1. Introduction
Piezoelectrics are seen as an important material group due to their place in a great variety of functional applications, ranging from simple sensors [
1,
2] to the interiors of the most advanced apparatuses, such as the atomic force microscope (AFM) [
3]. In general, piezoelectricity stands for a reversible process of acquiring an electrical charge in solid material by applying mechanical stress. Materials that generate a measurably useful current are known as piezoelectrics and include various single crystals, organic materials, and perovskite ceramics.
In engineering and various industries, lead-based perovskites are predominantly used when piezoelectric effects are pursued. The most notable example of excellent electromechanical properties is witnessed in Pb(Zr,Ti)O
3-based ceramics (PZT ceramics) [
4]. Therefore, such materials have been widely studied and optimized for commercial and scientific usage, resulting in relatively simple processing methods and industry prevalence [
5,
6]. However, with constantly increasing awareness about health-endangering materials, the necessity of alternative, nonhazardous solutions has emerged. Compositions based on K
0.5Na
0.5NbO
3 (KNN) are a possible alternative to replace lead-based piezoelectrics. Due to the significant piezoelectric response obtained by numerous teams [
5,
7,
8,
9], KNN-based materials have been widely researched, including in this study.
In a perovskite-type ABO
3 structure, A-site cations are either alkaline earth elements or rare-earth elements, with B-site cations being transition metals. Theoretical structure consists of A cations occupying cuboctahedral coordination positions in the middle of eight corner-sharing BO
6 octahedrons. Due to the specific structure and composition, perovskites have plenty of conductive properties used in material sciences, including significant piezoelectricity. The piezoelectric effect is directly derived from the noncentrosymmetric structure of materials. Such structures are electrically neutral, but lack symmetrical arrangement. Therefore, when mechanical stress is applied, and such structure shifts, it does not maintain a neutral charge [
4]. The high magnitude of electromechanical effects obtained in perovskites is derived from the ordering tendency present in those materials, mainly the shift of B cations [
4].
K
0.5Na
0.5NbO
3 has a similar structure to BaTiO
3, which consists of two phases, ferroelectric K
1-xNbO
3 and antiferroelectric Na
xNbO
3. Both phases have different ferroelectric transitions, crystallizing in a perovskite structure, with different symmetries. The phase transitions of KNN depend on the K/Na ratio [
10]. To synthesize the basic structure of KNN, K, and Na carbonates are used in conjunction with Nb
2O
5 [
5]:
The main challenge emerging during KNN sintering is the densification of the obtained polycrystals. There are a few possible reasons that may cause this issue. During the thermal processing of KNN, evaporation of sodium is commonly reported [
11,
12]. Oxygen vacancies tend to appear during sintering processes [
13]. Additionally, sintering of pure KNN is limited by lack of a liquid phase, resulting in a porous microstructure. Densification could be enhanced by the addition of various dopants. Rare-earth elements (REE) are reportedly used to address this issue due to the creation of a liquid phase and a high tolerance factor allowing for ion substitution in the perovskite structure [
14].
REE oxides are used to significantly improve the piezoelectric properties of KNN-based materials. It was reported that the addition of these oxides resulted in a stabilizing and lowering dissipation factor in piezoelectric ceramics because of A-site ion substitution. The addition of two REEs was highlighted for the purpose of this study, ytterbium and erbium. Studies by Li et al. indicated that Yb
3+ replaces both sodium and potassium (A site) and niobium (B site) in KNN structures, but each substitution can be promoted by changing the concentration of those cations. A low concentration (<0.25%) results in A-site substitutions, with Yb
3+ cations acting as donor ions, while increased concentration (>0.25%) results in B-site substitutions, with Yb
3+ cations acting as acceptor ions [
15]. When a Yb
3+ cation acts as a donor dopant, it causes the reduction of oxygen-vacancy concentration that appeared during sintering [
16]. Elimination of vacancies is desired in that case to maintain the electrical resistance of KNN material. This phenomenon may lead to the desired deformation of a crystal structure [
17]. The addition of Er
2O
3 has been investigated by Zhao et al. [
18,
19], and it enhances the photoluminescence properties of KNN ceramics. It also increases the coupling effects of mechanical-electrical luminescence. Er
2O
3 doping results in the appearance of a liquid phase during sintering, which is beneficial for material densification.
The aim of this study was to obtain a dense KNN-based material that could be used as a lead-free piezoelectric stress sensor. To achieve that two simultaneous approaches were investigated. First, 1 and 2 wt % amounts of Er
2O
3 and Yb
2O
3 as doping materials were proposed. Second, in order to increase the overall density of the material, uniaxial hot pressing in a graphite mold was employed instead of commonly used pressureless sintering. Such a process has a positive effect on texturing a polycrystal microstructure, which is desirable in this type of material due to the improvement of piezoelectric properties [
20,
21]. Additionally, it was expected that high pressure decreases sintering temperature, which, in turn, prevents alkali evaporation and reduces the effect of donor ions, increasing the KNN melting point. This allows to manufacture more stoichiometric K
0.5Na
0.5NbO
3 material after thermal processing [
22,
23].
