Highly Fluorinated Barium Titanate Nanoparticle Dispersion for Fabrication of Lithographically Patterned Thin Films
Abstract
1. Introduction
2. Materials and Methods
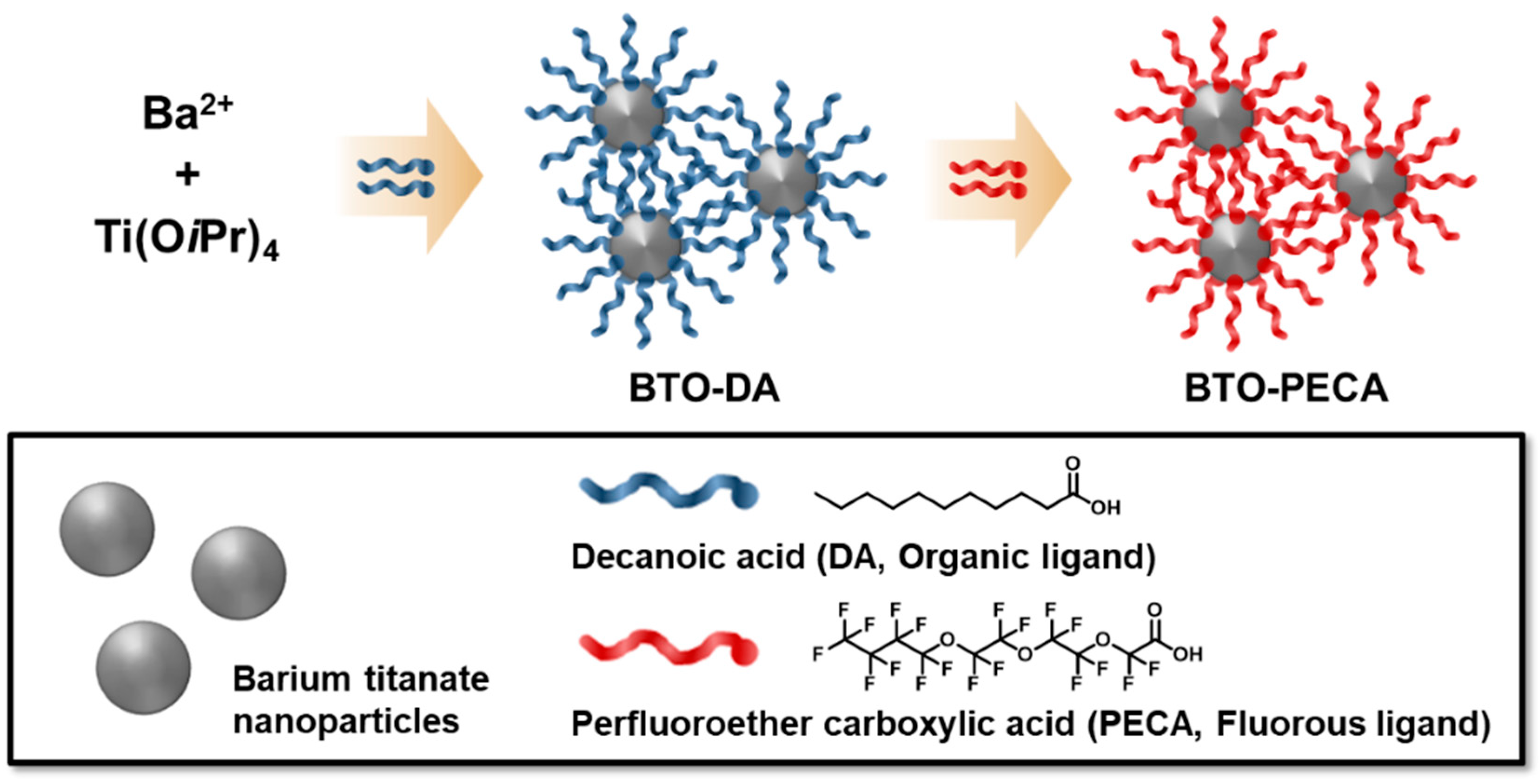
2.1. Synthesis of Decanoic Acid-Capped BTO Nanoparticles (BTO-DA)
2.2. Ligand-Exchange Reaction of BTO-DA to BTO-PECA (Perfluoro-3,6,9-trioxatridecanoic Acid-Capped BTO Nanoparticles)
