Characteristics of Hybrid Pigments Made from Alizarin Dye on a Mixed Oxide Host
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of Hybrid Pigments
2.3. Characterization of Hybrid Pigments
3. Results and Discussion
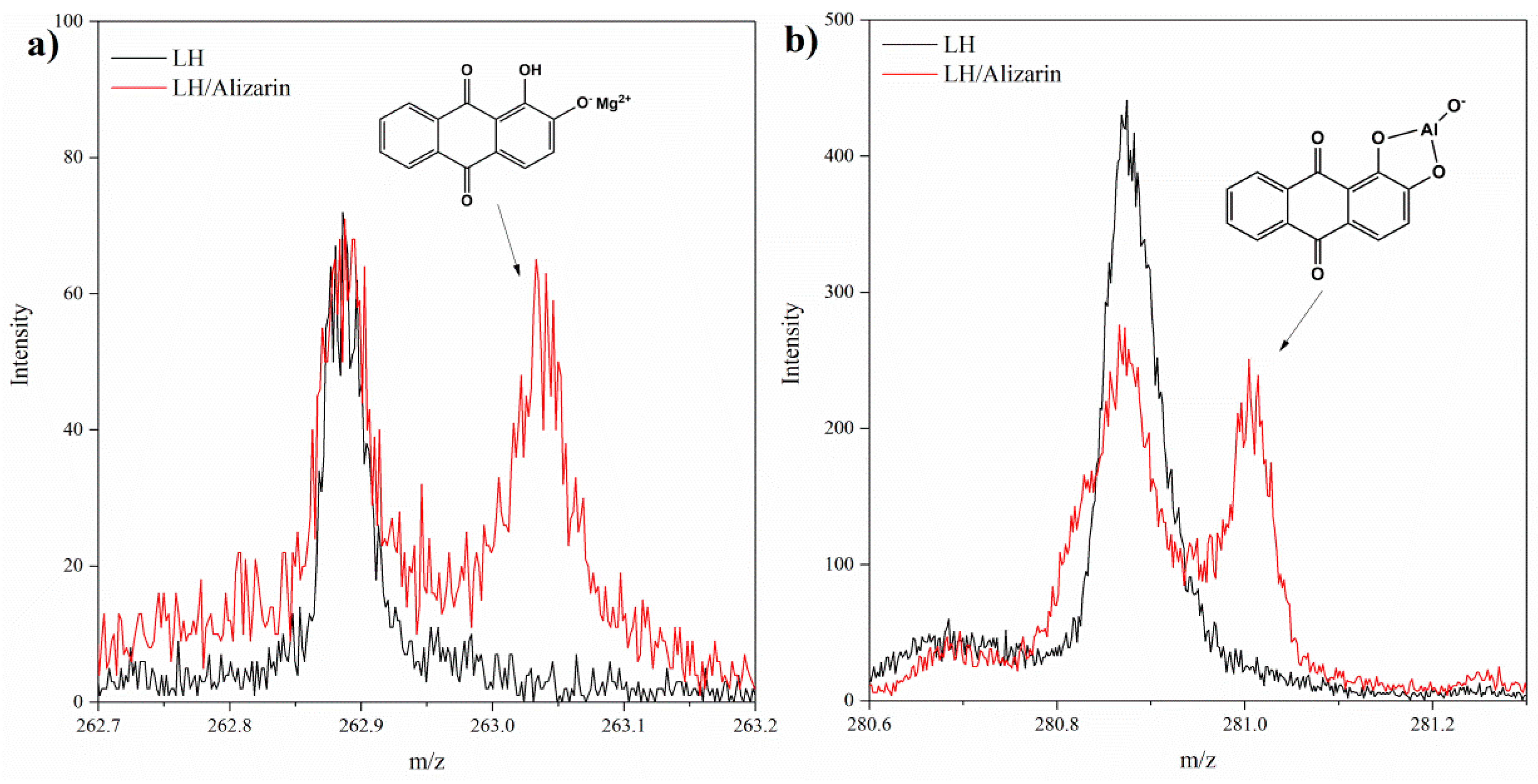
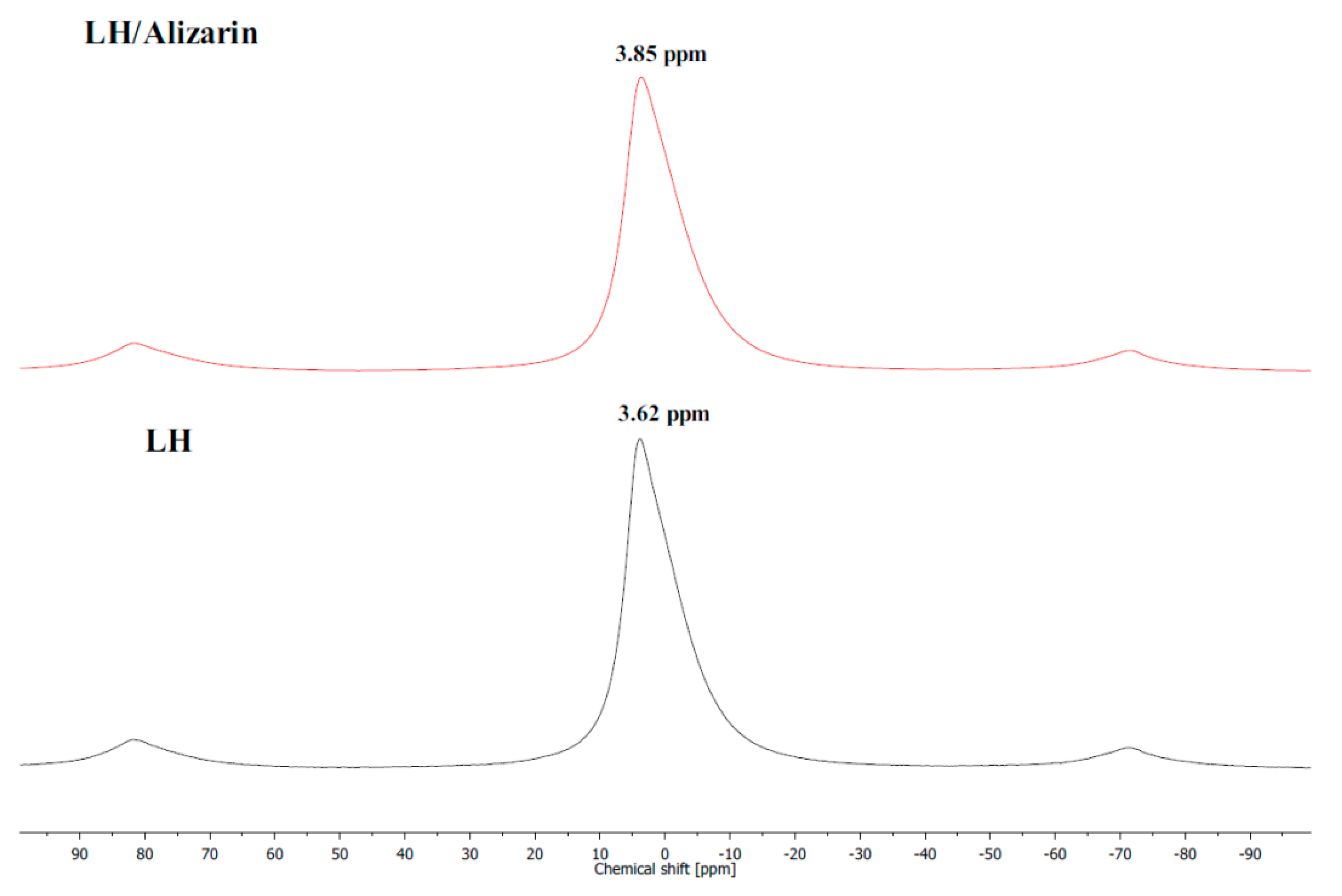
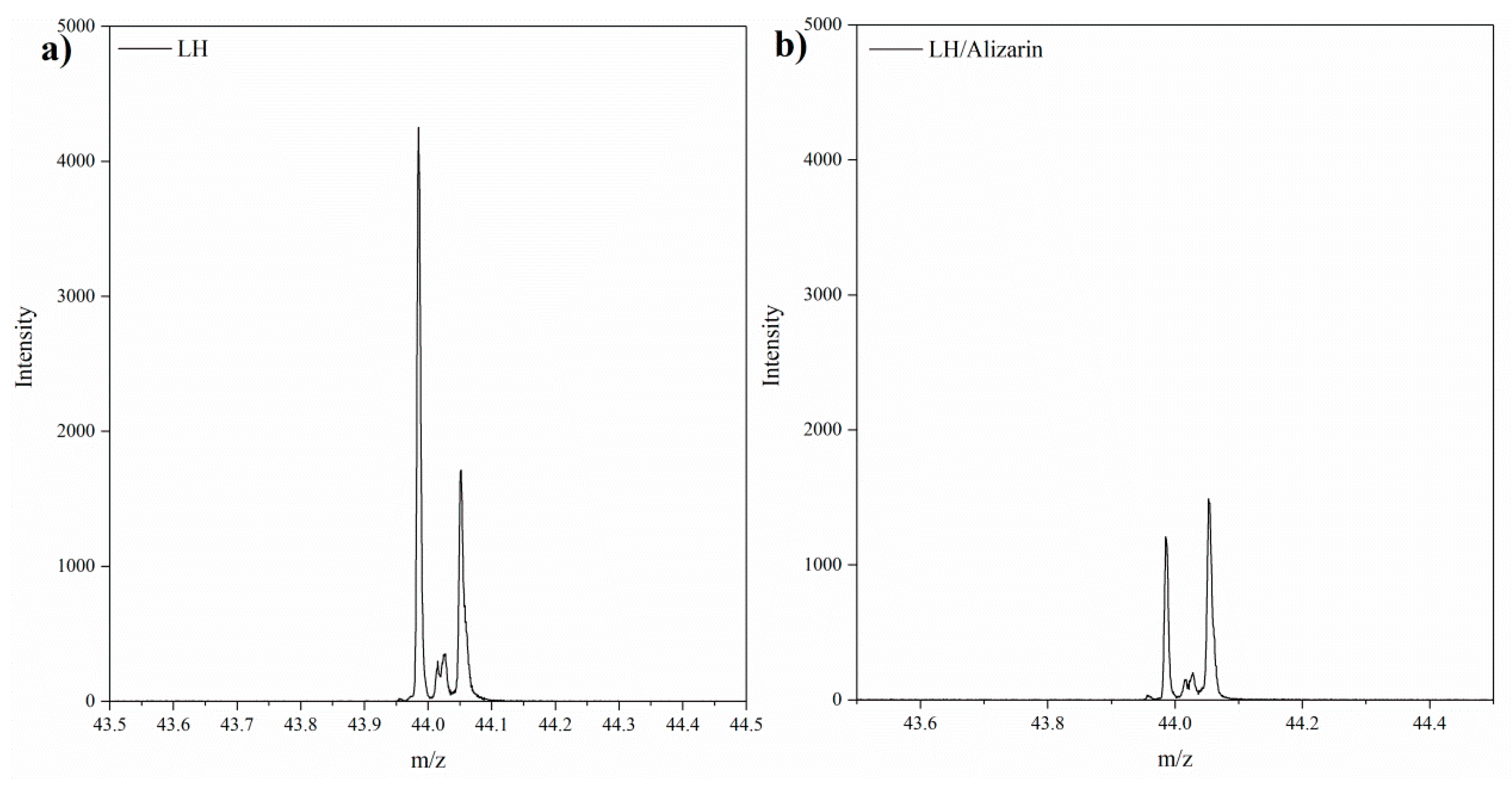
3.1. Secondary Ion Mass Spectrometry (TOF-SIMS) and 27Al NMR Analysis
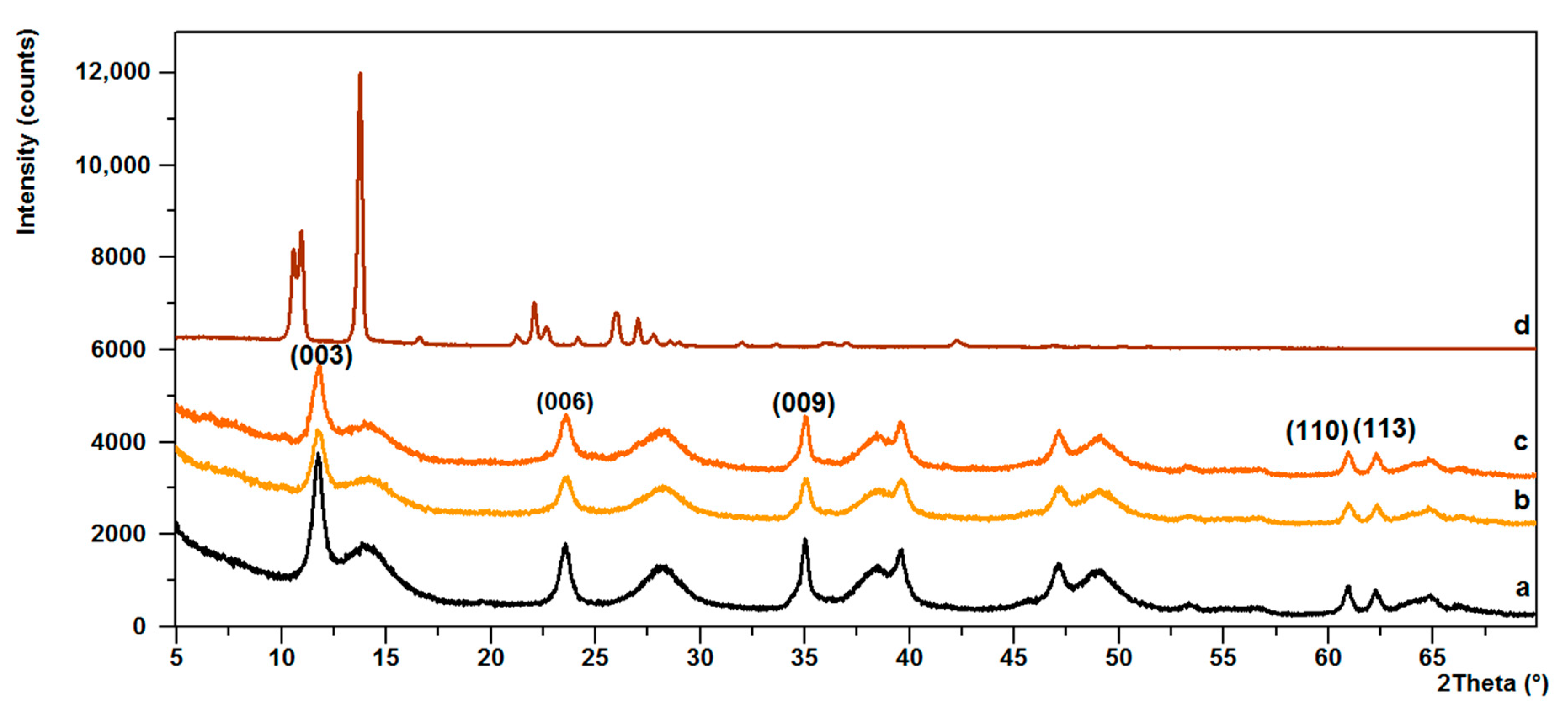
3.2. Powder X-ray Diffraction (PXRD)
3.3. Thermogravimetric Analysis (TG)
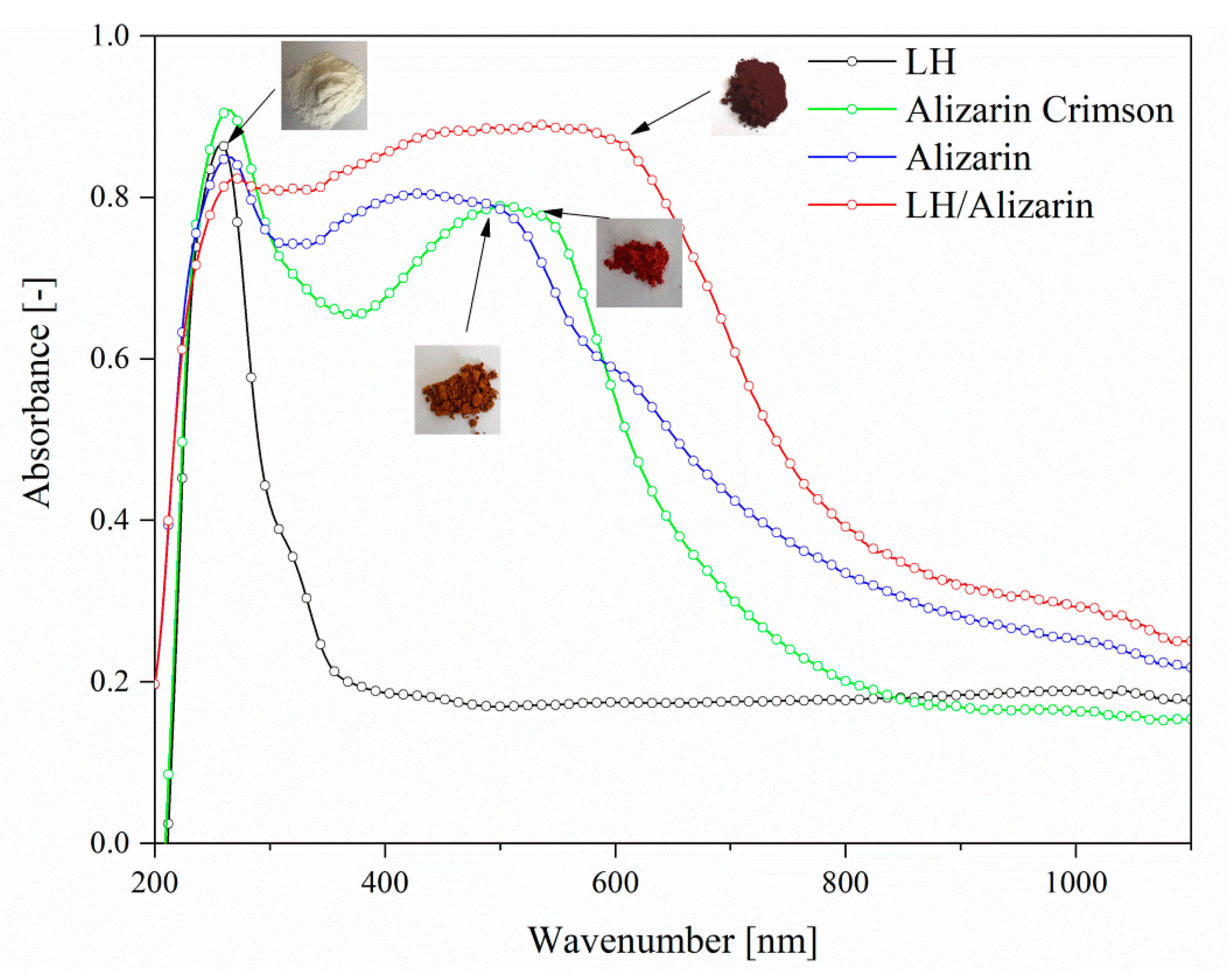
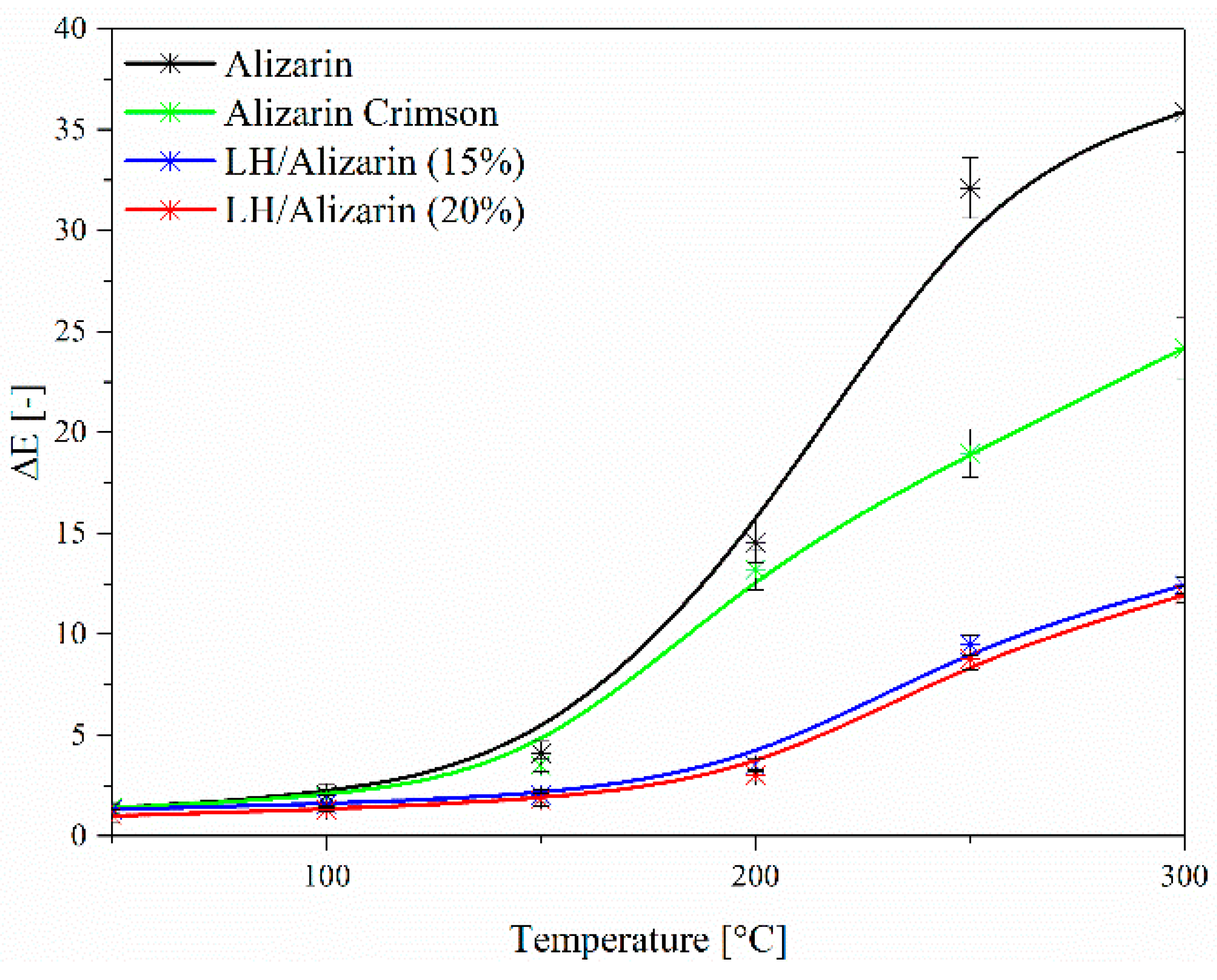
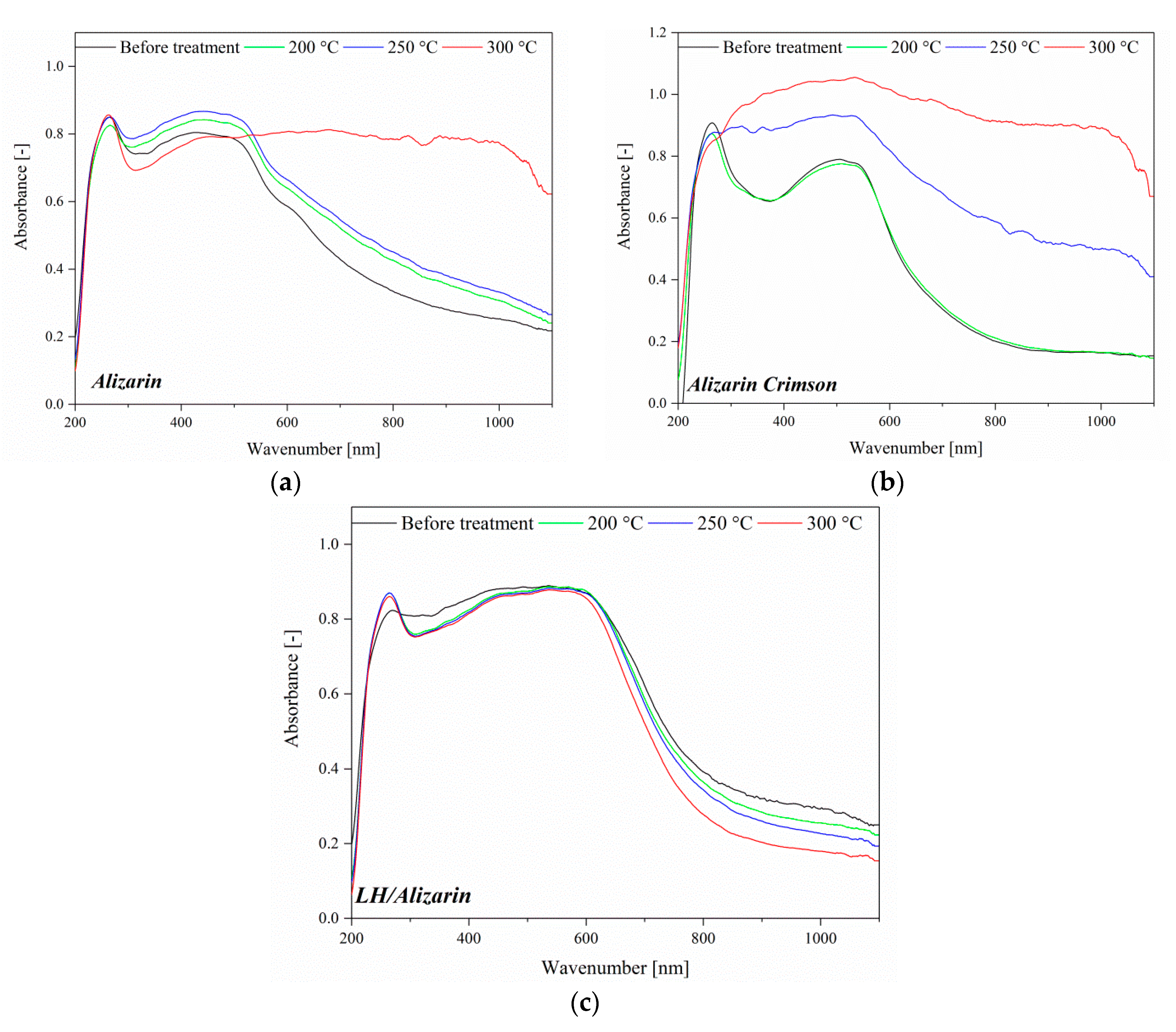
3.4. UV-VIS Spectroscopy and Color Stability
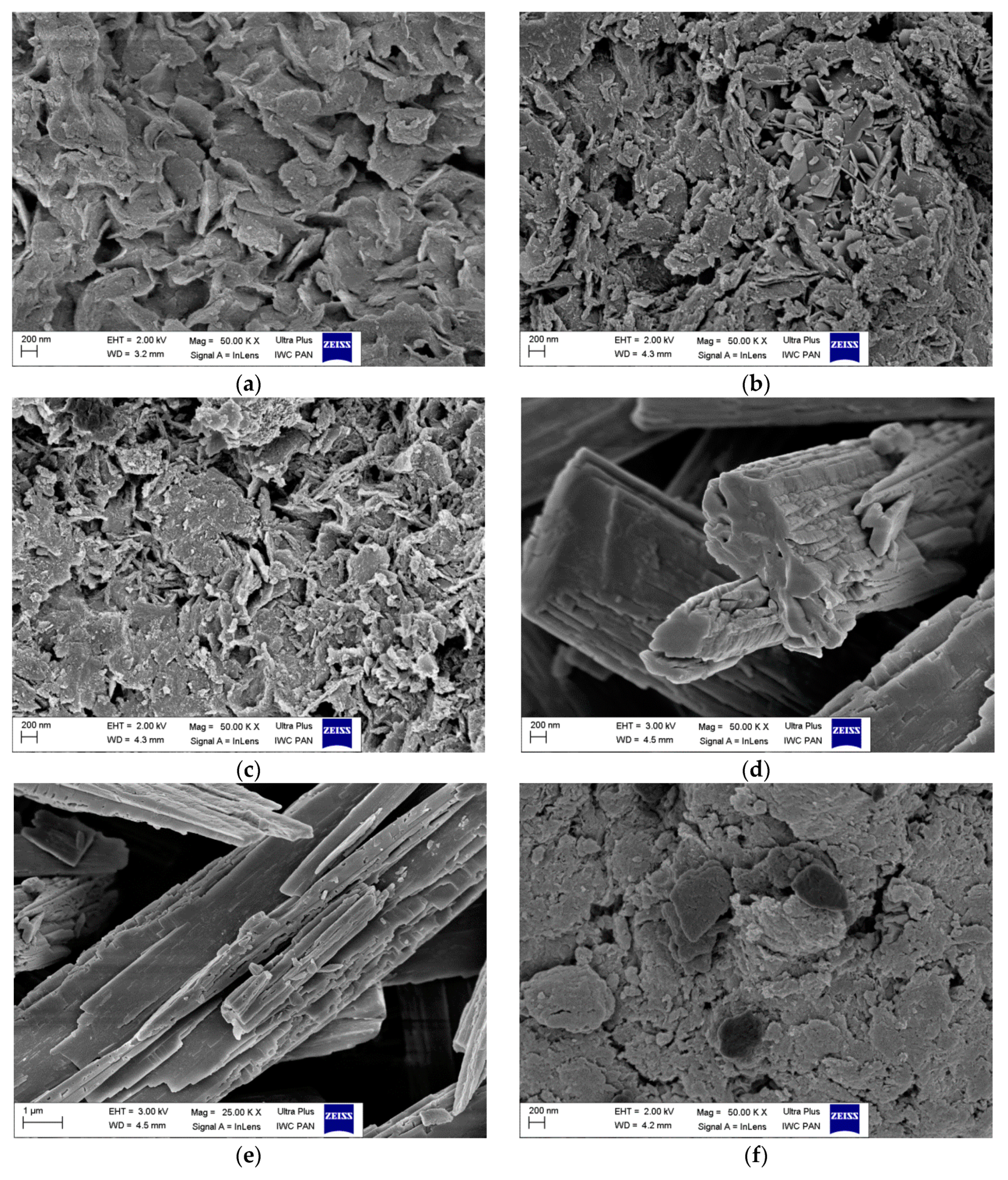
3.5. Scanning Electron Microscopy (SEM)
3.6. Solvent Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Velho, S.R.K.; Brum, L.F.W.; Petter, C.O.; dos Santos, J.H.Z.; Simunić, S.; Kappa, W.H. Development of structured natural dyes for use into plastics. Dyes Pigm. 2017, 136, 248–254. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Analysis of dyes in cosmetics: Challenges and recent developments. Cosmetics 2018, 5, 47. [Google Scholar] [CrossRef]
