Limits of Cation Solubility in AMg2Sb2 (A = Mg, Ca, Sr, Ba) Alloys
Abstract
1. Introduction
2. Methods
2.1. Synthesis
2.2. Structural Characterization
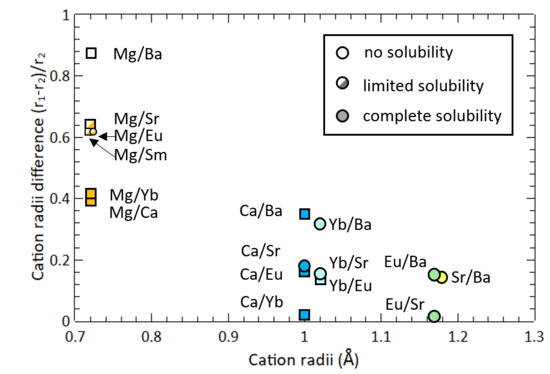
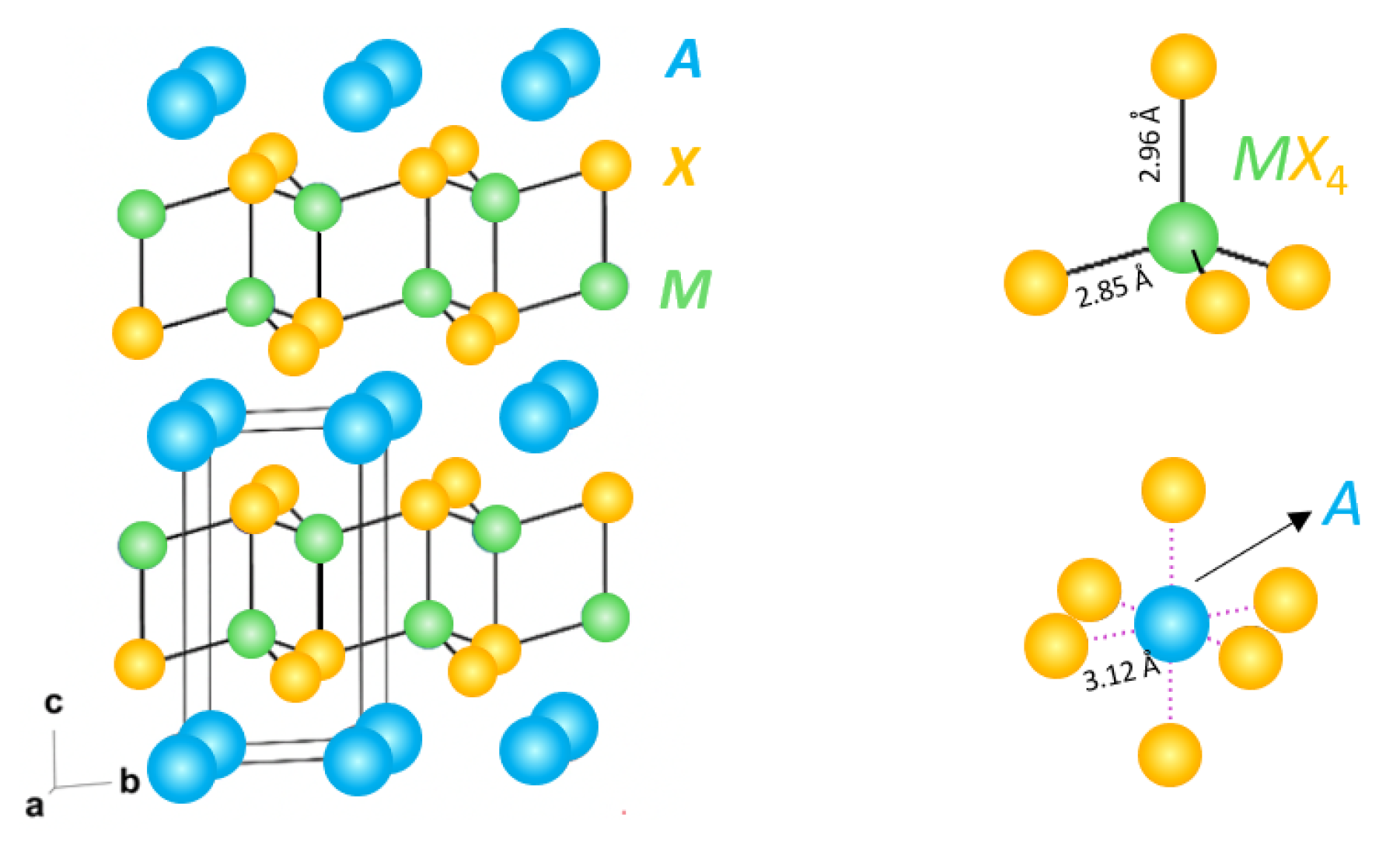
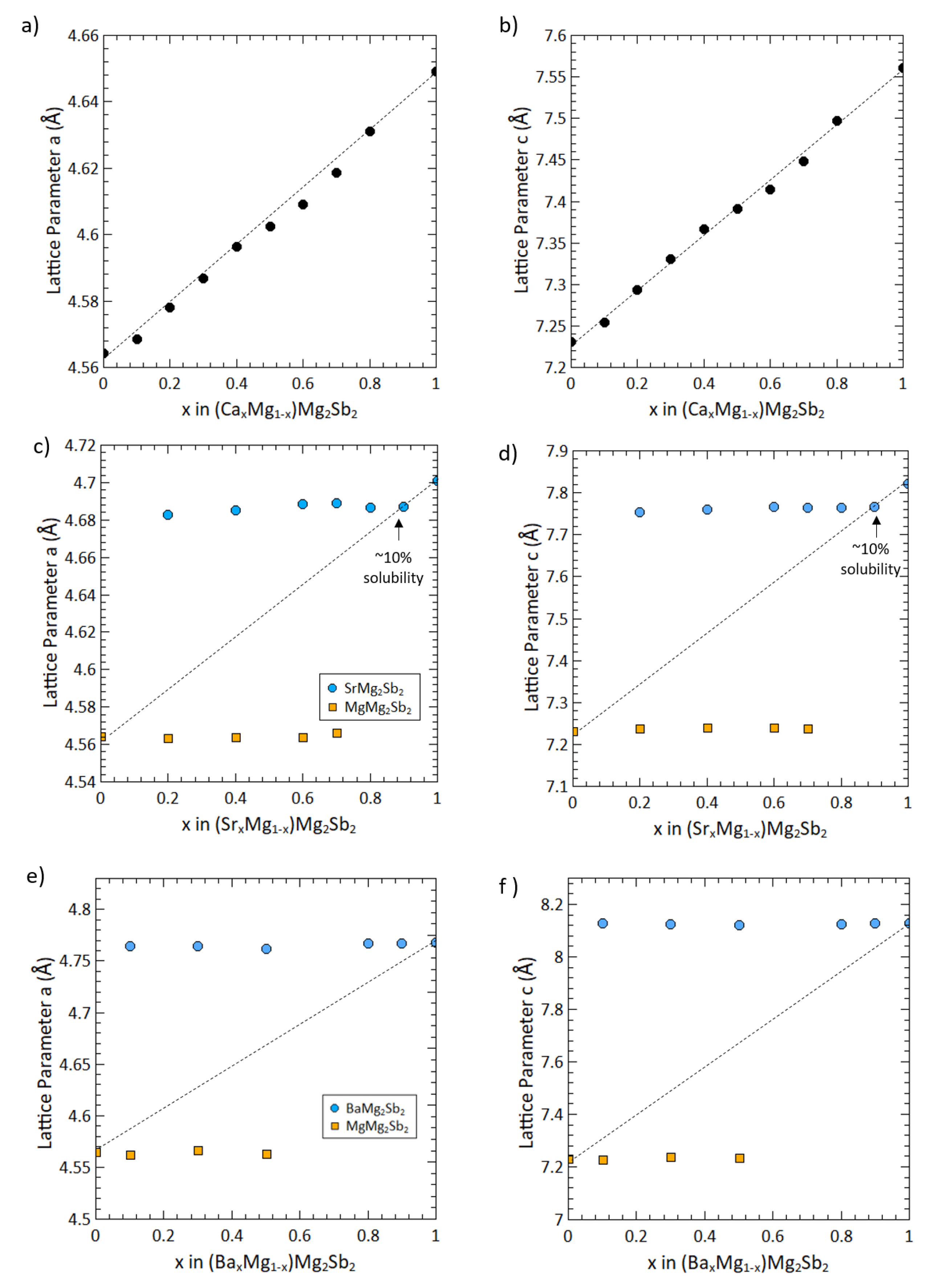
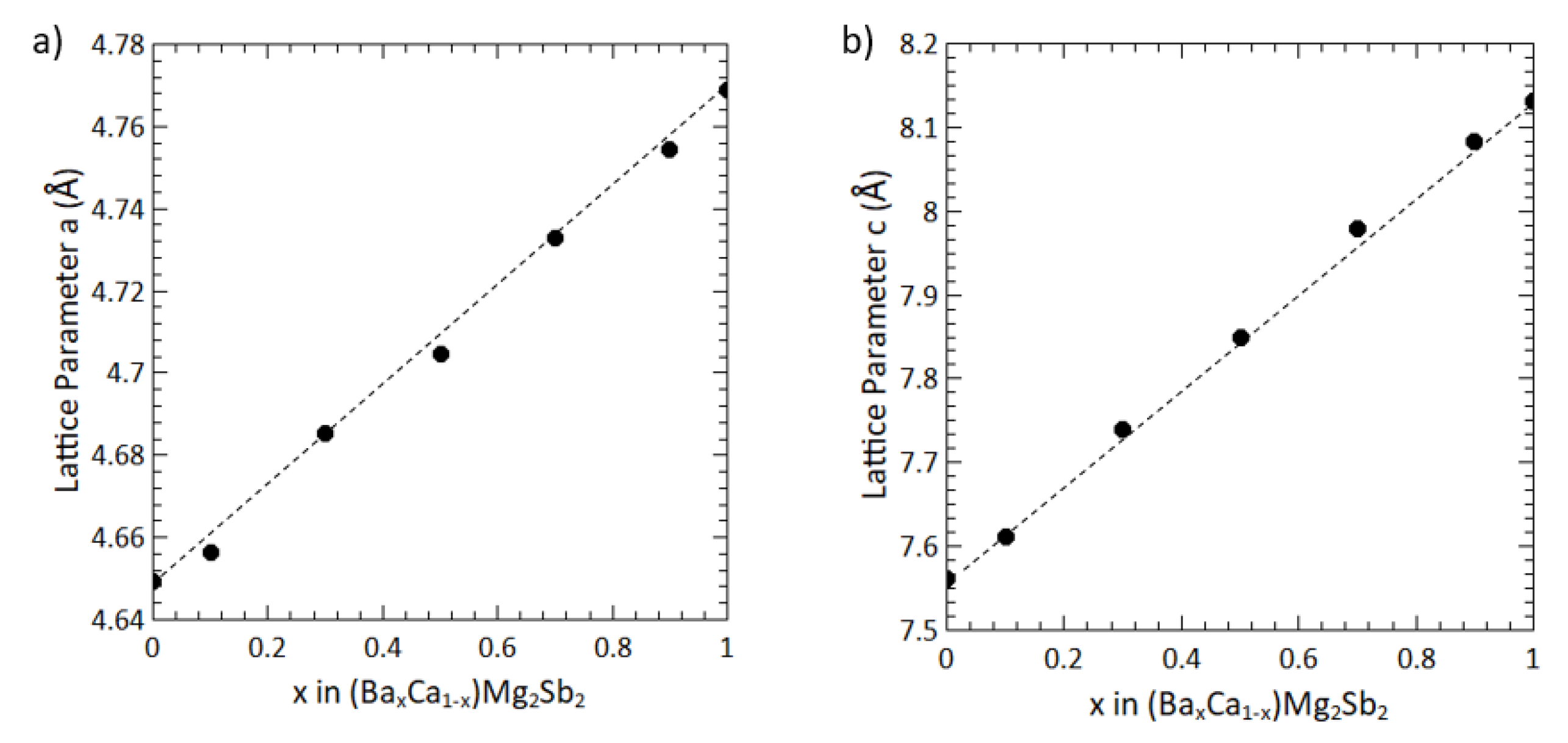
3. Results and Discussion
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.J.; Tang, M.B.; Chen, H.H.; Yang, X.X.; Zhao, J.T.; Burkhardt, U.; Grin, Y. Synthesis and high thermoelectric efficiency of Zintl phase YbCd2−xZnxSb2. Appl. Phys. Lett. 2009, 94, 092106. [Google Scholar] [CrossRef]
- Tamaki, H.; Sato, H.K.; Kanno, T. Isotropic Conduction Network and Defect Chemistry in Mg3+δSb2-Based Layered Zintl Compounds with High Thermoelectric Performance. Adv. Mater. 2016, 28, 10182–10187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, L.; Mamakhel, A.; Jørgensen, M.R.V.; Iversen, B.B. High-performance low-cost n-type Se-doped Mg3Sb2-based Zintl compounds for thermoelectric application. Chem. Mater. 2017, 29, 5371–5383. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Pedersen, S.H.; Yin, H.; Hung, L.T.; Iversen, B.B. Discovery of high-performance low-cost n-type Mg3Sb2-based thermoelectric materials with multi-valley conduction bands. Nat. Comm. 2017, 8, 13901. [Google Scholar] [CrossRef] [PubMed]
- Imasato, K.; Kang, S.D.; Ohno, S.; Snyder, G.J. Band engineering in Mg3Sb2 by alloying with Mg3Bi2 for enhanced thermoelectric performance. Mater. Horiz. 2018, 5, 59–64. [Google Scholar] [CrossRef]