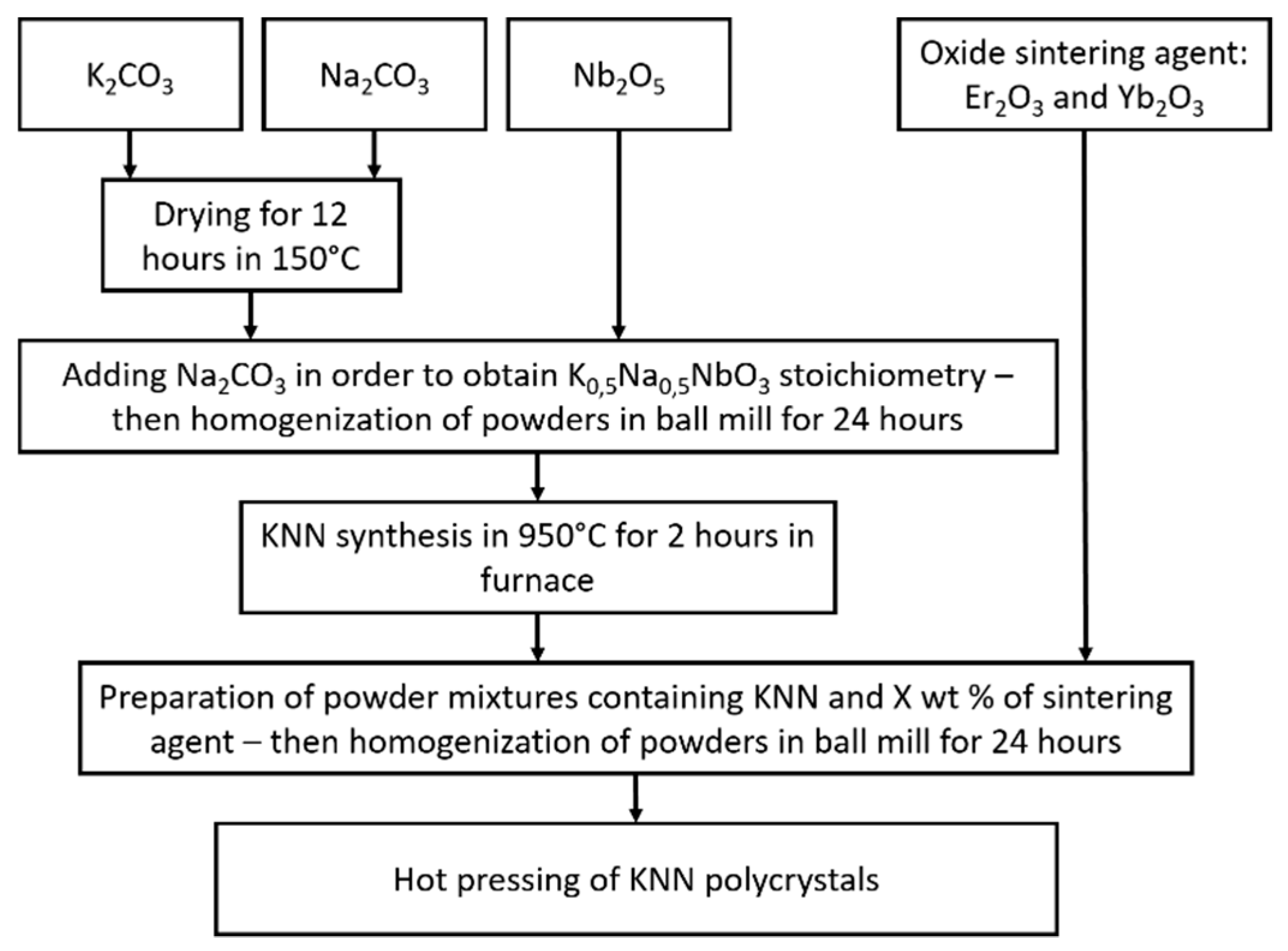
2. Materials and Methods
Experiments were performed with commercially available powders of Nb
2O
5 produced by Changsha Easchem Co. Ltd. (Changsha, China, 99.7% purity); K
2CO
3 and Na
2CO
3 powders were produced by Lach-Ner (Neratovice, Czech Republic; 99.5% purity). In order to synthesize KNN, these powders were used as reactants for processing. To remove residual water from the samples, K
2CO
3 and Na
2CO
3 powders were dried at 150 °C for 12 4 and then kept in a desiccator between each step of the preparation process. Subsequently, K
2CO
3, Na
2CO
3, and Nb
2O
5 powders were initially mixed in ceramic mortar with an additional 5 and 10 wt % of Na
2CO
3 in order to avoid sodium deficiency due to evaporation during synthesis. The prepared powder mixture was then dry-homogenized in a ball mill for 24 h with low rotary speed. Silicon nitride milling media were used. Due to their high wear resistance, samples were unlikely to feature any contamination from this process. Next, the mixture was placed in a furnace inside an Al
2O
3 crucible at 950 °C for 2 h to complete KNN synthesis. Set amounts of Er
2O
3 and Yb
2O
3 sintering agents, shown in
Table 1, were added to obtain KNN powder. Then, powders were additionally homogenized for 24 h in a rotary mill with the use of Si
3N
4 grinding media. Finally, circular green samples with a diameter of 25 mm and 3 mm thickness were prepared by hot pressing at 1000° C for 2 h of dwell time under 25 MPa pressure in a graphite mold in Ar gas flow. Due to differences in the atomic radius between K
+ (0.133 nm) and Na
+ (0.097 nm), sodium evaporates more easily during KNN sintering. A higher amount of K
+ ions present in the KNN structure leads to a larger distance of the crystal face and results in a 2θ decrease of KNN patterns, which was confirmed by Jiang [
24]. The procedure of KNN sample preparation is presented in
Figure 1.
