Green Synthesis of Composite Graphene Aerogels with Robust Magnetism for Effective Water Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis Methods
2.2.1. Synthesis of GO
2.2.2. Synthesis of M-CGAs
2.3. Characterization
2.4. Water Remediation Performance Evaluation
2.4.1. Adsorption Behavior
2.4.2. Photo-Fenton Catalytic Activity
2.4.3. Regeneration of M-CGAs
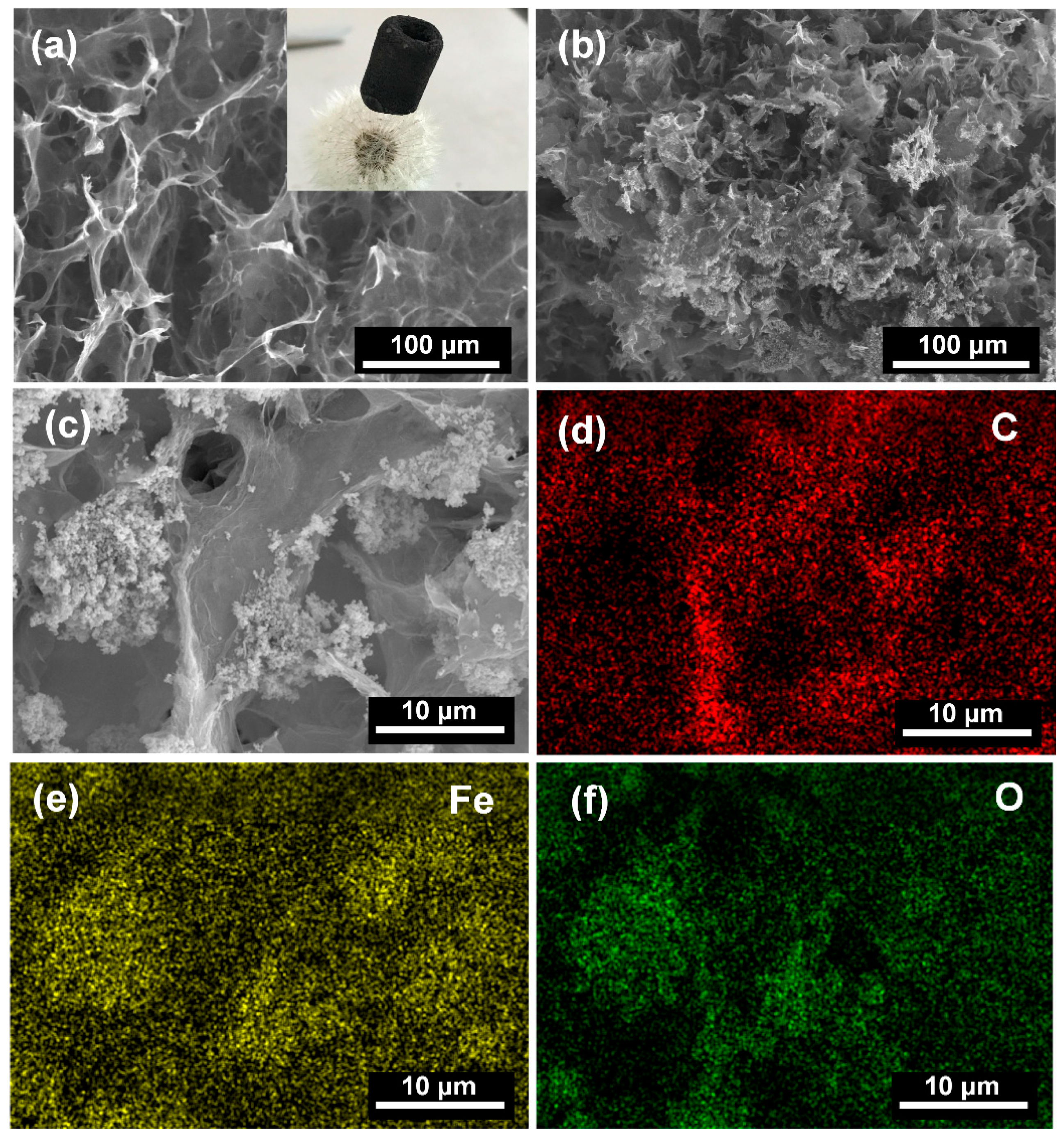
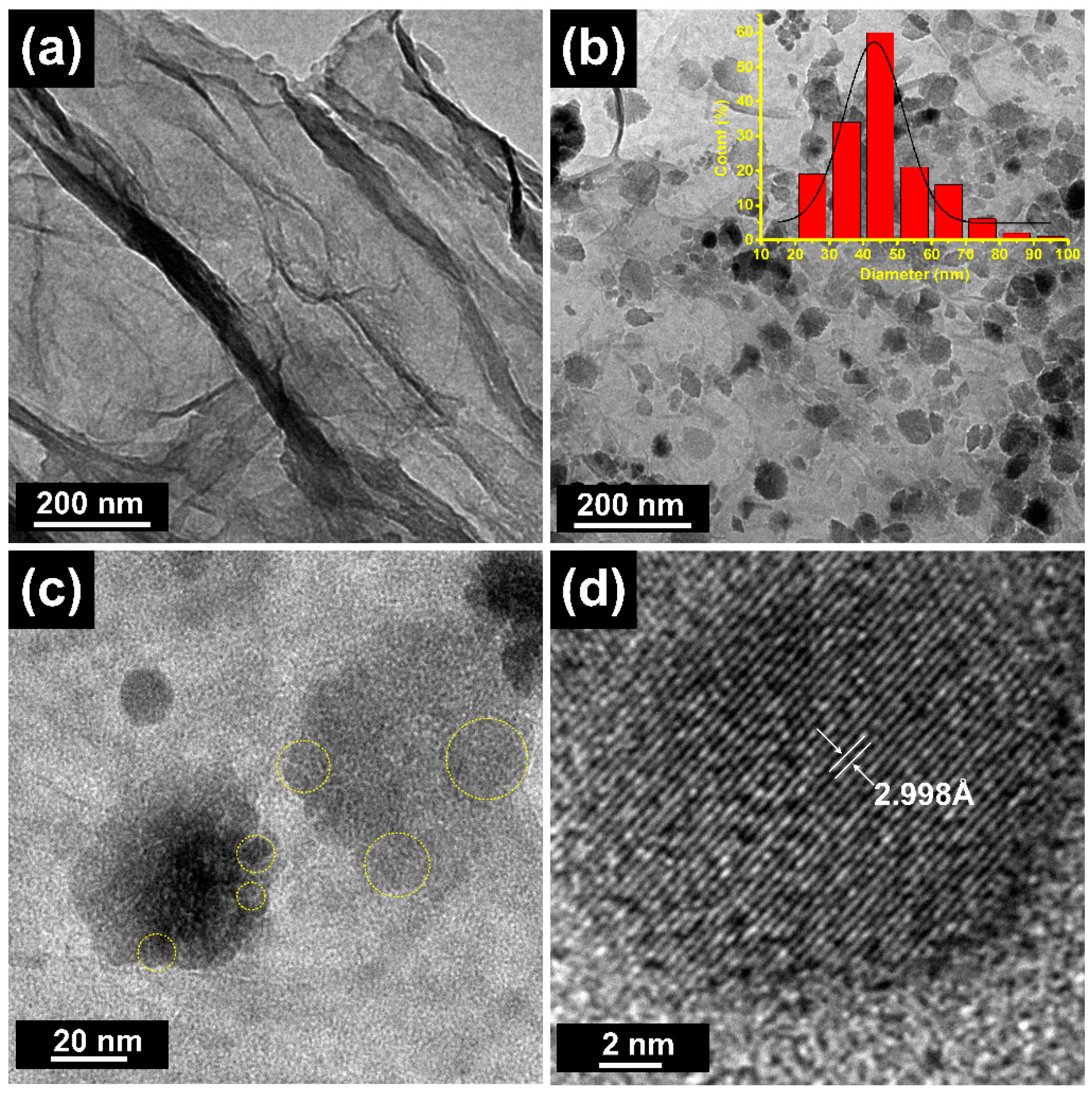
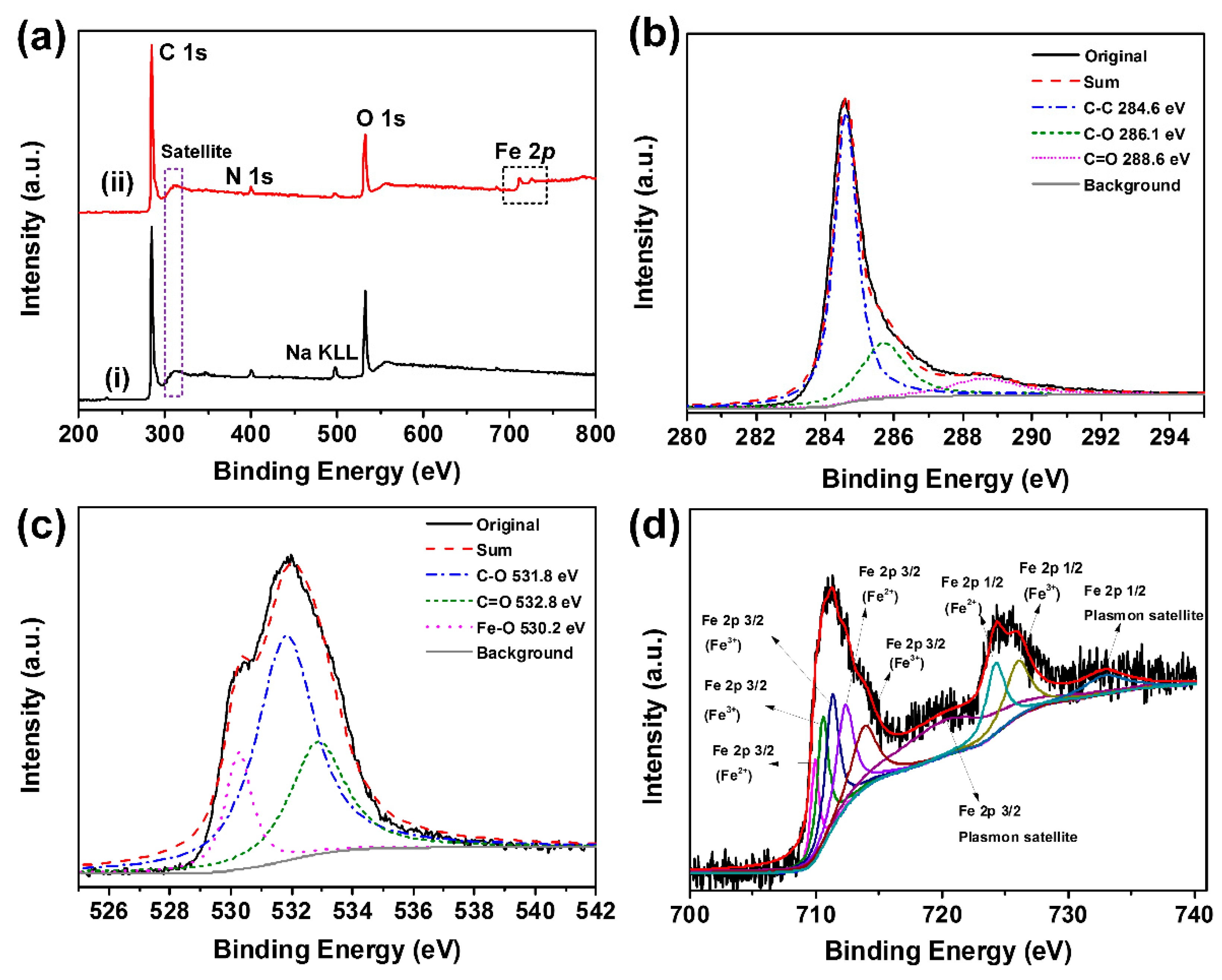
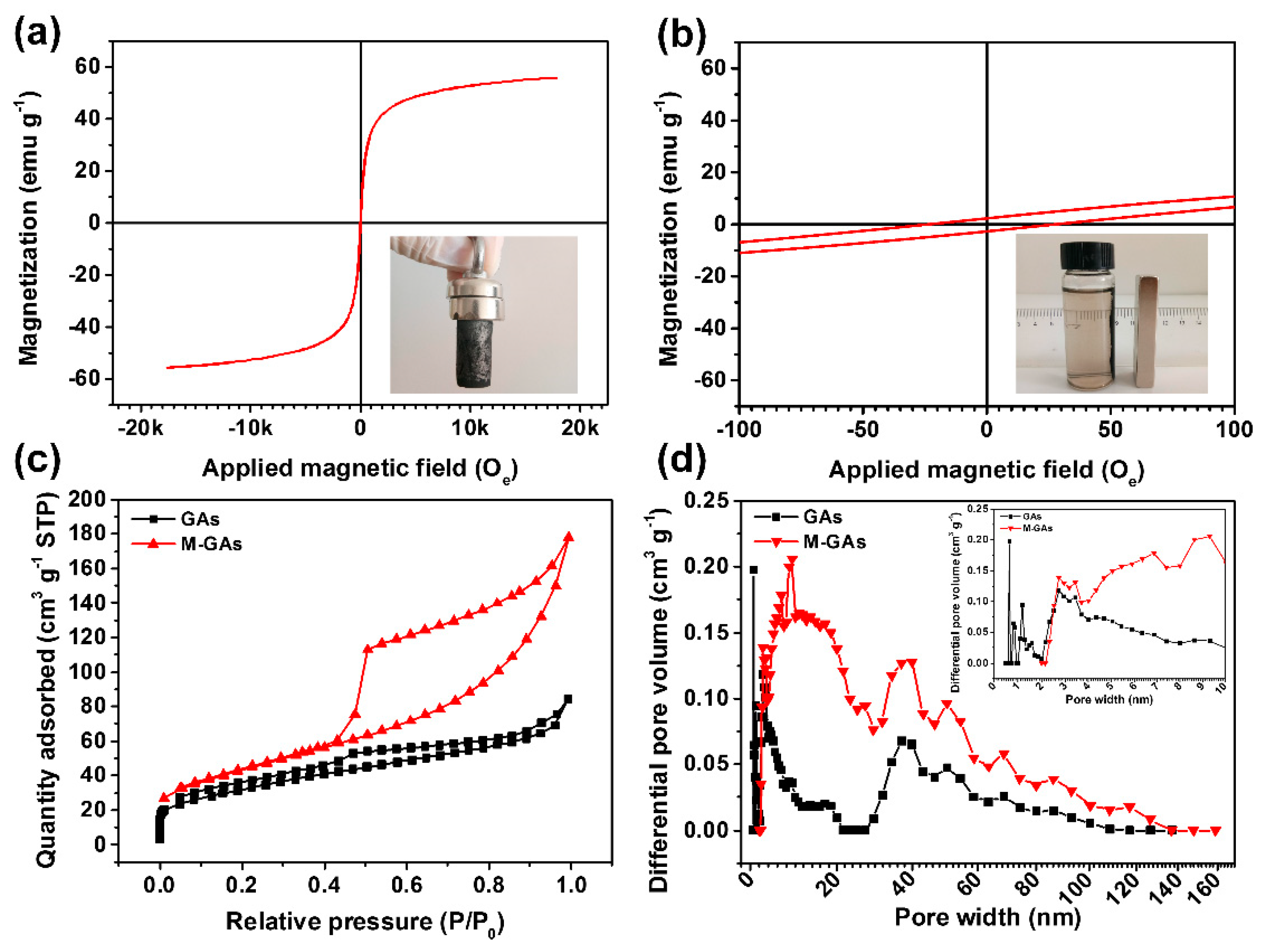
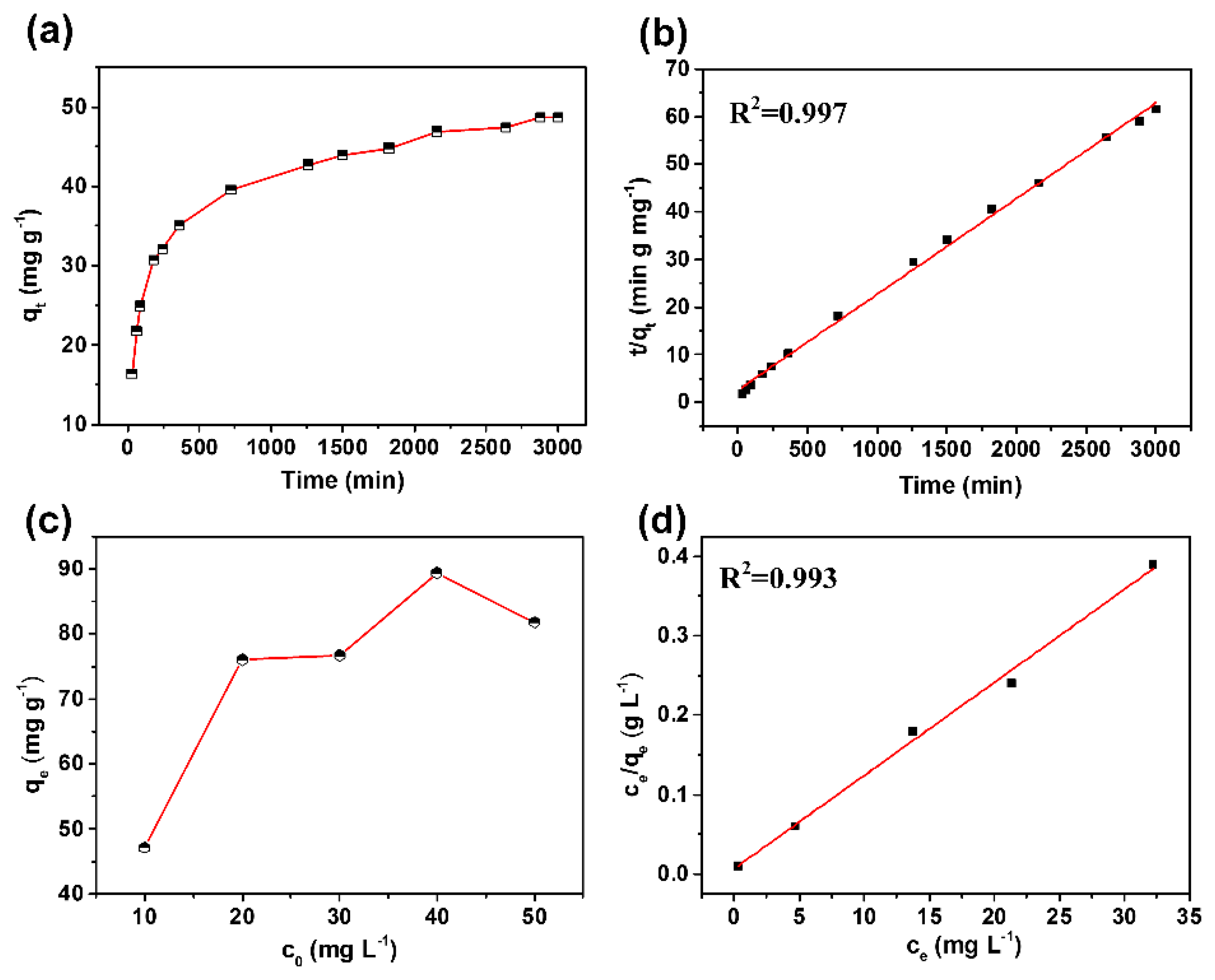
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, Y.; Park, S.J. Recent Advances in Carbonaceous Photocatalysts with Enhanced Photocatalytic Performances: A Mini Review. Materials 2019, 12, 1916. [Google Scholar] [CrossRef] [PubMed]
- Vocciante, M.; Finocchi, A.; D’Auris, A.D.; Conte, A.; Tonziello, J.; Pola, A.; Reverberi, A.P. Enhanced Oil Spill Remediation by Adsorption with Interlinked Multilayered Graphene. Materials 2019, 12, 2231. [Google Scholar] [CrossRef] [PubMed]
- Oribayo, O.; Feng, X.S.; Rempel, G.L.; Pan, Q.M. Synthesis of lignin-based polyurethane/graphene oxide foam and its application as an absorbent for oil spill clean-ups and recovery. Chem. Eng. J. 2017, 323, 191–202. [Google Scholar] [CrossRef]
