Abstract
The use of low-level laser therapy (LLLT) with biomodulatory effects on biological tissues, currently called photobiomodulation therapy (PBMT), assists in healing and reduces inflammation. The application of biomaterials has emerged in bone reconstructive surgery, especially the use of bovine bone due to its biocompatibility. Due to the many benefits related to the use of PBMT and bovine bones, the aim of this research was to review the literature to verify the relationship between PBMT and the application of bovine bone in bone reconstruction surgeries. We chose the PubMed/MEDLINE, Web of Science, and Scopus databases for the search by matching the keywords: “Bovine bone AND low-level laser therapy”, “Bovine bone AND photobiomodulation therapy”, “Xenograft AND low-level laser therapy”, and “Xenograft AND photobiomodulation therapy”. The initial search of the three databases retrieved 240 articles, 18 of which met all inclusion criteria. In the studies concerning animals (17 in total), there was evidence of PBMT assisting in biomaterial-related conduction, formation of new bone, bone healing, immunomarker expression, increasing collagen fibers, and local inflammation reduction. However, the results disagreed with regard to the resorption of biomaterial particles. The only human study showed that PBMT with bovine bone was effective for periodontal regeneration. It was concluded that PBMT assists the process in bone reconstruction when associated with bovine bone, despite divergences between applied protocols.
1. Introduction
Low-level laser therapy (LLLT) has been of interest to the scientific community since 1967, when Mester et al. [1] reported its effects on hair growth in rats. It was later verified that this therapy not only stimulated cellular components, but also modulated them, establishing photobiomodulation therapy (PBMT). Further, regenerative medicine has emerged in recent decades to develop adjuvant and assistive means in pathological processes, highlighting PBMT in relation to the anti-inflammatory, anti-allergic, healing and stimulating effects of tissue growth factors [2,3,4,5].
PBMT features electromagnetic energy technology with a wavelength spectrum of 600–1100 nm, with low energy density from a constant beam (0.04–60 J/cm2). Laser light sources include helium–neon (HeNe) and gallium–aluminum arsenide (GaAlAs), as these sources have excellent tissue penetration [4,6]. The therapeutic effects of PBMT are based on photochemical, photoelectric and photoenergetic reactions that affect cells by altering their metabolic functions. The modulatory effect is mainly related to cytochrome C oxidase, which, via photon absorption with mitochondrial reactions, generates increased adenosine triphosphate (ATP) [7,8]. The literature points to the effects of PBMT on tissues by its modulation of biological processes for cell differentiation and proliferation [9]. Its effects are related to the repair of muscle [10], nerves [11], bone [12], and burn injuries [13], besides the reduction of inflammatory cytokines and bacterial load due to photosensitive agents and biostimulation of blood vessels [10,13,14,15].
Most experimental and clinical studies describe that PBMT aids in the process of tissue regeneration, demonstrating biological modulatory effects on cell differentiation [9,16,17]. Photobiomodulation of bone tissue seems to increase the results of fracture repair [18], periodontal tissue [19], implant osseointegration [20] and bone reconstruction with or without biomaterials [15,21,22,23,24,25]. Its application in clinical practice with the purpose of assisting healing after bone graft reconstruction surgery [26] is still poorly described in the scientific literature.
Bone lesions with tissue loss can lead to changes in quality of life, especially when it concerns the face [27]. Patients requiring reconstructive surgery typically describe functional loss and physical, emotional, social and labor disturbances, as well as a financial change associated with these challenges [28]. The physiological bone remodeling process is naturally coordinated; however, imbalances may occur between bone deposition and removal. In extensive tissue defects, repair can become a challenge, requiring the use of bone grafts, implants or biomaterials. At this time, tissue engineering comes into play in helping the development of components that can lead to or assist in the reconstruction of lost tissue [29,30,31].
Bone graft material, regardless of its origin (autografts, allografts, alloplastic materials or xenografts), must have the biological, physical and chemical properties necessary for the tissue repair process. Emphasis is given to those materials that have osteointegration, osteoinduction, osteoconduction and osteogenesis capacities; however, only autologous material is capable of covering all four of these properties [32,33].
In instances of large bone loss, the need for a graft is imminent. In such a scenario, autologous bone is the first choice, but there are difficulties associated with potential morbidity of the donor site. In these cases, however, grafts tend to be absorbed before osteogenesis is complete [34]. The literature cites as necessary in the reconstruction of bone defects three simultaneous conditions: (i) osteoconductive properties; (ii) inductive properties; and (iii) the presence of bone-forming cells [35]. As an alternative material, bovine bone graft [15,20,36,37,38,39] is the most widely used due to its biocompatibility characteristics, as indicated in reconstructive areas related to traumatology, cranio-maxillary surgery, facial prosthetic rehabilitation, skeletal aging and esthetic aging [28]. Physical methods, such as low-intensity ultrasound (LIPUS) [40] and photobiomodulation therapy (PBMT), have the potential to improve the bone reconstruction process, acting or not in combination with bone grafts.
However, gaps still exist in explaining the mechanisms of PBMT and its relationship with the widely-used bovine bone. In this context, this systematic review research was based on the PICO [41,42] strategy, P: animals or humans with bone defects, I: The use of bovine bone as a scaffold and PBMT for bone defect repair, C: comparison to non-use of these components, and O: effect on bone repair. This PICO strategy was used to verify the relationship between PBMT and the use of bovine bone in bone reconstruction surgeries in different animals, based on the results presented by scientific studies already published in the PubMed/MEDLINE, Web of Science and Scopus databases.