X-ray diffraction analysis was performed using a PANalytical X-ray diffractor (XRD, Almelo, The Netherlands) equipped with Cu tube, and X-pert HighScore software (version 3.0e) was used in order to designate sample phase composition. Scanning-electron-microscopy (NOVA NANO SEM 200, FEI EUROPE COMPANY, Czech Republic, Brno) observations with energy-dispersive X-ray spectroscopy (EDS of EDAX, Brno, Czech Republic,) analysis were performed using NOVA NANO SEM 200 equipped with an EDAX EDS analyzer (FEI, Brno, Czech Republic). Helium density of the prepared samples was measured using gas pycnometer AccuPyc II 1340 (Micrometrics, Norcross, GA, USA). Material texturing was examined by ultrasonic measurement using a CT-3 apparatus (Unipan Ultrasonic, Warsaw, Poland) equipped with a 1 MHz ultrasonic head.
3. Results
Figure 2 shows the overall morphological appearance of the products after the synthesis of the initial reactant mixtures of a stoichiometric K/Na molar ratio. Coarse particles with size in the range of 4–10 µm and partially sintered fine grains with dimensions of about 1 µm are visible in
Figure 2A, B, respectively. In both cases, particles were strongly agglomerated. However, individual shapes of coarse grains are clearly distinguished. Particles shown in
Figure 2B were five to eight times finer in comparison to those shown in
Figure 2A.
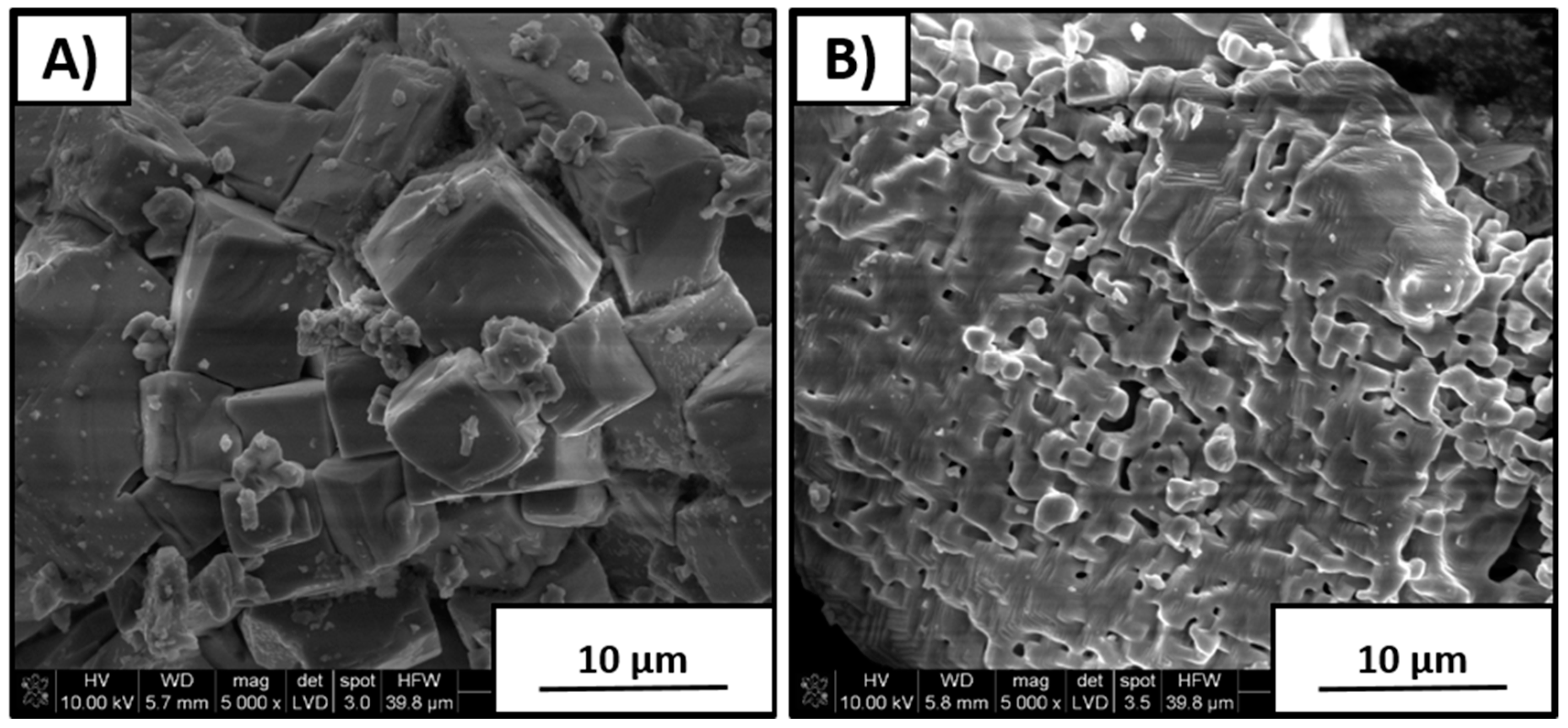
Figure 3 shows SEM images of material synthesized with 5 wt % excess Na
2CO
3 amount. It can be seen that morphology was different than that for the sample with stoichiometric K/Na composition. Coarse particles with 8–10 µm size were covered by a thin layer of very fine crystallites with a size of 1–2 µm. Extensive formation of agglomerates was observed, as well as that an excess amount of Na
2CO
3 positively affected the nucleation of fine particles. Such finer grain-size distribution is expected to improve the overall density of polycrystals due to more efficient milling needed prior to sintering.
