Application of the Microwave Technique in Continuous Flow Processing of Organophosphorus Chemical Reactions
Abstract
1. Introduction
1.1. Batch vs. Continuous Flow Systems
1.2. Batch Microwave Chemistry
1.3. Continuous Flow Microwave Attempts
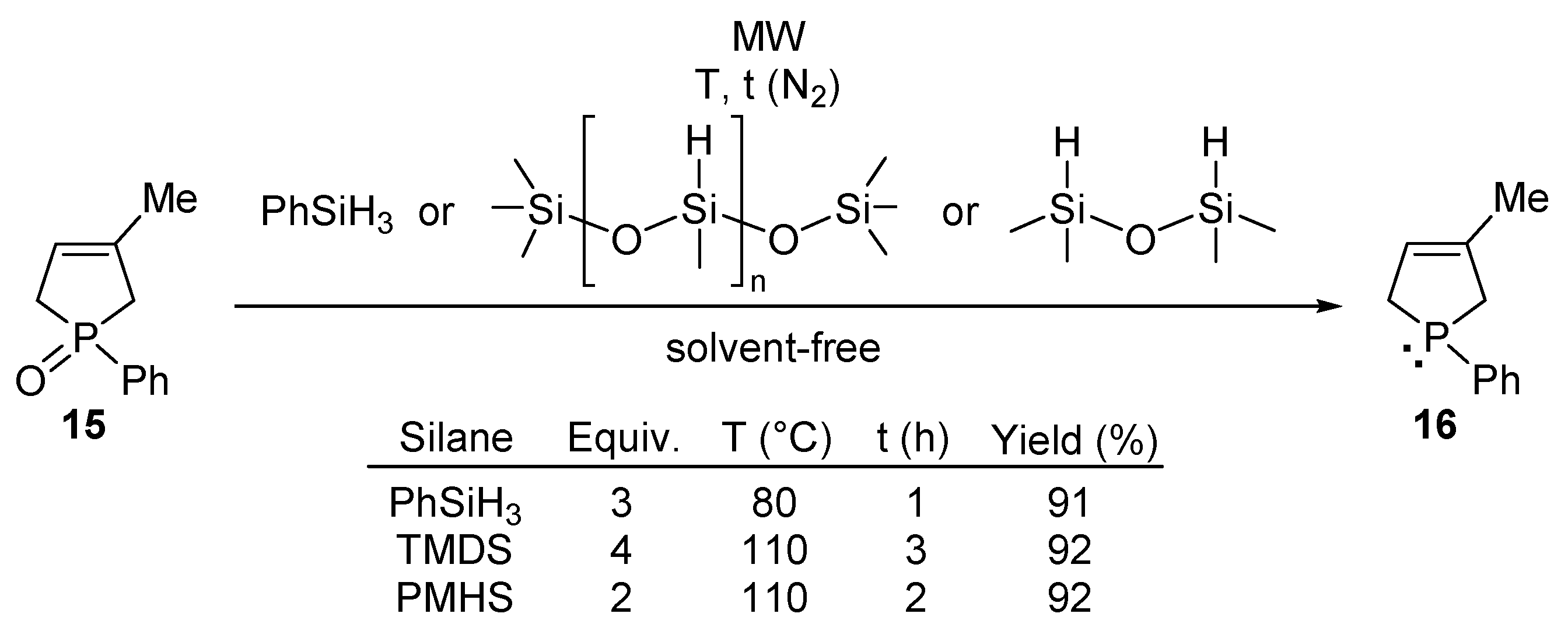
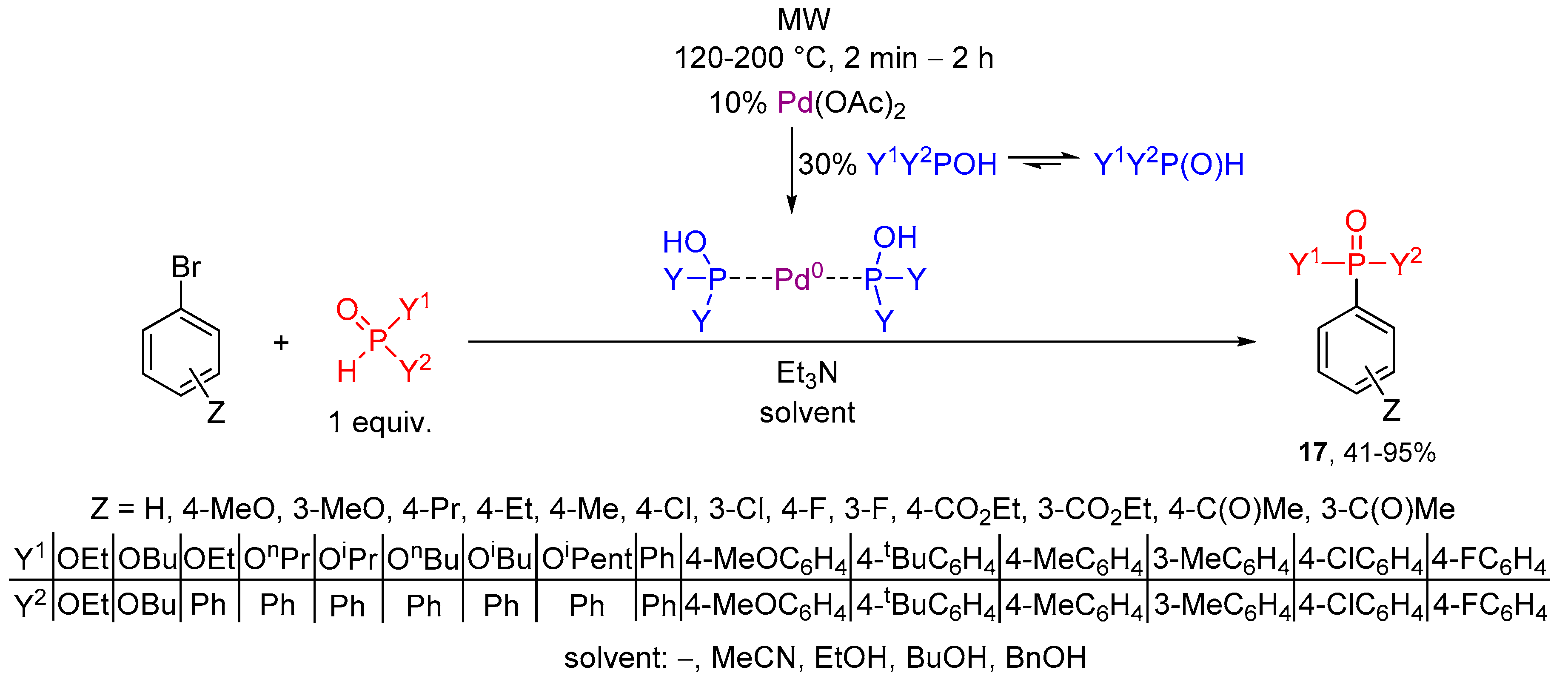
1.4. Microwaves in Batch Organophosphorus Syntheses