- Shuai, J.; Mao, J.; Song, S.; Zhu, Q.; Sun, J.; Wang, Y.; He, R.; Zhou, J.; Chen, G.; Singh, D.J.; et al. Tuning the carrier scattering mechanism to effectively improve the thermoelectric properties. Energy Environ. Sci. 2017, 10, 799–807. [Google Scholar] [CrossRef]
- Peng, W.; Chanakian, S.; Zevalkink, A. Crystal chemistry and thermoelectric transport of layered AM2X2 compounds. Inorg. Chem. Front. 2018, 5, 1744–1759. [Google Scholar] [CrossRef]
- Burdett, J.K.; Miller, G.J. Fragment formalism in main-group solids: applications to aluminum boride (AlB2), calcium aluminum silicide (CaAl2Si2), barium-aluminum (BaAl4), and related materials. Chem. Mater. 1990, 2, 12–26. [Google Scholar] [CrossRef]
- Mao, J.; Wu, Y.; Song, S.; Zhu, Q.; Shuai, J.; Liu, Z.; Pei, Y.; Ren, Z. Defect Engineering for Realizing High Thermoelectric Performance in n-Type Mg3Sb2-Based Materials. ACS Energy Lett. 2017, 2, 2245–2250. [Google Scholar] [CrossRef]
- Imasato, K.; Ohno, S.; Kang, S.D.; Snyder, G.J. Improving the thermoelectric performance in Mg3+xSb1.5Bi0.49Te0.01 by reducing excess Mg. APL Mater. 2018, 6, 016106. [Google Scholar] [CrossRef]
- Kanno, T.; Tamaki, H.; Sato, H.K.; Kang, S.D.; Ohno, S.; Imasato, K.; Kuo, J.J.; Snyder, G.J.; Miyazaki, Y. Enhancement of average thermoelectric figure of merit by increasing the grain-size of Mg3.2Sb1.5Bi0.49Te0.01. Appl. Phys. Lett. 2018, 112, 033903. [Google Scholar] [CrossRef]
- Gorai, P.; Ortiz, B.R.; Toberer, E.S.; Stevanović, V. Investigation of n-type doping strategies for Mg3Sb2. J. Mater. Chem. A 2018, 6, 13806–13815. [Google Scholar] [CrossRef]