2. Materials and Methods
This systematic review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist, as well as previously published systematic reviews [43,44].
For this study, we searched three databases, PubMed/MEDLINE, Web of Science, and Scopus, during September 2019, using the following terms as keywords: “Bovine bone AND low level laser therapy”, “Bovine bone AND photobiomodulation therapy”, “Xenograft AND low-level laser therapy” and “Xenograft AND photobiomodulation therapy”, with no restriction on publication time.
The search results were initially screened by title and then abstract to sort articles into included and excluded folders. Eligibility criteria were applied impartially by the authors regardless of the results presented by each article.
Eligibility criteria:
Inclusion criteria were:
- Use of bovine bone as a scaffold and PBMT in bone reconstructions;
- Human or animal studies;
- Publications in the English language only and which allowed full access to the text.
- Each included article should present data regarding: wavelength, output power, energy density, application protocol (points, frequency and days).
Exclusion criteria were:
- Duplicate articles;
- Excluded because title was not related to aim;
- Did not use bovine bone;
- Use of other languages (not English);
- No access;
- Literature review;
- Data absence: wavelength (nm), output power (mW); energy density (J/cm2); quantity of radiation.
First, we verified the works that presented titles and abstracts that related to the theme of the initial research, using the two variables: bovine bone as a scaffold and PBMT. The next step was to evaluate and restrict those articles that used bovine bone as a scaffold in animals or humans. The methodology, results and relevance were considered to list the selection of articles.
Analysis and integration of reflective and consistent texts on the subject were performed. The search scheme is presented in Figure 1, according to the PRISMA flow diagram [42,44].

Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram delineating the search performed in the PubMed/MEDLINE, Web of Science and Scopus databases.
3. Results
3.1. Inclusion of Studies, Quality of Studies, and Test Subjects
The initial search retrieved 240 articles from the three databases, after which 146 articles were excluded because they were duplicated and 36 were excluded due to their titles being unrelated to the theme. The abstracts of 58 articles were read, resulting in the further exclusion of 37 papers as they either did not use bovine bone, did not provide access or were a literature review article, and therefore did not meet the inclusion criteria. This left 21 articles elected for full analysis. After full reading of these 21 articles, three more papers were deleted due to incomplete data. Therefore, in the end, 18 articles related to the theme were included, 17 of which were related to animals and only 1 to humans.
Table 1 and Table 2 present the main details of the selected animal and human studies, respectively.

Table 1.
Summary of the main photobiomodulation therapy (PBMT) parameters used in animals studies.

Table 2.
Summary of the main PBMT parameters used in the human study.
Evaluating the 17 articles that involved animal experiments, the total population of test subjects was 663. This total population was made up of 27 rabbits and 636 rats, divided into control groups with a total of 157 animals and intervention groups with 506 animals. The control group animals were always characterized as “empty cavity” or “clot”, while the intervention groups contained animals that underwent treatment. Nine studies used male animals [12,15,18,20,22,33,37,46,47] and seven used male and female animals [48,49,50,51,52,53,54], while only one study did not describe the gender of the subjects [45].
The periods chosen for analysis ranged from a minimum of 7 days [20] to a maximum of 90 days [15]. There appeared to be a preference seems for studies conducted up to 30 days, with 13 articles falling into this category [18,20,22,37,45,46,48,49,50,51,52,53,54], while four articles [12,15,33,47] evaluated results after this period.
Considering all the articles included in this review, the application of PBMT in bone lesions was verified in rats in 15 articles, five of which involved the calvaria [12,20,22,45,47], nine the femur [37,46,48,49,50,51,52,53,54] and one the mandibular branch [15]. The two articles employing rabbits involved the calvaria [33] and tibia [18]. The only article in humans [36] was on the alveolar bone due to periodontal disease. The use of bovine bone in its inorganic phase was observed in 10 studies [12,15,20,22,33,37,47,52,53,54] versus six using the organic phase [18,46,48,49,50,51]; only one study [45] did not offer a distinction. Bovine bone was associated with another component in 11 articles: fibrin sealant [12]; bone morphogenic proteins (BMP), collagen binder and bovine biological membrane [46,50]; hydroxyapatite/β-tricalcium phosphate (HA/βTCP) [15]; internal rigid fixation (IRF), BMP, collagen bone and decalcified cortical osseous membrane [18]; BMP and collagen gel [48]; decalcified cortical osseous membrane [51,52,54]; and collagen membrane [45].
The wavelength parameter employed in the studies covered a wide range of values, from 618 to 830 nm. This included one study for each of 618 nm [37], 660 nm [47], 780 nm [22] and 790 nm [18], three studies with 808 nm [15,20,45], two studies with 810 nm [33,36], and nine studies with 830 nm [12,46,48,49,50,51,52,53,54], as shown in Figure 2.