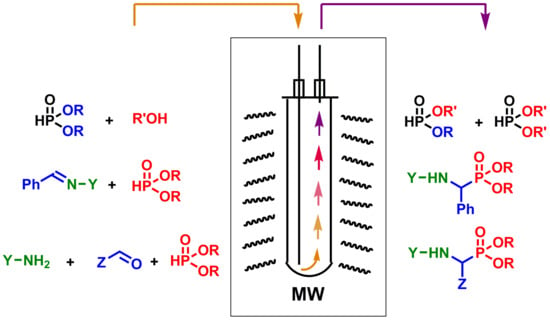
2. Microwave-Assisted Continuous Flow Applications
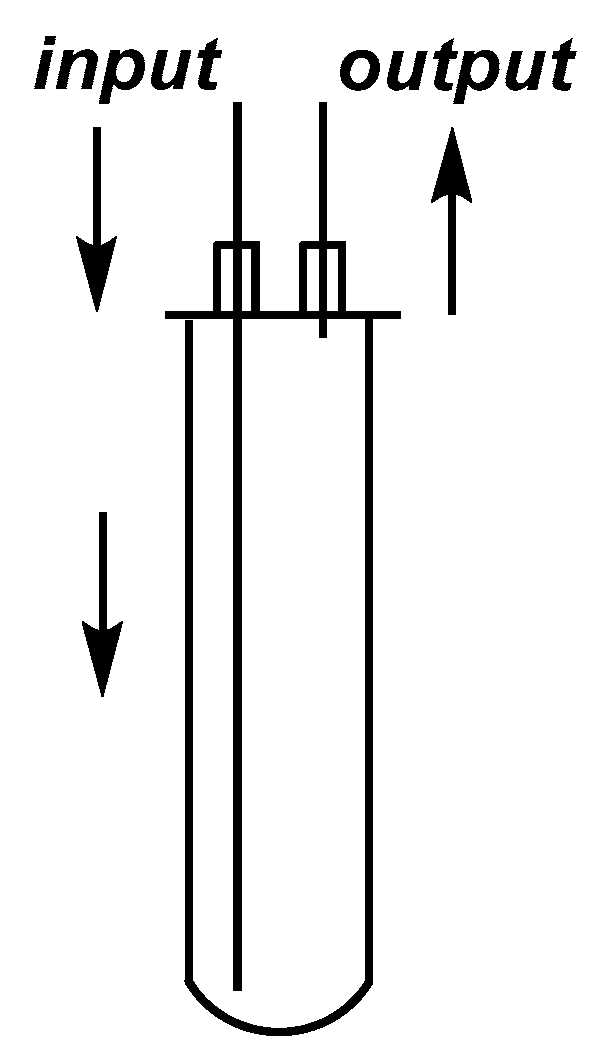
2.1. Development of the Continuous Flow Microwave Device
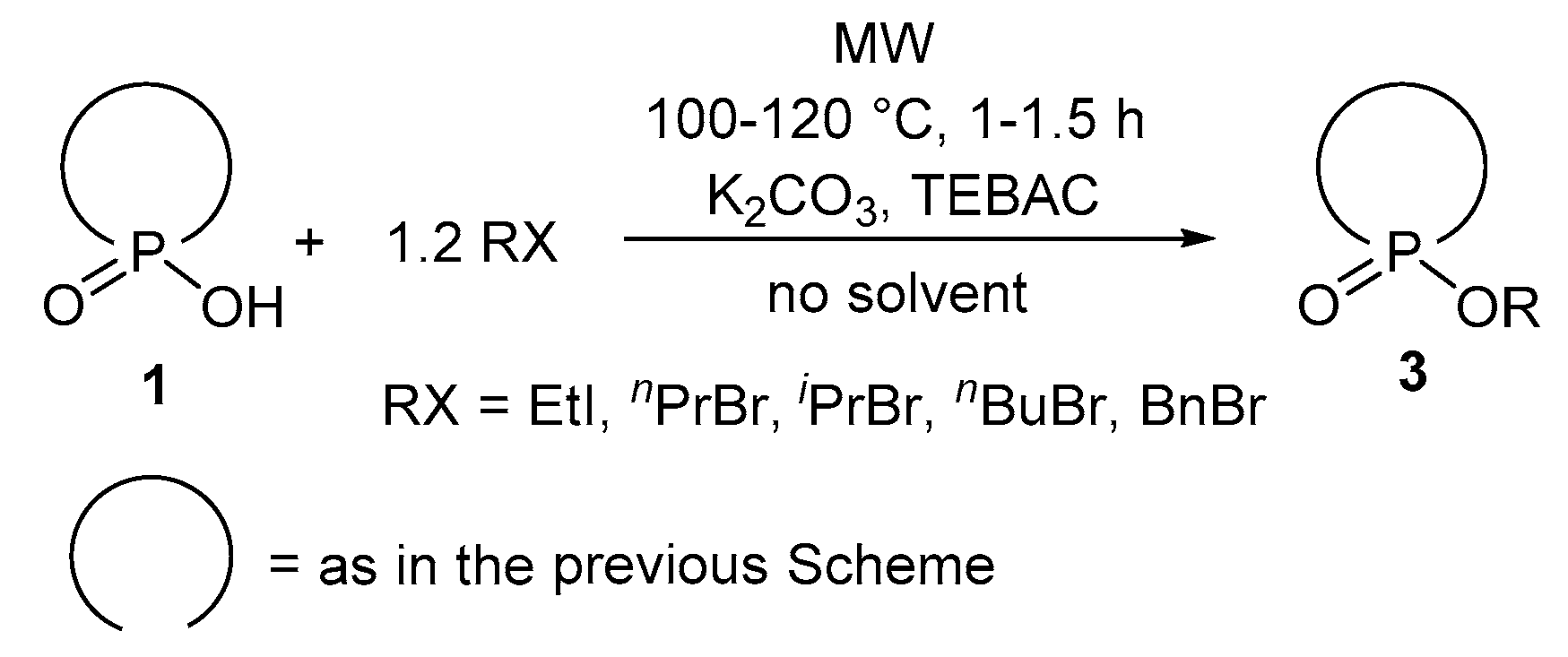
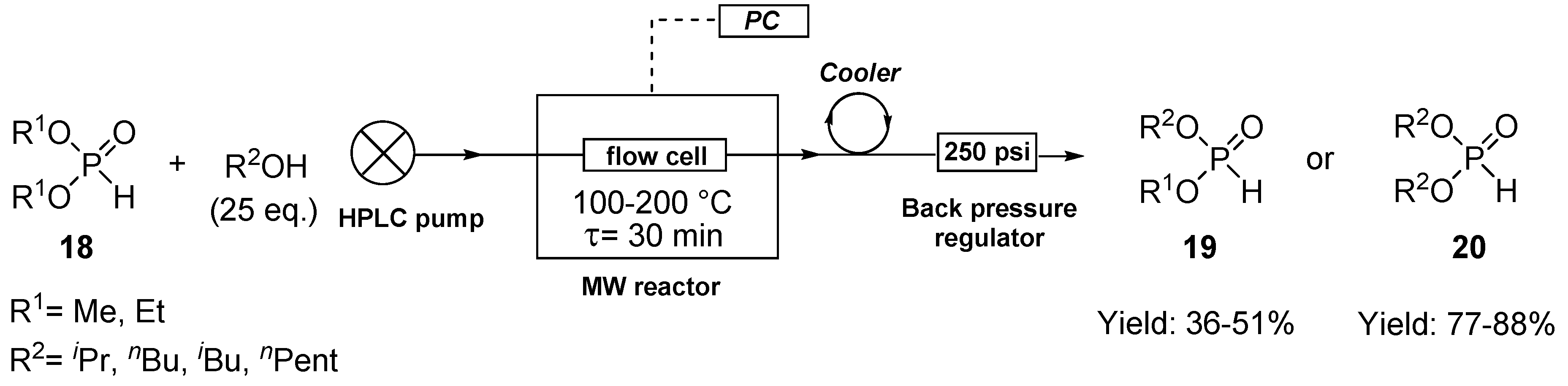
2.2. Elaboration of the Continuous Flow Transesterification of Dialkyl Phosphites