- Christie, R.M.; Mackay, J.L. Metal salt azo pigments. Color. Technol. 2008, 124, 133–144. [Google Scholar] [CrossRef]
- Fournier, F.; Viguerie, L.; Balme, S.; Janot, J.M.; Walter, P.; Jaber, M. Physico-chemical characterization of lake pigments based on montmorillonite and carminic acid. Appl. Clay Sci. 2016, 130, 12–17. [Google Scholar] [CrossRef]
- De Santis, D.; Moresi, M. Production of alizarin extracts from Rubia tinctorum and assessment of their dyeing properties. Ind. Crops Prod. 2007, 26, 151–162. [Google Scholar] [CrossRef]
- Gedik, G.; Avinc, O.; Yavas, A.; Khoddami, A. A novel eco-friendly colorant and dyeing method for poly(ethylene terephthalate) substrate. Fiber Polym. 2014, 15, 261–272. [Google Scholar] [CrossRef]
- Weiser, H.B.; Porter, E.E. The physical chemistry of color lake formation III alizarin lakes. J. Phys. Chem. 1927, 31, 1824–1839. [Google Scholar] [CrossRef]
- Kiel, E.G.; Heertjes, P.M. Metal complexes of alizarin I. The structure of the calcium-aluminum lake of alizarin. J. Soc. Colour. 1963, 79, 21–27. [Google Scholar] [CrossRef]