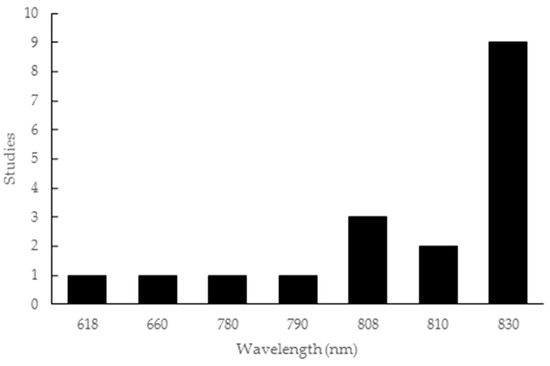
Figure 2.
Wavelength parameters used in the articles included in this review.
Regarding the type of laser used in the studies, eight studies employed GaAlAs lasers (44.44%), one study cites the use of a light-emitting diode (LED) (5.55%), two specified the use of a diode laser (11.11%) and 7 researches did not mention the type of laser (38.88%). The seven studies that did not mention the type of laser used describe the application of the 830nm wavelength, which corresponds to the infrared range (Figure 3).

Figure 3.
Laser types used in the articles included in this review.
The energy density employed in the studies ranged from 2 to 354 J/cm2, with one study only citing the total energy (24 J/cm2) without specifying the energy per point. Eleven studies used 4 J/cm2 and two used 6 J/cm2, while energy densities of 8.3 J/cm2, 30.85 J/cm2 and 354 J/cm2 were applied in one study each (Figure 4).
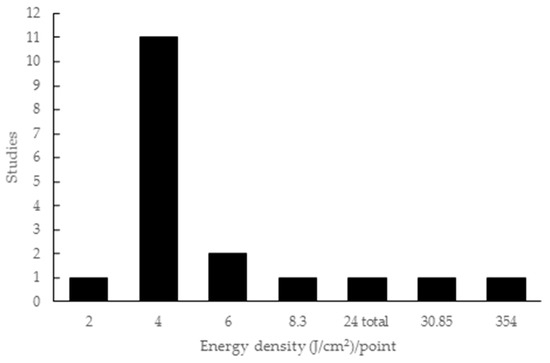
Figure 4.
Energy density parameters used in the articles included in this review.
3.2. Outcome Measures Used in the Included Studies
Table 3 presents the outcome measures, characteristics of the test subjects, and results obtained from the studies included in this review. Ten studies evaluated the primary outcome measure of bone density using four major methods: µCT, histological analysis of percent volume density of bone (v/v), plain X-rays and the multimodal CMS/SS OCT system. Five studies evaluated the secondary outcome measure of expression of markers, most commonly examining expression of receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG) and receptor activator of nuclear factor-κB (RANK), through histopathological analysis, inflammatory process detection and Raman spectroscopy, and measurement of hydroxyapatite deposition.

Table 3.
Data from included studies regarding outcome measures, subject attributes, and results.
4. Discussion
In recent decades, there has been a significant increase in the incidence of craniomaxillofacial and orthopedic disorders, although this has been simultaneous with remarkable progress in the development of biomaterials for reconstruction of lost bone tissue [55].
However, even though there is a wide variety of bone substitutes with satisfactory bone-filling results, histological evidence and biological behavior have only been reported for bovine bone derivatives. Thus, these xenografts have transformed reconstructive surgery and significantly improved clinical outcomes [56].
In addition, noninvasive, adjuvant methods in tissue regeneration have been associated with grafting techniques in an attempt to overcome some practical limits and further improve the repair results of defects filled with biomaterial. Given this context, we performed a review of the scientific literature in order to elucidate the relationship of PBMT with bovine bone when the latter is used as scaffolding for bone reconstruction.
Scientific research related to tissue engineering aims to investigate the process of bone reconstruction using scaffolds, as these are necessary as an auxiliary means for growth of new bone tissue [57]. Efforts to minimize complications and the time needed to heal by improving the process and enhancing biocompatibility has led to the emergence of PBMT-associated biomaterial application in the world literature [12,31]. Bovine bone is listed as the most frequently used type of graft in the literature for the reconstructive bone process [15,20,36,37,38,39].
Rats accounted for 95.92% of the total animals used in the articles evaluated, showing a preference for these animals in empirical study. One advantage of using rats is their easy handling due to their size, and they are generally chosen for preclinical studies in bone reconstruction biomaterial tests—being the main choice in in vivo studies in regenerative processes [58,59].
The use of male animals in nine of the studies examined suggests a preference of gender for test subjects. This decision is supported in the literature, as it avoids the possible influence of female inhibitory hormones in relation to bone tissue, in addition to the lower risk of fracture and greater bone mass [60,61].
Concerning the use of bovine bone, a preference for its inorganic phase (10 papers) was identified [12,15,20,22,33,37,47,52,53,54], although no differences in the process of bone healing when associated with a laser were reported, while six studies [18,46,48,49,50,51] used bone with an organic matrix. Bovine bone matrix has been widely used as a heterogeneous graft in orthopedic surgeries and craniofacial reconstructive procedures with satisfactory osteoconductive properties [32,33,34]. However, previous studies have shown differences between the effectiveness of inorganic and organic bovine matrices in the bone repair process. Some researches advocate for the use of inorganic material due to the absence of proteins and cells, which decreases the risk of immunogenic reactions. Further, this material provides a large amount of hydroxyapatite, which is a major component in normal bones [62]. Other researches elect to use organic material for the permanence of its protein scaffold, mainly comprised of type I collagen, which may initially favor formation of the extracellular matrix [15,63].
