Photodegradation and Biodegradation of Poly(Lactic) Acid Containing Orotic Acid as a Nucleation Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Scanning Electron and Optical Microscopy
2.2.1. Scanning Electron Microscopy (SEM)
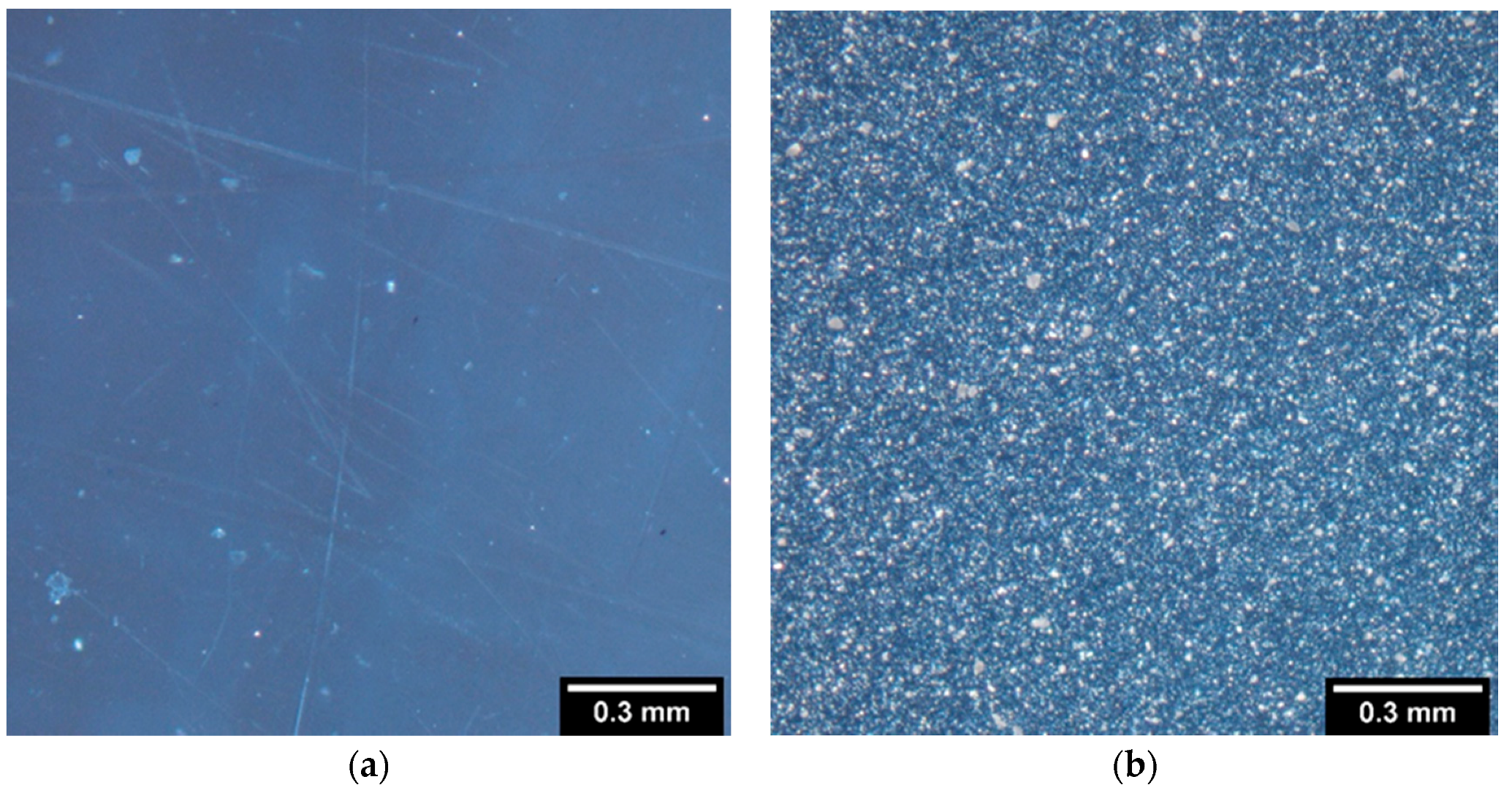
2.2.2. Polarized Light Microscopy
2.3. Differential Scanning Calorimetry (DSC)
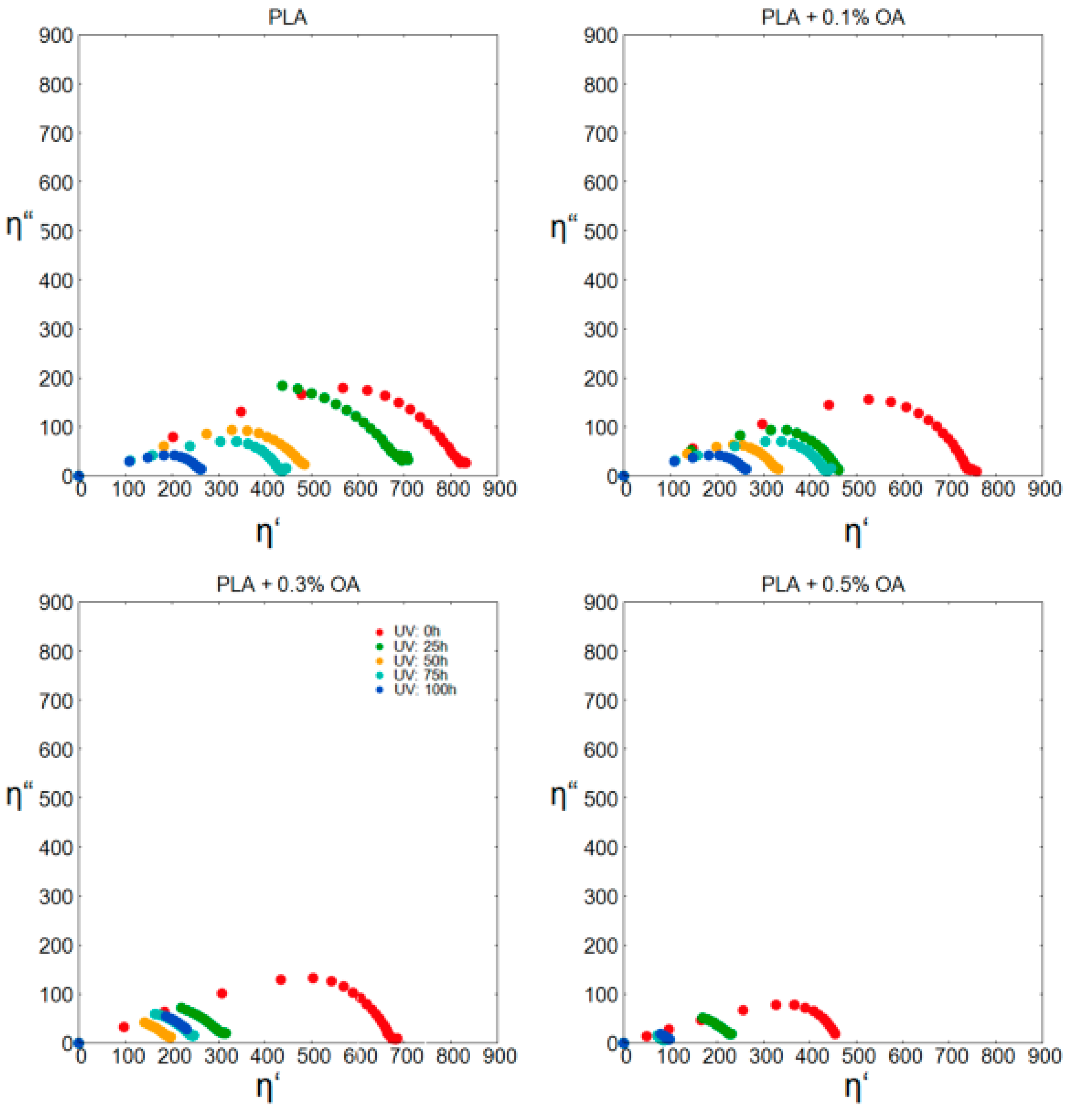
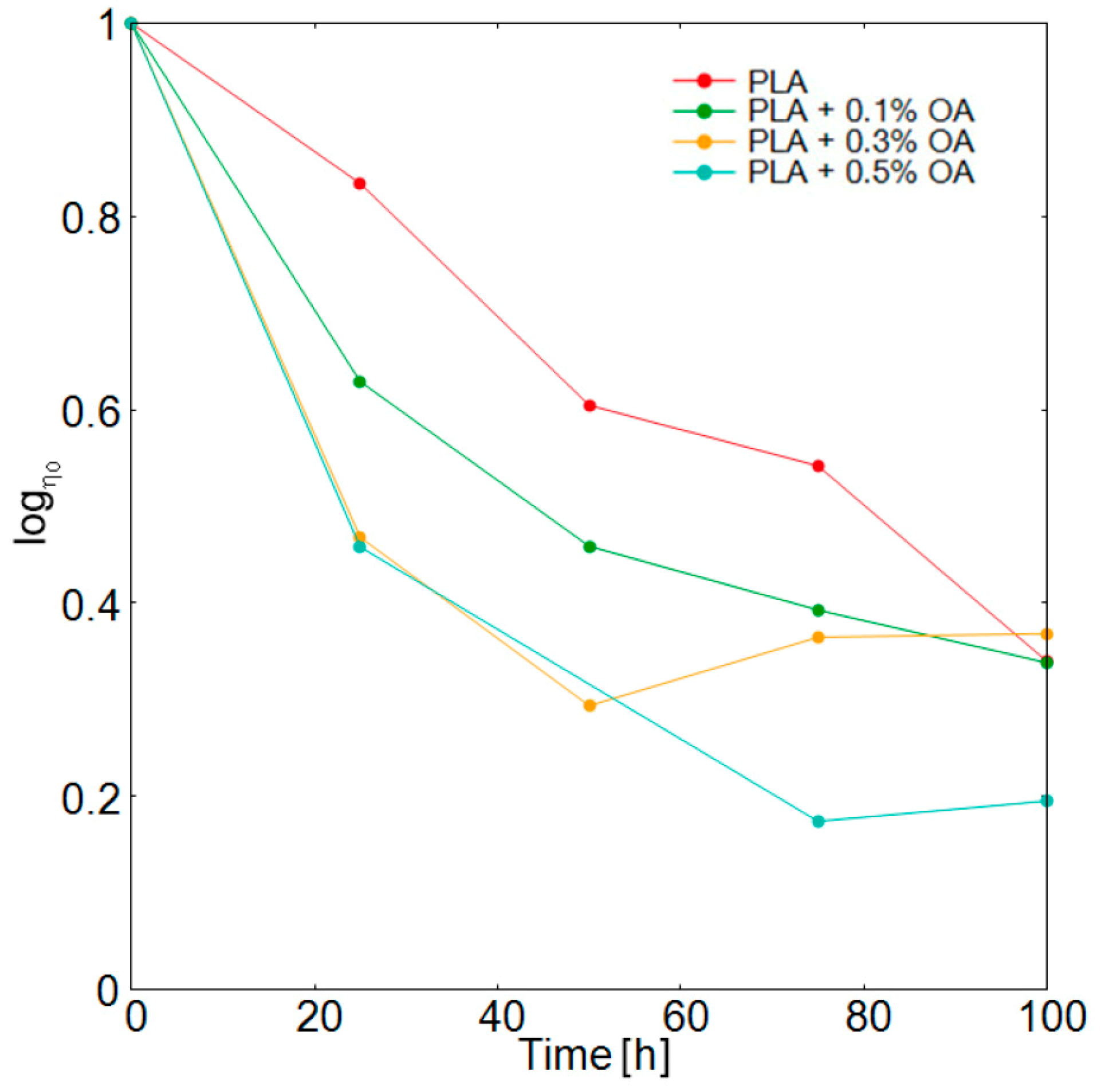
2.4. Melt Rheology Study
2.5. Photooxidation
2.6. Spectroscopic Characterization
2.7. Biodegradation Testing
3. Results and Discussion
3.1. Preparation of Samples
3.2. Thermal Properties
3.3. Photodegradation and Subsequent Analysis
3.4. Biodegradation in Compost
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blanco, I. Lifetime prediction of polymer: To bet, or not bet—Is This the Question? Materials 2018, 11, 1383. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Luan, Y.; Yang, L.; Sun, H. A Perspective on Reversibility in Controlled Polymerization Systems: Recent Progress and New Opportunities. Molecules 2018, 23, 2870. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerin, B.S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2019, 89, 61–75. [Google Scholar] [CrossRef]
- Blanco, I. End-life prediction of commercial PLA used for food packaging through short term TGA experiments: Real chance or law reliability? Chin. J. Polym. Sci. 2014, 32, 681–689. [Google Scholar] [CrossRef]
