Improving the Tribological Performance of MAO Coatings by Using a Stable Sol Electrolyte Mixed with Cellulose Additive
Abstract
1. Introduction
- The carbide and nitride oxide that must be mixed into the metal matrices are so hard and brittle that they may be broken in the course of mixing or in the consolidation processes.
- The additive may not be uniformly dispersed into the electrolyte.
- A chemical reaction between the metal matrix and the coating may occur during the exposure to elevating temperature, which leads to poor mechanical properties of the composites.
- The particle sizes are typically in tens to hundreds of microns, which considerably reduces the ductility and toughness, as well as ineffectively utilizing the strength and stiffness of the reinforcement.
- The electrolyte is unstable and cannot be used in actual industrial production.
2. Experimental Procedure
2.1. Materials
2.2. Experiment Process
2.3. Characterization
3. Results and Discussion
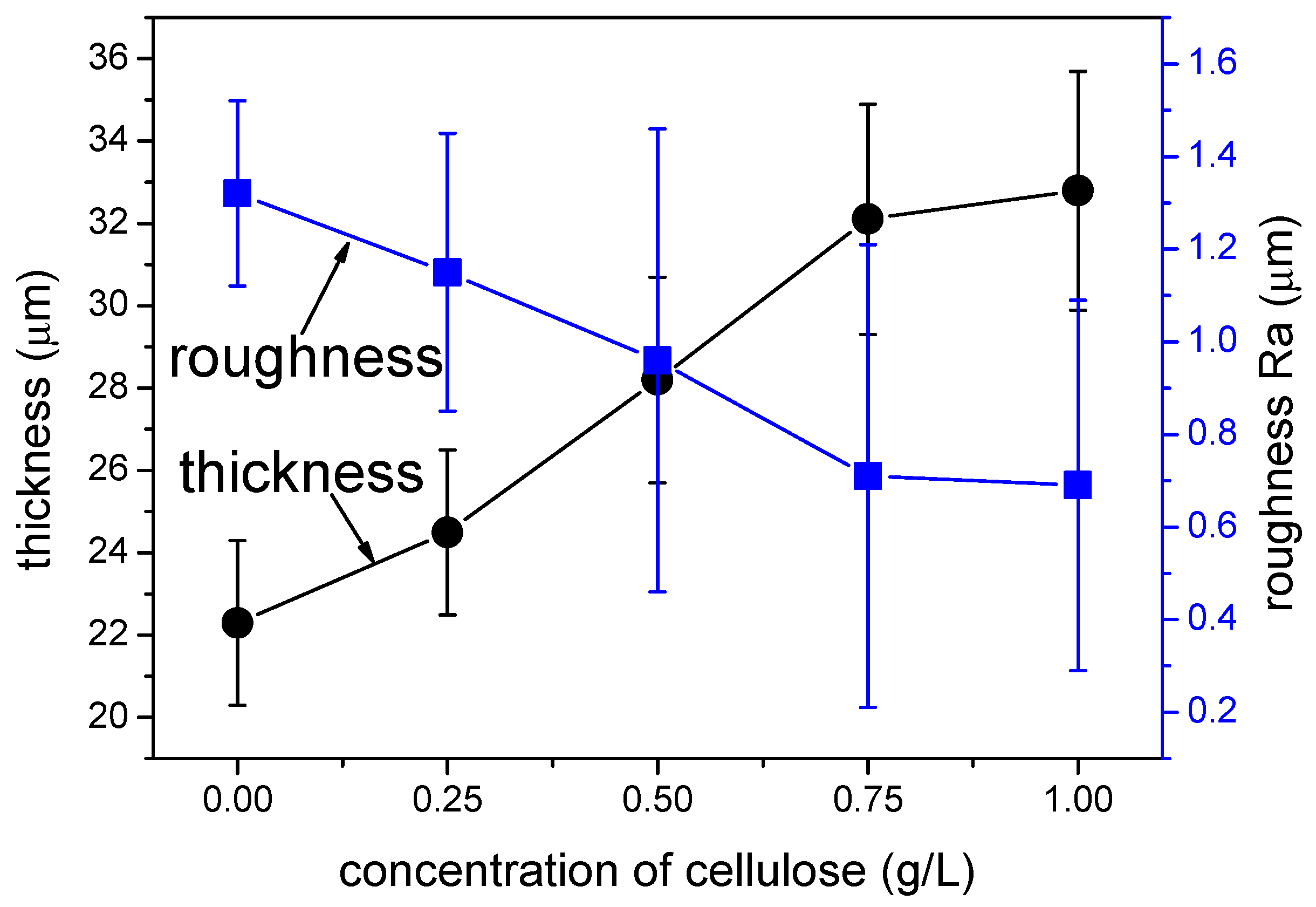