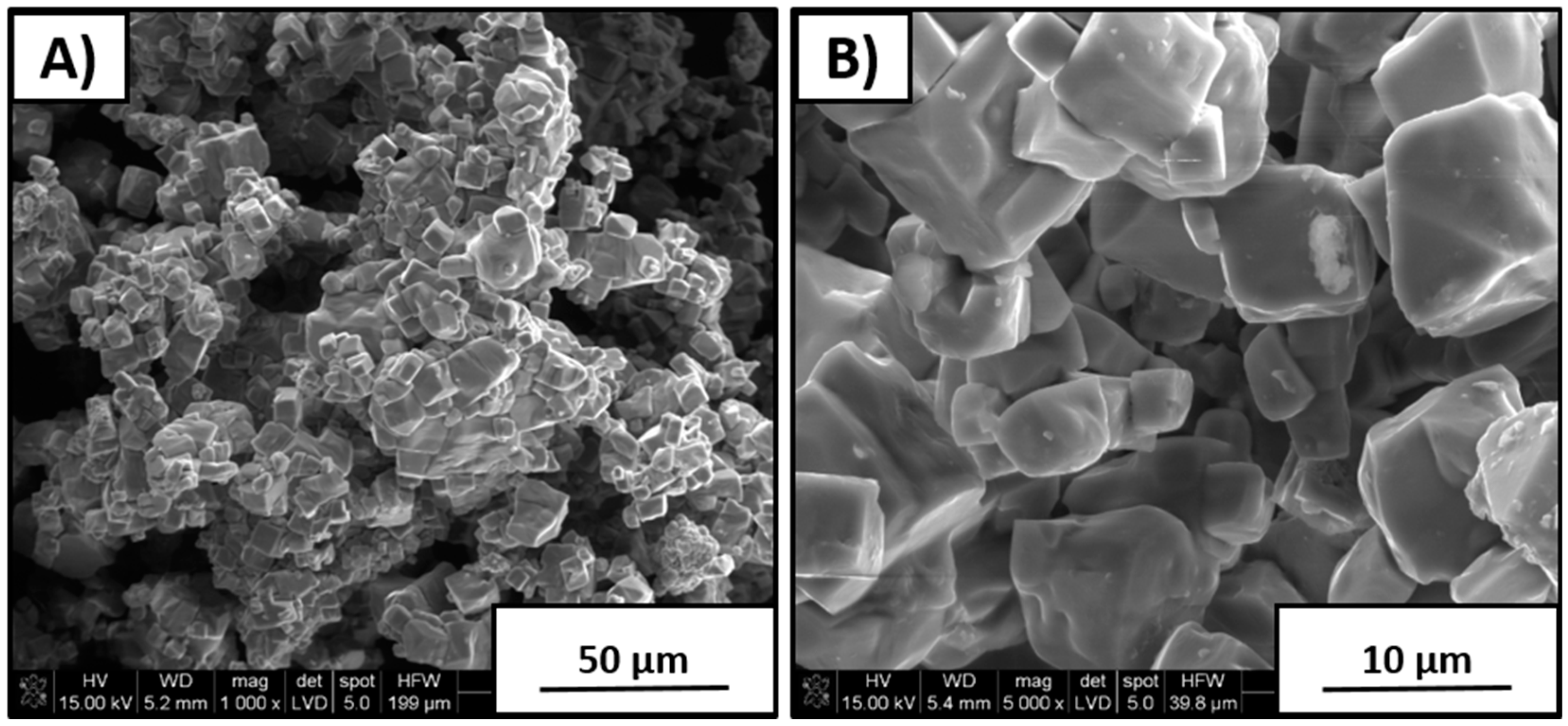
As seen in
Figure 4, in the case of 10% sodium carbonate addition, powder morphology was significantly more uniform than that of the rest of the synthesized powders. It was characterized by a large amount of agglomerates formed from the cubical shapes of the particles. Deviation in size of the single crystallites varied I the range of 5–10 µm. Overabundance of 10 wt % Na
2CO
3 allowed for more homogeneous grain growth during KNN synthesis at 950 °C.