2.3. Layer Preparation for Dielectric-Constant Measurement
2.4. Pattern Fabrication Using Electron-Beam (E-Beam) Lithography and Photolithography
3. Results and Discussion
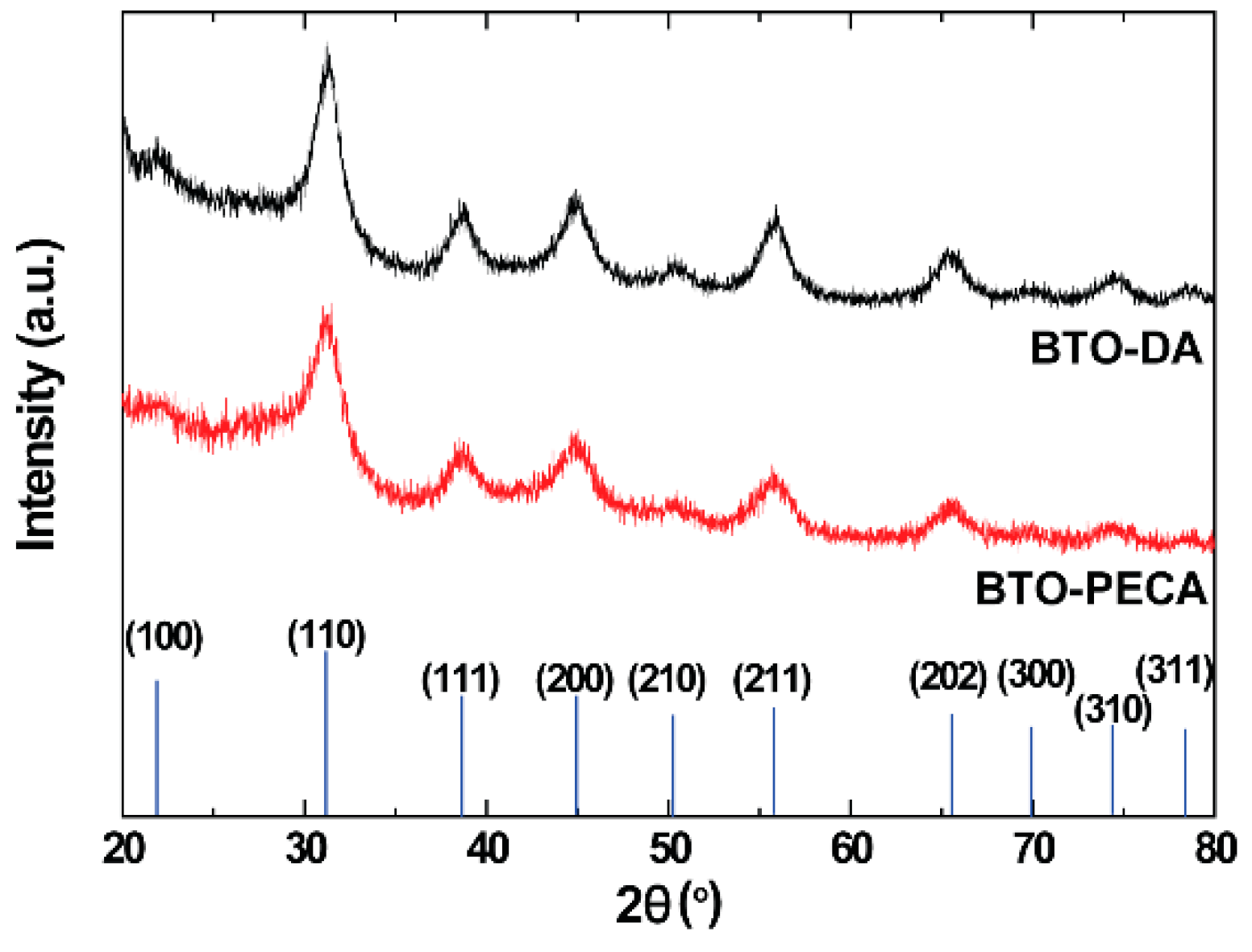
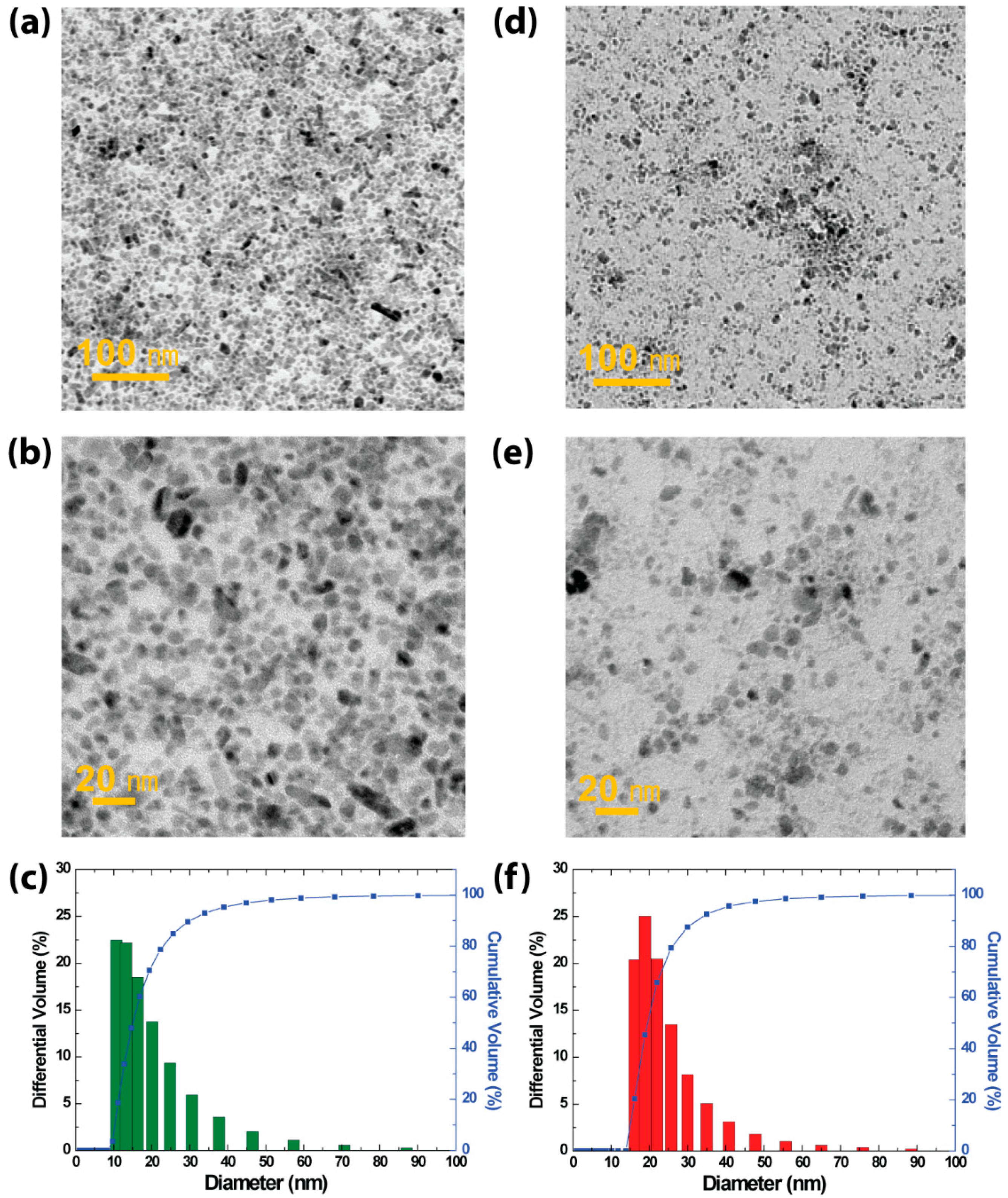
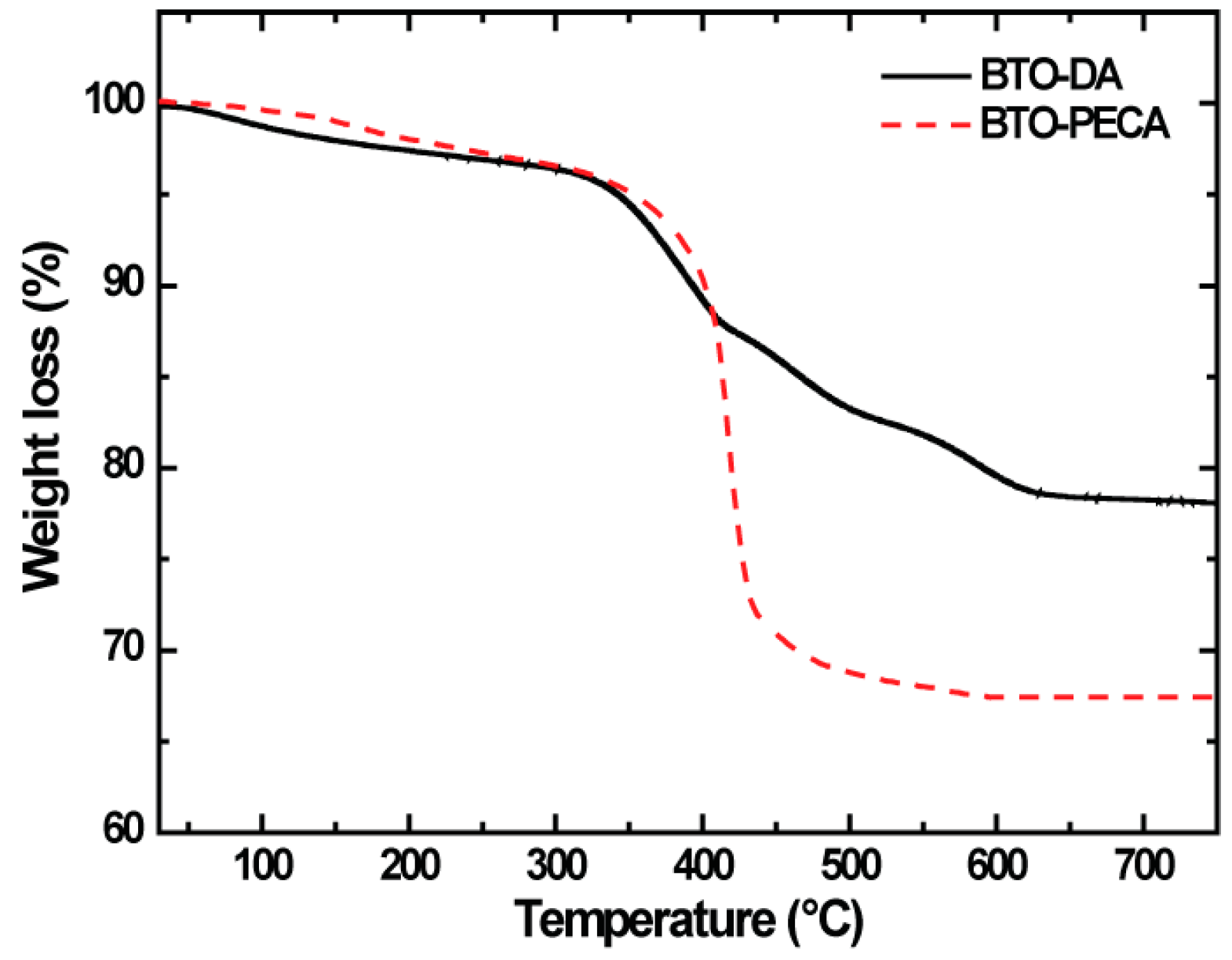
3.1. Structural and Morphological Properties of BTO Nanoparticles