2.3. Continuous Flow Synthesis of α-Aminophosphonates by the aza-Pudovik Reaction
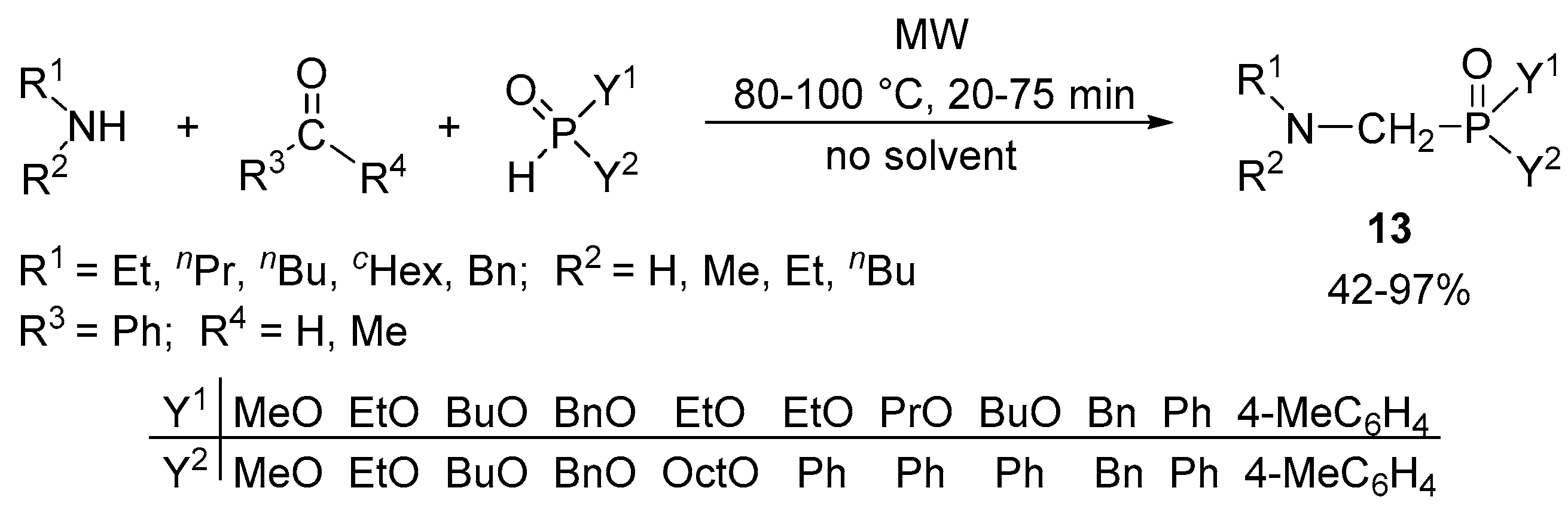
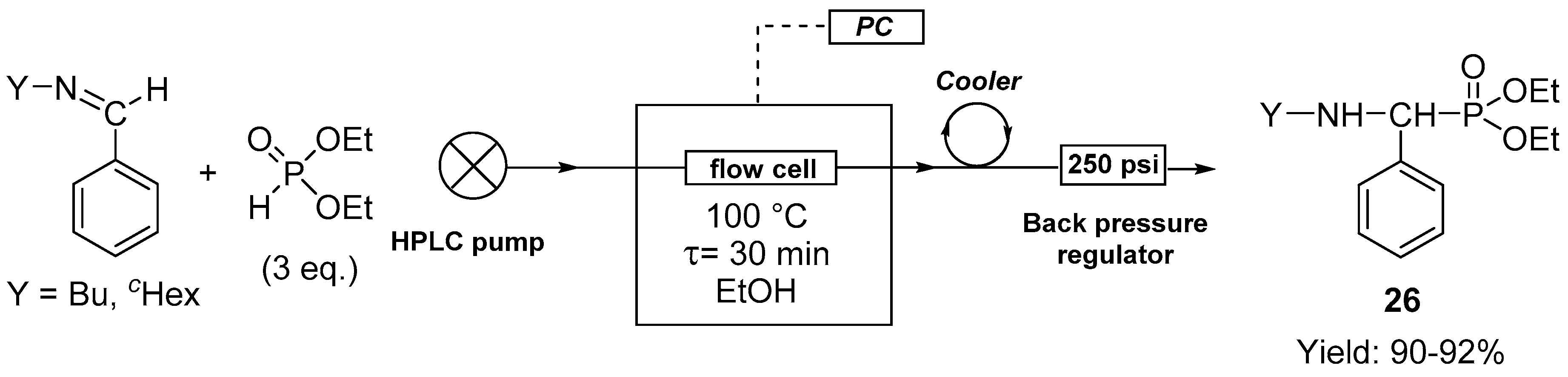
2.4. The Synthesis of α-Aminophosphonates by Continuous Flow Kabachnik–Fields Reaction
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH: Weinheim, Germany, 2000; ISBN 978-3-527-30385-4.