- Rudnik, E. Compostable Polymer Materials, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780080560847. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Gregorova, A.; Sedlarik, V.; Pastorek, M.; Jachandra, H.; Stelzer, F. Effect of compatibilizing agent on the properties of highly crystalline composites based on poly(lactic acid) and wood flour and/or mica. J. Polym. Environ. 2011, 19, 372–381. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly(lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature review of poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Bastioli, C. Effect of molecular weight and crystallinity on poly(lactic acid) mechanical properties. J. Appl. Polym. Sci. 1996, 59, 37–43. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, T.; Xu, P.; Zhao, X.; Li, X.; Dong, W.; Ma, P.; Chen, M. Crystallization modification of poly(lactide) by using nucleating agents and stereocomplexation. E-Polymers 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymers 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
- Ma, P.; Xu, Y.; Wang, D.; Dong, Q.; Chen, M. Rapid crystallization of poly(lactic acid) by using tailor-made oxalamide derivatives as novel soluble-type nucleating agents. Ind. Eng. Chem. Res. 2014, 53, 12888–12892. [Google Scholar] [CrossRef]
- Cicala, G.; Giordano, D.; Tosto, C.; Filippone, G.; Recca, A.; Blanco, I. Polylactide (PLA) filament a biobased solution for additive manufacturing: Correlating rheology and thermomechanical properties with printing quality. Materials 2018, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Okamoto, M.; Okamoto, H.; Nakano, M.; Usuki, A.; Matsuda, M. Morphology and crystallization kinetics in a mixture of low-molecular weight aliphatic amide and polylactide. Polymers 2006, 47, 1340–1347. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, W.; Deng, H.; Zhang, Q.; Qianf, F. Control of crystal morphology in poly(l-lactide) by adding nucleating agent. Macromolecules 2011, 44, 1233–1237. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bhattacharya, S. Orotic acid as a useful supramolecular synthon for the fabrication of an OPV based hydrogel: Stoichiometry dependent injectable behavior. Chem. Commun. 2015, 51, 6765–6768. [Google Scholar] [CrossRef]
- Jacquel, N.; Tajima, K.; Nakamura, N.; Miyagawa, T.; Pan, P.; Inoue, Y. Effect of orotic acid as a nucleating agent on the crystallization of bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymers. J. Appl. Polym. Sci. 2009, 114, 1287–1294. [Google Scholar] [CrossRef]
- Jacquel, N.; Tajima, K.; Nakamura, N.; Kawichi, H.; Pan, P.; Inoue, Y. Nucleation mechanism of polyhydroxybutyrate and poly(hydroxybutyrate-co-hydroxyhexanoate) crystallized by orotic acid as a nucleating agent. J. Appl. Polym. Sci. 2010, 115, 709–715. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, Z. Effect of Orotic Acid on the Crystallization Kinetics and Morphology of Biodegradable Poly(l-lactide) as an Efficient Nucleating Agent. Ind. Eng. Chem. Res. 2011, 50, 12299–12303. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, P.; Xu, P.; Wang, R.; Dong, W.; Chen, M.; Joziasse, C. The crystallization behavior of poly(lactic acid) with different types of nucleating agents. Int. J. Biol. Macromol. 2018, 106, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Tsui, A.; Frank, C.W. Comparison of anhydrous and monohydrated forms of orotic acid as crystal nucleating agents for poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polymer 2014, 55, 6364–6372. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyauchi, S. Enzymatic hydrolysis of poly(lactide)s: Effects of molecular weight, L-lactide content, and enantiomeric and diastereoisomeric polymer blending. Biomacromolecules 2001, 2, 597–604. [Google Scholar] [CrossRef]
- Tsuji, H.; Takai, H.; Fukuda, N.; Takikawa, H. Non-Isothermal Crystallization Behavior of Poly(l-lactic acid) in the Presence of various Additives. Macromol. Mater. Eng. 2006, 291, 325–335. [Google Scholar] [CrossRef]
- Stloukal, P.; Koutny, M.; Sedlarik, V.; Kucharczyk, P. Mathematical Models and Methods in Modern Science. In Proceedings of the 2nd International Conference on Development, Energy, Environment, Economics, Factors Influencing Encapsulation Efficiency of Biologically Active Compound into PLA Submicroparticles, Puerto De La Cruz, Tenrife, Spain, 10–12 December 2011; Mastorakis, N., Mladenov, V., Travieso-Gonzalez, C.M., Kohler, M., Eds.; WSEAS Press: Cambridge, UK, 2011. [Google Scholar]