3.1. Thickness and Roughness of the MAO Coating
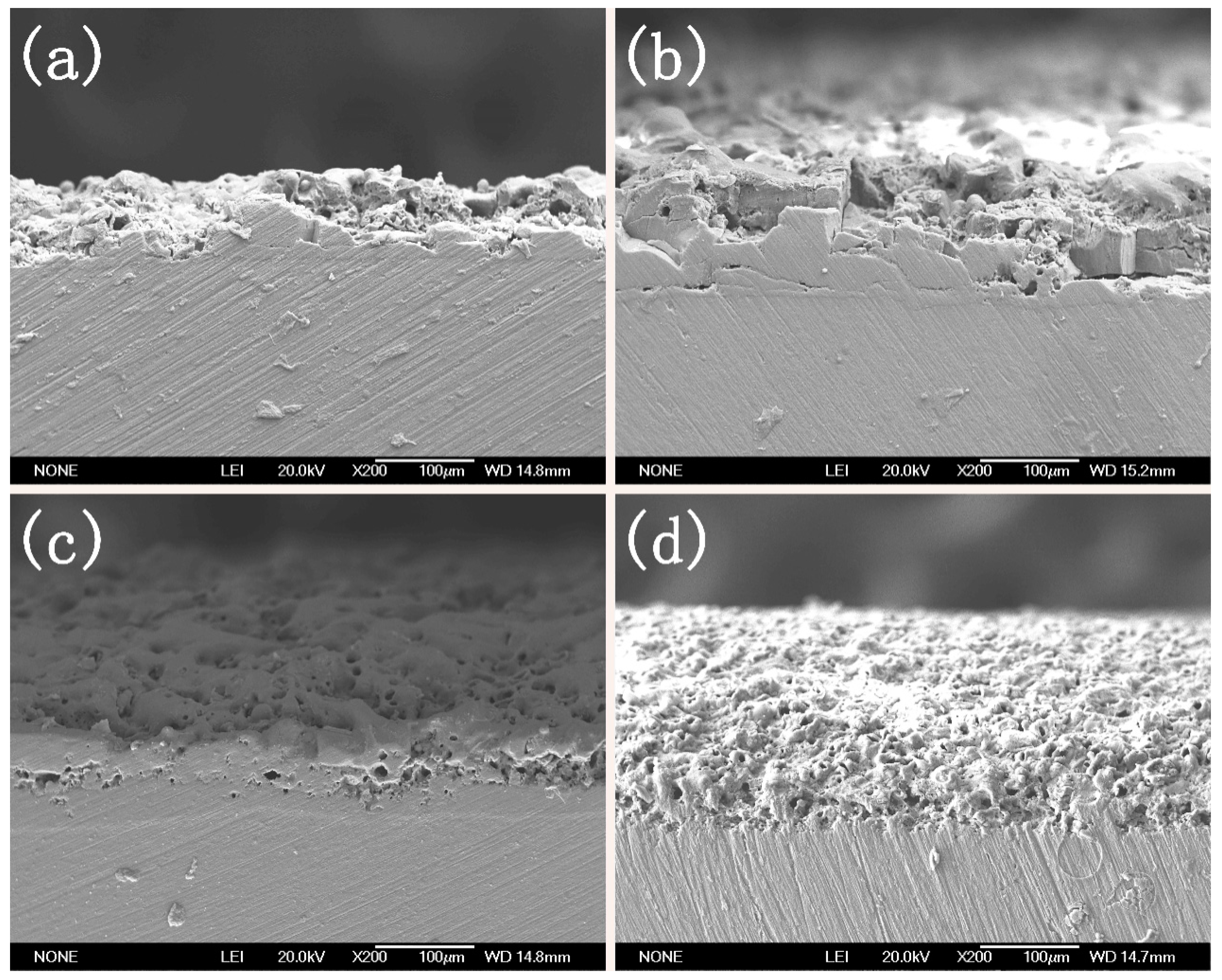
3.2. Microstructure of the MAO Coating
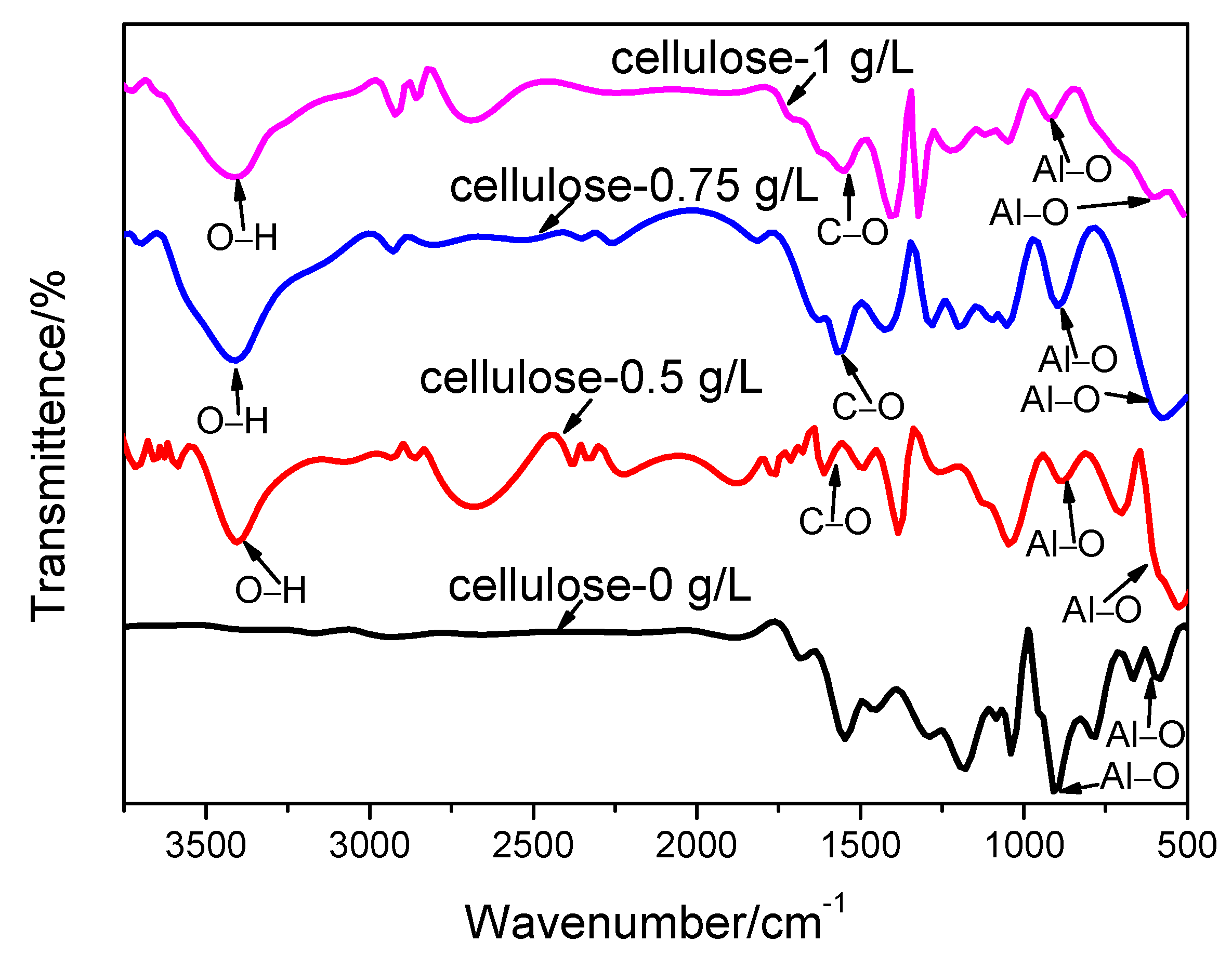
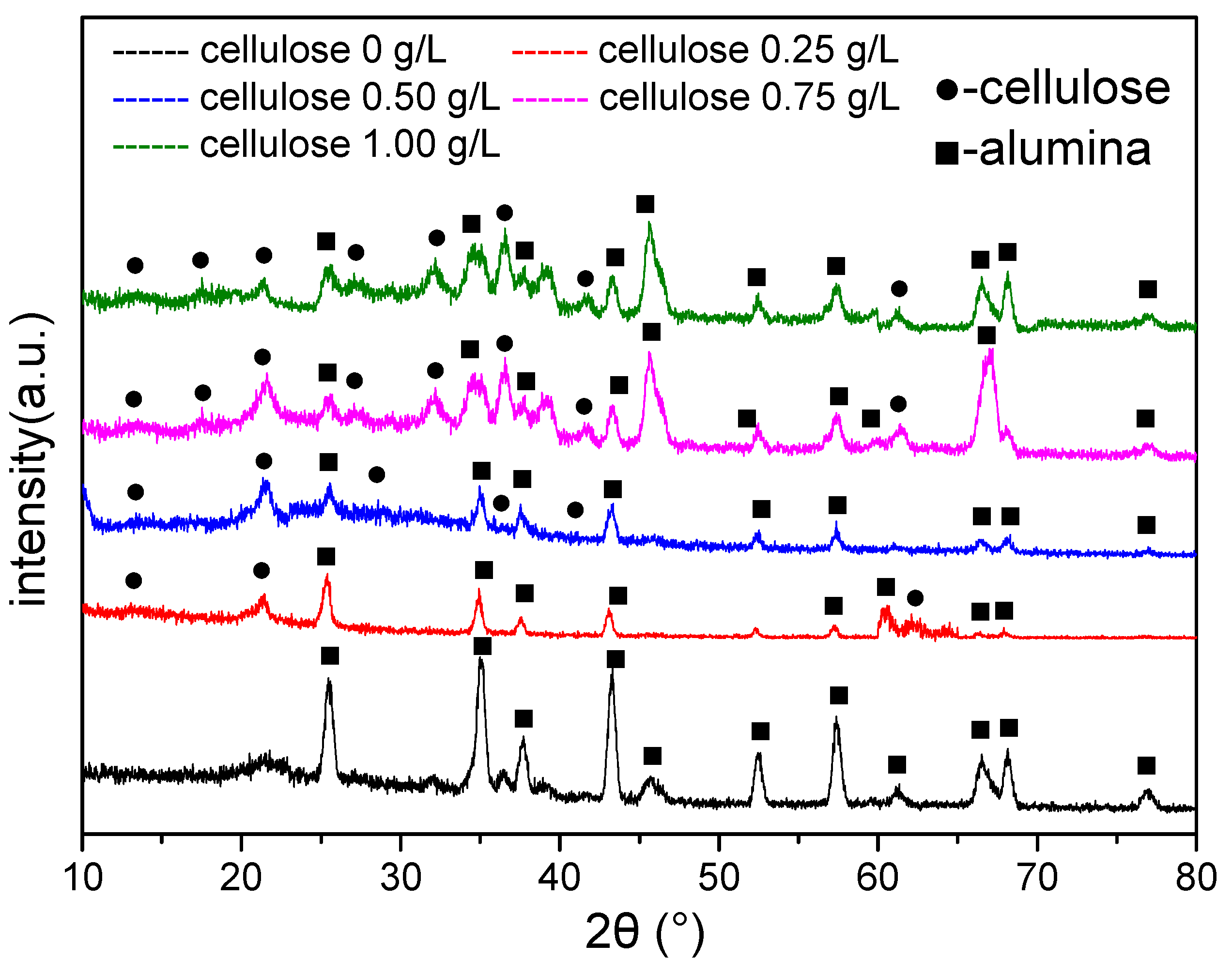
3.3. Phase Structure of the Coating
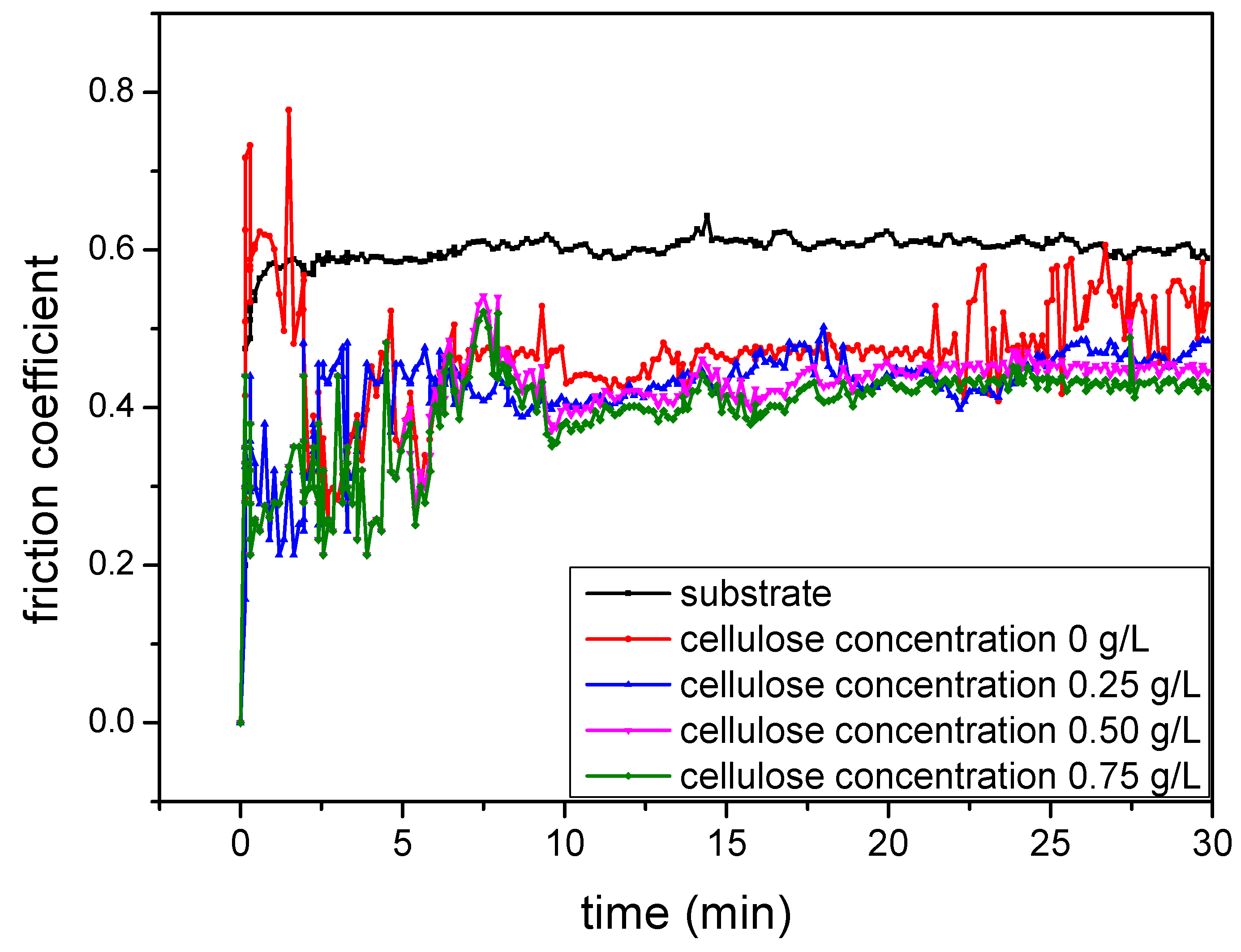
3.4. Tribological Performances of the MAO Coatings