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.C.; Seema, H.; Saleh, M.; Le, N.H.; Mahesh, K.; Chandra, V.; Kim, K.S. Environmental applications using graphene composites: Water remediation and gas adsorption. Nanoscale 2013, 5, 3149–3171. [Google Scholar] [CrossRef]
- Zhu, J.H.; Wei, S.Y.; Gu, H.B.; Rapole, S.B.; Wang, Q.; Luo, Z.P.; Haldolaarachchige, N.; Young, D.P.; Guo, Z.H. One-Pot Synthesis of Magnetic Graphene Nanocomposites Decorated with Core@Double-shell Nanoparticles for Fast Chromium Removal. Environ. Sci. Technol. 2012, 46, 977–985. [Google Scholar] [CrossRef]
- Sun, H.Y.; Xu, Z.; Gao, C. Multifunctional, Ultra-Flyweight, Synergistically Assembled Carbon Aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Noe, G.T.; Mishra, Y.K. Gated Graphene Enabled Tunable Charge–Current Configurations in Hybrid Plasmonic Metamaterials. ACS Appl. Electron. Mater. 2019, 1, 637–641. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Ramezani, Z. Gated graphene island-enabled tunable charge transfer plasmon terahertz metamodulator. Nanoscale 2019, 11, 8091–8095. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Zhao, Q.C.; Zheng, Z.; Hu, W.P.; Zhang, J. 3D Self-Supporting Porous Magnetic Assemblies for Water Remediation and Beyond. Adv. Energy Mater. 