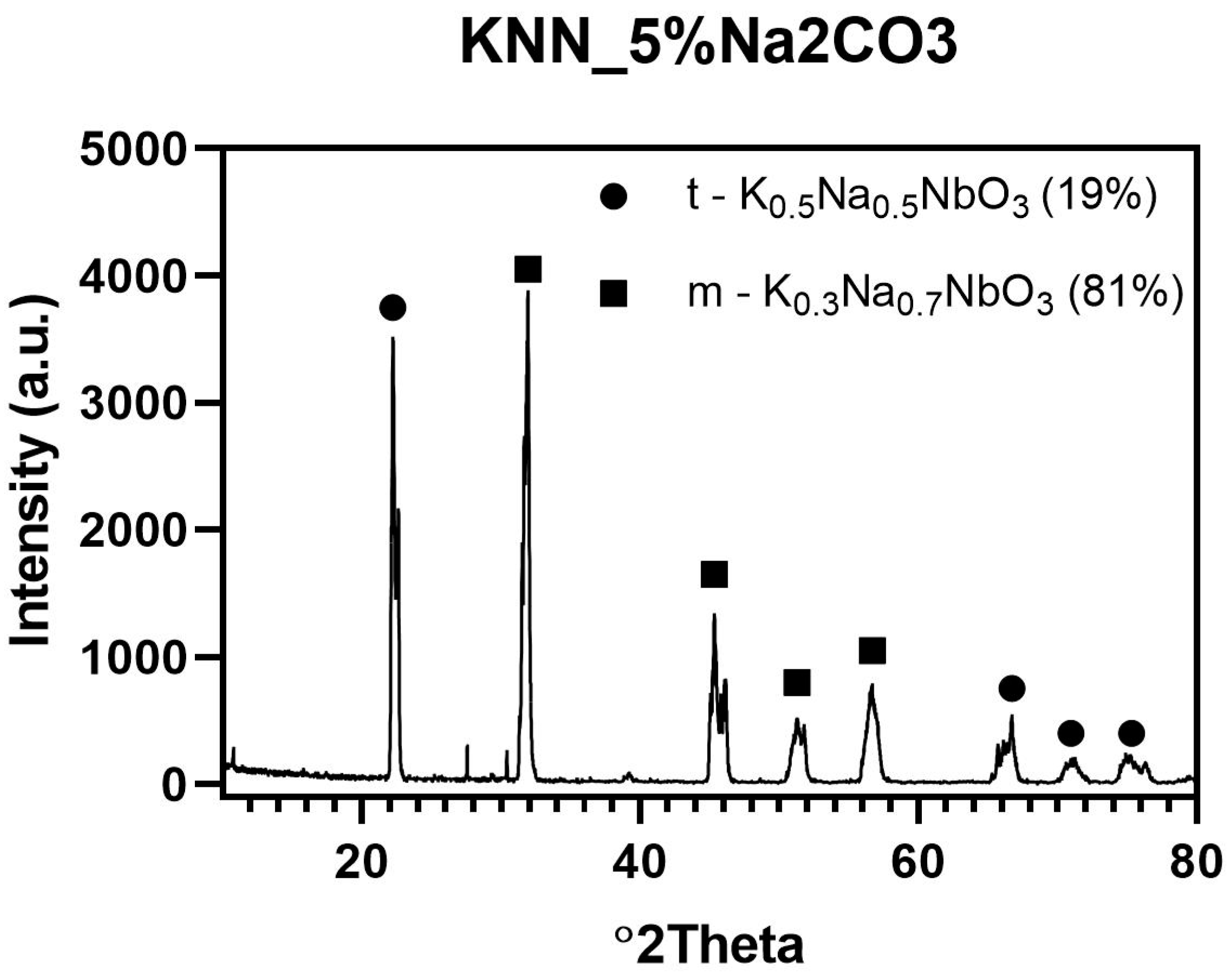
Initial evaluation of the morphology of the synthesized KNN powders showed that the sample with 5 wt % overabundance of Na
2CO
3 was better suited for further processing. In order to check the phase composition of this powder, qualitative and quantitative XRD analysis was performed as shown in
Figure 5. The presence of two different KNN phases was detected, tetragonal K
0.5Na
0.5NbO
3 and monoclinic K
0.3Na
0.7NbO
3. It was shown that synthesis conditions were correctly chosen to complete the reaction between reagents. After KNN synthesis, only about 19 wt % of tetragonal K
0.5Na
0.5NbO
3 was obtained. Therefore, monoclinic K
0.3Na
0.7NbO
3 (81%) are expected to transform into orthorhombic KNN phase after the sintering process with Er
2O
3 and Yb
2O
3 dopants, according to literature data [
4].
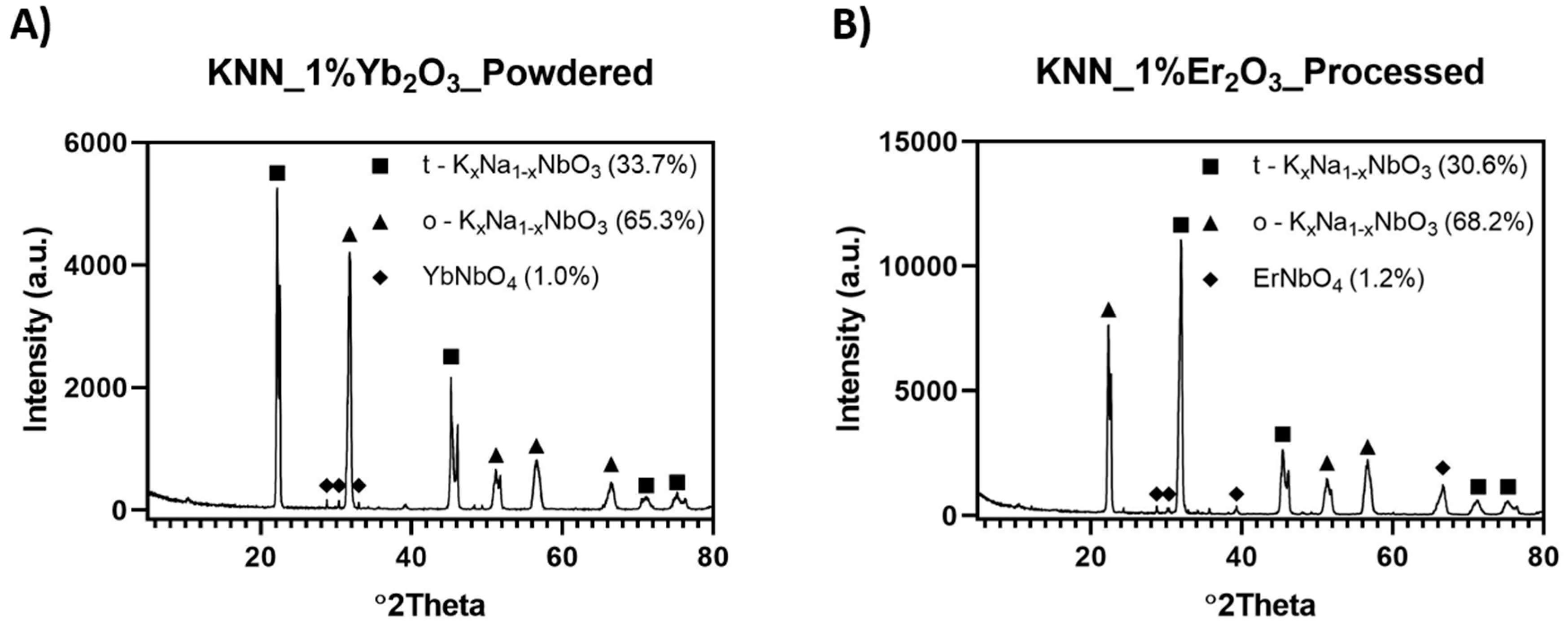
The addition of a sintering agent in the form of rare metals oxides Er
2O
3 and Yb
2O
3 had a significant effect on the phase composition of the samples, as shown in
Figure 6.
When qualitatively compared, the same phases were present in both samples, with an additional ErNbO
4 and Er
2O
3 phase in the material doped by Er
2O
3. The quantity of erbium niobate increased and Er
2O
3 decreased with the addition of erbium oxide to the starting mixture. A similar increase in case of ytterbium niobate was seen in samples with ytterbium oxide introduced. However, quantitative analysis indicated that Yb
2O
3 substantially changed the sintering process in terms of acquiring tetragonal and orthorhombic KNN phases. Qualitative XRD phase-composition analysis showed that the synthetized powder obtained at 950 °C was composed of two KNN phases and contaminations, and it is presented in
Table 2. A proportionally increasing amount of tetragonal and orthorhombic KNN was observed. The presence of dipotassium carbonate sesquihydrate (K
2CO
3·1.5H
2O) was observed in each sample, which formed as the result of water absorption due to the hygroscopic properties of K
2CO
3. Water could be absorbed during extraction from the hot pressing (HP) apparatus or removal of the graphite foil layer used during the HP process, but the latter was performed in isopropanol to prevent such a reaction. K
2CO
3 appeared in the dense sintered material due to the reaction between the graphite form or graphite foil and the green KNN sample. XRD measurements were conducted on the material surface, and graphite was nonetheless not found in further EDS analysis of the fractured samples (
Table 3), which may suggest that this effect occurred mainly on the material surface. The internal part of the sample was also free of water in comparison to the surface. Results indicated that Yb
2O
3 addition had a better effect on the final composition of the sintered samples than that of Er
2O
3. The overall amount of tetragonal and orthorhombic KNN phases was higher in samples containing Yb
2O
3. In Samples Y1 and Y2, the total weight percentage of the KNN phases exceeded 50% of the material. The appearance of orthorhombic KNN was the result of the phase transformation of the monoclinic phase detected in the powder after synthesis at 950 °C.