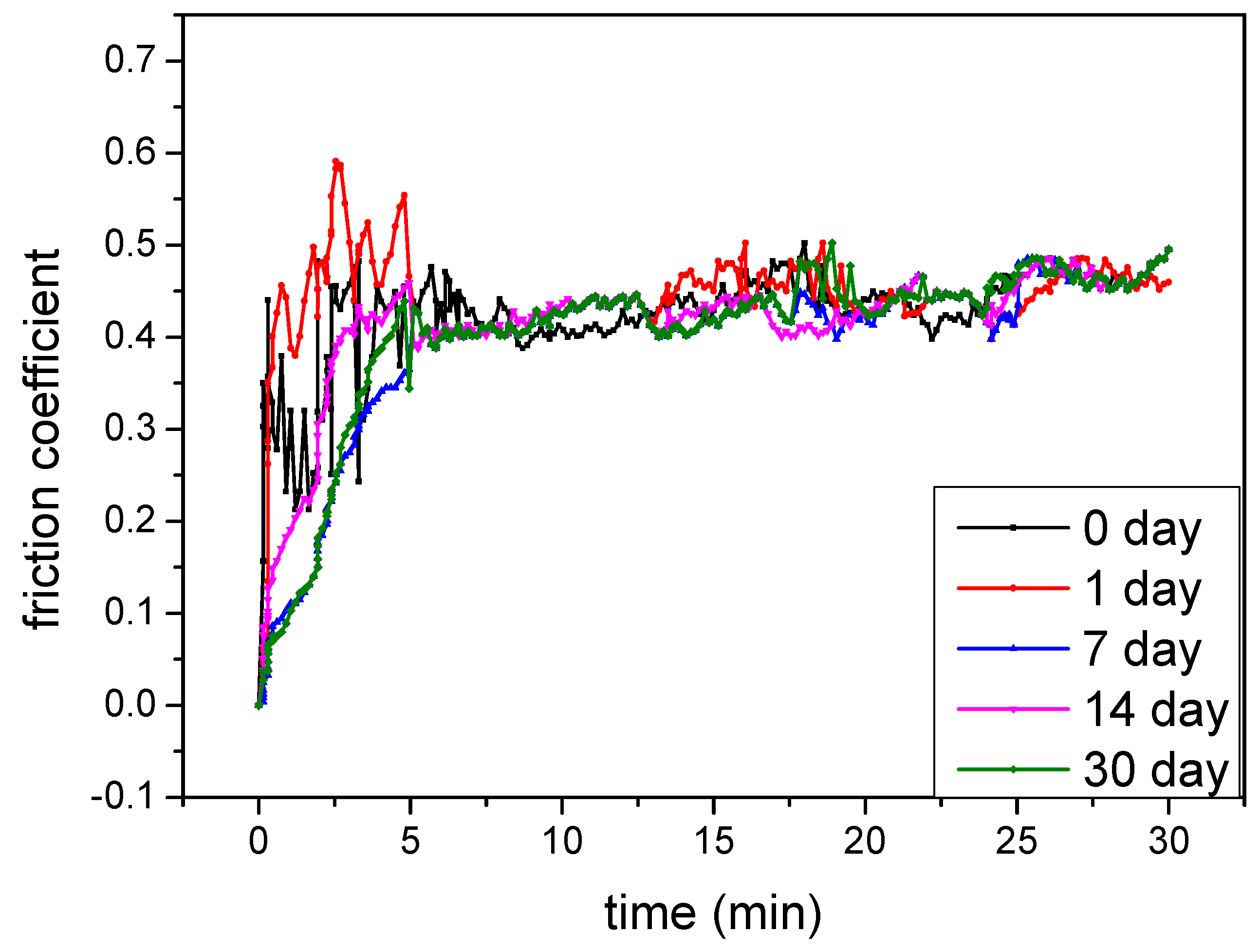
3.5. Stability of the Electrolyte
4. Conclusions
- The tribological performances of MAO coatings are improved by mixing 0.75 g/L of cellulose into the electrolyte. The thickness of the coating increases while the roughness decreases with the increase in cellulose content.