- Carta, L.; Biczysko, M.; Bloino, J.; Licari, D.; Barone, V. Environmental and complexation effects on the structures and spectroscopic signatures of organic pigments relevant to cultural heritage: The case of alizarin and alizarin-Mg(II)/Al(III) complexes. Phys. Chem. Chem. Phys. 2014, 16, 2897–2911. [Google Scholar] [CrossRef] [PubMed]
- Guillermin, D.; Debroise, T.; Trigueiro, P.; de Viguerie, L.; Rigaud, B.; Morlet-Savary, F.; Balme, S.; Janot, J.M.; Tielens, F.; Michot, L.; et al. New pigments based on carminic acid and smectites: A molecular investigation. Dyes Pigm. 2019, 160, 971–982. [Google Scholar] [CrossRef]
- Adriaens, A. Non-destructive analysis and testing of museum objects: An overview of 5 years of research. Spectrochim. Acta B 2005, 60, 1503–1516. [Google Scholar] [CrossRef]
- Canamares, M.V.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Surface-enhanced Raman scattering study of adsorption of the anthraquinone pigment alizarin on Ag nanoparticles. J. Raman Spectrosc. 2004, 35, 921–927. [Google Scholar] [CrossRef]
- Baran, A.; Wrzosek, B.; Bukowska, J.; Proniewicz, L.M.; Baranska, M. Analysis of alizarin by surface-enhanced and FT-Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 436–441. [Google Scholar] [CrossRef]
- Millani, C.; Romani, A.; Favaro, G. A spectrophotometric and fluorimetric study of some anthraquinoid and indigoid colorants used in artistic paintings. Spectrochim. Acta A 1998, 54, 581–588. [Google Scholar] [CrossRef]
- Tangaraj, V.; Janot, J.M.; Jaber, M.; Bechelany, M.; Balme, S. Adsorption and photophysical properties of fluorescent dyes over montmorillonite and saponite modified by surfactant. Chemosphere 2017, 184, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Brai, M.; Camaiti, M.; Casieri, C.; De Luca, F.; Fantazzini, P. Nuclear magnetic resonance for cultural heritage. Magn. Reson. Imaging 2007, 25, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Kanezaki, E. Unexchangeable interlayer anions; synthesis and characterization of Zn/Al- and Mg/Al-layered double hydroxides with interlayer alizarin red S. J. Incl. Phenom. Macro Chem. 2003, 46, 89–95. [Google Scholar] [CrossRef]