- Imasato, K.; Wood, M.; Kuo, J.J.; Snyder, G.J. Improved stability and high thermoelectric performance through cation site doping in n-type La-doped Mg3Sb1.5Bi0.5. J. Mater. Chem. A 2018, 6, 19941–19946. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Madsen, G.K.; Fischer, K.F.; Zhang, W.; Shi, X.; Iversen, B.B. Designing high-performance layered thermoelectric materials through orbital engineering. Nat. Commun. 2016, 7, 10892. [Google Scholar] [CrossRef] [PubMed]
- Shuai, J.; Geng, H.; Lan, Y.; Zhu, Z.; Wang, C.; Liu, Z.; Bao, J.; Chu, C.W.; Sui, J.; Ren, Z. Higher thermoelectric performance of Zintl phases (Eu0.5Yb0.5)1−xCaxMg2Bi2 by band engineering and strain fluctuation. Proc. Natl. Acad. Sci. USA 2016, 113, E4125–E4132. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Petretto, G.; Rignanese, G.M.; Hautier, G.; Zevalkink, A. An unlikely route to low lattice thermal conductivity: Small atoms in a simple layered structure. Joule 2018, 2, 1879–1893. [Google Scholar] [CrossRef]
- Xin, J.; Li, G.; Auffermann, G.; Borrmann, H.; Schnelle, W.; Gooth, J.; Zhao, X.; Zhu, T.; Felser, C.; Fu, C. Growth and transport properties of Mg3X2 (X = Sb, Bi) single crystals. Mater. Today Phys. 2018, 7, 61–68. [Google Scholar] [CrossRef]
- Maccioni, M.B.; Farris, R.; Fiorentini, V. Ab initio thermal conductivity of thermoelectric Mg3Sb2: Evidence for dominant extrinsic effects. Phys. Rev. B 2018, 98, 220301. [Google Scholar] [CrossRef]
- Wubieneh, T.A.; Wei, P.C.; Yeh, C.C.; Chen, S.Y.; Chen, Y.Y. Thermoelectric Properties of Zintl Phase Compounds of Ca1−xEuxZn2Sb2 (0 ≤ x ≤ 1). J. Electron. Mater. 2016, 45, 1942–1946. [Google Scholar] [CrossRef]
- Shuai, J.; Wang, Y.; Liu, Z.; Kim, H.S.; Mao, J.; Sui, J.; Ren, Z. Enhancement of thermoelectric performance of phase pure Zintl compounds Ca1−xYbxZn2Sb2, Ca1−xEuxZn2Sb2, and Eu1−xYbxZn2Sb2 by mechanical alloying and hot pressing. Nano Energy 2016, 25, 136–144. [Google Scholar] [CrossRef]