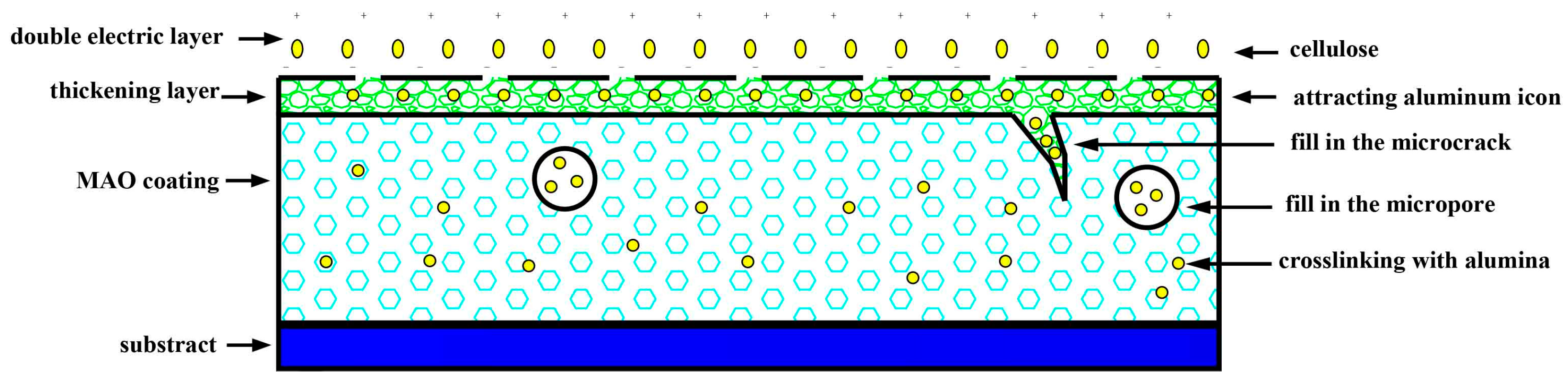
- The coating compositions are thereby analyzed by FTIR and XRD, which prove the presence of cellulose in the coating. Moreover, the coating microstructure is observed through SEM, which reveals that the coating has a compact structure; meanwhile, the coating compositions at micropores, microcracks and normal positions are examined through EDS, suggesting that part of the cellulose fills in the microcracks and micropores, and part of it cross-links with the Al3+.
- The tribological performances of the coatings at different cellulose concentrations are evaluated using a ball-on-disk tester under dry sliding conditions. After different storage periods, they are compared at the same electrolyte, revealing that MAO coatings with consistent quality can be produced in this electrolyte.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeon, G.T.K.; Kim, Y.; Moon, J.H.; Lee, C.; Kim, W.J.; Kim, S.J. Effect of Al 6061 Alloy Compositions on Mechanical Properties of the Automotive Steering Knuckle Made by Novel Casting Process. Metals 2018, 8, 857. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xiong, B.Q.; Li, X.W.; Yan, L.Z.; Li, Z.H.; Zhang, Y.A.; Liu, H.W. Deep drawing of 6A16 aluminum alloy for automobile body with various blank-holder forces. Rare Met. 2019, 38, 946–953. [Google Scholar] [CrossRef]
- Kovalov, D.; Fekete, B.; Engelhardt, G.R.; Macdonal, D.D. Prediction of corrosion fatigue crack growth rate in alloys. Part I: General corrosion fatigue model for aero-space aluminum alloys. Corros. Sci. 2018, 141, 22–29. [Google Scholar] [CrossRef]
- Lenihan, D.; Ronan, W.; O’Donoghue, P.E.; Leen, S.B. A review of the integrity of metallic vehicle armour to projectile attack. Proc. Inst. Mech. Eng. Part L-J. Mater. 2019, 233, 73–94. [Google Scholar] [CrossRef]
- Benea, L.; Dumitrascu, V. Enhancement in sustained friction and wear resistance of nanoporous aluminum oxide films obtained by controlled electrochemical oxidation process. Rsc. Adv. 2019, 9, 25056–25063. [Google Scholar] [CrossRef]
- Sardar, S.; Pradhan, S.K.; Karmakar, S.K.; Das, D. Modeling of Abraded Surface Roughness and Wear Resistance of Aluminum Matrix Composites. J. Tribol. 2019, 141, 071601. [Google Scholar] [CrossRef]
- Ghafaripoor, M.; Raeissi, K.; Santamaria, M.; Hakimizad, A. The corrosion and tribocorrosion resistance of PEO composite coatings containing alpha-Al2O3 particles on 7075 Al alloy. Surf. Coat. Tech. 2018, 349, 470–479. [Google Scholar] [CrossRef]
- Stark, L.M.; Smid, I.; Segall, A.E.; Eden, T.J.; Potter, J. Self-Lubricating Cold-Sprayed Coatings Utilizing Microscale Nickel-Encapsulated Hexagonal Boron Nitride. Tribol. Trans. 2012, 55, 624–630. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.X.; Zheng, Z.C.; Ma, Y.L.; Andrej, A.; Chen, X.B.; Xie, Z.H.; Su, D.E.; Pan, F.S. Fabrication and characterization of an actively protective Mg-Al LDHs/Al2O3 composite coating on magnesium alloy AZ31. Appl. Surf. Sci. 2019, 487, 558–568. [Google Scholar] [CrossRef]
- Blomqvist, A.; Århammar, C.; Pedersen, H.; Silvearv, F.; Norgren, S.; Ahuja, R. Understanding the catalytic effects of H2S on CVD-growth of alpha-alumina: Thermodynamic gas-phase simulations and density functional theory. Surf. Coat. Tech. 2011, 206, 1771–1779. [Google Scholar] [CrossRef]
- SINGH, A.; HARIMKAR, S.P. Laser Surface Engineering of Magnesium Alloys: A Review. JOM-US. 2012, 64, 716–733. [Google Scholar] [CrossRef]
- Chen, J.M.; Yang, J.; Zhao, X.Q.; An, Y.L.; Hou, G.L.; Chen, J.; Zhou, H.D. Preparation and properties of poly-(p)-oxybenzoyl/aluminum bronze composite coating by atmosphere plasma spraying. Surf. Coat. Tech. 2014, 253, 261–267. [Google Scholar] [CrossRef]