- Verney, V.; Michel, A. Representation of the rheological properties of polymer melts in terms of complex fluidity. Rheol. Acta 1989, 28, 54–60. [Google Scholar] [CrossRef]
- Marek, A.A.; Verney, V. Rheological behavior of polyolefins during UV irradiation at high temperature as a coupled degradative process. Eur. Polym. J. 2015, 72, 1–11. [Google Scholar] [CrossRef]
- Palade, L.; Lehermeier, H.J.; Dorgan, J.R. Melt rheology of high l-content poly(lactic acid). Macromolecules 2001, 34, 1384–1390. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Janzen, J.; Clayton, M.P.; Sukhendu, H.; Knauss, D. Melt rheology of variable l-content poly(lactic acid). J. Rheol. 2005, 49, 607. [Google Scholar] [CrossRef]
- Stloukal, P.; Jandikova, G.; Koutny, M.; Sedlařík, V. Carbodiimide additive to control hydrolytic stability and biodegradability of PLA. Polym. Test. 2016, 54, 19–28. [Google Scholar] [CrossRef]
- Avenel, C.; Gardette, J.-L.; Therias, S.; Disdier, A.; Raccurt, O. Accelerated aging test of solar mirrors: Comparison of different UV chambers. AIP Conf. Proc. 2017, 1850, 130001. [Google Scholar]
- Pekařová, S.; Dvořáčková, M.; Stloukal, P.; Ingr, M.; Šerá, J.; Koutny, M. Quantitation of the inhibition effect of model compounds representing plant biomass degradation products on methane production. BioResources 2017, 12, 2421–2432. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of Poly(l-lactide): Molecular weight dependence. Macromolecules 2014, 40, 6898–6905. [Google Scholar] [CrossRef]