During this review, an array of different protocol elements was observed. A range of wavelength parameters—from 618 to 830 nm [12,37,46,48,49,50,51,52,53,54]—was used, along with variation in energy density, application time and type of laser used, even with similar types of lesions. Most articles used the infrared light spectrum [12,15,18,20,22,33,45,46,48,49,51,52,53,54], including the study on humans [36], with promotion of new (local) formations and increased protein and genes of osteoblastic factors. PBMT involves radiation from the red to infrared regions, with the latter being most cited in the literature as effective in the early stages of bone repair during the reconstruction process. This is because, at the early stages, there is a large amount of differentiating cells, and reduction of these cells at a late time of repair reduces the PBMT-related osteostimulatory potential [25,48,64].
Regarding the evaluation time of the experiments performed in the analyzed articles, a preference for periods up to 30 days was observed, as the literature shows more modulatory effects of PBMT during the early stages of the bone repair process. Specifically, effects such as greater proliferation of osteoblasts, collagen fibers, and mesenchymal cells, less inflammation, and greater expression of immunomarkers have been reported [12,15,18,20,37,65].
The therapeutic effects of PBMT is dependent on the mode of application, time, frequency and number of sessions of irradiation and dosing, as well as the biologically-dependent relationship of energy density and intensity. PBMT presents conflicting results in the literature, especially with regard to these modulatory effects, as the parameters (wavelength, power density, treatment dose, method and number of applications) are greatly diversified [66,67,68]. When verifying that PBMT has a major effect on mitochondria, the parameter of wavelength appears to have a major influence on the therapeutic process, with the visible (red) wavelengths activating the mitochondrial respiratory chain and the non-visible (infrared) wavelengths acting on the cell membrane. Two experiments with beneficial cellular effects of laser application can be exemplified, where greater collagen production from fibroblasts and osteoid matrix originating from osteoblasts was observed [50,66,69].
The presence of more organized collagen fibers when bovine bone grafts are associated with PBMT has been reported, relating to a biostimulatory effect on collagen production [47,48,49,50,51,52,53,54], as well as improving osteoblastic activity with the release of calcium hydroxyapatite [18,47]. This relationship with osteoblast activity seems to be related to an increase in alkaline phosphatase (ALP), bone morphogenetic protein 2 (BMP2), runt-related transcription factor 2 (Runx2) and Jagged1 differentiation genes, and osteocalcin (OCN) [15], up to a period of 30 days. Kim et al. [20] pointed to an increase of receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG) and receptor activator of nuclear factor-κB (RANK) in the first 7 days, as already mentioned in previous studies relating bovine bones and lasers [33,70].
When using PBMT with 660 nm [47] and 618 nm [37], studies mentioned that, despite the increase of new bone, there was no resorption of bovine bone particles, while at 780 nm [22] and 808 nm [15], the biomaterial resorption occurred partially. It has been reported that osteoconductive biomaterials reduce local bone formation, which, by not being absorbed eventually, replace the new bone [63]. Oliveira et al. [15] found 60% more bone in a group without a biomaterial (control); however, computed microtomography showed that, in the groups with bovine bone, there was a greater amount of mineralized tissue. This suggests that, clinically, the use of osteoconductive biomaterials is important for maintaining morphology and function, rather than for new bone formation itself.
Most studies used infrared spectrum wavelengths, with GaAlAs being cited in eight studies [12,15,20,22,36,45,46,47]. Seven studies [48,49,50,51,52,53,54] did not state which type of laser they used, but did describe the application of 830 nm in the infrared range. The infrared spectrum is the most widely used in reconstructive processes, as it shows less energy loss when penetrating tissues, with about 37% reaching 2 mm deep and, at larger thicknesses, the maximum loss can be as little as 162.92 mW per cm2 [12,71].
Bovine biomaterial is widely used and has good results in bone reconstruction processes, such as enlargement of the maxillary sinus or preparation for dental implants [72,73]. Of the articles included in this review, 17 cite positive results regarding the association of bovine bone with PBMT. However, Bosco et al. [47] concluded that PBMT stimulates bone formation regardless of the presence of biomaterial. The presence or absence of a membrane plus a biomaterial also did not seem to have an influence on the biostimulatory effects of the laser in three other studies [51,52,54]. This is in contrast to the results reported by Ghahroudi et al. [33], wherein greater bone neoformation was found when it was associated with both a biomaterial and PBMT, and the bovine bone group alone was better than a laser alone.
A critical review of the studies elected for examination showed that PBMT was associated with the promotion of new bone at lesion sites [12,15,18,20,22,33,36,37,46,47,48,51,52,53,54], increased deproteinized bovine bone (DBB) and HA/βTCP osteoconduction [15], osteoblast proteins and genes [15], increased levels of calcium hydroxyapatite (CHA) [18], metabolism and expression of immunomarkers [20] and aided the treatment of periodontal disease [36], but divergent results were found regarding particle resorption.
A lack of persistence in the standardization of methodology employed by authors was observed, with instances of absence of important data, such as output power, energy density and application time, a pattern also observed in reviews relating PBMT to other types of lesions (such as nervous) [5]. It is extremely important to highlight the scarcity of publications addressing PBMT. This complementary treatment method is cited in the literature in association with the widely-used bovine bone scaffolds in bone reconstruction, with both having good results and clinical applicability.