- Gu, Y.H.; Ma, H.J.; Yue, W.; Tian, B.; Chen, L.L.; Mao, D.L. Microstructure and corrosion model of MAO coating on nano grained AA2024 pretreated by ultrasonic cold forging technology. J. Alloy. Compd. 2016, 681, 120–127. [Google Scholar] [CrossRef]
- Tonelli, L.; Pezzato, L.; Dolcet, P.; Dabalà, M.; Martini, C. Effects of graphite nano-particle additions on dry sliding behaviour of plasma-electrolytic-oxidation-treated EV31A magnesium alloy against steel in air. Wear 2018, 404, 122–132. [Google Scholar] [CrossRef]
- Yi, P.; Yue, W.; Liang, J.; Hou, B.B.; Sun, J.H.; Gu, Y.H.; Liu, J.X. Effects of nanocrystallized layer on the tribological properties of micro-arc oxidation coatings on 2618 aluminum alloy under high temperatures. Int. J. Adv. Manuf. Tech. 2018, 96, 1635–1646. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Ge, Y.F.; Liu, G.L.; Gao, F.; Li, P.Y. Investigation of tribological properties of micro-arc oxidation ceramic coating on Mg alloy under dry sliding condition. Ceram. Int. 2018, 44, 16164–16172. [Google Scholar] [CrossRef]
- Demirbas, C.; Ayday, A. The influence of Nano-TiO2 and Nano-Al2 O3 Particles in Silicate Based Electrolytes on Microstructure and Mechanical Properties of Micro Arc Coated Ti6Al4V Alloy. Mater. Res. 2018, 21, e20180092. [Google Scholar] [CrossRef]
- Dudarev, N.; Gallyamova, R. The Cnfluence of Chemical Composition of Aluminum Alloys on the Quality of Oxide Layers Formed by Microarc Oxidation. Mater. Today-Proc. 2019, 11, 89–94. [Google Scholar] [CrossRef]
- Tran, Q.P.; Kuo, Y.C.; Sun, J.K.; He, J.L.; Chin, T.S. High quality oxide-layers on Al-alloy by micro-arc oxidation using hybrid voltages. Surf. Coat. Tech. 2016, 303, 61–67. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Q.; Xin, T.Z.; Yi, C.G.; Jiang, Z.H.; Wang, F.P. Influence of Co(CH3COO)(2) concentration on thermal emissivity of coatings formed on titanium alloy by micro-arc oxidation. Curr. Appl. Phys. 2012, 12, 284–290. [Google Scholar] [CrossRef]
- Muhaffffel, F.; Cimenoglu, H. Development of corrosion and wear resistant micro arc oxidation coating on a magnesium alloy. Surf. Coat. Tech. 2019, 357, 822–832. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Sobolev, A.; Wolicki, I.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. Coating Formation on Ti-6Al-4V Alloy by Micro Arc Oxidation in Molten Salt. Materials 2018, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.G.; Zhou, Z.B.; Cai, H.; Wang, X.L.; Wang, Y. Preparation and Properties of a Flexible Al2O3/Al/Al2O3 Composite. Adv. Mater. Sci. Eng. 2018, 2018, 4739267. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Jiang, Z.Q.; Tang, S.G.; Dong, W.B.; Tong, Q.; Li, W.Z. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 aluminum alloy and the coating properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Balaji, R.; Pushpavanam, M.; Kumar, K.Y.; Subramanian, K. Electrodeposition of bronze-PTFE composite coatings and study on their tribological characteristics. Surf. Coat. Tech. 2006, 201, 3205–3211. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: review of the literature. Burn 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Burg, K.J.L.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Fukumasa, O.; Tagashira, R.; Tachino, K.; Mukunoki, H. Spraying of MgO fifilms with a well-controlled plasma jet. Surf. Coat. Tech. 2003, 169, 579–582. [Google Scholar] [CrossRef]
- Li, Z.W.; Di, S.C. Effect of Anode Pulse-Width on the Microstructure and Wear Resistance of Microarc Oxidation Coatings. Metals 2017, 7, 243. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.Z.; Zhang, J. Effect of ZrO2 particle on the performance of micro-arc oxidation coatings on Ti6Al4V. Appl. Surf. Sci. 2015, 342, 183–190. [Google Scholar] [CrossRef]