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef] [PubMed]
- Glasnov, T. Continuous-Flow Chemistry in the Research Laboratory; Springer International Publishing: Basel, Switzerland, 2016; ISBN 978-3-319-32194-3. [Google Scholar]
- Vaccaro, L. Sustainable Flow Chemistry: Methods and Applications; Wiley: Weinheim, Germany, 2017; ISBN 978-3-527-338528. [Google Scholar]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battiloccihio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef] [PubMed]
- Bakeev, K.A. Process Analytical Technology: Spectroscopic Tools and Implemented Strategies for the Chemical and Pharmaceutical Industries; Wiley-VCH: Chichester, UK, 2010; ISBN 978-0-470-72207-7. [Google Scholar]
- May, S.A. Flow Chemistry, Continuous Processing, and Continuous Manufacturing: A Pharmaceutical Perspective. J. Flow Chem. 2017, 7, 137–145. [Google Scholar] [CrossRef]
- Strying, P.; Parracho, A.I.R. From discovery to production: Scale-out of continuous flow meso reactors. Beilstein J. Org. Chem. 2009, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Pashkova, A.; Greiner, L. Towards Small-Scale Continuous Chemical Production: Technology Gaps and Challenges. Chem. Ing. Tech. 2011, 83, 1337–1342. [Google Scholar] [CrossRef]
- Cravotto, G.; Carnaroglio, D. Microwave Chemistry; De Gruyter: Berlin, Germany, 2017; ISBN 978-3-11-047993-5. [Google Scholar]
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave Chemical and Materials Processing; Springer: Singapore, 2018; ISBN 978-981-10-6465-4. [Google Scholar]
- Bálint, E.; Keglevich, G. The Spread of the Application of the Microwave Technique in Organic Synthesis. In Milestones in Microwave Chemistry; Keglevich, G., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–10. [Google Scholar]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Giguere, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 1986, 27, 4945–4948. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A.; Dallinger, D. Microwaves in Organic and Medicinal Chemistry, 2nd ed.; Wiley: Weinheim, Germany, 2012; Volume 52, ISBN 978-3-527-33185-7. [Google Scholar]
- Zhao, X.; Zhang, Z.; Wang, L.; Xi, K.; Cao, Q.; Wang, D.; Yang, Y.; Du, Y. Excellent microwave absorption property of Graphene-coated Fe nanocomposites. Sci. Rep. 2013, 3, 3421. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, J.; Pu, B.; Chen, H.; Li, Y.; Wang, Z.; Niu, X. Excellent microwave absorption of lead halide perovskites with high stability. J. Mater. Chem. C 2018, 6, 4201–4207. [Google Scholar] [CrossRef]
- Gong, J.; Yang, F.; Shao, Q.; He, X.; Zhang, X.; Liu, S.; Tang, L.; Deng, Y. Microwave absorption performance of methylimidazolium ionic liquids: Towards novel ultra-wideband metamaterial absorbers. RSC Adv. 2017, 7, 41980–41988. [Google Scholar] [CrossRef]
- Kiss, N.Z.; Bálint, E.; Keglevich, G. Microwave-Assisted Syntheses in Organic Chemistry. In Milestones in Microwave Chemistry; Keglevich, G., Ed.; Springer: Cham, Switzerland, 2016; pp. 11–45. [Google Scholar]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern Microwave Methods in Solid-State Inorganic Materials Chemistry: From Fundamentals to Manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, G.; Sallay, P.; Greiner, I. Continuous flow microwave reactors. Hung. Chem. J. 2008, 63, 278–283. [Google Scholar]
- de la Hoz, A.; Díaz-Ortiz, A. Nonconventional Techniques in Sustainable Flow Chemistry. In Sustainable Flow Chemistry: Methods and Applications; Vaccaro, L., Ed.; Wiley: Weinheim, Germany, 2017; pp. 219–248. [Google Scholar]
- Estel, L.; Poux, M.; Benamara, N.; Polaert, I. Continuous flow-microwave reactor: Where are we? Chem. Eng. Process. 2016, 113, 56–64. [Google Scholar] [CrossRef]
- Wang, C.S. Processing parameters of continuous microwave heating of ethylene-propylene terpolymer. Rubber Chem. Technol. 1984, 57, 134–144. [Google Scholar] [CrossRef]
- Gunasekaran, S. Grain drying using continuous and pulsed microwave energy. Dry. Technol. 1990, 8, 1039–1047. [Google Scholar] [CrossRef]
- Strauss, C.R. A Strategic, ‘Green’ Approach to Organic Chemistry with Microwave Assistance and Predictive Yield Optimization as Core, Enabling Technologies. Aust. J. Chem. 1999, 52, 83–96. [Google Scholar] [CrossRef]
- Baxendale, I.; Hayward, J.; Ley, S. Microwave reactions under continuous flow conditions. Comb. Chem. High Throughput Screen. 2007, 10, 802–836. [Google Scholar] [CrossRef] [PubMed]
- Öhrngren, P.; Fardost, A.; Russo, F.; Schanche, J.-S.; Fagrell, M.; Larhed, M. Evaluation of a nonresonant microwave applicator for continuous-flow chemistry applications. Org. Proc. Res. Dev. 2012, 16, 1053–1063. [Google Scholar] [CrossRef]