- Mahmud-Ali, A.; Fitz-Binder, Ch.; Bechtold, T. Aluminium based dye lakes for plant extracts for textile coloration. Dyes Pigm. 2012, 94, 533–540. [Google Scholar] [CrossRef]
- Kiel, E.G.; Heertjes, P.M. Metal complexes of alizarin IV—The structure of the potassium and calcium salts of alizarin and of 3-nitroalizarin. Color. Technol. 1963, 79, 363–367. [Google Scholar] [CrossRef]
- Perez, E.; Ibarra, I.A.; Guzman, A.; Lima, E. Hybrid pigments resulting from several guest dyes onto γ-alumina host: A spectroscopic analysis. Spectrochim. Acta A 2017, 172, 174–181. [Google Scholar] [CrossRef]
- Trigueiro, P.; Pereira, F.A.R.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.M.; Santos, I.M.G.; Fonseca, M.G.; Walter, P.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes Pigm. 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Ghannam, L.; Garay, H.; Billon, L. Sensitive colored hybrid inorganic/organic pigments based on polymer-coated microsized particles. Macromolecules 2008, 41, 7374–7382. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Moszyński, D.; Kozanecki, M.; Zaborski, M. Characterization and properties of new color-tunable hybrid pigments based on layered double hydroxides (LDH) and 1,2-dihydroxyanthraquinone dye. J. Ind. Eng. Chem. 2018. [Google Scholar] [CrossRef]
- Soubayrol, P.; Dana, G.; Man, P.P. Aluminium-27 solid state NMR study of aluminium coordination complexes of alizarin. Magn. Reson. Chem. 1996, 8, 638–645. [Google Scholar] [CrossRef]
- Doskocz, M.; Kubas, K.; Frackowiak, A.; Gancarz, R. NMR and ab initio studies of Mg2+, Ca2+, Zn2+, Cu2+ alizarin complexes. Polyhedron 2009, 28, 2201–2205. [Google Scholar] [CrossRef]
- Vyalikh, A.; Massiot, D.; Scheler, U. Structural characterisation of aluminium layered double hydroxides by 27Al solid-state NMR. Solid State Nucl. Magn. Reson. 2009, 36, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, K.; Blanc, S.; Mathe, C.; Dupin, J.C.; Vieillescazes, C. Spectroscopic and chromatographic analysis of yellow flavonoidic lakes: Quercitin chromophore. Appl. Clay Sci. 2011, 53, 598–607. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Yun, S.K.; Pinnavaia, T.J. Water content and particle texture of synthetic hydrotalcite-like layered double hydroxides. Chem. Mater. 1995, 7, 348–354. [Google Scholar] [CrossRef]
- Costa, F.R.; Leuteritz, A.; Wagenknecht, U.; Jehnichen, D.; Häußler, L.; Heinrich, G. Intercalation of Mg-Al layered double hydroxide by anionic surfactants: Preparation and characterization. Appl. Clay Sci. 2008, 38, 153–164. [Google Scholar] [CrossRef]
- Fain, V.Y.; Zaitsev, B.E.; Ryabov, M.A. Metal complexes with 1,5- and 1,8-dihydroxy-9,10-anthraquinones: Electronic absorption spectra and structure of ligands. Russ. J. Coord. Chem. 2004, 30, 360–364. [Google Scholar] [CrossRef]
- Komiha, N.; Kabbaj, O.K.; Chraibi, M. A density functional study of alizarin two of its isomers and its transition metals and rare-earth complexes. J. Mol. Struct. 2002, 594, 135–145. [Google Scholar] [CrossRef]
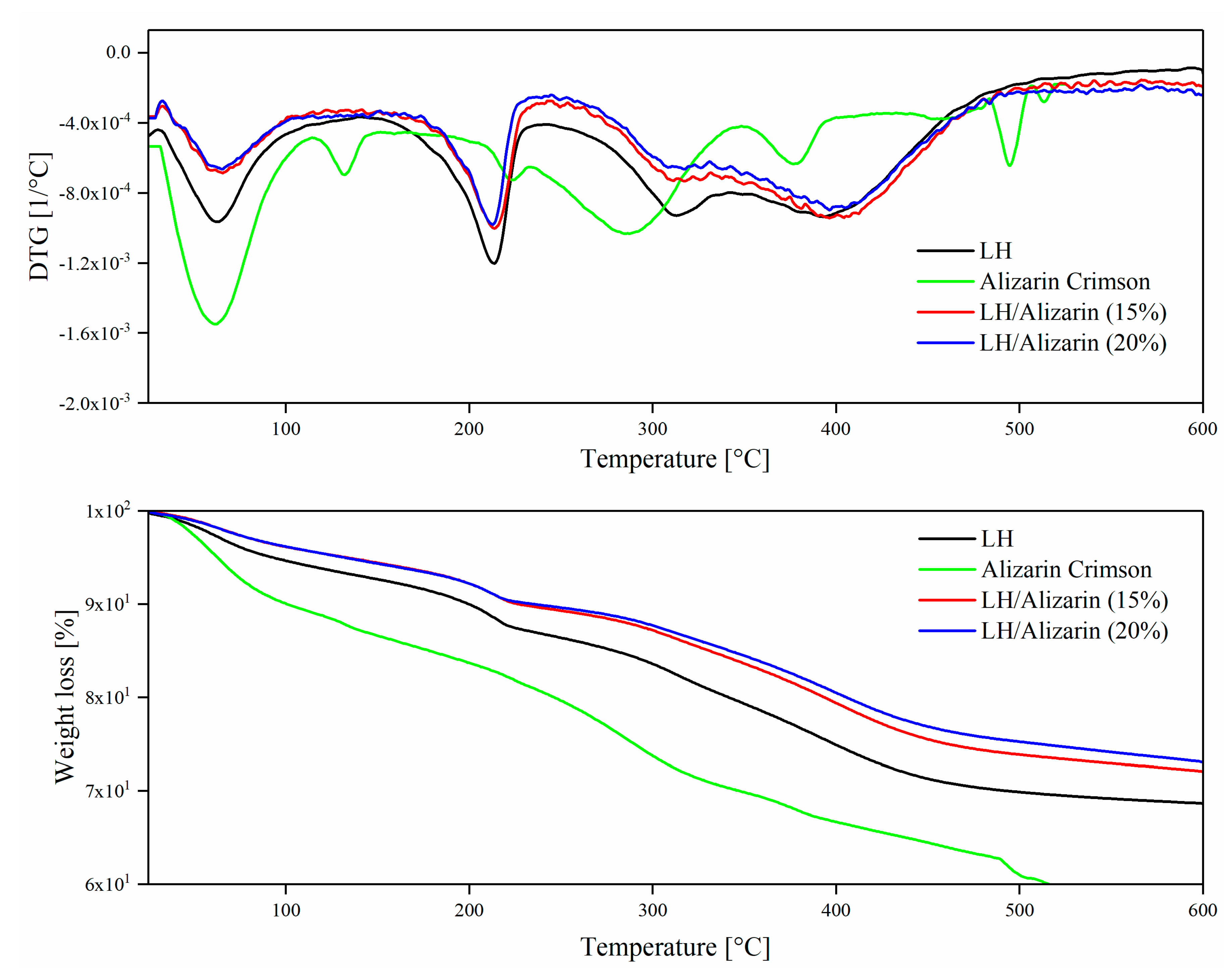


| Sample | 1 T05% (°C) | T10% (°C) | T20% (°C) | T30% (°C) |
|---|---|---|---|---|
| LH | 93 | 199 | 342 | 493 |
| Alizarin Crimson | 72 | 120 | 301 | 427 |