5. Conclusions
At the end of this review, it can be verified that the data presented in recent literature shows potential to improve the bone reconstructive process using PBMT together with bovine bone as a scaffold. A variability of parameters seems to be common in studies using PBMT, as well as a lack of parameters, generating doubts regarding reproducibility and, consequently, the production of satisfactory results.
Author Contributions
M.P.d.O.R. wrote the paper and performed search in database; K.T.P. and B.B.D.C. assisted in data search and analysis of articles; C.H.B.R., J.P.G.P and G.D.J. analyzed the data; D.V.B. reviewed the manuscript and R.L.B. mentored and revised the paper.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mester, E.; Szende, B.; Gartner, P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. 1968, 9, 621–626. [Google Scholar]
- Migliario, M.; Pittarella, P.; Fanuli, M.; Rizzi, M.; Renò, F. Laser-induced osteoblast proliferation is mediated by ROS production. Lasers Med. Sci. 2014, 29, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Mitani, A.; Fukuda, M.; Mogi, M.; Osawa, K.; Takahashi, S.; Aino, M.; Iwamura, Y.; Miyajima, S.; Yamamoto, H.; et al. Irradiation with a low-level diode laser induces the developmental endothelial locus-1 gene and reduces proinflammatory cytokines in epithelial cells. Lasers Med. Sci. 2014, 29, 987–994. [Google Scholar] [CrossRef] [PubMed]
- AlGhamdi, K.; Kumar, A.; Moussa, N. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012, 27, 237–249. [Google Scholar] [CrossRef]
- Rosso, M.; Buchaim, D.; Kawano, N.; Furlanette, G.; Pomini, K.; Buchaim, R. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef]
- Khadra, M.; Kasem, N.; Haanaes, H.; Ellingsen, J.; Lyngstadaas, S. Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 693–700. [Google Scholar] [CrossRef]
- Angeletti, P.; Pereira, M.; Gomes, H.; Hino, C.; Ferreira, L. Effect of low-level laser therapy (GaAlAs) on bone regeneration in midpalatal anterior suture after surgically assisted rapid maxillary expansion. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e38–e46. [Google Scholar] [CrossRef]
- Morries, L.; Cassano, P.; Henderson, T. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015, 20, 2159–2175. [Google Scholar]
- Pyo, S.; Song, W.; Kim, I.; Park, B.; Kim, C.; Shin, S.; Chung, I.; Kim, Y. Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-β1 in hypoxic-cultured human osteoblasts. Lasers Med. Sci. 2013, 28, 543–550. [Google Scholar] [CrossRef]
- De Paiva, P.; Tomazoni, S.; Johnson, D.; Vanin, A.; Albuquerque-Pontes, G.; Machado, C.; Casalechi, H.; de Carvalho, P.; Leal-Junior, E. Photobiomodulation therapy (PBMT) and/or cryotherapy in skeletal muscle restitution, what is better? A randomized, double-blinded, placebo-controlled clinical trial. Lasers Med. Sci. 2016, 31, 1925–1933. [Google Scholar] [CrossRef]
- Rosso, M.P.D.O.; Rosa Júnior, G.M.; Buchaim, D.V.; German, I.J.S.; Pomini, K.T.; de Souza, R.G.; Pereira, M.; Favaretto Júnior, I.A.; Bueno, C.R.D.S.; Gonçalves, J.B.D.O.; et al. Stimulation of morphofunctional repair of the facial nerve with photobiomodulation, using the end-to-side technique or a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2017, 175, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Pomini, K.; Buchaim, D.; Andreo, J.; Rosso, M.; Della Coletta, B.; German, Í.; Biguetti, A.; Shinohara, A.; Rosa Júnior, G.; Cosin Shindo, J.; et al. Fibrin sealant derived from human plasma as a scaffold for bone grafts associated with photobiomodulation therapy. Int. J. Mol. Sci. 2019, 20, 1761. [Google Scholar] [CrossRef] [PubMed]
- Brassolatti, P.; Bossini, P.; Oliveira, M.; Kido, H.; Tim, C.; Almeida-Lopes, L.; Retto Da Silva De Avó, L.; Araújo-Moreira, F.; Parizotto, N. Comparative effects of two different doses of low-level laser therapy on wound healing third-degree burns in rats. Microsc. Res. Tech. 2016, 79, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Arany, P. Craniofacial wound healing with photobiomodulation therapy: New insights and current challenges. J. Dent. Res. 2016, 95, 977–984. [Google Scholar] [CrossRef]
- de Oliveira, G.; Aroni, M.; Medeiros, M.; Marcantonio, E.; Marcantonio, R. Effect of low-level laser therapy on the healing of sites grafted with coagulum, deproteinized bovine bone, and biphasic ceramic made of hydroxyapatite and β-tricalcium phosphate. In vivo study in rats. Lasers Surg. Med. 2018, 50, 651–660. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D.; Gao, X.; Wu, S. Low-power laser irradiation promotes cell proliferation by activating PI3K/Akt pathway. J. Cell. Physiol. 2009, 219, 553–562. [Google Scholar] [CrossRef]
