-co-MPS)-grafted multimodal porous silica nanoparticles as reverse thermoresponsive drug delivery system. Asian J. Pharm. Sci. 2017, 12, 279–284. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/shell gel beads with embedded halloysite nanotubes for controlled grug release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Lisuzzo, L.; Milioto, S.; Parisi, F. Selective adsorption of oppositely charged PNIPAAM on halloysite surfaces: A route to thermo-responsive nanocarriers. Nanotechnology 2018, 29, 325702. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Shetake, N.G.; Barick, B.K.; Barick, K.C.; Pandey, B.N.; Priyadarsini, K.I.; Hassan, P.A. pH sensitive surfactant-stabilized Fe3O4 magnetic nanocarriers for dual drug delivery. Colloid Surf. B 2018, 162, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, M.; Luitel, H.N.; Gunawardhana, N.; Inoue, M.; Yusa, S.; Watari, T.; Nakashima, K. Synthesis of magnetic α-Fe2O3 and Fe3O4 hollow nanospheres for sustained release of ibuprofen. Mater. Lett. 2012, 73, 4–7. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.Q.; Sun, K.K.; Zhang, R.; Fan, L.; Liang, K.K.; Mao, L.B. Controllable preparation and drug loading properties of core-shell microspheres Fe3O4@MOFs/GO. Mater. Lett. 2016, 162, 207–210. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, J. The progress in the research of magnetic iron oxide drug nano-carrier. Prog. Mod. Biomed. 2011, 11, 3386–3389. [Google Scholar]
- Zhao, H.; Cui, H.J.; Fu, M.L. A general and facile method for improving carbon coat on magnetic nanoparticles with a thickness control. J. Colloid Interface Sci. 2016, 461, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Asgharinezhad, A.A.; Ebrahimzadeh, H. Poly(2-aminobenzothiazole)-coated graphene oxide/magnetite nanoparticles composite as an efficient sorbent for determination of non-steroidal anti-inflammatory drugs in urine sample. J. Chromatogr. A 2016, 1435, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, L.; Gai, L.; Wang, Y.; Hou, Y.; Liu, H. Conjugation of methotrexate onto dedoped Fe3O4/PPy nanospheres to produce magnetic targeting drug with controlled drug release and targeting specificity for HeLa cells. Synth. Met. 2015, 207, 18–25. [Google Scholar] [CrossRef]