2016, 6, 1600473. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Q.L.; Tan, C.L.; Lim, T.T.; Huang, L.; Zhang, H. Carbon-Based Sorbents with Three-Dimensional Architectures for Water Remediation. Small 2015, 11, 3319–3336. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, Y.; Heo, Y.J.; Park, S.J. Advanced Design and Synthesis of Composite Photocatalysts for the Remediation of Wastewater: A Review. Catalysts 2019, 9, 122. [Google Scholar] [CrossRef]
- Hong, J.Y.; Yun, S.; Wie, J.J.; Zhang, X.; Dresselhaus, M.S.; Kong, J.; Park, H.S. Cartilage-inspired superelastic ultradurable graphene aerogels prepared by the selective gluing of intersheet joints. Nanoscale 2016, 8, 12900–12909. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.P.; Lu, X.; Han, Z. Synthesis and Electro-Magneto-Mechanical Properties of Graphene Aerogels Functionalized with Co-Fe-P Amorphous Alloys. Micromachines 2016, 7, 117. [Google Scholar] [CrossRef]
- Tang, M.Y.; Wu, T.; Na, H.Y.; Zhang, S.; Li, X.X.; Pang, X.B. Fabrication of graphene oxide aerogels loaded with catalytic AuPd nanoparticles. Mater. Res. Bull. 2015, 63, 248–252. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Sharma, S.; Naushad, M.; Mola, G.T.; Dhiman, P.; Stadler, F.J. Aerogels and metal-organic frameworks for environmental remediation and energy production. Environ. Chem. Lett. 2018, 16, 797–820. [Google Scholar] [CrossRef]
- Mao, J.J.; Ge, M.Z.; Huang, J.Y.; Lai, Y.K.; Lin, C.J.; Zhang, K.Q.; Meng, K.; Tang, Y.X. Constructing multifunctional MOF@rGO hydro-/aerogels by the self-assembly process for customized water remediation. J. Mater. Chem. A 2017, 5, 11873–11881. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Niu, Z.X.; Yuan, W.Z. Multifunctional magnetic superhydrophobic carbonaceous aerogel with micro/nano-scale hierarchical structures for environmental remediation and energy storage. Appl. Surf. Sci. 2019, 480, 851–860. [Google Scholar] [CrossRef]
- Li, N.; Jiang, H.L.; Wang, X.L.; Wang, X.; Xu, G.J.; Zhang, B.B.; Wang, L.J.; Zhao, R.S.; Lin, J.M. Recent advances in graphene-based magnetic composites for magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2018, 102, 60–74. [Google Scholar] [CrossRef]
- Fang, H.; Meng, F.T.; Yan, J.; Chen, G.Y.; Zhang, L.S.; Wu, S.D.; Zhang, S.C.; Wang, L.Z.; Zhang, Y.X. Fe3O4 hard templating to assemble highly wrinkled graphene sheets into hierarchical porous film for compact capacitive energy storage. RSC Adv. 2019, 9, 20107–20112. [Google Scholar] [CrossRef]