- Khadra, M.; Lyngstadaas, S.; Haanaes, H.; Mustafa, K. Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 2005, 26, 3503–3509. [Google Scholar] [CrossRef]
- Lopes, C.; Pacheco, M.; Silveira, L.; Cangussú, M.; Pinheiro, A. The effect of the association of near infrared laser therapy, bone morphogenetic proteins, and guided bone regeneration on tibial fractures treated with internal rigid fixation: A Raman spectroscopic study. J. Biomed. Mater. Res. A 2010, 94, 1257–1263. [Google Scholar] [CrossRef]
- Aykol, G.; Baser, U.; Maden, I.; Kazak, Z.; Onan, U.; Tanrikulu-Kucuk, S.; Ademoglu, E.; Issever, H.; Yalcin, F. The Effect of Low-Level Laser Therapy as an Adjunct to Non-Surgical Periodontal Treatment. J. Periodontol. 2011, 82, 481–488. [Google Scholar] [CrossRef]
- Kim, Y.; Song, W.; Kim, S.; Kim, G.; Hwang, D.; Shin, S.; Kim, U.; Kim, J.; Chung, I. Expression of receptor activator of nuclear factor -κB ligand, receptor activator of nuclear factor -κB, and osteoprotegerin, following low-level laser treatment on deproteinized bovine bone graft in rats. Lasers Med. Sci. 2009, 24, 577–584. [Google Scholar] [CrossRef]
- Da Silva, J.P.; da Silva, M.A.; Almeida, A.P.; Lombardi Junior, I.; Matos, A.P. Laser therapy in the tissue repair process: A literature review. Photomed. Laser Surg. 2010, 28, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Esper, L.; Sbrana, M.; Oliveira, P.; Valle, A.; Almeida, A. Effect of Low-Level Laser on Bone Defects Treated with Bovine or Autogenous Bone Grafts: In Vivo Study in Rat Calvaria. BioMed Res. Int. 2014, 2014, 104230. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.; de Magalhães Júnior, E.; Magalhães, C.; Ferreira, C.; Marques, A.; Pinheiro, A. New bone formation around implants inserted on autologous and xenografts irradiated or not with IR laser light: A histomorphometric study in rabbits. Braz. Dent. J. 2013, 24, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.; Turrioni, A.; Soares, D.; Bagnato, V.; Hebling, J.; de Souza Costa, C. Low-level laser therapy for osteonecrotic lesions: Effects on osteoblasts treated with zoledronic acid. Support. Care Cancer 2014, 22, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Yildirimturk, S.; Sirin, Y.; Soluk Tekkesin, M.; Gurler, G.; Firat, D. The effects of low-level laser therapy on the healing of bone defects in streptozotocin-induced diabetic rats: A histological and morphometric evaluation. J. Cosmet. Laser Ther. 2017, 19, 397–403. [Google Scholar] [CrossRef]
- Leja, C.; Geminiani, A.; Caton, J.; Romanos, G. Thermodynamic effects of laser irradiation of implants placed in bone: An in vitro study. Lasers Med. Sci. 2013, 28, 1435–1440. [Google Scholar] [CrossRef]
- Caran, E.; Barone, T.; Barone, J.; Lopes, N.; Alves, M.; França, C. Facial reconstruction surgery 10 years after treatment for hemangiopericytoma: Planning considerations and clinical outcomes. J. Cosmet. Laser Ther. 2014, 16, 201–204. [Google Scholar] [CrossRef]
- Malard, O.; Espitalier, F.; Bordure, P.; Daculsi, G.; Weiss, G.; Corre, P. Biomaterials for tissue reconstruction and bone substitution of the ear, nose and throat, face and neck. Expert Rev. Med. Devices 2007, 4, 729–739. [Google Scholar] [CrossRef]
- Orsi, P.; Landim-Alvarenga, F.; Justulin, L.; Kaneno, R.; De Assis Golim, M.; Dos Santos, D.; Creste, C.; Oba, E.; Maia, L.; Barraviera, B.; et al. A unique heterologous fibrin sealant (HFS) as a candidate biological scaffold for mesenchymal stem cells in osteoporotic rats. Stem Cell Res. Ther. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Lienemann, P.; Metzger, S.; Kiveliö, A.; Blanc, A.; Papageorgiou, P.; Astolfo, A.; Pinzer, B.; Cinelli, P.; Weber, F.; Schibli, R.; et al. Longitudinal in vivo evaluation of bone regeneration by combined measurement of multi-pinhole SPECT and micro-CT for tissue engineering. Sci. Rep. 2015, 5, 10238. [Google Scholar] [CrossRef]
- Buchaim, R.; Rosso, M.; Andreo, J.; Buchaim, D.; Okamoto, R.; Rodrigues, A.; Shinohara, A.; Roque, J.; Roque, D.; Rosa Junior, G.; et al. A New Anionic Bovine Tendon as Scaffold for the Repair of Bone Defects: A Morphological, Histomorphometric and Immunohistochemical Study A New Anionic Bovine Tendon as Scaffold for the Repair of Bone Defects: A Morphological, Histomorphometric and Imm. Br. J. Med. Med. Res. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Fellah, B.; Gauthier, O.; Weiss, P.; Chappard, D.; Layrolle, P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials 2008, 29, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Rasouli Ghahroudi, A.; Rokn, A.; Kalhori, K.; Khorsand, A.; Pournabi, A.; Pinheiro, A.; Fekrazad, R. Effect of low-level laser therapy irradiation and Bio-Oss graft material on the osteogenesis process in rabbit calvarium defects: A double blind experimental study. Lasers Med. Sci. 2014, 29, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Cruess, R. Bone and cartilage transplantation surgery. J. Bone Jt. Surg. Am. 1982, 64, 270–279. [Google Scholar] [CrossRef]