- Asgharinezhad, A.A.; Karami, S.; Ebrahimzadeh, H.; Shekari, N.; Jalilian, N. Polypyrrole/magnetic nanoparticles composite as an efficient sorbent for dispersive micro-solid-phase extraction of antidepressant drugs from biological fluids. Int. J. Pharm. 2015, 494, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Dai, J.; Song, X.; Wang, G.; Yang, W. Facile synthesis of Fe3O4/SiO2 composite nanoparticles from primary silica particles. Colloid. Surf. A 2008, 317, 450–456. [Google Scholar] [CrossRef]
- Bogush, G.H.; Zukoski, C.F. Studies of the kinetics of the precipitation of uniform silica particles through the hydrolysis and condensation of silicon alkoxides. J. Colloid. Interface Sci. 1991, 142, 1–18. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Ren, F.; Qiu, G.; Tang, M. Controllable fabrication of SiO2/polypyrrole core-shell particles and polypyrrole hollow spheres. Mater. Chem. Phys. 2008, 109, 5–9. [Google Scholar] [CrossRef]
- Dehghani, E.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Fabricating cauliflower-like and dumbbell-like Janus particles: Loading and simultaneous release of DOX and ibuprofen. Colloid Surf. B 2019, 173, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Dziadkowiec, J.; Mansa, R.; Quintela, A.; Rocha, F.; Detellier, C. Preparation, characterization and application in controlled release of Ibuprofen-loaded Guar Gum/Montmorillonite Bionanocomposites. Appl. Clay Sci. 2017, 135, 52–63. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric nanoparitcles containing diazepam: Preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.C.; Patil, S.A.; Kuchekar, B.S.; Chabukswar, A.R. Preparation and characterizaiton of metformin hydrochloride–compritol 888 ATO solid dispersion. J. Young Pharm. 2011, 3, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, C.; Kang, X.; Yang, D.; Yang, P.; Hou, Z.; Lin, J. Synthesis of a multifunctional nanocomposite with magnetic, mesoporous, and near-IR absorption properties. J. Phys. Chem. C 2010, 114, 16343–16350. [Google Scholar] [CrossRef]