- Rydfjord, J.; Svensson, F.; Fagrell, M.; Savmarker, J.; Thulin, M.; Larhed, M. Temperature measurements with two different IR sensors in a continuous-flow microwave heated system. Beilstein J. Org. Chem. 2013, 9, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, L.; Proverbio, E. Influence of process parameters in microwave continuous synthesis of zeolite LTA. Microporous Mesoporous Mater. 2008, 112, 481–493. [Google Scholar] [CrossRef]
- Khadilkar, B.M.; Madyar, V.R. Scaling up of dihydropyridine ester synthesis by using aqueous hydrotrope solutions in a continuous microwave reactor. Org. Process Res. Dev. 2001, 5, 452–455. [Google Scholar] [CrossRef]
- Pillai, U.R.; Sahle-Demessie, E.; Varma, R.S. Hydrodechlorination of chlorinated benzenes in a continuous microwave reactor. Green Chem. 2004, 6, 295–298. [Google Scholar] [CrossRef]
- Correa, R.; Gonzalez, G.; Dougar, V. Emulsion polymerization in a microwave reactor. Polymer 1998, 39, 1471–1474. [Google Scholar] [CrossRef]
- Cáceres, A.; Jaimes, M.; Chávez, G.; Bravo, B.; Ysambertt, F.; Márquez, N. Continuous system with microwave irradiation to obtain alkyl benzoates. Talanta 2005, 68, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Vámosi, P.; Matsuo, K.; Masuda, T.; Sato, K.; Narumi, T.; Takeda, K.; Mase, N. (2018) Rapid Optimization of Reaction Conditions Based on Comprehensive Reaction Analysis Using a Continuous Flow Microwave Reactor. Chem. Rec. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Bonnet, C.; Estel, L.; Ledoux, A.; Mazari, B.; Louis, A. Study of the thermal repartition in a microwave reactor: Application to the nitrobenzene hydrogenation. Chem. Eng. Proc. 2004, 43, 1435–1440. [Google Scholar] [CrossRef]
- Bo, L.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. Degradation of p-nitrophenol in aqueous solution by microwave assisted oxidation process through a granular activated carbon fixed bed. Water Res. 2006, 40, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Bagley, M.C.; Jenkins, R.L.; Lubinu, M.C.; Mason, C.; Wood, R. A simple continuous flow microwave reactor. J. Org. Chem. 2005, 70, 7003–7006. [Google Scholar] [CrossRef] [PubMed]
- Kabza, K.G.; Chapados, B.R.; Getswicki, J.; McGrath, J.L. Microwave-induced esterification using heterogeneous acid catalyst in a low dielectric constant medium. J. Org. Chem. 2000, 65, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Haswell, S.J.; Fletcher, P.D.I. Efficiency, monitoring and control of microwave heating within a continuous flow capillary reactor. Sens. Actuator B Chem. 2005, 105, 516–520. [Google Scholar] [CrossRef]
- Shore, G.; Morin, S.; Organ, M.G. Catalysis in capillaries by Pd thin films using microwave-assisted continuous-flow organic synthesis (MACOS). Angew. Chem. Int. Ed. 2006, 45, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Comer, E.; Organ, M.G. A microreactor for microwave-assisted capillary (continuous flow) organic synthesis (MACOS). J. Am. Chem. Soc. 2005, 127, 8160–8167. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Kaval, N.; Tomar, S.; Van der Eycken, E.; Parmar, V.S. Transition metal-catalyzed carbon–carbon bond formation Suzuki, Heck, and Sonogashira reactions using microwave and microtechnology. Org. Process Res. Dev. 2008, 12, 468–474. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Kappe, C.O. Microwave-assisted synthesis under continuous-flow conditions. Macromol. Rapid Commun. 2007, 28, 395–410. [Google Scholar] [CrossRef]
- Ullah, F.; Samarakoon, T.; Rolfe, A.; Kurtz, R.D.; Hanson, P.R.; Organ, M.G. Scaling out by microwave-assisted, continuous flow organic synthesis (MACOS): Multi-gram synthesis of bromo- and fluoro-benzofused sultams benzthiaoxazepine-1,1-dioxides. Chem. Eur. J. 2010, 16, 10959–10962. [Google Scholar] [CrossRef] [PubMed]
- Dressen, M.H.C.L.; van de Kruijs, B.H.P.; Meuldijk, J.; Vekemans, J.A.J.M.; Hulshof, L.A. Flow processing of microwave-assisted (heterogeneous) organic reactions. Org. Process Res. Dev. 2010, 14, 351–361. [Google Scholar] [CrossRef]
- Bergamelli, F.; Iannelli, M.; Marafie, J.A.; Moseley, J.D. A commercial continuous flow microwave reactor evaluated for scale-up. Org. Process Res. Dev. 2010, 14, 926–930. [Google Scholar] [CrossRef]
- Bagley, M.C.; Fusillo, V.; Jenkins, R.L.; Lubinu, M.C.; Mason, C. Continuous flow processing from microreactors to mesoscale: The Bohlmann-Rahtz cyclodehydration reaction. Org. Biomol. Chem. 2010, 8, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Moseley, J.D.; Lawton, S.J. Initial results from a commercial continuous flow microwave reactor for scale-up. Chem. Today 2007, 25, 16–19. [Google Scholar]