- Stauropoulos, A.; Kostopoulos, L.; Nyengaard, J.; Karring, T. Deproteinized bovine bone (Bio-Oss) and bio active glass (Biogram) arrest bone formation when used as an adjust to guided tissue regeneration (GTR): An experimental study in the rat. J. Clin. Periodontol. 2003, 7, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S. Low Level Laser Therapy in the Treatment of Intra-Osseous Defect- A Case Report. J. Clin. Diagn. Res. 2016, 10, 10–12. [Google Scholar] [CrossRef]
- Havlucu, U.; Bölükbaşı, N.; Yeniyol, S.; Çetin, Ş.; Özdemir, T. Effects of Light-Emitting Diode Photobiomodulation Therapy and BioOss as Single and Combined Treatment in an Experimental Model of Bone Defect Healing in Rats. J. Oral Implantol. 2014, 41, e110–e117. [Google Scholar] [CrossRef]
- Fernández-Bodereau, E.; Dedossi, G.; Asencio, V.; Fernández-Domínguez, M.; Gehrke, S.; Aragoneses, J.; Calvo-Guirado, J. Comparison of different bone filling materials and resorbable membranes by means of micro-tomography. A preliminary study in Rabbits. Materials 2019, 12, 1197. [Google Scholar] [CrossRef]
- Leventis, M.; Fairbairn, P.; Mangham, C.; Galanos, A.; Vasiliadis, O.; Papavasileiou, D.; Horowitz, R. Bone healing in rabbit calvaria defects using a synthetic bone substitute: A histological and micro-CT comparative study. Materials 2018, 11, 4. [Google Scholar] [CrossRef]
- Pomini, K.T.; Andreo, J.C.; De Rodrigues, A.C.; De Gonçalves, J.B.O.; Daré, L.R.; German, I.J.S.; Rosa, G.M., Jr.; Buchaim, R.L. Effect of low-intensity pulsed ultrasound on bone regeneration biochemical and radiologic analyses. J. Ultrasound Med. 2014, 33, 713–717. [Google Scholar] [CrossRef]
- Santos, C.; Pimenta, C.; Nobre, M. A estratégia PICO para a construção da pergunta de pesquisa e busca de evidências. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.; Ioannidis, J.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Santinoni, C. dos S.; Oliveira, H.F.F.; Batista, V.E. de S.; Lemos, C.A.A.; Verri, F.R. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: A systematic review. J. Photochem. Photobiol. B Biol. 2017, 169, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. referred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Luca, R.; Todea, C.; Duma, V.; Bradu, A.; Podoleanu, Ag. Quantitative assessment of rat bone regeneration using complex master-slave optical coherence tomography. Quant. Imaging Med. Surg. 2019, 9, 782–798. [Google Scholar] [CrossRef]
- Gerbi, M.; Miranda, J.; De Arruda, J.; Moreno, L.; Carneiro, V.; Brasilino, N.; Menezes, R.; Brugnera, A.; Pinheiro, A. Photobiomodulation Therapy in Bone Repair Associated with Bone Morphogenetic Proteins and Guided Bone Regeneration: A Histomorphometric Study. Photomed. Laser Surg. 2018, 36, 581–588. [Google Scholar] [CrossRef]
- Bosco, A.; Faleiros, P.; Carmona, L.; Garcia, V.; Theodoro, L.; de Araujo, N.; Nagata, M.; de Almeida, J. Effects of low-level laser therapy on bone healing of critical-size defects treated with bovine bone graft. J. Photochem. Photobiol. B Biol. 2016, 163, 303–310. [Google Scholar] [CrossRef]
- Gerbi, M.; Marques, A.; Ramalho, L.; Ponzi, E.; Carvalho, C.; Santos, R.; Oliveira, P.C.; Nóia, M.; Pinheiro, A.L.B. Infrared laser light further improves bone healing when associated with bone morphogenic proteins: An in vivo study in a rodent model. Photomed. Laser Surg. 2008, 26, 55–60. [Google Scholar] [CrossRef]
- Márquez Martínez, M.; Pinheiro, A.; Ramalho, L. Effect of IR laser photobiomodulation on the repair of bone defects grafted with organic bovine bone. Lasers Med. Sci. 2008, 23, 313–317. [Google Scholar] [CrossRef]
- Pinheiro, A.; Gerbi, M.; Ponzi, E.; Ramalho, L.; Marques, A.; Carvalho, C.; Santos, R.; Oliveira, P.; Nóia, M. Infrared laser light further improves bone healing when associated with bone morphogenetic proteins and guided bone regeneration: An in vivo study in a rodent model. Photomed. Laser Surg. 2008, 26, 167–174. [Google Scholar] [CrossRef]