- Gao, R.; Hao, Y.; Zhang, L.; Cui, X.; Liu, D.; Zhang, M.; Tang, Y.; Zheng, Y. A facile method for protein imprinting on directly carboxyl-functionalized magnetic nanoparticles using non-covalent template immobilization strategy. Chem. Eng. J. 2016, 284, 139–148. [Google Scholar] [CrossRef]
- Fan, H.; Li, B.; Feng, Y.; Qiu, D.; Song, Y. Multifunctional Fe3O4@SiO2@GdVO4:Eu3+ core-shell nanocompositen for a potential drug carrier. Ceram. Int. 2016, 42, 13326–13330. [Google Scholar] [CrossRef]
- Yao, T.; Cui, T.; Fang, X.; Yu, J.; Cui, F.; Wu, J. Preparation of yolk/shell Fe3O4@polypyrrle composites and their applications as catalyst supports. Chem. Eng. J. 2013, 225, 230–236. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, M.; Wu, J.; Wang, L.; He, H. Preparation of Fe3O4@PPy magnetic nanoparticles as solid-phase extraction sorbents for preconcentration and separation of phthalic acid esters in water by gas chromatography-mass spectrometry. J. Chromatogr. B 2016, 1011, 33–44. [Google Scholar] [CrossRef]
- Xin, T.; Ma, M.; Zhang, H.; Gu, J.; Wang, S.; Liu, M.; Zhang, Q. A facile approach for the synthesis of magnetic separable Fe3O4@TiO2, core-shell nanocomposites as highly recyclable photocatalysts. Appl. Surf. Sci. 2014, 288, 51–59. [Google Scholar] [CrossRef]
- Huang, S.; Fan, Y.; Cheng, Z.; Kong, D.; Yang, P.; Quan, Z.; Zhang, C.; Lin, J. Magnetic mesoporous silica spheres for drug targeting and controlled release. J. Phys. Chem. C 2009, 113, 1775–1784. [Google Scholar] [CrossRef]
- Ji, C.; Li, J.; Hou, C.; Huo, D.; Yang, M.; Zhang, L. Mesoporous hollow silica shells modified with functional diamine groups show high-performance absorption capacity and selective colorimetric response to copper ions in aqueous solutions. Sens. Actuators B 2017, 240, 718–725. [Google Scholar] [CrossRef]
- Umegaki, T.; Hoshino, M.; Watanuki, Y.; Kojima, Y. Preparation of hollow mesoporous silica spheres with immobilized silicomolybdic acid and their catalytic activity for the hydrolytic dehydrogenation of ammonia borane. Micropor. Micropor. Mater. 2016, 223, 152–156. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Enhanced removal of Cr(VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. J. Hazard. Mater. 2011, 190, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mao, H.; Zhang, W. Fabrication of core-shell Fe3O4/polypyrrole and hollow polypyrrole microspheres. Polym. Compos. 2009, 30, 847–854. [Google Scholar] [CrossRef]