- Xiao, H.W.; Li, P.; Ren, X.; Jiang, T.; Yeh, J.T. Isothermal crystallization kinetics and crystal structure of poly(lactic acid): Effect of triphenyl phosphate and talc. J. Appl. Polym. Sci. 2010, 118, 3558–3569. [Google Scholar] [CrossRef]
- Lemaire, J.; Arnaud, R.; Lacoste, J. The prediction of the long-term photoageing of solid polymers. Acta Polym. 1988, 39, 27–32. [Google Scholar] [CrossRef]
- Bocchini, S.; Fukushima, K.; Blasio, A.D.; Fina, A.; Frache, A.; Geobaldo, F. Polylactic acid and polylactic acid-based nanocomposite photooxidation. Biomacromolecules 2010, 11, 2919–2926. [Google Scholar] [CrossRef]
- Marra, A.; Cimmino, S.; Silvestre, C. Effect of TiO2 and ZnO on PLA degradation in various media. Adv. Mater. Sci. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Stloukal, P.; Verney, V.; Commereuc, S.; Rychly, J.; Matisova-Rychla, L.; Pis, V.; Koutny, M. Assessment of the interrelation between photooxidation and biodegradation of selected polyesters after artificial weathering. Chemosphere 2012, 88, 1214–1219. [Google Scholar] [CrossRef]

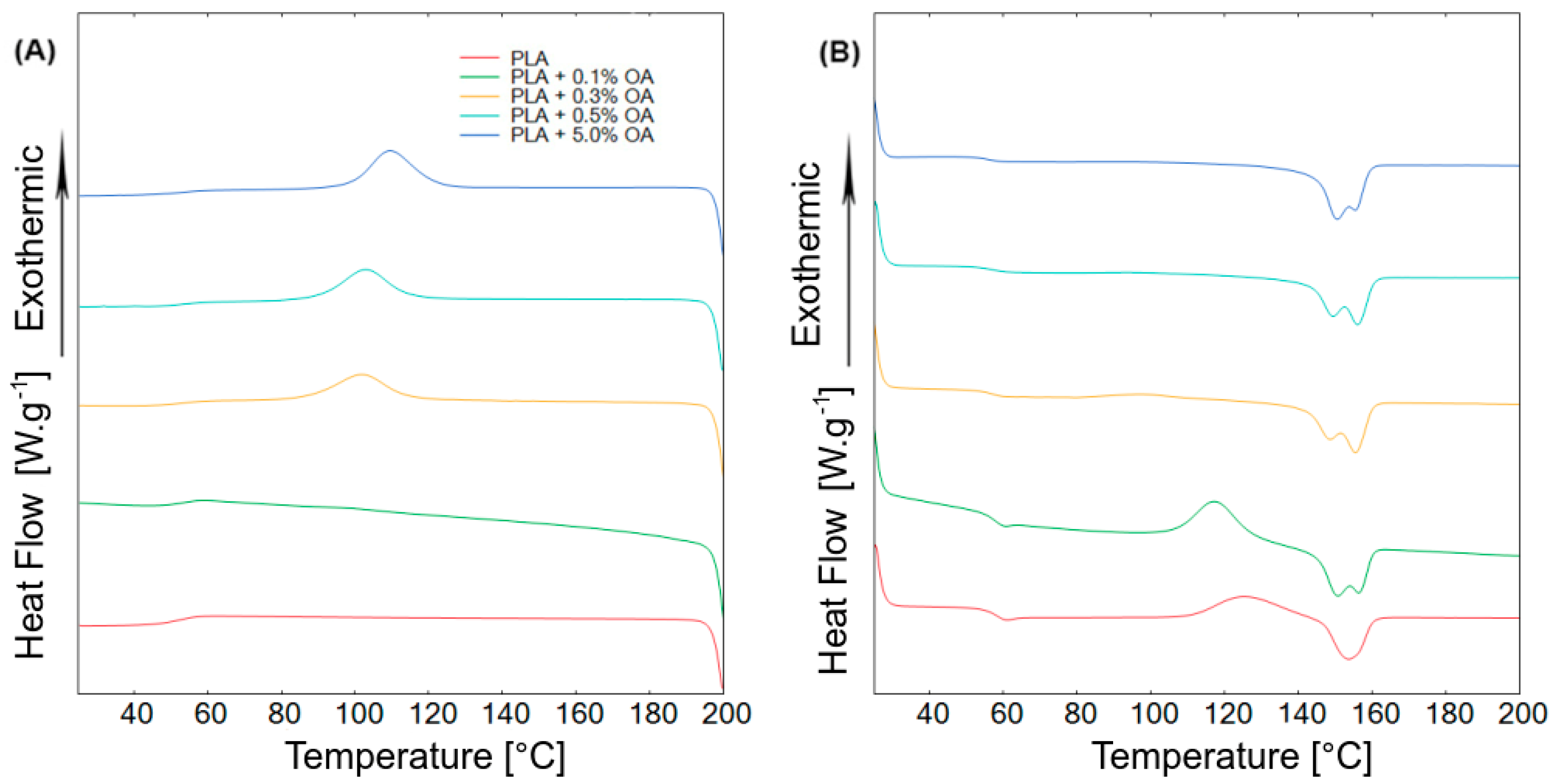


| Material | Tg (°C) | Tm1 (°C) | Tm2 (°C) | Tc (°C) | Hg (J·g−1·K−1) | Hc (J·g−1) | χc (%) |
|---|---|---|---|---|---|---|---|
| PLA | 57 | 153 | nd | nd | 0.53 | 0 | 0 |
| PLA + 0.1% OA | 58 | 150 | 156 | 99 | 0.40 | 1.8 | 2 |
| PLA + 0.3% OA | 59 | 148 | 155 | 102 | 0.29 | 26.2 | 28 |
| PLA + 0.5% OA | 59 | 149 | 156 | 103 | 0.26 | 28.6 | 31 |
| PLA + 5.0% OA | 59 | 150 | 155 | 110 | 0.27 | 31.8 | 34 |
| Parameter | PLA | PLA + 0.3% OA | ||
|---|---|---|---|---|
| UV (h) | 0 | 75 | 0 | 75 |
| CO2_Max a (%) | 86 ± 2.5 | 95 ± 1.1 | 85 ± 2.1 | 112 ± 5.1 |
| k b (day−1) | 1.9 ± 0.09 | 1.8 ± 0.04 | 1.4 ± 0.04 | 1.5 ± 0.05 |
| C c, (days) | 28.4 ± 1.2 | 18.5 ± 0.57 | 19.9 ± 0.92 | 14.7 ± 1.4 |
| R2 | 0.991 | 0.994 | 0.996 | 0.991 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salač, J.; Šerá, J.; Jurča, M.; Verney, V.; Marek, A.A.; Koutný, M. Photodegradation and Biodegradation of Poly(Lactic) Acid Containing Orotic Acid as a Nucleation Agent. Materials 2019, 12, 481. https://doi.org/10.3390/ma12030481
Salač J, Šerá J, Jurča M, Verney V, Marek AA, Koutný M. Photodegradation and Biodegradation of Poly(Lactic) Acid Containing Orotic Acid as a Nucleation Agent. Materials. 2019; 12(3):481. https://doi.org/10.3390/ma12030481
Chicago/Turabian StyleSalač, Jan, Jana Šerá, Martin Jurča, Vincent Verney, Adam A. Marek, and Marek Koutný. 2019. "Photodegradation and Biodegradation of Poly(Lactic) Acid Containing Orotic Acid as a Nucleation Agent" Materials 12, no. 3: 481. https://doi.org/10.3390/ma12030481
APA StyleSalač, J., Šerá, J., Jurča, M., Verney, V., Marek, A. A., & Koutný, M. (2019). Photodegradation and Biodegradation of Poly(Lactic) Acid Containing Orotic Acid as a Nucleation Agent. Materials, 12(3), 481. https://doi.org/10.3390/ma12030481