The existence of K
2CO
3 in the hot-pressed materials was also examined in the volume of the material by two methods. First, the selected material was gently pulverized in a ceramic SiC mortar, followed by XRD analysis, which is shown in
Figure 7A. Second, the surface layer of KNN + 1% Er
2O
3 was mechanically removed, and the result is illustrated in
Figure 7B. This confirmed that K
2CO
3 was not present in the sample volume. Phase analysis showed the existence of tetragonal and orthorhombic KNN with the addition of ErNbO
4 or YbNbO
4. This indicated that K
2CO
3 only formed on the material surface.
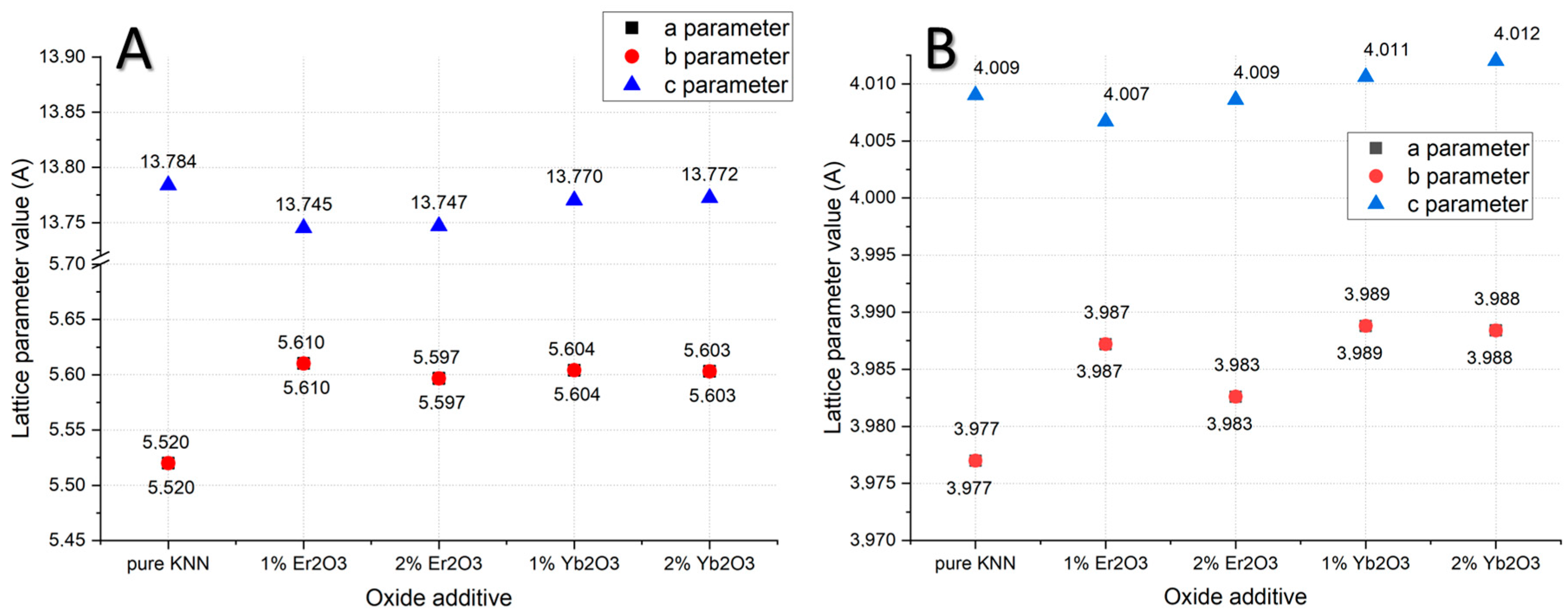
XRD data analysis with a comparison to the reference material showed differences in the lattice-parameter values of KNN structures induced by erbium and ytterbium dopants (
Figure 7). The lattice parameters of pure orthorhombic KNN were a,b = 5.5200 Å, and c = 13.7840 Å. In the case of 1% Yb
2O
3 addition, they were a,b = 5.6042 Å, and c = 13.7703 Å. For 2% Yb
2O
3 addition, they were a, b = 5.6031 Å, and c = 13.7727 Å. Therefore, lattice-structure deformation was visible in the shrinkage of the “a” and “b” parameters, and in the elongation of the “c” parameter. A similar situation was observed in erbium doped samples with a visibly higher shrinkage of the “c” parameter (
Figure 8A). In case of a tetragonal structure, the “c” parameter was elongated with the addition of Yb
2O
3, differing from samples in the orthorhombic structure (
Figure 8B). In both structures, higher additions of dopants led to a decrease of the “a” lattice parameter, which agreed with data from the literature [
15].
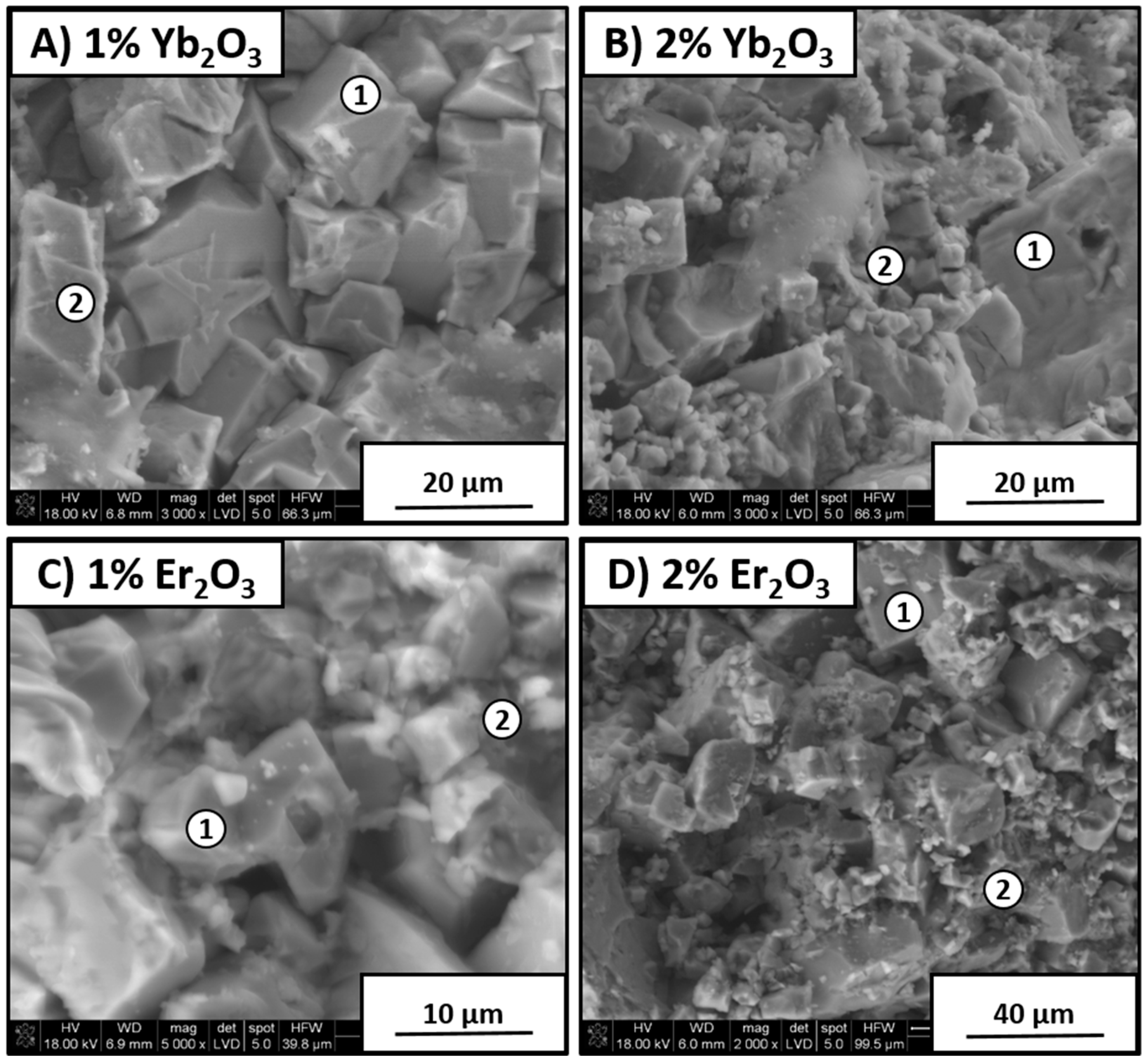
Figure 9 shows samples with 1 and 2 wt % addition of Er
2O
3 and Yb
2O
3 sintering agents. It can be seen that both types of material had similar morphology. In the case of samples containing 1 wt % oxide additions, grains were bigger than those for materials with 2 wt % additions. This indicates that more of the sintering agent caused fine KNN grains to appear. This was confirmed by EDS analysis, as shown in
Table 3.