- Abbas, M.; Abdel-Hamed, M.O.; Chen, J. Efficient one-pot sonochemical synthesis of thickness-controlled silica-coated superparamagnetic iron oxide (Fe3O4/SiO2) nanospheres. Appl. Phys. A 2017, 123, 775. [Google Scholar] [CrossRef]
- Abbas, M.; Torati, S.R.; Kim, C. A novel approach for the synthesis of ultrathin silica-coated iron oxide nanocubes decorated with silver nanodots (Fe3O4/SiO2/Ag) and their superior catalytic reduction of 4-nitroaniline. Nanoscale 2015, 7, 12192–12204. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Lu, Y.J.; Chen, J.P. Magnetic graphene oxide as a carrier for targeted delivery of chemotherapy drugs in cancer therapy. J. Magn. Magn. Mater. 2017, 427, 34–40. [Google Scholar] [CrossRef]
- Shen, L.; Qiao, Y.; Guo, Y.; Zhao, J. Synthesis and magnetic properties of Fe3O4 nanoparticles from the blast furnace flue dust. Optoelectron. Adv. Mat. 2013, 7, 525–529. [Google Scholar]
- He, Y.; Li, H.; Ou, L.; Ding, F.; Zhan, Z.; Zhong, Y. Preparation and characterisation of water-based aluminium pigments modified with SiO2 and polymer brushes. Corros. Sci. 2016, 111, 802–810. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Liu, D.; Guo, J.; Liu, P. Synthesis and electrochemical performance of CeO2/PPy nanocomposites: Interfacial effect. Ind. Eng. Chem. Res. 2016, 55, 866–874. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Formation and characterization of β-cyclodextrin (β-CD)-polyethyleneglycol (PEG)-polyethyleneimine (PEI) coated Fe3O4 nanoparticles for loading and releasing 5-Fluorouracil drug. Biomed. Pharmacother. 2016, 80, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lindner, W.D.; Lippold, B.C. Drug release from hydrocolloid Embeddings with high or low susceptibility to hydrodynamic stress. Pharm. Res. 1995, 12, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Balcerzak, J.; Mucha, M. Analysis of model drug release kinetics from complex matrices of polylactide-chitosan. Prog. Chem. Appl. Chin Its Deriv. 2010, XV, 117–126. [Google Scholar]
- Zhang, Y.; Yu, J.; Bomba, H.N.; Zhu, Y.; Gu, Z. Mechanical force-triggered drug delivery. Chem. Rev. 2016, 116, 12536–12563. [Google Scholar] [CrossRef] [PubMed]
- Kariminia, S.; Shamsipur, A.; Shamsipur, M. Analytical characteristics and application of novel chitosan coated magnetic nanoparticles as an rfficient drug delivery system for ciprofloxacin. Enhanced drug release kinetics by low-frequency ultrasounds. J. Pharmaceut. Biomed. 2016, 129, 450–457. [Google Scholar] [CrossRef] [PubMed]

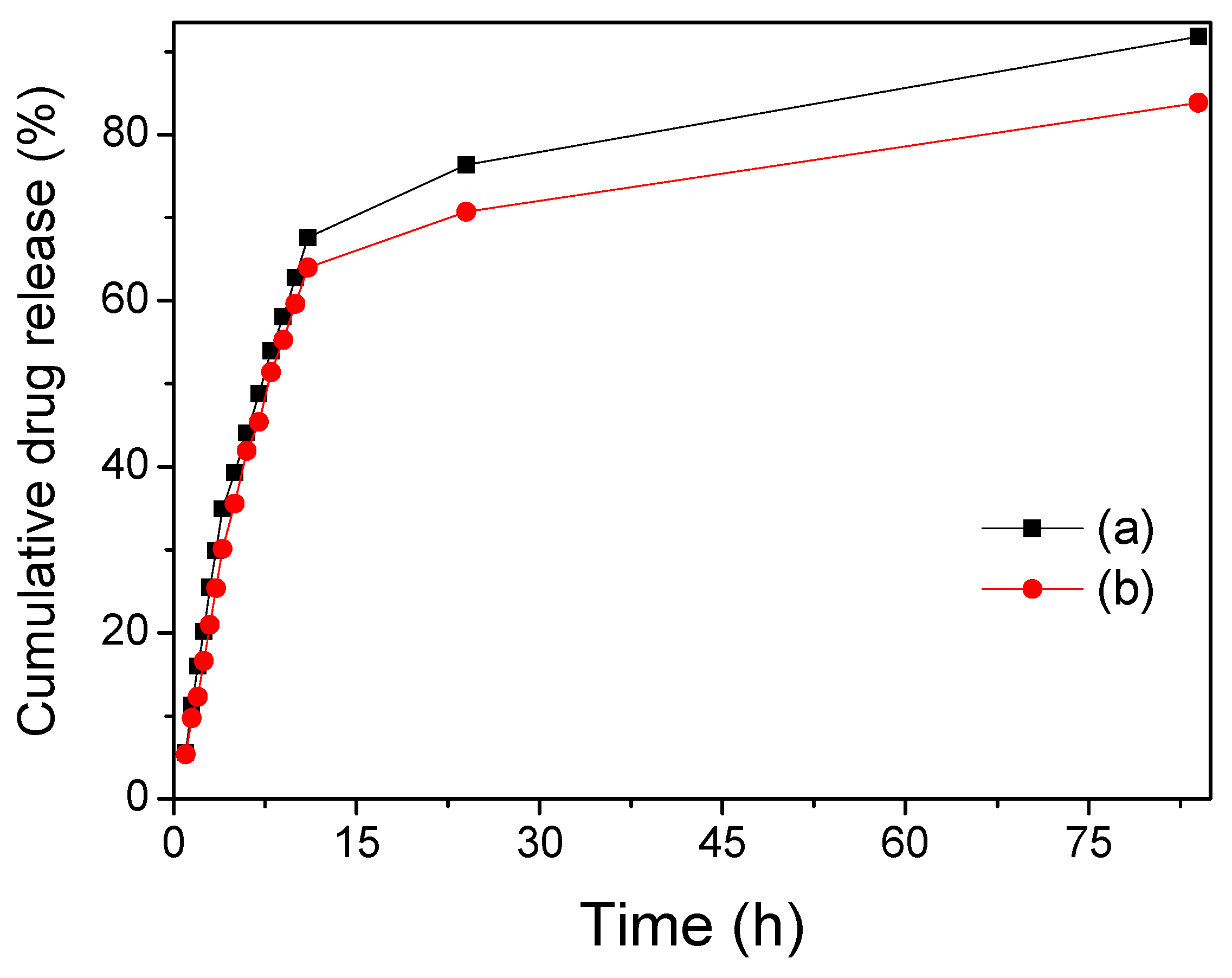
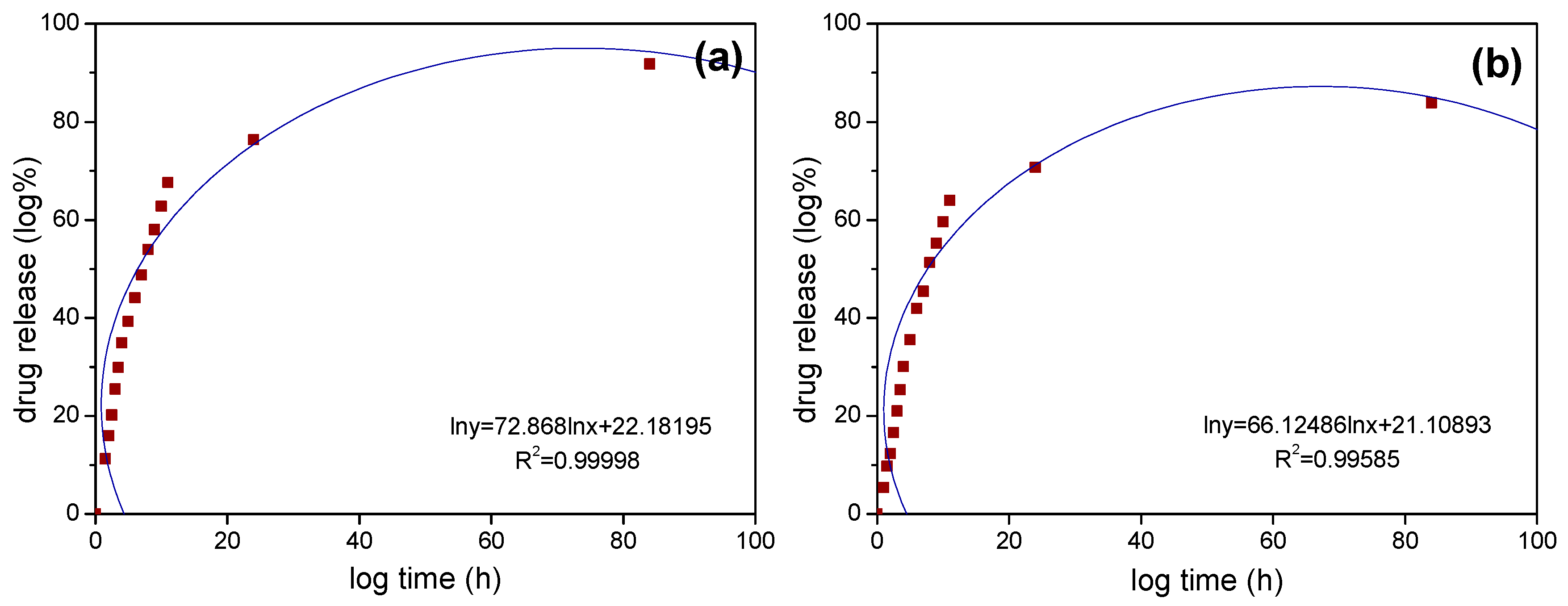
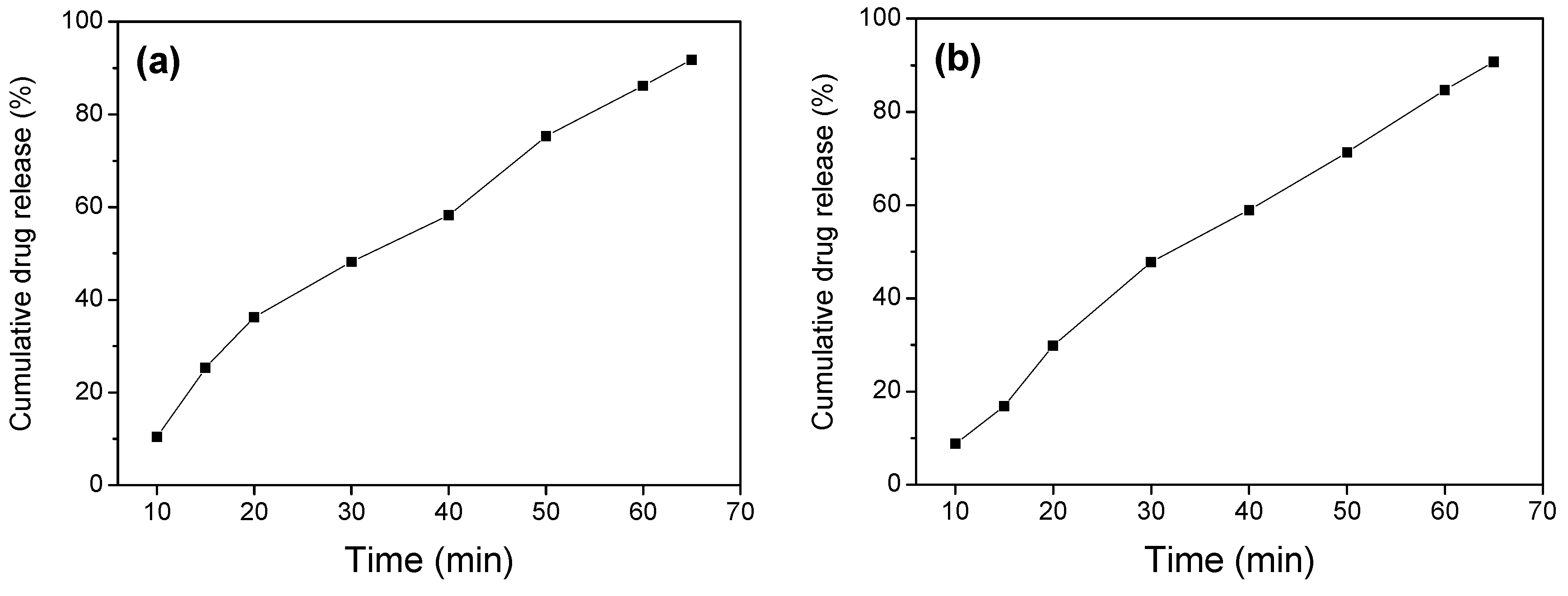

| Sample | Fe3O4/SiO2 | Fe3O4/SiO2/PPy |
|---|---|---|
| Concentration of IBU (mg/mL) | 1 | 1 |
| Drug loading (mg) | 3.329 | 3.640 |
| Encapsulation efficiency (%) | 13.32 | 14.56 |
| Drug loading efficiency (%) | 33.29 | 36.40 |
| Drug release efficiency (%) | 91.82 (stirring for 84 h) | 83.86 (stirring for 84 h) |
| 91.82 (ultrasound for 65 min) | 90.73 (ultrasound for 65 min) |
| Sample | R2 | k | n |
|---|---|---|---|
| Fe3O4/SiO2 | 0.99998 | 22.36 | 0.3409 |
| Fe3O4/SiO2/PPy | 0.99585 | 19.89 | 0.3606 |
| Sample | R2 | k | n |
|---|---|---|---|
| Fe3O4/SiO2 | 1 | 2.165 | 0.9008 |
| Fe3O4/SiO2/PPy | 1 | 1.398 | 0.9907 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Li, B.; Qiao, Y.; Song, J. Monodisperse Fe3O4/SiO2 and Fe3O4/SiO2/PPy Core-Shell Composite Nanospheres for IBU Loading and Release. Materials 2019, 12, 828. https://doi.org/10.3390/ma12050828
Shen L, Li B, Qiao Y, Song J. Monodisperse Fe3O4/SiO2 and Fe3O4/SiO2/PPy Core-Shell Composite Nanospheres for IBU Loading and Release. Materials. 2019; 12(5):828. https://doi.org/10.3390/ma12050828
Chicago/Turabian StyleShen, Lazhen, Bei Li, Yongsheng Qiao, and Jinping Song. 2019. "Monodisperse Fe3O4/SiO2 and Fe3O4/SiO2/PPy Core-Shell Composite Nanospheres for IBU Loading and Release" Materials 12, no. 5: 828. https://doi.org/10.3390/ma12050828
APA StyleShen, L., Li, B., Qiao, Y., & Song, J. (2019). Monodisperse Fe3O4/SiO2 and Fe3O4/SiO2/PPy Core-Shell Composite Nanospheres for IBU Loading and Release. Materials, 12(5), 828. https://doi.org/10.3390/ma12050828