- Benaskar, F.; Hessel, V.; Krtschil, U.; Löb, P.; Stark, A. Intensification of the capillary-based Kolbe-Schmitt synthesis from resorcinol by reactive ionic liquids, microwave heating or a combination thereof. Org. Process Res. Dev. 2009, 13, 970–982. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Barnard, T.M.; Stencel, L.M. Batch and continuous-flow preparation of biodiesel derived from butanol and facilitated by microwave heating. Energy Fuels 2008, 22, 2005–2008. [Google Scholar] [CrossRef]
- Smith, C.J.; Iglesias-Siguenza, F.J.; Baxendale, I.R.; Ley, S.V. Flow and batch mode focused microwave synthesis of 5-amino-4-cyanopyrazoles and their further conversion to 4-aminopyrazolopyrimidines. Org. Biomol. Chem. 2007, 5, 2758–2761. [Google Scholar] [CrossRef] [PubMed]
- Organ, M.G.; Hanson, P.R.; Rolfe, A.; Samarakoon, T.B.; Ullah, F. Accessing stereochemically rich sultams via microwave-assisted, continuous flow organic synthesis (MACOS) scale-out. J. Flow Chem. 2011, 1, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Morschhäuser, R.; Krull, M.; Kayser, C.; Boberski, C.; Bierbaum, R.; Püschner, P.A.; Glasnov, T.N.; Kappe, C.O. Microwave-assisted continuous flow synthesis on industrial scale. Green Process Synth. 2012, 1, 281–290. [Google Scholar] [CrossRef]
- Guenin, E.; Meziane, D. Microwave Assisted Phosphorus Organic Chemistry: A Review. Curr. Org. Chem. 2011, 15, 3465–3485. [Google Scholar] [CrossRef]
- Kiss, N.Z.; Ludányi, K.; Drahos, L.; Keglevich, G. Novel Synthesis of Phosphinates by the Microwave-Assisted Esterification of Phosphinic Acids. Synth. Commun. 2009, 39, 2392–2404. [Google Scholar] [CrossRef]
- Keglevich, G.; Bálint, E.; Kiss, N.Z.; Jablonkai, E.; Hegedűs, L.; Grün, A.; Greiner, I. Microwave-Assisted Esterification of Phosphinic Acids. Curr. Org. Chem. 2011, 15, 1802–1810. [Google Scholar] [CrossRef]
- Keglevich, G.; Kiss, N.Z.; Mucsi, Z.; Körtvélyesi, T. Insights into a surprising reaction: The microwave-assisted direct esterification of phosphinic acids. Org. Biomol. Chem. 2012, 10, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.Z.; Böttger, É.; Drahos, L.; Keglevich, G. Microwave-assisted direct esterification of cyclic phosphinic acids. Heteroat. Chem. 2013, 24, 283–288. [Google Scholar] [CrossRef]
- Mucsi, Z.; Kiss, N.Z.; Keglevich, G. A quantum chemical study on the mechanism and energetics of the direct esterification, thioesterification and amidation of 1-hydroxy-3-methyl-3-phospholene 1-oxide. RSC Adv. 2014, 4, 11948–11954. [Google Scholar] [CrossRef]
- Kiss, N.Z.; Keglevich, G. Microwave-assisted direct esterification of cyclic phosphinic acids in the presence of ionic liquids. Tetrahedron Lett. 2016, 57, 971–974. [Google Scholar] [CrossRef]
- Bálint, E.; Jablonkai, E.; Bálint, M.; Keglevich, G. Alkylating esterification of 1-hydroxy-3-phospholene oxides under solventless MW conditions. Heteroat. Chem. 2010, 21, 211–214. [Google Scholar] [CrossRef]
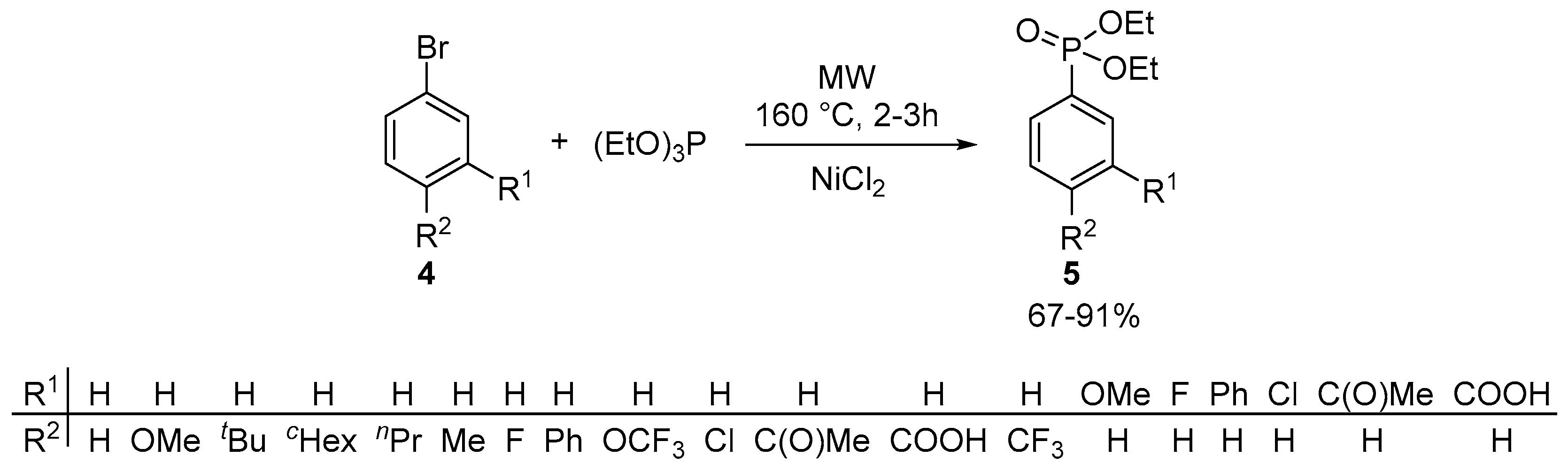
- Keglevich, G.; Grün, A.; Bölcskei, A.; Drahos, L.; Kraszni, M.; Balogh, G.T. Synthesis and proton dissociation properties of arylphosphonates: A microwave-assisted catalytic arbuzov reaction with aryl bromides. Heteroat. Chem. 2012, 23, 574–582. [Google Scholar] [CrossRef]
- Dargó, G.; Bölcskei, A.; Grün, A.; Béni, S.; Szántó, Z.; Lopata, A.; Keglevich, G.; Balogh, G.T. Proton dissociation properties of arylphosphonates: Determination of accurate Hammett equation parameters. J. Pharm. Biomed. Anal. 2017, 143, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Greiner, I.; Grün, A.; Ludányi, K.; Keglevich, G. Solid–liquid two-phase alkylation of tetraethyl methylenebisphosphonate under microwave irradiation. Heteroat. Chem. 2011, 22, 11–14. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Blastik, Z.; Greiner, I. Solid-liquid phase alkylation of P=O-functionalized CH acidic compounds utilizing phase transfer catalysis and microwave irradiation. Heteroat. Chem. 2011, 22, 174–179. [Google Scholar] [CrossRef]
- Grün, A.; Blastik, Z.; Drahos, L.; Keglevich, G. Microwave-assisted alkylation of diethyl ethoxycarbonylmethylphosphonate under solventless conditions. Heteroat. Chem. 2012, 23, 241–246. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A. Microwave Irradiation as a Substitute for Phase Transfer Catalyst in CAlkylation Reactions. Curr. Green Chem. 2015, 2, 254–263. [Google Scholar] [CrossRef]