- Lin, X.Z.; Zhu, M.H.; Cai, Z.B.; Zheng, J.F.; Peng, J.F. Microstructure and Reciprocating Sliding Tribological Performance of Micro-arc Oxidation Coating Prepared on Al-Si Alloy. Adv. Mater. Res. 2010, 97–101, 1518–1526. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Aoki, Y.; Habazaki, H. Two-step plasma electrolytic oxidation of Ti–15V–3Al–3Cr–3Sn for wear-resistant and adhesive coating. Surf. Coat. Tech. 2011, 205, 4732–4740. [Google Scholar] [CrossRef]
- Li, X.; Luan, B.L. Discovery of Al2O3 particles incorporation mechanism in plasma electrolytic oxidation of AM60B magnesium alloy. Mater. Lett. 2012, 86, 88–91. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, Y.; Jiang, Z.; Wang, F.; Wu, Z. Preparation and structure of ceramic coatings containing zirconium oxide on Ti alloy by plasma electrolytic oxidation. J. Mater. Process. Tech. 2008, 205, 303–307. [Google Scholar] [CrossRef]
- Huang, S.Q.; Wu, L.J.; Li, T.Z.; Xu, D.Y.; Lin, X.L.; Wu, C.D. Facile preparation of biomass lignin-based hydroxyethyl cellulose superabsorbent hydrogel for dye pollutant removal. Int. J. Biol. Macromol. 2019, 137, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.B.; Guo, B.G.; Yan, F.Y.; Tian, J.; Liang, J. Tribological Properties of Micro-arc Oxidation Coatings on Al Alloy. J. Mater. Sci. Eng. 2004, 22, 564–567. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.L.; Yu, D.Z.; Wang, F.P.; Zhao, L.C. Wear resistance of micro-arc oxidation coatings on biomedical NiTi alloy. J. Alloy. Compd. 2009, 487, 391–394. [Google Scholar] [CrossRef]
- Zhu, M.H.; Cai, Z.B.; Lin, X.Z.; Zheng, J.F.; Luo, J.; Zhou, Z.R. Fretting wear behaviors of micro-arc oxidation coating sealed by grease. Wear 2009, 267, 299–307. [Google Scholar] [CrossRef]
- Gao, D.D.; Dou, J.H.; Hu, C.; Yu, H.J.; Chen, C.Z. Corrosion behaviour of micro-arc oxidation coatings on Mg-2Sr prepared in poly (ethylene glycol)-incorporated electrolytes. Rsc. Adv. 2018, 8, 3846–3857. [Google Scholar] [CrossRef]


| Point | C K (at. %) | O K (at. %) | Al K (at. %) |
|---|---|---|---|
| 1 | 4.21 | 45.31 | 42.48 |
| 2 | 3.16 | 48.43 | 45.41 |
| 3 | 3.83 | 46.54 | 43.63 |
| 4 | 2.43 | 49.84 | 46.73 |
| Cellulose Concentration g/L | Micro-Hardness HV0.1 | Weight Loss mg | Wear Track Depth µm | Wear Track Width µm | Wear Rate 10−5 mm3/N·m |
|---|---|---|---|---|---|
| 0 | 1260 | 16 | 6.42 | 679.61 | 2.30 |
| 0.25 | 1230 | 14 | 4.59 | 537.49 | 1.13 |
| 0.50 | 1240 | 12 | 4.31 | 522.32 | 1.03 |
| 0.75 | 1230 | 11 | 3.54 | 486.26 | 0.84 |
| 1.00 | 1220 | 10 | 3.13 | 482.01 | 0.82 |
| Stability of the Electrolyte Day | Micro-Hardness HV0.1 | Weight Loss mg | Thickness µm | Roughness µm | Wear Rate 10−5 mm3/N·m |
|---|---|---|---|---|---|
| 0 | 1230 | 11 | 32.1 | 0.66 | 0.84 |
| 1 | 1230 | 15 | 32.2 | 0.72 | 0.79 |
| 7 | 1220 | 10 | 31.9 | 0.68 | 0.91 |
| 14 | 1230 | 11 | 32.1 | 0.69 | 0.89 |
| 30 | 1220 | 11 | 31.9 | 0.67 | 0.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Jiang, B.; Ji, D. Improving the Tribological Performance of MAO Coatings by Using a Stable Sol Electrolyte Mixed with Cellulose Additive. Materials 2019, 12, 4226. https://doi.org/10.3390/ma12244226
Song W, Jiang B, Ji D. Improving the Tribological Performance of MAO Coatings by Using a Stable Sol Electrolyte Mixed with Cellulose Additive. Materials. 2019; 12(24):4226. https://doi.org/10.3390/ma12244226
Chicago/Turabian StyleSong, Wei, Bailing Jiang, and Dongdong Ji. 2019. "Improving the Tribological Performance of MAO Coatings by Using a Stable Sol Electrolyte Mixed with Cellulose Additive" Materials 12, no. 24: 4226. https://doi.org/10.3390/ma12244226
APA StyleSong, W., Jiang, B., & Ji, D. (2019). Improving the Tribological Performance of MAO Coatings by Using a Stable Sol Electrolyte Mixed with Cellulose Additive. Materials, 12(24), 4226. https://doi.org/10.3390/ma12244226