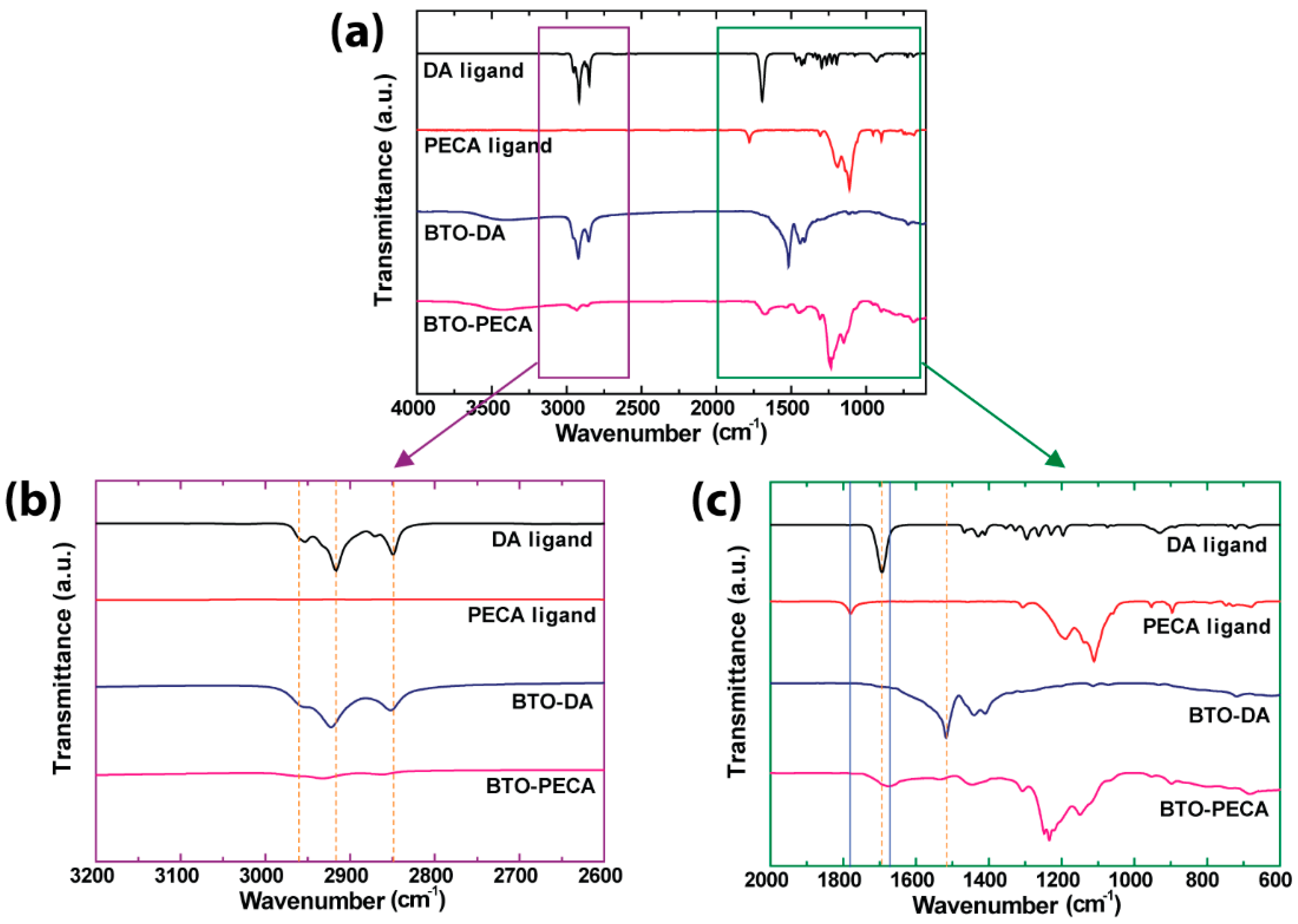
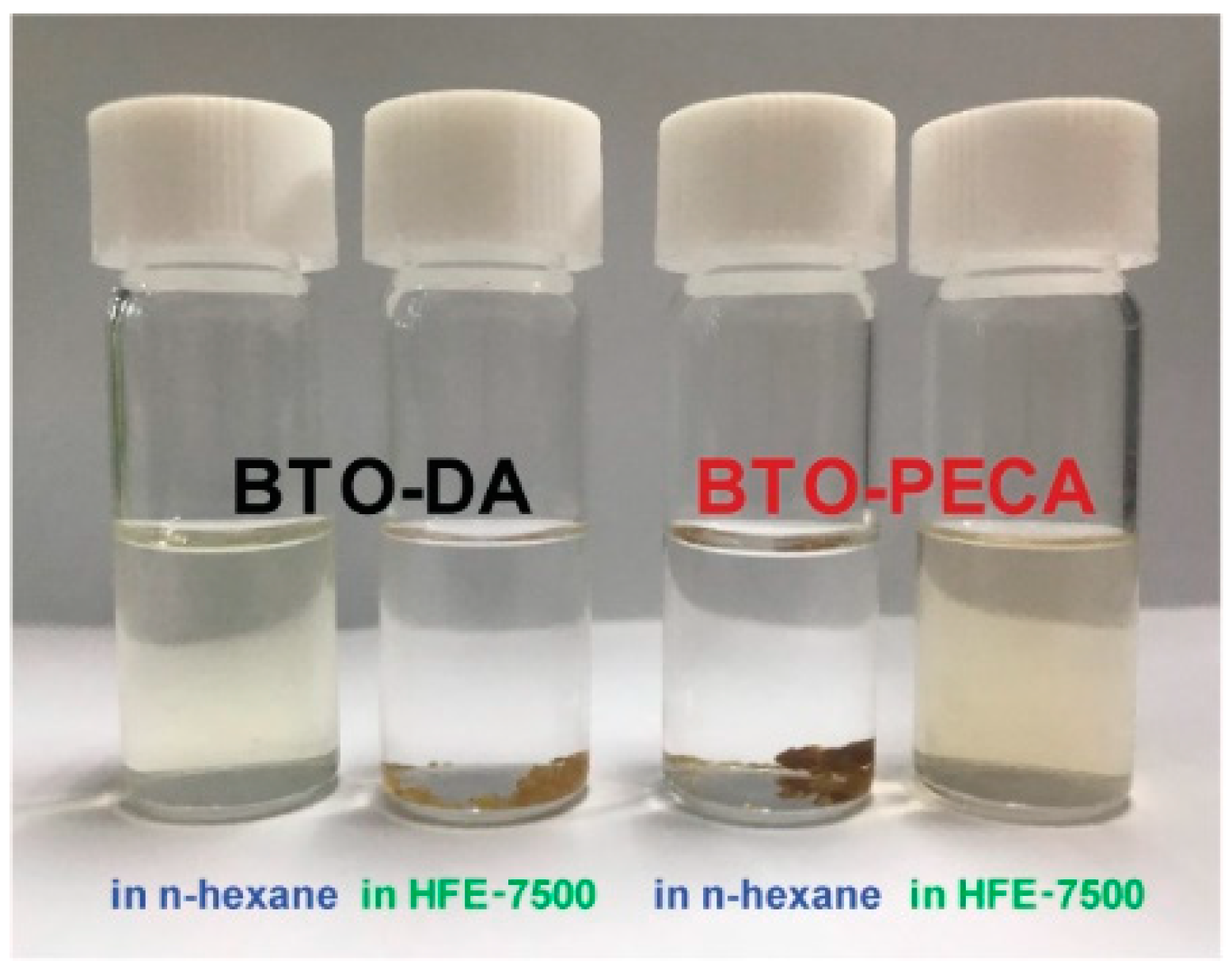
3.2. Characterization of BTO Nanoparticle Ligands
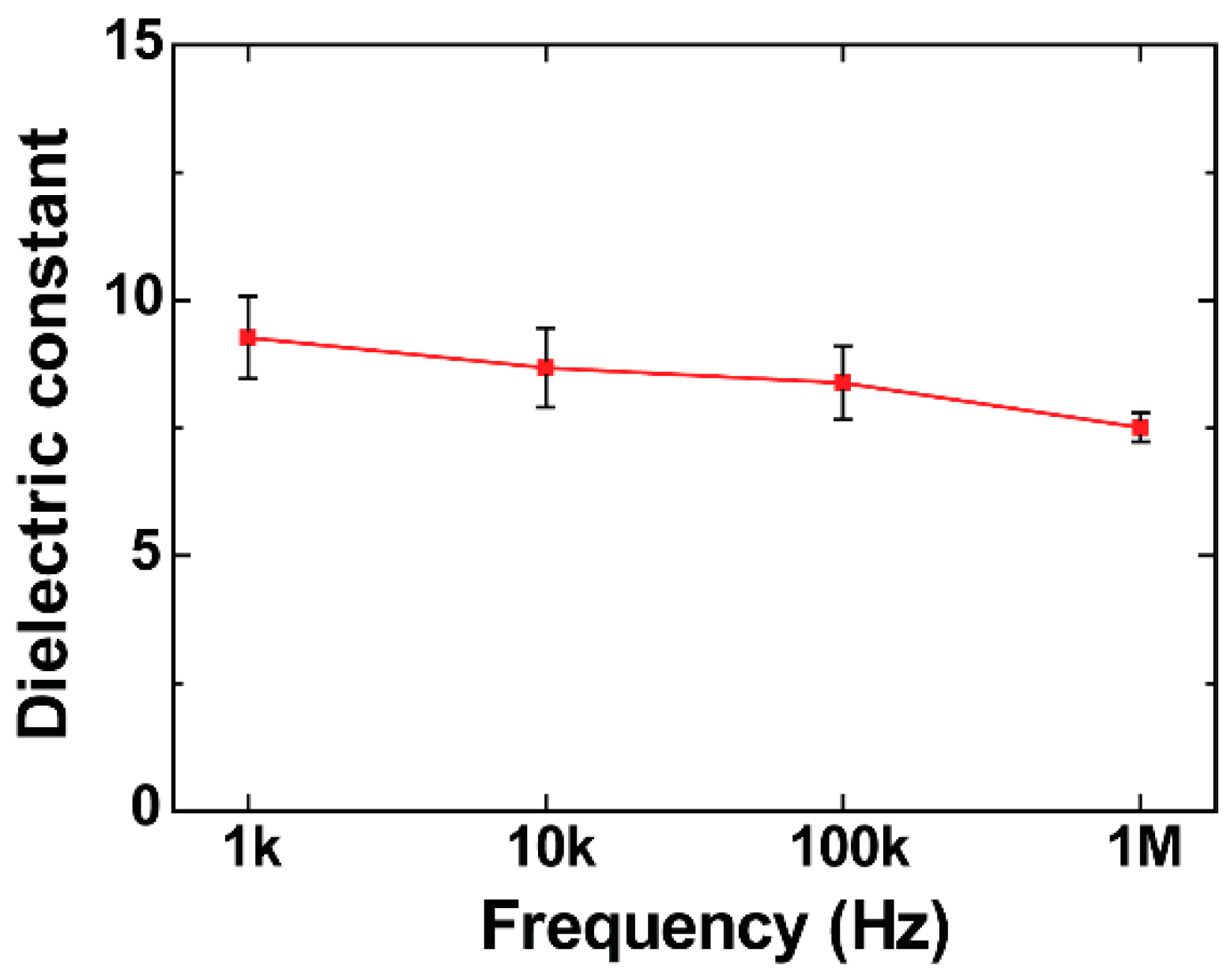
3.3. Dielectric Constant of BTO-PECA Films
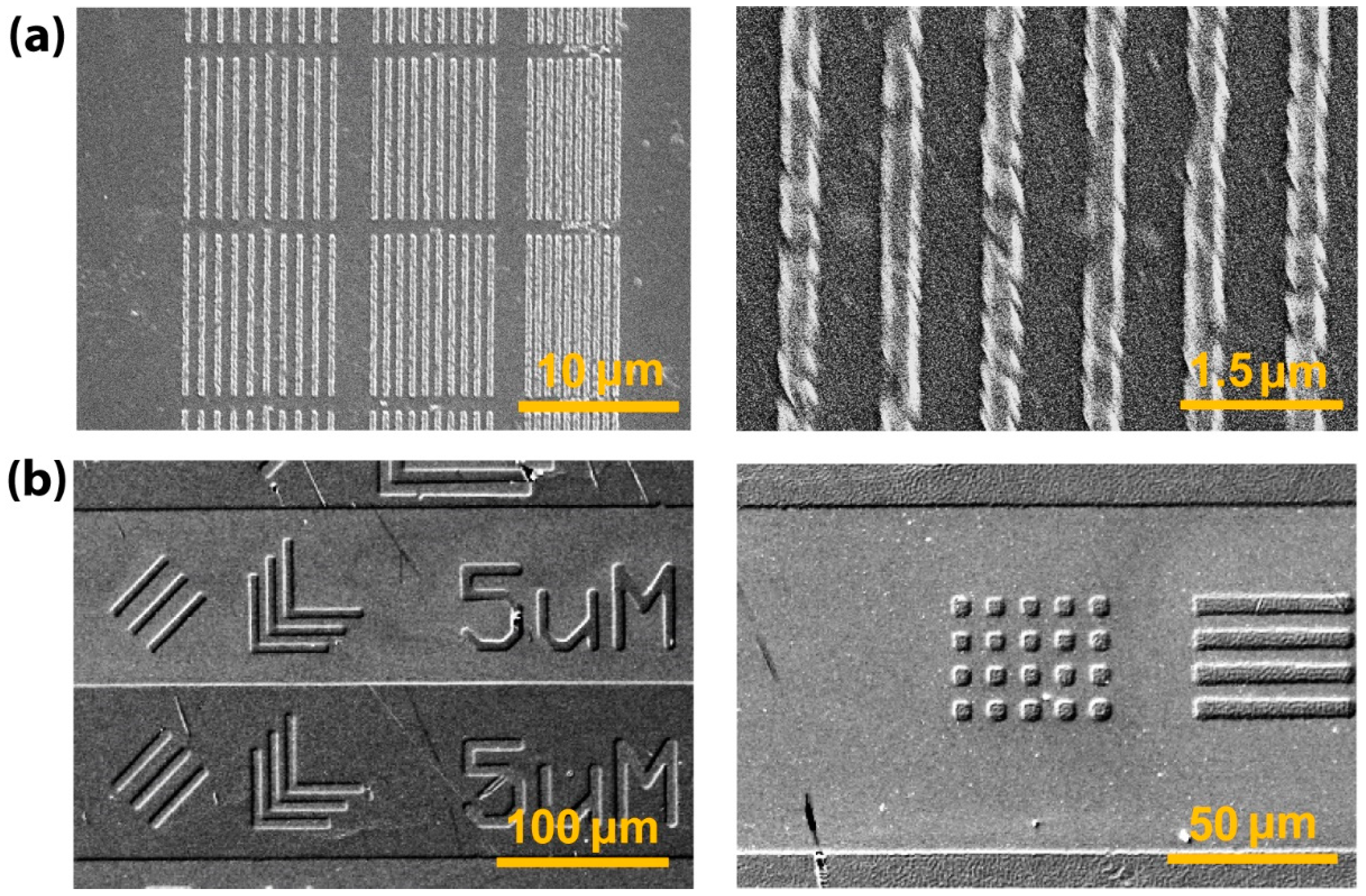
3.4. Lithographic Patterning of BTO-PECA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Don