- Marquez de Martinez Gerbi, M.; Barbosa Pinheiro, A.; de Assis Limeira, F., Jr.; Marzola, C.; Pedreira Ramalho, L.; Arruda Carneiro Ponzi, E.; Olveira Soares, A.; Bandeira de Carvalho, L.; Vieira Lima, H.; Oliveira Goncalves, T.; et al. Assessment of bone repair associated to the use of organic bovine bone and membrane irradiated with 830nm. Lasers Dent. IX 2003, 4950, 156. [Google Scholar]
- de Assis Limeira, F., Jr.; Barbosa Pinheiro, A.; Marquez de Martinez Gerbi, M.; Pedreira Ramalho, L.; Marzola, C.; Carneiro Ponzi, E.; Soares, A.; Bandeira de Carvalho, L.; Vieira Lima, H.; Oliveira Gon‡alves, T.; et al. Assessment of bone repair following the use of anorganic bone graft and membrane associated or not to 830-nm laser light. Lasers Dent. IX 2003, 4950, 30. [Google Scholar]
- Pinheiro, A.; Limeira Júnior, F.; Gerbi, M.; Ramalho, L.; Marzola, C.; Ponzi, E. Effect of low level laser therapy on the repair of bone defects grafted with inorganic bovine bone. Braz. Dent. J. 2003, 14, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.; Limeira, F.; Gerbi, M.; Ramalho, L.; Marzola, C.; Ponzi, E.; Soares, A.; De Carvalho, L.; Lima, H.; Gonçalves, T. Effect of 830-nm Laser Light on the Repair of Bone Defects Grafted with Inorganic Bovine Bone and Decalcified Cortical Osseous Membrane. J. Clin. Laser Med. Surg. 2003, 21, 383–388. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.; Epari, D.; Steck, R.; Glatt, V.; Dickinson, I.; Choong, P.; Schuetz, M.; Hutmacher, D. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Nat. Publ. Gr. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Rocha, L.; Goissis, G.; Rossi, M. Biocompatibility of anionic collagen matrix as scaffold for bone healing. Biomaterials 2002, 23, 449–456. [Google Scholar] [CrossRef]
- Gomes, P.; Fernandes, M. Rodent models in bone-related research: The relevance of calvarial defects in the assessment of bone regeneration strategies. Lab. Anim. 2011, 45, 14–24. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Li, L.; Qin, L.; Wang, X.; Lai, Y. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Venken, K.; Calleweart, F.; Boonem, S.; Vanderschueren, D. Sex hormones, their conseptors and bone health. Osteoporos. Int. 2008, 19, 1517–1525. [Google Scholar] [CrossRef]
- Oury, F. A crosstalk between bone and gonads. Ann. N. Y. Acad. Sci. 2012, 1260, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, G.; Araujo, M.; Hayacibara, R.; Sukekava, F.; Lindhe, J. Healing of extraction sockets and surgically produced—Augmented and non-augmented—Defects in the alveolar ridge. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, R.; Kesic, L.; Pesevska, S. Influence of low-level laser therapy on biomaterial osseointegration: A mini-review. Lasers Med. Sci. 2009, 24, 447–451. [Google Scholar] [CrossRef]
- Marques, L.; Holgado, L.A.; Francischone, L.A.; Ximenez, J.P.B.; Okamoto, R.; Kinoshita, A. New LLLT protocol to speed up the bone healing process—Histometric and immunohistochemical analysis in rat calvarial bone defect. Lasers Med. Sci. 2015, 30, 1225–1230. [Google Scholar] [CrossRef]
- Pinheiro, A.; Gerbi, M. Photoengineering of bone repair processes. Photomed. Laser Surg. 2006, 24, 47–49. [Google Scholar] [CrossRef]
- Vladimirov, Y.; Osipov, A.; Klebanov, G. Photobiological principles of therapeutic applications of laser radiation. Biochemistry 2004, 69, 81–90. [Google Scholar] [CrossRef]
- Brugnera, A.; dos Santos, A.; Bologna, E.; Ladalardo, T. Atlas of Applied Laser Therapy to Clinical Dentistry.; Quintessence Editoria LTDA: São Paulo, Brazil, 2006. [Google Scholar]
- Rabie, A.; Chay, S. Clinical applications of composite intramembranous bone grafts. Am. J. Orthod. Dentofac. Orthop. 2000, 117, 375–383. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Hwang, D.; Kim, S.; Kwon, Y.; Shin, S.; Kim, U.; Kim, J.; Chung, I. Effect of low-level laser treatment after installation of dental titanium implant-immunohistochemical study of RANKL, RANK, OPG: An experimental study in rats. Lasers Surg. Med. 2007, 39, 441–450. [Google Scholar] [CrossRef]
- Basford, J. Low intensity laser therapy: Still not an established clinical tool. Lasers Surg. Med. 1995, 16, 331–342. [Google Scholar] [CrossRef]
- Torroni, A.; Marianetti, T.; Romandini, M.; Gasparini, G.; Cervelli, D.; Pelo, S. Mandibular reconstruction with different techniques. J. Craniofac. Surg. 2015, 26, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, E.; Heard, R.; Janakievski, J.; Mandelaris, G.; Nevins, M.; Pickering, S.; Richardson, C.; Pope, B.; Toback, G.; Velásquez, D.; et al. A randomized, controlled, multicentre clinical trial of post-extraction alveolar ridge preservation. J. Clin. Periodontol. 2016, 43, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).