| LH/Alizarin (15%) | 133 | 227 | 393 | 616 |
| LH/Alizarin (20%) | 132 | 235 | 406 | 620 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Szynkowska, M.I.; Zaborski, M. Characteristics of Hybrid Pigments Made from Alizarin Dye on a Mixed Oxide Host. Materials 2019, 12, 360. https://doi.org/10.3390/ma12030360
Marzec A, Szadkowski B, Rogowski J, Maniukiewicz W, Szynkowska MI, Zaborski M. Characteristics of Hybrid Pigments Made from Alizarin Dye on a Mixed Oxide Host. Materials. 2019; 12(3):360. https://doi.org/10.3390/ma12030360
Chicago/Turabian StyleMarzec, Anna, Bolesław Szadkowski, Jacek Rogowski, Waldemar Maniukiewicz, Małgorzata Iwona Szynkowska, and Marian Zaborski. 2019. "Characteristics of Hybrid Pigments Made from Alizarin Dye on a Mixed Oxide Host" Materials 12, no. 3: 360. https://doi.org/10.3390/ma12030360
APA StyleMarzec, A., Szadkowski, B., Rogowski, J., Maniukiewicz, W., Szynkowska, M. I., & Zaborski, M. (2019). Characteristics of Hybrid Pigments Made from Alizarin Dye on a Mixed Oxide Host. Materials, 12(3), 360. https://doi.org/10.3390/ma12030360