The sample density is presented in
Table 4. All prepared materials were characterized by high relative density above 95%, with the highest of 99.4% in the addition of 2% Er
2O
3. The 5 wt % excess of Na
2CO
3 caused non-uniform grain-size distribution after the synthesis process. The addition of rare-earth sintering agents of Er
2O
3 and Yb
2O
3 with high pressure and temperature during hot pressing allowed good powder compaction, which led to obtaining dense polycrystals.
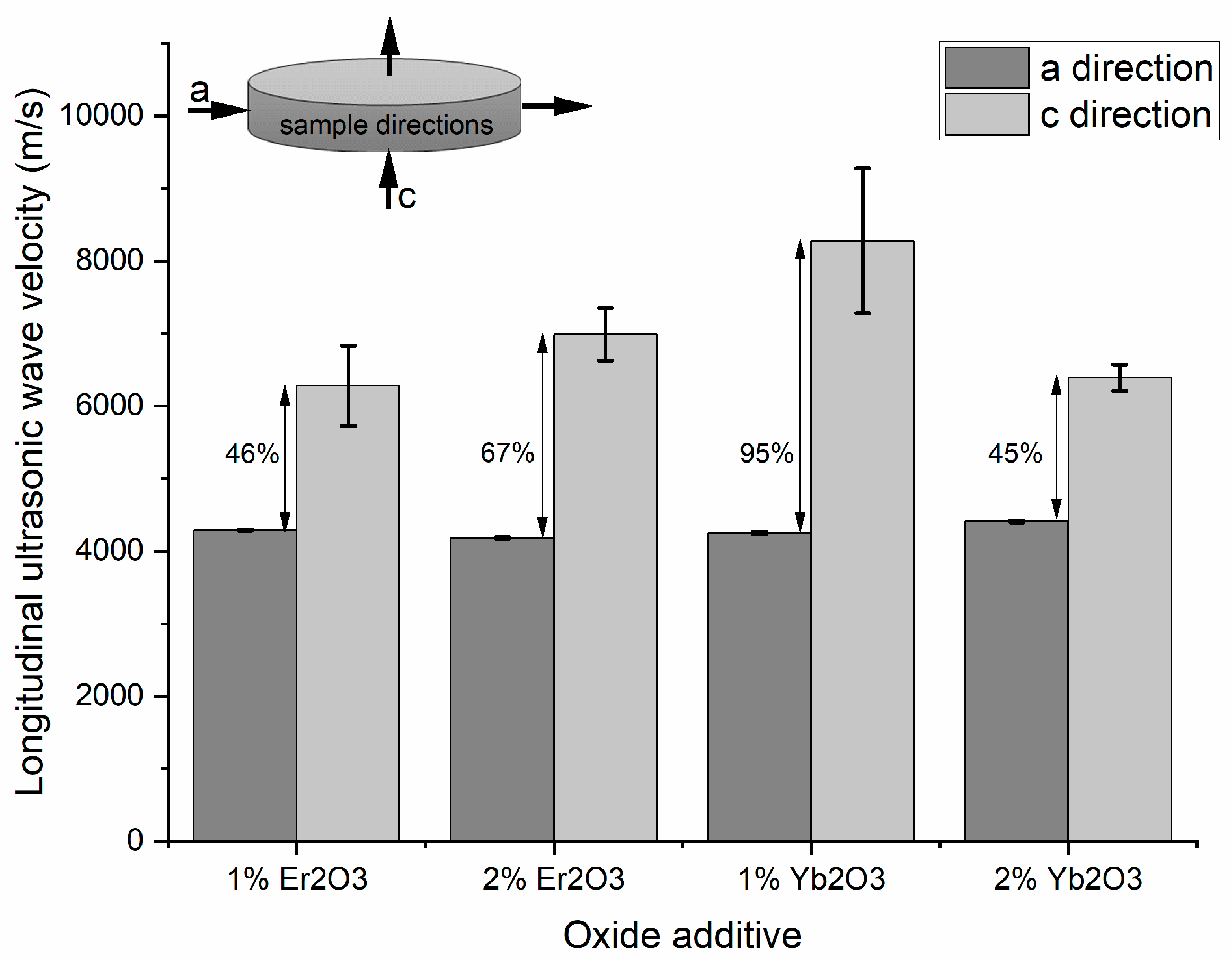
KNN polycrystal analysis revealed the anisotropic feature of their microstructure. Ultrasonic measurements were conducted in two different directions on each sample, as indicated in
Figure 10. The difference in longitudinal ultrasonic-wave velocity measured in the “a” and “c” directions was higher than 45% and reached almost 95%. Wave velocities differed significantly for each sample, so texturing of the material as an effect of applied pressure during the hot-pressing process was observed.