- Gascoin, F.; Ottensmann, S.; Stark, D.; Haïle, S.M.; Snyder, G.J. Zintl phases as thermoelectric materials: tuned transport properties of the compounds CaxYb1−xZn2Sb2. Adv. Funct. Mater. 2005, 15, 1860–1864. [Google Scholar] [CrossRef]
- Cao, Q.G.; Zhang, H.; Tang, M.B.; Chen, H.H.; Yang, X.X.; Grin, Y.; Zhao, J.T. Zintl phase Yb1−xCaxCd2Sb2 with tunable thermoelectric properties induced by cation substitution. J. Appl. Phys. 2010, 107, 053714. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, L.; Tang, M.B.; Man, Z.; Chen, H.; Yang, X.; Baitinger, M.; Grin, Y.; Zhao, J.T. Thermoelectric properties of YbxEu1−xCd2Sb2. J. Chem. Phys. 2010, 133, 194701. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.; Bobev, S. Zintl phases with group 15 elements and the transition metals: A brief overview of pnictides with diverse and complex structures. J. Solid State Chem. 2019, 270, 346–359. [Google Scholar] [CrossRef]
- Zhou, T.; Mao, J.; Jiang, J.; Song, S.; Zhu, H.; Zhu, Q.; Zhang, Q.; Ren, W.; Wang, Z.; Wang, C.; et al. Large reduction of thermal conductivity leading to enhanced thermoelectric performance in p-type Mg3Bi2-YbMg2Bi2 solid solutions. J. Mater. Chem. C 2019, 7, 434–440. [Google Scholar] [CrossRef]
- Shuai, J.; Liu, Z.; Kim, H.S.; Wang, Y.; Mao, J.; He, R.; Sui, J.; Ren, Z. Thermoelectric properties of Bi-based Zintl compounds Ca1−xYbxMg2Bi2. J. Mater. Chem. A 2016, 4, 4312–4320. [Google Scholar] [CrossRef]
- Pomrehn, G.S.; Zevalkink, A.; Zeier, W.G.; van de Walle, A.; Snyder, G.J. Defect-controlled electronic properties in AZn2Sb2 Zintl phases. Angew. Chem. Int. Ed. Engl. 2014, 53, 3422–3426. [Google Scholar] [CrossRef]