- Grün, A.; Bálint, E.; Keglevich, G. Solid-Liquid Phase C-Alkylation of Active Methylene Containing Compounds under Microwave Conditions. Catalysts 2015, 5, 634–652. [Google Scholar] [CrossRef]
- Grün, A.; Blastik, Z.; Drahos, L.; Keglevich, G. Dialkylation of diethyl ethoxycarbonylmethylphosphonate under microwave and solventless conditions. Heteroat. Chem. 2014, 25, 107–113. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrényi, A. Eco-Friendly Accomplishment of the Extended Kabachnik–Fields Reaction; a Solvent- and Catalyst-Free Microwave-Assisted Synthesis of α-Aminophosphonates and α-Aminophosphine Oxides. Lett. Org. Chem. 2008, 5, 616–622. [Google Scholar] [CrossRef]
- Keglevich, G.; Bálint, E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules 2012, 17, 12821–12835. [Google Scholar] [CrossRef] [PubMed]
- Tajti, Á.; Bálint, E.; Keglevich, G. Synthesis of ethyl octyl α-aminophosphonate derivatives. Curr. Org. Synth. 2016, 13, 638–645. [Google Scholar] [CrossRef]
- Bálint, E.; Tóth, R.E.; Keglevich, G. Synthesis of alkyl α-aminomethyl-phenylphosphinates and N,N-bis(alkoxyphenylphosphinylmethyl)amines by the microwave-assisted Kabachnik–Fields reaction. Heteroat. Chem. 2016, 27, 323–335. [Google Scholar] [CrossRef]
- Bálint, E.; Tajti, Á.; Kalocsai, D.; Mátravölgyi, B.; Karaghiosoff, K.; Czugler, M.; Keglevich, G. Synthesis and utilization of optically active α-aminophosphonate derivatives by Kabachnik-Fields reaction. Tetrahedron 2017, 73, 5659–5667. [Google Scholar] [CrossRef]
- Bálint, E.; Tripolszky, A.; Jablonkai, E.; Karaghiosoff, K.; Czugler, M.; Mucsi, Z.; Kollár, L.; Pongrácz, P.; Keglevich, G. Synthesis and use of α-aminophosphine oxides and N,N-bis(phosphinoylmethyl)amines—A study on the related ring platinum complexes. J. Organomet. Chem. 2016, 801, 111–121. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrényi, A.; Szöllősy, Á.; Drahos, L. Synthesis of bis(phosphonatomethyl)-,bis(phosphinatomethyl)-, and bis(phosphinoxidomethyl)amines, as well as related ring bis(phosphine) platinum complexes. Synth. Commun. 2011, 41, 2265–2272. [Google Scholar] [CrossRef]
- Bálint, E.; Fazekas, E.; Pintér, G.; Szöllősy, Á.; Holczbauer, T.; Czugler, M.; Drahos, L.; Körtvélyesi, T.; Keglevich, G. Synthesis and utilization of the bis(>P(O)CH2)amine derivatives obtained by the double Kabachnik–Fields reaction with cyclohexylamine; Quantum chemical and X-ray study of the related bidentate chelate platinum complexes. Curr. Org. Chem. 2012, 16, 547–554. [Google Scholar] [CrossRef]
- Bálint, E.; Fazekas, E.; Pongrácz, P.; Kollár, L.; Drahos, L.; Holczbauer, T.; Czugler, M.; Keglevich, G. N-Benzyl and N-aryl bis(phospha-Mannich adducts): Synthesis and catalytic activity of the related bidentate chelate platinum complexes in hydroformylation. J. Organomet. Chem. 2012, 717, 75–82. [Google Scholar] [CrossRef]
- Li, Y.H.; Das, S.; Zhou, S.L.; Junge, K.; Beller, M. General and Selective Copper-Catalyzed Reduction of Tertiary and Secondary Phosphine Oxides: Convenient Synthesis of Phosphines. J. Am. Chem. Soc. 2012, 134, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Keglevich, G. The reduction of tertiary phosphine oxides by silanes. Curr. Org. Chem. 2017, 21, 569–585. [Google Scholar] [CrossRef]
- Keglevich, G.; Kovács, T.; Csatlós, F. The deoxygenation of phosphine oxides under green chemical conditions. Heteroat. Chem. 2015, 26, 199–205. [Google Scholar] [CrossRef]
- Kovács, T.; Urbanics, A.; Csatlós, F.; Binder, J.; Falk, A.; Uhlig, F.; Keglevich, G. A Study on the Deoxygenation of Phosphine Oxides by Different Silane Derivatives. Curr. Org. Synth. 2016, 13, 148–153. [Google Scholar] [CrossRef]
- Kovács, T.; Urbanics, A.; Csatlós, F.; Keglevich, G. A study on the deoxygenation of trialkyl-, dialkyl-phenyl- and alkyl-diphenyl phosphine oxides by hydrosilanes. Heteroat. Chem. 2017, 28, e21376. [Google Scholar] [CrossRef]
- Hirao, T.; Masunaga, T.; Yamada, N.; Ohshiro, Y.; Agawa, T. Palladium-catalyzed New Carbon-Phosphorus Bond Formation. Bull. Chem. Soc. Jpn. 1982, 55, 909–913. [Google Scholar] [CrossRef]
- Hirao, T.; Masunaga, T.; Ohshiro, Y.; Agawa, T. A Novel Synthesis of Dialkyl Arenephosphonates. Synthesis 1981, 1981, 56–57. [Google Scholar] [CrossRef]
- Jablonkai, E.; Keglevich, G. P-ligand-free, microwave-assisted variation of the Hirao reaction under solvent-free conditions; The P-C coupling reaction of >p(O)H species and bromoarenes. Tetrahedron Lett. 2013, 54, 4185–4188. [Google Scholar] [CrossRef]
- Keglevich, G.; Jablonkai, E.; Balázs, L.B. A “green” variation of the Hirao reaction: The P–C coupling of diethyl phosphite, alkyl phenyl-H-phosphinates and secondary phosphine oxides with bromoarenes using a P-ligand-free Pd(OAc)2catalyst under microwave and solvent-free conditions. RSC Adv. 2014, 4, 22808–22816. [Google Scholar] [CrossRef]
- Keglevich, G.; Henyecz, R.; Mucsi, Z.; Kiss, N.Z. The Palladium Acetate-Catalyzed Microwave-Assisted Hirao Reaction without an Added Phosphorus Ligand as a “Green” Protocol: A Quantum Chemical Study on the Mechanism. Adv. Synth. Catal. 2017, 359, 4322–4331. [Google Scholar] [CrossRef] [PubMed]
- Ergan, B.T.; Bayaromoglu, M. Poly (l-lactic acid) synthesis using continuous microwave irradiation–simultaneous cooling method. Chem. Eng. Commun. 2018, 205, 1665–1677. [Google Scholar] [CrossRef]
- Chen, S.-T.; Chiou, S.-H.; Wang, K.-T. Preparative scale organic synthesis using a kitchen microwave oven. J. Chem. Soc. Chem. Commun. 1990, 807–809. [Google Scholar] [CrossRef]
- Pipus, G.; Plazl, I.; Koloini, T. Esterification of benzoic acid in microwave tubular flow reactor. Chem. Eng. J. 2000, 76, 239–245. [Google Scholar] [CrossRef]
- Cablewski, T.; Faux, A.F.; Strauss, C.R. Development and Application of a Continuous Microwave Reactor for Organic Synthesis. J. Org. Chem. 1994, 59, 3408–3412. [Google Scholar] [CrossRef]
- Krull, M.; Moschhaeuser, R. Continuous Method for Producing Esters of Aromatic Carboxylic Acids. U.S. Patent 0088918, 12 April 2012. [Google Scholar]
- Tajti, Á.; Tóth, N.; Bálint, E.; Keglevich, G. Esterification of benzoic acid in a continuous flow microwave reactor. J. Flow Chem. 2018, 8, 11–19. [Google Scholar] [CrossRef]
- Bálint, E.; Tajti, Á.; Drahos, L.; Ilia, G.; Keglevich, G. Alcoholysis of dialkyl phosphites under Microwave conditions. Curr. Org. Chem. 2013, 17, 555–562. [Google Scholar] [CrossRef]
- Bálint, E.; Tripolszky, A.; Tajti, Á. Synthesis of α-aminophosphonates by the Kabachnik–Fields reaction. In Organophosphorus Chemistry; Keglevich, G., Ed.; De Gruyter: Berlin, Germany, 2018; pp. 108–147. [Google Scholar]
- Keglevich, G.; Bálint, E.; Tajti, Á.; Mátravölgyi, B.; Balogh, G.T.; Bálint, M.; Ilia, G. Microwave-assisted alcoholysis of dialkyl phosphites by ethylene glycol and ethanolamine. Pure Appl. Chem. 2014, 86, 1723–1728. [Google Scholar] [CrossRef]
- Tajti, Á.; Keglevich, G.; Bálint, E. Microwave-assisted alcoholysis of dialkyl H-phosphonates by diols and amino alcohols. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 769–775. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Yuan, Y.-Y.; Du, J.-Z.; Yang, X.-Z.; Wang, J. Recent Progress in Polyphosphoesters: From Controlled Synthesis to Biomedical Applications. Macromol. Biosci. 2009, 9, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Troev, K.D. Polyphosphoesters: Chemistry and Application; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Bálint, E.; Tajti, Á.; Tóth, N.; Keglevich, G. Continuous Flow Alcoholysis of Dialkyl H-Phosphonates with Aliphatic Alcohols. Molecules 2018, 23, 1618. [Google Scholar] [CrossRef] [PubMed]
- Bálint, E.; Tajti, Á.; Ladányi-Pára, K.; Tóth, N.; Mátravölgyi, B.; Keglevich, G. Continuous flow synthesis of α-aryl-α-aminophosphonates. Pure Appl. Chem. 2019, 91, 67–76. [Google Scholar] [CrossRef]
- Bálint, E.; Tajti, Á.; Ádám, A.; Csontos, I.; Karaghiosoff, K.; Czugler, M.; Ábrányi-Balogh, P.; Keglevich, G. The synthesis of α-aryl-α-aminophosphonates and α-aryl-α-aminophosphine oxides by the microwave-assisted Pudovik reaction. Beilstein J. Org. Chem. 2017, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]




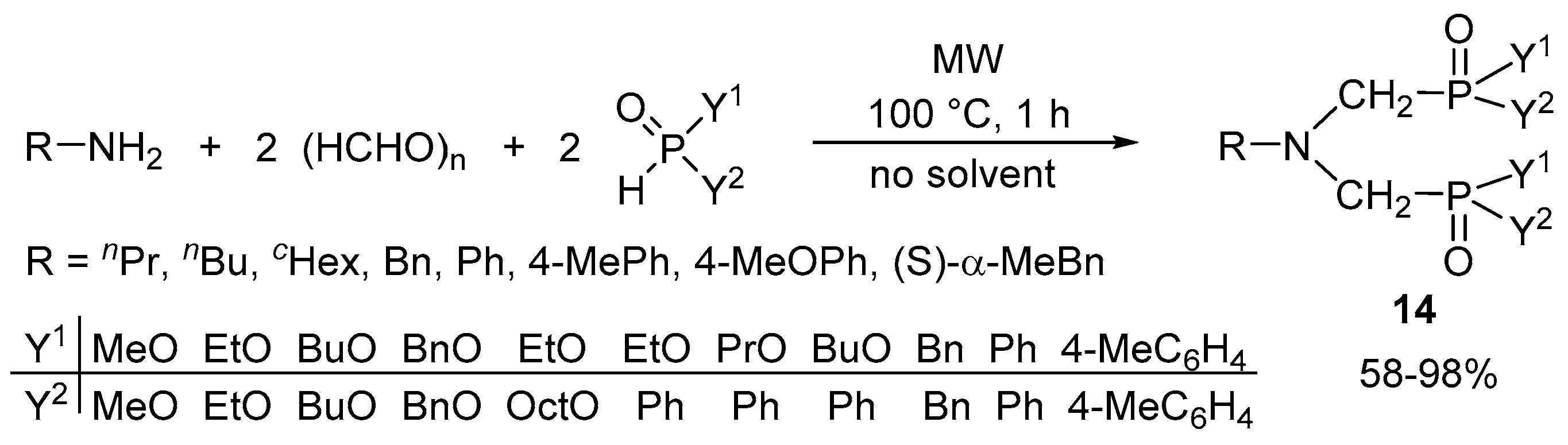



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bálint, E.; Tajti, Á.; Keglevich, G. Application of the Microwave Technique in Continuous Flow Processing of Organophosphorus Chemical Reactions. Materials 2019, 12, 788. https://doi.org/10.3390/ma12050788
Bálint E, Tajti Á, Keglevich G. Application of the Microwave Technique in Continuous Flow Processing of Organophosphorus Chemical Reactions. Materials. 2019; 12(5):788. https://doi.org/10.3390/ma12050788
Chicago/Turabian StyleBálint, Erika, Ádám Tajti, and György Keglevich. 2019. "Application of the Microwave Technique in Continuous Flow Processing of Organophosphorus Chemical Reactions" Materials 12, no. 5: 788. https://doi.org/10.3390/ma12050788
APA StyleBálint, E., Tajti, Á., & Keglevich, G. (2019). Application of the Microwave Technique in Continuous Flow Processing of Organophosphorus Chemical Reactions. Materials, 12(5), 788. https://doi.org/10.3390/ma12050788