- Scheibe, B.; Mrowczynski, R.; Michalak, N.; Zaleski, K.; Matczak, M.; Kempinski, M.; Pietralik, Z.; Lewandowski, M.; Jurga, S.; Stobiecki, F. Anchoring Fe3O4 nanoparticles in a reduced graphene oxide aerogel matrix via polydopamine coating. Beilstein J. Nanotechnol. 2018, 9, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yin, D.; Wang, B.; Zhang, Q.W. Synthesis of Three-Dimensional Fe3O4/Graphene Aerogels for the Removal of Arsenic Ions from Water. J. Nanomater. 2015, 16, 250. [Google Scholar] [CrossRef]
- Chen, W.F.; Li, S.R.; Chen, C.H.; Yan, L.F. Self-Assembly and Embedding of Nanoparticles by In Situ Reduced Graphene for Preparation of a 3D Graphene/Nanoparticle Aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef]
- Sui, Z.Y.; Meng, Q.H.; Li, J.T.; Zhu, J.H.; Cui, Y.; Han, B.H. High surface area porous carbons produced by steam activation of graphene aerogels. J. Mater. Chem. A 2014, 2, 9891–9898. [Google Scholar] [CrossRef]
- Geng, Z.G.; Lin, Y.; Yu, X.X.; Shen, Q.H.; Ma, L.; Li, Z.Y.; Pan, N.; Wang, X.P. Highly efficient dye adsorption and removal: A functional hybrid of reduced graphene oxide—Fe3O4 nanoparticles as an easily regenerative adsorbent. J. Mater. Chem. 2012, 22, 3527–3535. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Woo, K.; Lee, H.J.; Ahn, J.P.; Park, Y.S. Sol–Gel Mediated Synthesis of Fe2O3 Nanorods. Adv. Mater. 2003, 15, 1761–1764. [Google Scholar] [CrossRef]
- Darezereshki, E. One-step synthesis of hematite (α-Fe2O3) nano-particles by direct thermal-decomposition of maghemite. Mater. Lett. 2011, 65, 642–645. [Google Scholar] [CrossRef]
- Yang, Y.M.; Hu, G.W.; Chen, F.J.; Liu, J.; Liu, W.S.; Zhang, H.L.; Wang, B.D. An atom-scale interfacial coordination strategy to prepare hierarchically porous Fe3O4-graphene frameworks and their application in charge and size selective dye removal. Chem. Commun. 2015, 51, 14405–14408. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shen, Y.; Xie, A.; Zhang, W. Green synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2010, 322, 1828–1833. [Google Scholar] [CrossRef]
- Tanhaei, M.; Mahjoub, A.; Nejat, R. Three-Dimensional Graphene-Magnetic Palladium Nanohybrid: A Highly Efficient and Reusable Catalyst for Promoting Organic Reactions. Catal. Lett. 2018, 148, 1549–1561. [Google Scholar] [CrossRef]
- Shen, D.Z.; Liu, J.; Gan, L.H.; Huang, N.Z.; Long, M.N. Green Synthesis of Fe3O4/Cellulose/Polyvinyl Alcohol Hybride Aerogel and Its Application for Dye Removal. J. Polym. Environ. 2018, 26, 2234–2242. [Google Scholar] [CrossRef]
- Dang, B.K.; Chen, Y.P.; Wang, H.W.; Chen, B.; Jin, C.D.; Sun, Q.F. Preparation of High Mechanical Performance Nano-Fe3O4/Wood Fiber Binderless Composite Boards for Electromagnetic Absorption via a Facile and Green Method. Nanomaterials 2018, 8, 52. [Google Scholar] [CrossRef]
- Ge, J.L.; Fan, G.; Si, Y.; He, J.X.; Kim, H.Y.; Ding, B.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.Y. Elastic and hierarchical porous carbon nanofibrous membranes incorporated with NiFe2O4 nanocrystals for highly efficient capacitive energy storage. Nanoscale 2016, 8, 2195–2204. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Yao, Y.J.; Xu, F.F.; Chen, M.; Xu, Z.X.; Zhu, Z.W. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef]
- Ge, Y.L.; Zhang, Y.F.; Yang, Y.; Xie, S.; Liu, Y.; Maruyama, T.; Deng, Z.Y.; Zhao, X.L. Enhanced adsorption and catalytic degradation of organic dyes by nanometer iron oxide anchored to single-wall carbon nanotubes. Appl. Surf. Sci. 2019, 488, 813–826. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Ahmad, I.; Israr, M. Graphene/Fe3O4 nanocomposite: Interplay between photo-Fenton type reaction, and carbon purity for the removal of methyl orange. Ceram. Int. 2018, 44, 2643–2648. [Google Scholar] [CrossRef]
- Castilla, S.; Terres, B.; Autore, M.; Viti, L.; Li, J.; Nikitin, A.Y.; Vangelidis, I.; Watanabe, K.; Taniguchi, T.; Lidorikis, E.; et al. Fast and Sensitive Terahertz Detection Using an Antenna-Integrated Graphene pn Junction. Nano Lett. 2019, 19, 2765–2773. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Hu, S.; Yang, Z.; Zhang, X.; Ge, J. Green Synthesis of Composite Graphene Aerogels with Robust Magnetism for Effective Water Remediation. Materials 2019, 12, 4106. https://doi.org/10.3390/ma12244106
Liu Q, Hu S, Yang Z, Zhang X, Ge J. Green Synthesis of Composite Graphene Aerogels with Robust Magnetism for Effective Water Remediation. Materials. 2019; 12(24):4106. https://doi.org/10.3390/ma12244106
Chicago/Turabian StyleLiu, Qixia, Shiqi Hu, Zhilian Yang, Xueyan Zhang, and Jianlong Ge. 2019. "Green Synthesis of Composite Graphene Aerogels with Robust Magnetism for Effective Water Remediation" Materials 12, no. 24: 4106. https://doi.org/10.3390/ma12244106
APA StyleLiu, Q., Hu, S., Yang, Z., Zhang, X., & Ge, J. (2019). Green Synthesis of Composite Graphene Aerogels with Robust Magnetism for Effective Water Remediation. Materials, 12(24), 4106. https://doi.org/10.3390/ma12244106