- Zevalkink, A.; Zeier, W.G.; Cheng, E.; Snyder, J.; Fleurial, J.P.; Bux, S. Nonstoichiometry in the Zintl phase Yb1−xZn2Sb2 as a route to thermoelectric optimization. Chem. Mater. 2014, 26, 5710–5717. [Google Scholar] [CrossRef]
- Artioli, G.; Monaco, H.L.; Viterbo, D.; Ferraris, G.; Gilli, G.; Zanotti, G.; Catti, M. Fundamentals of Crystallography; Giacovazzo, C., Ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Shannon, R.T.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Crystallogr. Sect. B Struct. Crystallogr. Crys. Chem. 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161. [Google Scholar] [CrossRef]
- Goodman, D.; Bennett, L.; Watson, R. Valency effects and relative solubilities in transition metal alloys. Scripta Metallurgica 1983, 17, 91–96. [Google Scholar] [CrossRef]
- Raynor, G.V., XII. Atomic and ionic radii.—III. Polarization effects in alloys. Lond. Edinburgh Dublin Philos. Mag. J. Sci. 1938, 26, 152–165. [Google Scholar] [CrossRef]
- Hume-Rothery, W.; Raynor, G.V., XI. Atomic and ionic radii.—II. Application to the theory of solid solubility in alloys. Lond. Edinburgh Dublin Philos. Mag. J. Sci. 1938, 26, 143–152. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Yang, Y.; Wang, J.; Liu, C. Atomic-size effect and solid solubility of multicomponent alloys. Scripta Mater. 2015, 94, 28–31. [Google Scholar] [CrossRef]
- Gupta, S.; Ganguli, A.K.; Corbett, J.D. Mg5.23Sm0.77Sb4: An ordered superstructure derived from the Mg3Sb2 structure type. Inorg. Chem. 2006, 45, 8175–8178. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Mao, J.; Bordelon, M.; He, R.; Wang, Y.; Shuai, J.; Sun, J.; Lei, X.; Ren, Z.; Chen, S.; et al. Joint effect of magnesium and yttrium on enhancing thermoelectric properties of n-type Zintl Mg3+δY0.02Sb1.5Bi0.5. Mater. Today Phys. 2019, 8, 25–33. [Google Scholar] [CrossRef]

| (CaMg)MgSb | x = 0 | x = 0.1 | x = 0.2 | x = 0.3 | x = 0.4 | x = 0.5 | x = 0.6 | x = 0.7 | x = 0.8 | x = 1 |
| Temperature () | 850 | 810 | 790 | 770 | 750 | 730 | 710 | 690 | 670 | 650 |
| (SrMg)MgSb | x = 0 | x = 0.1 | x = 0.2 | x = 0.4 | x = 0.6 | x = 0.7 | x = 0.8 | x = 0.9 | x = 1 | - |
| Temperature () | 850 | 800 | 750 | 750 | 750 | 750 | 750 | 750 | 700 | - |
| (BaMg)MgSb | x = 0 | x = 0.3 | x = 0.5 | x = 0.8 | x = 0.9 | x = 1 | - | - | - | - |
| Temperature () | 850 | 750 | 700 | 700 | 700 | 700 | - | - | - | - |
| (BaCa)MgSb | x = 0 | x = 0.1 | x = 0.3 | x = 0.5 | x = 0.7 | x = 0.9 | x = 1 | - | - | - |
| Temperature () | 650 | 700 | 700 | 700 | 700 | 700 | 700 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Zevalkink, A. Limits of Cation Solubility in AMg2Sb2 (A = Mg, Ca, Sr, Ba) Alloys. Materials 2019, 12, 586. https://doi.org/10.3390/ma12040586
Peng W, Zevalkink A. Limits of Cation Solubility in AMg2Sb2 (A = Mg, Ca, Sr, Ba) Alloys. Materials. 2019; 12(4):586. https://doi.org/10.3390/ma12040586
Chicago/Turabian StylePeng, Wanyue, and Alexandra Zevalkink. 2019. "Limits of Cation Solubility in AMg2Sb2 (A = Mg, Ca, Sr, Ba) Alloys" Materials 12, no. 4: 586. https://doi.org/10.3390/ma12040586
APA StylePeng, W., & Zevalkink, A. (2019). Limits of Cation Solubility in AMg2Sb2 (A = Mg, Ca, Sr, Ba) Alloys. Materials, 12(4), 586. https://doi.org/10.3390/ma12040586