Park, Y.; Lim, J.A.; Lee, H.S.; Cho, K. Interface engineering in organic transistors. Mater. Today 2007, 10, 46–54. [Google Scholar] [CrossRef]
- Pei, M.; Ko, J.S.; Shin, H.; Cho, M.; Baek, J.; Kim, G.; Youk, J.H.; Yang, H. Self-Assembly of Pentacene on Sub-nm Scale Surface Roughness-Controlled Gate Dielectrics. Macromol. Res. 2018, 26, 942–949. [Google Scholar] [CrossRef]
- Li, F.M.; Nathan, A.; Wu, Y.L.; Ong, B.S. Organic thin-film transistor integration using silicon nitride gate dielectric. Appl. Phys. Lett. 2007, 90, 133514. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.H.; Im, S. Pentacene thin-film transistors with Al2O3+x gate dielectric films deposited on indium-tin-oxide glass. Appl. Phys. Lett. 2003, 83, 2689–2691. [Google Scholar] [CrossRef]
- Halik, M.; Klauk, H.; Zschieschang, U.; Schmid, G.; Dehm, C.; Schütz, M.; Maisch, S.; Effenberger, F.; Brunnbauer, M.; Stellacci, F. Low-voltage organic transistors with an amorphous molecular gate dielectric. Nature 2004, 431, 963–966. [Google Scholar] [CrossRef]
- Park, Y.M.; Desai, A.; Salleo, A.; Jimison, L. Solution-Processable Zirconium Oxide Gate Dielectrics for Flexible Organic Field Effect Transistors Operated at Low Voltages. Chem. Mater. 2013, 25, 2571–2579. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Kawase, T.; Friend, R.H.; Shimoda, T.; Inbasekaran, M.; Wu, W.; Woo, E.P. High-Resolution Inkjet Printing of All-Polymer Transistor Circuits. Science 2000, 290, 2123–2126. [Google Scholar] [CrossRef]
- Rogers, J.A.; Bao, Z.; Makhija, A.; Braun, P. Printing Process Suitable for Reel-to-Reel Production of High-Performance Organic Transistors and Circuits. Adv. Mater. 1999, 11, 741–745. [Google Scholar] [CrossRef]
- Yoon, M.-H.; Yan, H.; Facchetti, A.; Marks, T.J. Low-Voltage Organic Field-Effect Transistors and Inverters Enabled by Ultrathin Cross-Linked Polymers as Gate Dielectrics. J. Am. Chem. