Electrophoretic Deposition of Quantum Dots and Characterisation of Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Colloidal Quantum Dots
2.3. Fabrication of TiO2 Working Electrode
2.4. Electrophoretic Deposition Process
2.5. Successive Ionic Layer Adsorption and Reaction (SILAR) Method for ZnS Coating of TiO2 Electrode
2.6. Instrumentation
3. Results and Discussion
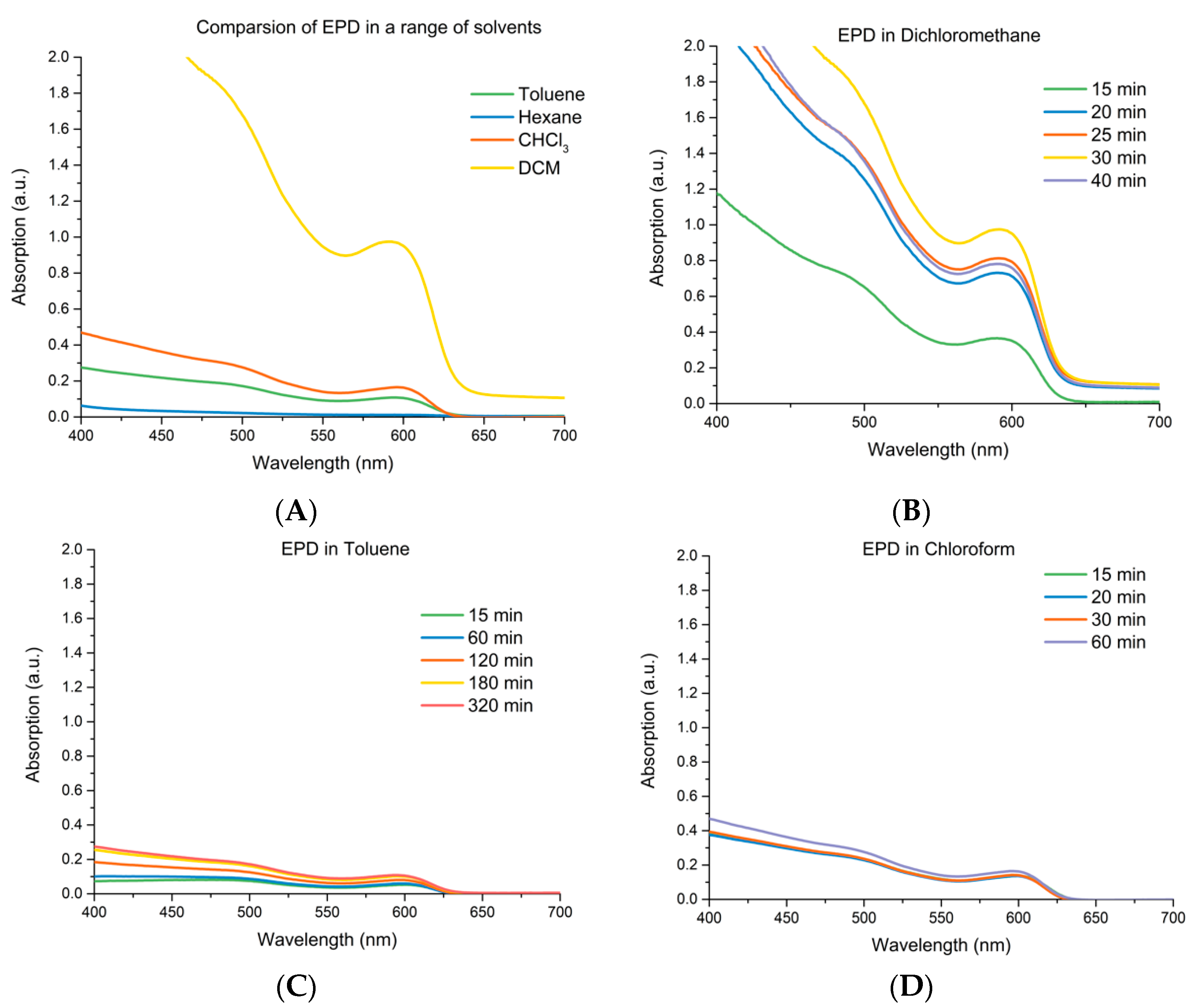
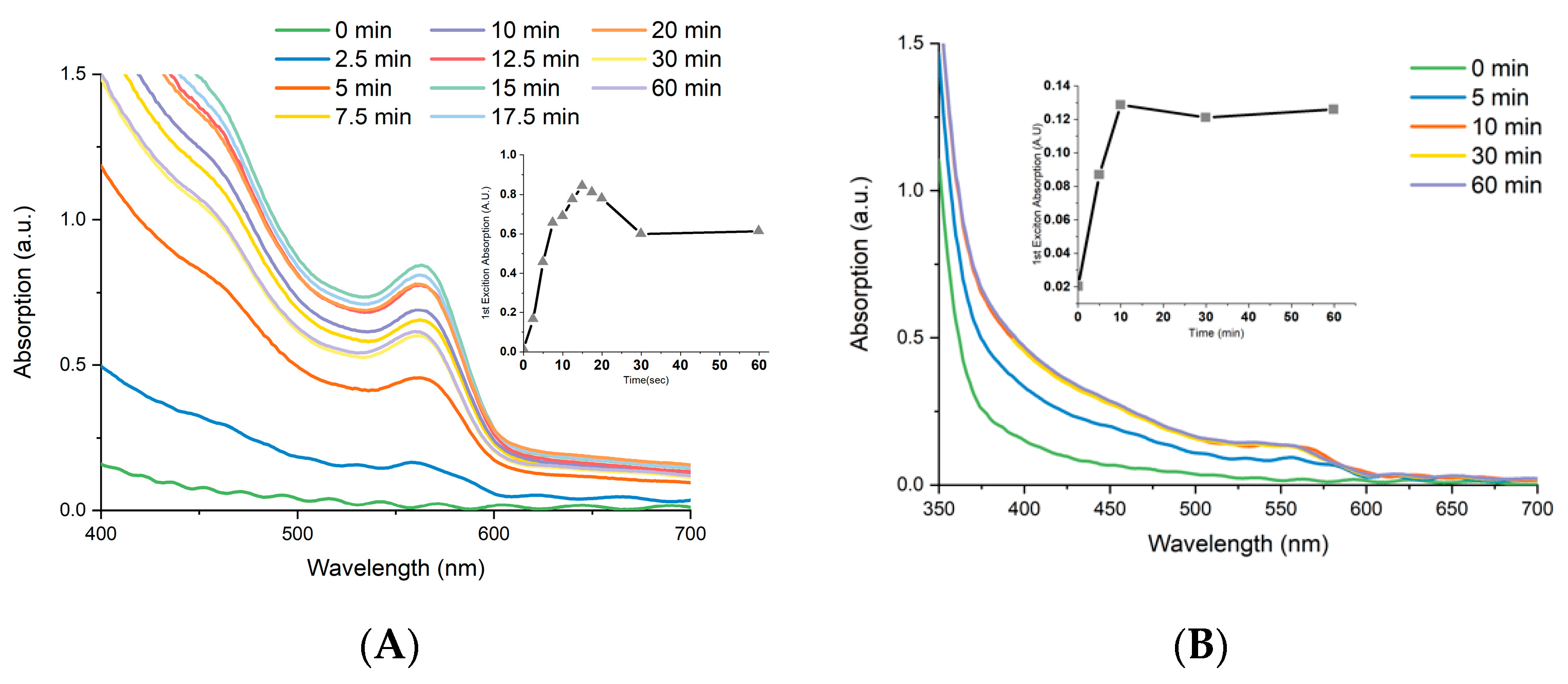
3.1. EPD of CdSe CQDs
3.2. Effects of EPD Duration upon Total Loading
3.3. Repeatability
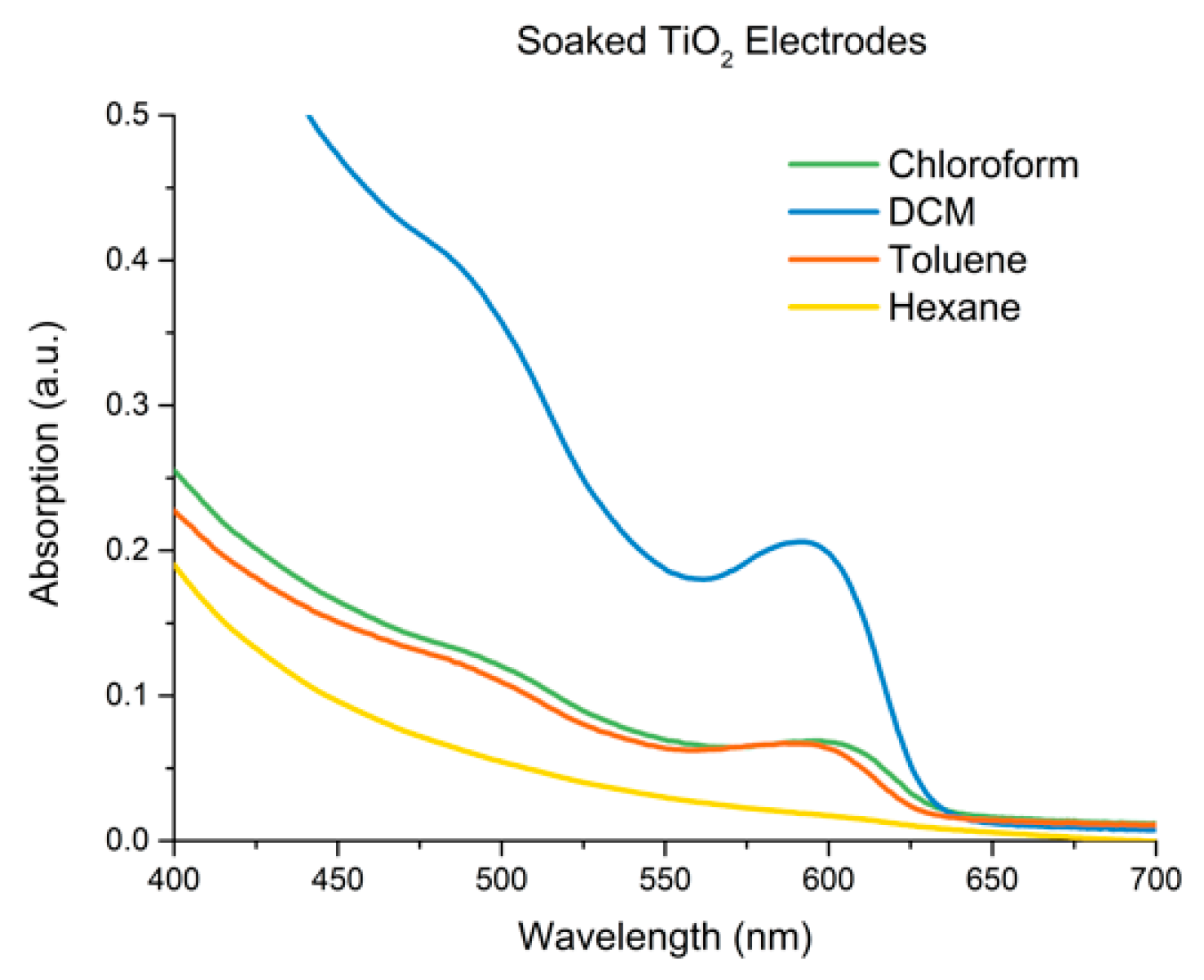
3.4. Adherence and Comparison to Controls
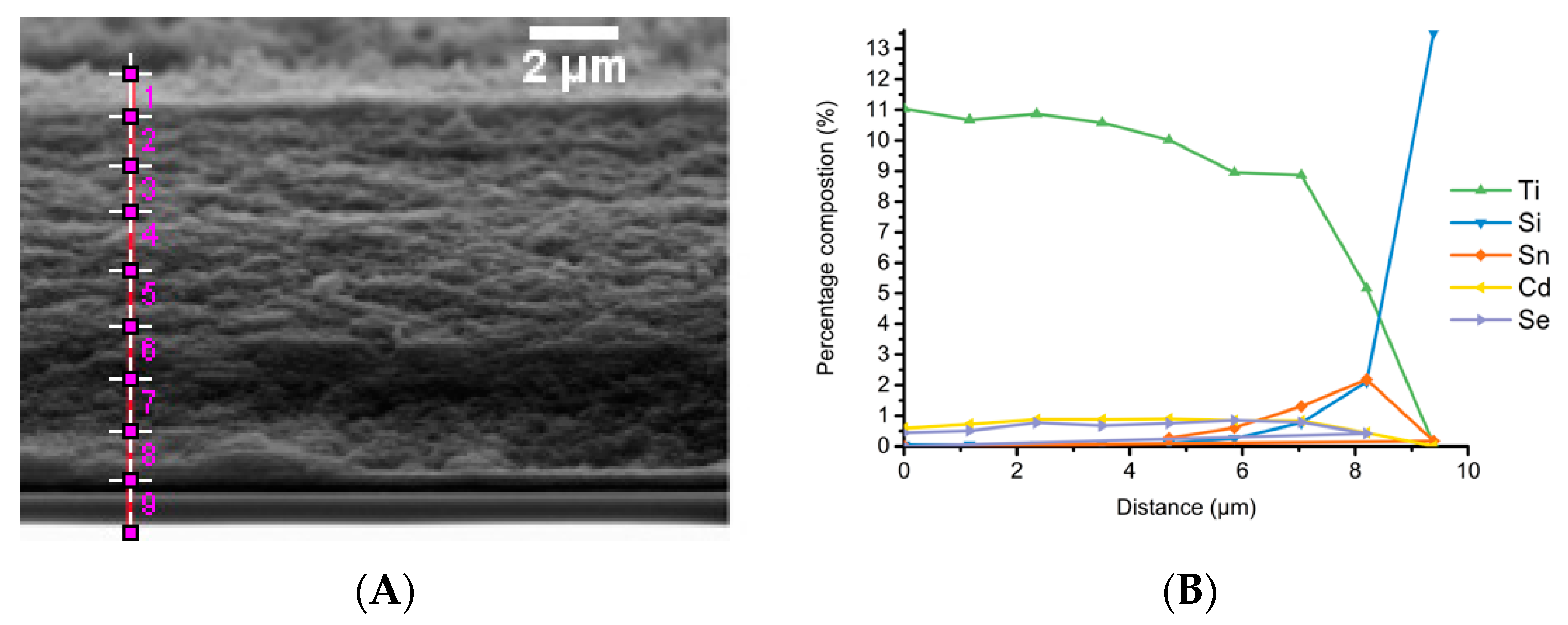
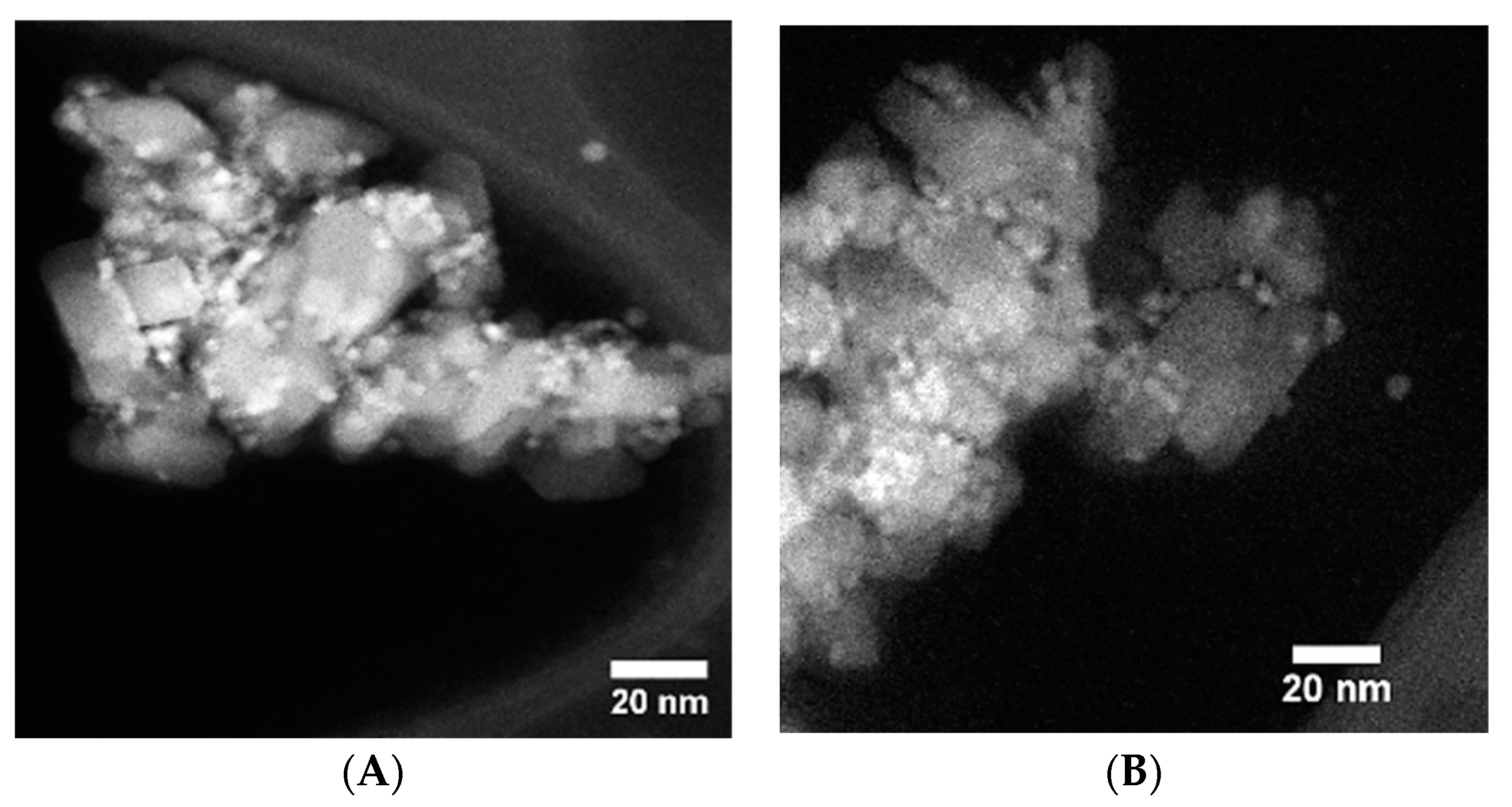
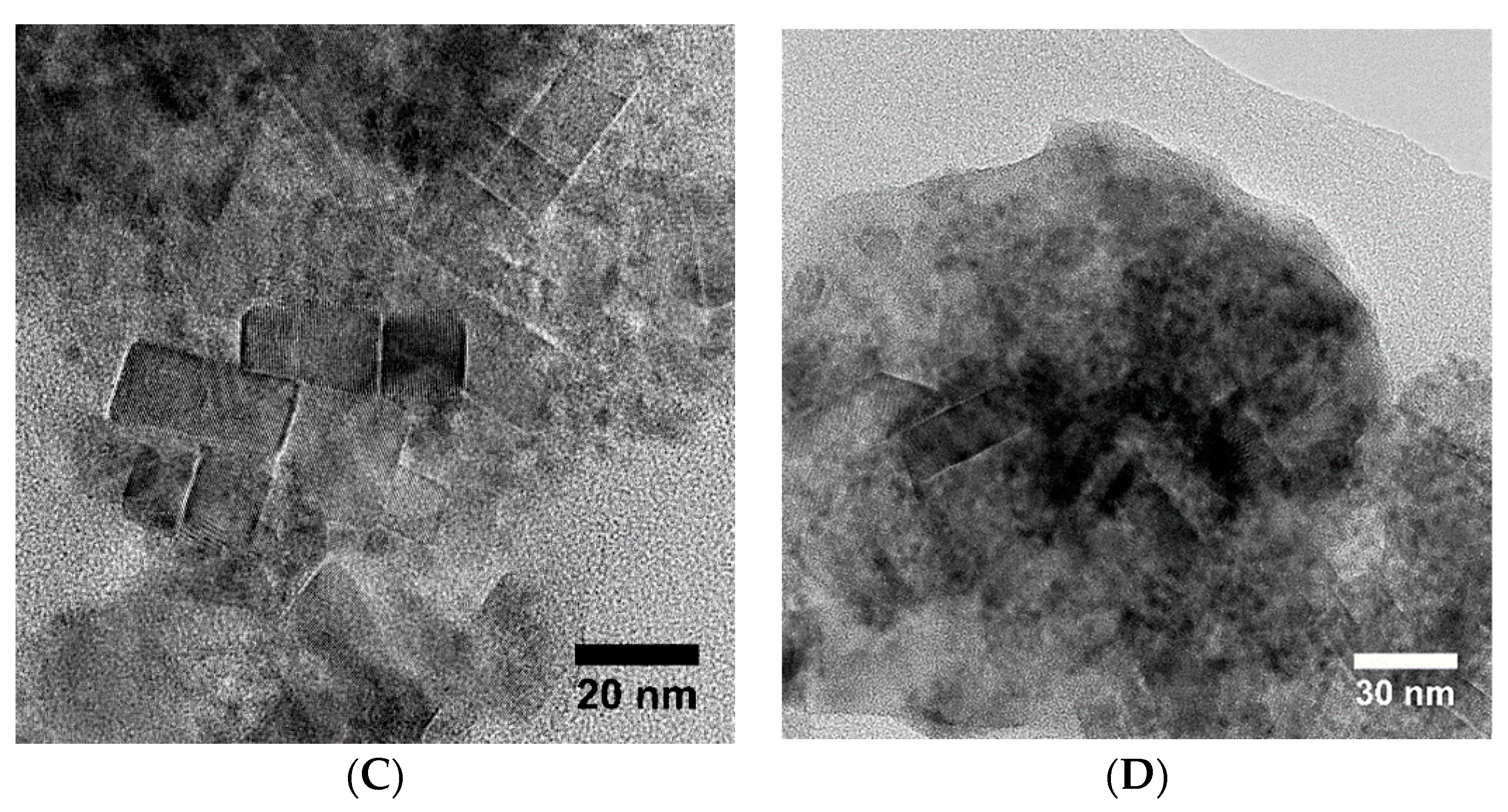
3.5. Electron Microscopy Analysis
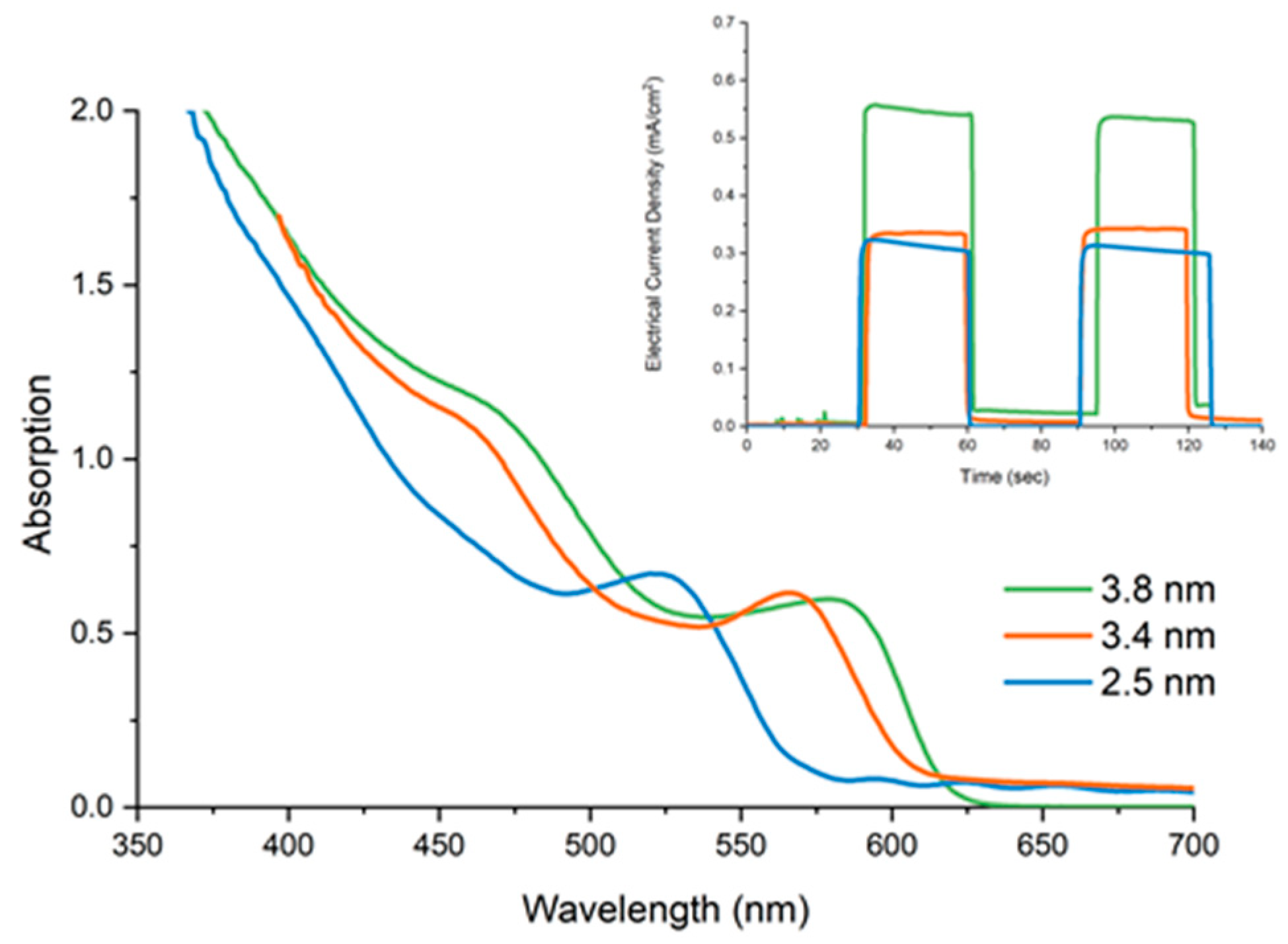
3.6. Photoresponsivity
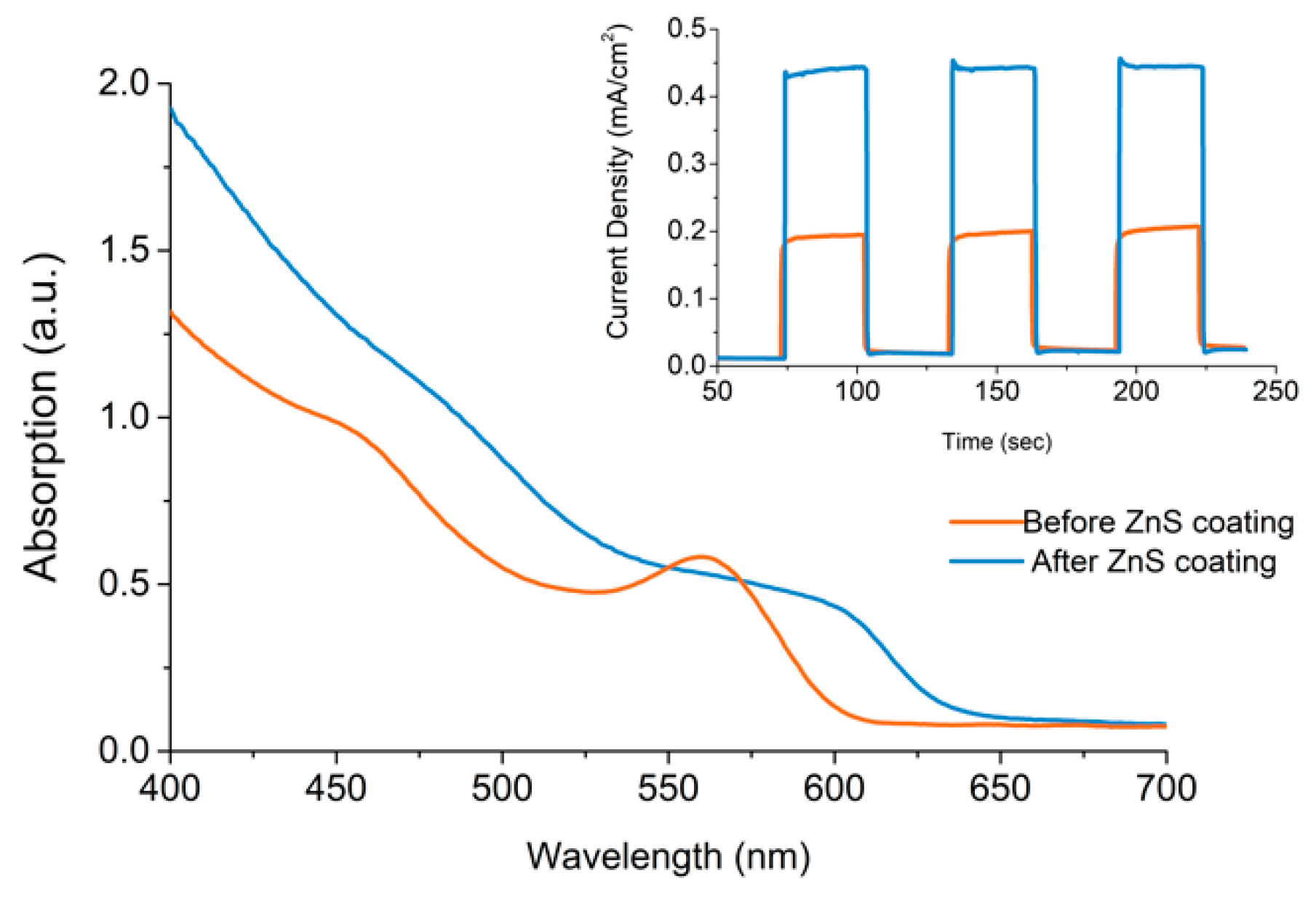
3.7. ZnS Treatment of Electrodes
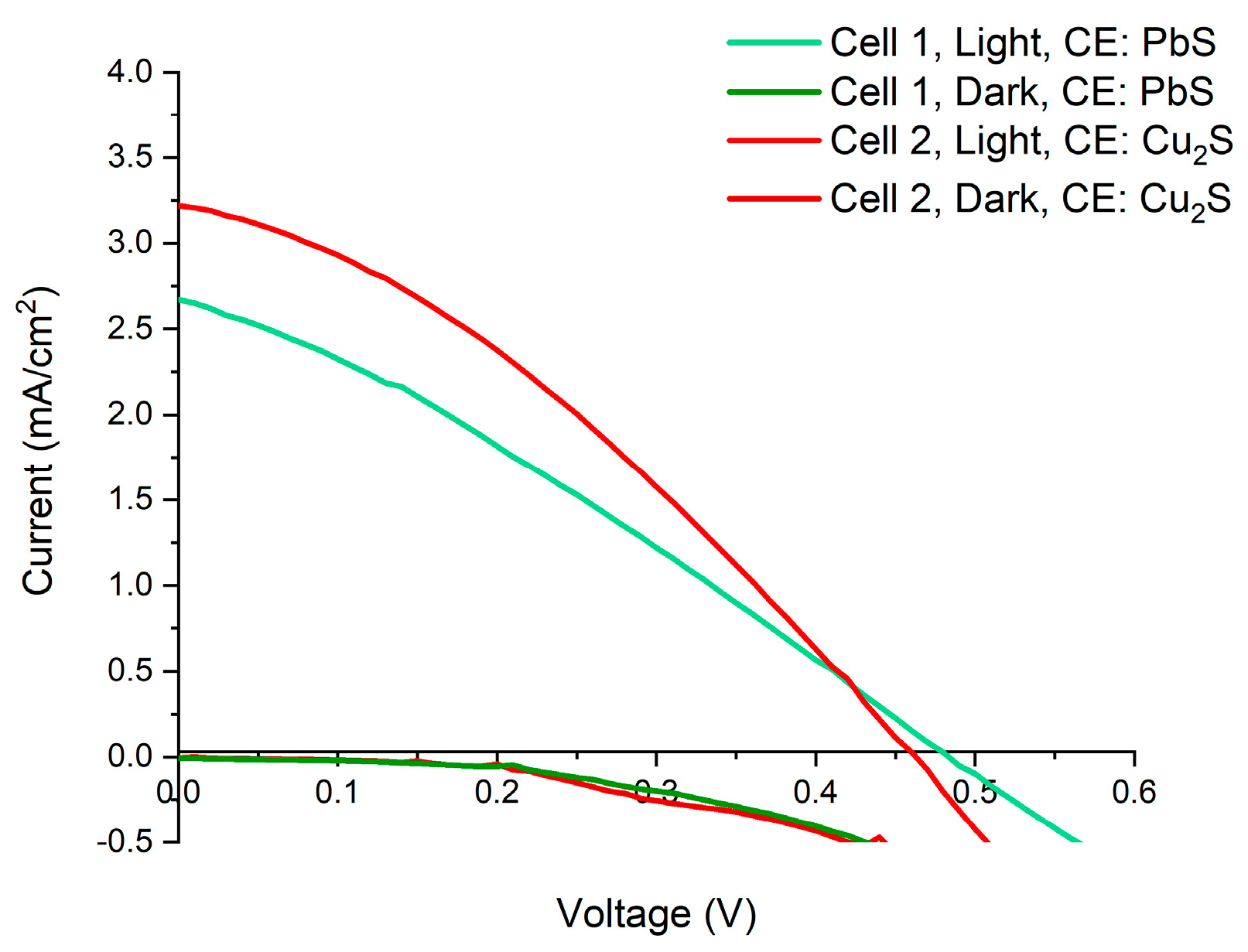
3.8. Open Cell Measurements
3.9. Demonstration of EPD with a Range of Alternatively Ligand Capped CdSe CQDs and Other Core and Core/Shell CQDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (epd). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Brennan, L.J.; Purcell-Milton, F.; McKenna, B.; Watson, T.M.; Gun’ko, Y.K.; Evans, R.C. Large area quantum dot luminescent solar concentrators for use with dye-sensitised solar cells. J. Mater. Chem. A 2018, 6, 2671–2680. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Li, T.; Zhou, D.; Zhang, K.; Sheng, Y.; Cui, J.; Zhang, H.; Yang, B. Electrophoretic deposition of fluorescent cu and au sheets for light-emitting diodes. Nanoscale 2016, 8, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Woo, J.Y.; Lee, D.C.; Lee, J.; Jeong, S.; Kim, D. Continuous purification of colloidal quantum dots in large-scale using porous electrodes in flow channel. Sci. Rep. 2017, 7, 43581. [Google Scholar] [CrossRef] [PubMed]
- Zillner, E.; Kavalakkatt, J.; Eckhardt, B.; Dittrich, T.; Ennaoui, A.; Lux-Steiner, M. Formation of a heterojunction by electrophoretic deposition of CdTe/CdSe nanoparticles from an exhaustible source. Thin Solid Films 2012, 520, 5500–5503. [Google Scholar] [CrossRef]
- Song, K.W.; Costi, R.; Bulović, V. Electrophoretic deposition of CdSe/ZnS quantum dots for light-emitting devices. Adv. Mater. 2013, 25, 1420–1423. [Google Scholar] [CrossRef]
- Jun, H.K.; Careem, M.A.; Arof, A.K. Quantum dot-sensitized solar cells—perspective and recent developments: A review of cd chalcogenide quantum dots as sensitizers. Renew. Sustain. Energy Rev. 2013, 22, 148–167. [Google Scholar] [CrossRef]
- Salant, A.; Shalom, M.; Hod, I.; Faust, A.; Zaban, A.; Banin, U. Quantum dot sensitized solar cells with improved efficiency prepared using electrophoretic deposition. ACS Nano 2010, 4, 5962–5968. [Google Scholar] [CrossRef]
- Blanton, S.A.; Leheny, R.L.; Hines, M.A.; GuyotSionnest, P. Dielectric dispersion measurements of CdSe nanocrystal colloids: Observation of a permanent dipole moment. Phys. Rev. Lett. 1997, 79, 865–868. [Google Scholar] [CrossRef]
- Shim, M.; Guyot-Sionnest, P. Permanent dipole moment and charges in colloidal semiconductor quantum dots. J. Chem. Phys. 1999, 111, 6955–6964. [Google Scholar] [CrossRef]
- Jia, S.; Banerjee, S.; Herman, I.P. Mechanism of the electrophoretic deposition of CdSe nanocrystal films: Influence of the nanocrystal surface and charge. J. Phys. Chem. C 2008, 112, 162–171. [Google Scholar] [CrossRef]
- Jasieniak, J.; Mulvaney, P. From cd-rich to se-rich—the manipulation of CdSe nanocrystal surface stoichiometry. JACS 2007, 129, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.H.Y.; Evans, C.M.; Swartz, B.D.; Neukirch, A.J.; Young, J.; Prezhdo, O.V.; Krauss, T.D. Colloidal semiconductor quantum dots with tunable surface composition. Nano Lett. 2012, 12, 4465–4471. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Y.; Li, X.B.; Gao, Y.J.; Li, J.; Wu, H.L.; Zhang, L.P.; Tung, C.H.; Wu, L.Z. Surface stoichiometry manipulation enhances solar hydrogen evolution of CdSe quantum dots. J. Mater. Chem. A 2018, 6, 6015–6021. [Google Scholar] [CrossRef]
- Cirillo, M.; Strubbe, F.; Neyts, K.; Hens, Z. Thermal charging of colloidal quantum dots in apolar solvents: A current transient analysis. ACS Nano 2011, 5, 1345–1352. [Google Scholar] [CrossRef]
- Baker, D.R.; Kamat, P.V. Tuning the emission of CdSe quantum dots by controlled trap enhancement. Langmuir 2010, 26, 11272–11276. [Google Scholar] [CrossRef]
- Salant, A.; Shalom, M.; Tachan, Z.; Buhbut, S.; Zaban, A.; Banin, U. Quantum rod-sensitized solar cell: Nanocrystal shape effect on the photovoltaic properties. Nano Lett. 2012, 12, 2095–2100. [Google Scholar] [CrossRef]
- Brown, P.; Kamat, P.V. Quantum dot solar cells. Electrophoretic deposition of CdSe–c60 composite films and capture of photogenerated electrons with nc60 cluster shell. J. Am. Chem. Soc. 2008, 130, 8890–8891. [Google Scholar] [CrossRef]
- Chen, J.; Lei, W.; Li, C.; Zhang, Y.; Cui, Y.; Wang, B.; Deng, W. Flexible quantum dot sensitized solar cell by electrophoretic deposition of CdSe quantum dots on zno nanorods. Phys. Chem. Chem. Phys. 2011, 13, 13182–13184. [Google Scholar] [CrossRef]
- Santra, P.K.; Kamat, P.V. Tandem-layered quantum dot solar cells: Tuning the photovoltaic response with luminescent ternary cadmium chalcogenides. J. Am. Chem. Soc. 2012, 135, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Lhuillier, E.; Hease, P.; Ithurria, S.; Dubertret, B. Selective electrophoretic deposition of CdSe nanoplatelets. Chem. Mater. 2014, 26, 4514–4520. [Google Scholar] [CrossRef]
- Smith, N.J.; Emmett, K.J.; Rosenthal, S.J. Photovoltaic cells fabricated by electrophoretic deposition of CdSe nanocrystals. Appl. Phys. Lett. 2008, 93, 3. [Google Scholar] [CrossRef]
- Parsi Benehkohal, N.; González-Pedro, V.; Boix, P.P.; Chavhan, S.; Tena-Zaera, R.; Demopoulos, G.P.; Mora-Seró, I. Colloidal PbS and pbses quantum dot sensitized solar cells prepared by electrophoretic deposition. J. Phys. Chem. C 2012, 116, 16391–16397. [Google Scholar] [CrossRef]
- Esparza, D.; Bustos-Ramirez, G.; Carriles, R.; Lopez-Luke, T.; Zarazua, I.; Martinez-Benitez, A.; Torres-Castro, A.; De la Rosa, E. Studying the role of CdS on the tio2 surface passivation to improve cdsete quantum dots sensitized solar cell. J. Alloy. Compd. 2017, 728, 1058–1064. [Google Scholar] [CrossRef]
- Santra, P.K.; Nair, P.V.; George Thomas, K.; Kamat, P.V. Cuins2-sensitized quantum dot solar cell. Electrophoretic deposition, excited-state dynamics, and photovoltaic performance. J. Phys. Chem. Lett. 2013, 4, 722–729. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Liu, Z.; Xie, Y.-H. The conversion of cuins2/ZnS core/shell structure from type i to quasi-type ii and the shell thickness-dependent solar cell performance. J. Colloid Interface Sci. 2019, 546, 276–284. [Google Scholar] [CrossRef]
- Poulose, A.C.; Veeranarayanan, S.; Varghese, S.H.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Functionalized electrophoretic deposition of CdSe quantum dots onto tio2 electrode for photovoltaic application. Chem. Phys. Lett. 2012, 539–540, 197–203. [Google Scholar] [CrossRef]
- Purcell-Milton, F.; Chiffoleau, M.; Gun’ko, Y.K. Investigation of quantum dot–metal halide interactions and their effects on optical properties. J. Phys. Chem. C 2018, 122, 25075–25084. [Google Scholar] [CrossRef]
- Available online: www.awt-europe (accessed on 20 September 2019).
- Pan, Z.; Zhang, H.; Cheng, K.; Hou, Y.; Hua, J.; Zhong, X. Highly efficient inverted type-i CdS/CdSe core/shell structure qd-sensitized solar cells. ACS Nano 2012, 6, 3982–3991. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Verweij, H. Theory of cast formation in electrophoretic deposition. J. Am. Ceram. Soc. 1999, 82, 1451–1455. [Google Scholar] [CrossRef]
- Hamaker, H.C. Formation of a deposit by electrophoresis. Trans. Faraday Soc. 1940, 35, 279–287. [Google Scholar] [CrossRef]
- Rosenholm, J.B. Evaluation of particle charging in non-aqueous suspensions. Adv. Colloid Interface Sci. 2018, 259, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wu, C.; Xu, H. Particle size and zeta potential of carbon black in liquid media. Carbon 2007, 45, 2806–2809. [Google Scholar] [CrossRef]
- Stenutz, R. Dielectric Constants and Refractive Index. 2013. Available online: http://www.stenutz.eu/chem/solv23.php (accessed on 20 September 2019).
- Wypych, G. (Ed.) Copyright. In Handbook of Solvents, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2019. [Google Scholar]
- Ash, M.; Ash, I. Handbook of Solvents, 3rd ed.; Synapse Information Resources, Inc.: Endicott, NY, USA, 2018. [Google Scholar]
- Wang, H.; Bai, Y.; Zhang, H.; Zhang, Z.; Li, J.; Guo, L. CdS quantum dots-sensitized tio2 nanorod array on transparent conductive glass photoelectrodes. J. Phys. Chem. C 2010, 114, 16451–16455. [Google Scholar] [CrossRef]
- Shalom, M.; Dor, S.; Rühle, S.; Grinis, L.; Zaban, A. Core/CdS quantum dot/shell mesoporous solar cells with improved stability and efficiency using an amorphous Tio2 coating. J. Phys. Chem. C 2009, 113, 3895–3898. [Google Scholar] [CrossRef]
- Bang, J.H.; Kamat, P.V. Quantum dot sensitized solar cells. A tale of two semiconductor nanocrystals: CdSe and CdTe. ACS Nano 2009, 3, 1467–1476. [Google Scholar] [CrossRef]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe−Tio2 architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef]
- Lee, H.J.; Chen, P.; Moon, S.-J.; Sauvage, F.; Sivula, K.; Bessho, T.; Gamelin, D.R.; Comte, P.; Zakeeruddin, S.M.; Seok, S.I.; et al. Regenerative PbS and CdS quantum dot sensitized solar cells with a cobalt complex as hole mediator. Langmuir 2009, 25, 7602–7608. [Google Scholar] [CrossRef]
- McDaniel, H.; Fuke, N.; Makarov, N.S.; Pietryga, J.M.; Klimov, V.I. An integrated approach to realizing high-performance liquid-junction quantum dot sensitized solar cells. Nat. Commun. 2013, 4, 10. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Chang, C.-H. Efficient polysulfide electrolyte for CdS quantum dot-sensitized solar cells. J. Power Sour. 2008, 185, 584–588. [Google Scholar] [CrossRef]
- Huang, G.-W.; Chen, C.-Y.; Wu, K.-C.; Ahmed, M.O.; Chou, P.-T. One-pot synthesis and characterization of high-quality CdSe/znx (x=s, se) nanocrystals via the cdo precursor. J. Cryst. Growth 2004, 265, 250–259. [Google Scholar] [CrossRef]
- Reiss, P.; Bleuse, J.; Pron, A. Highly luminescent CdSe/ZnSe core/shell nanocrystals of low size dispersion. Nano Lett. 2002, 2, 781–784. [Google Scholar] [CrossRef]
- Shen, Q.; Kobayashi, J.; Diguna, L.J.; Toyoda, T. Effect of ZnS coating on the photovoltaic properties of CdSe quantum dot-sensitized solar cells. J. Appl. Phys. 2008, 103, 084304. [Google Scholar] [CrossRef]
- Shankar, K.; Bandara, J.; Paulose, M.; Wietasch, H.; Varghese, O.K.; Mor, G.K.; LaTempa, T.J.; Thelakkat, M.; Grimes, C.A. Highly efficient solar cells using tio2 nanotube arrays sensitized with a donor-antenna dye. Nano Lett. 2008, 8, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purcell-Milton, F.; Curutchet, A.; Gun’ko, Y. Electrophoretic Deposition of Quantum Dots and Characterisation of Composites. Materials 2019, 12, 4089. https://doi.org/10.3390/ma12244089
Purcell-Milton F, Curutchet A, Gun’ko Y. Electrophoretic Deposition of Quantum Dots and Characterisation of Composites. Materials. 2019; 12(24):4089. https://doi.org/10.3390/ma12244089
Chicago/Turabian StylePurcell-Milton, Finn, Antton Curutchet, and Yurii Gun’ko. 2019. "Electrophoretic Deposition of Quantum Dots and Characterisation of Composites" Materials 12, no. 24: 4089. https://doi.org/10.3390/ma12244089
APA StylePurcell-Milton, F., Curutchet, A., & Gun’ko, Y. (2019). Electrophoretic Deposition of Quantum Dots and Characterisation of Composites. Materials, 12(24), 4089. https://doi.org/10.3390/ma12244089





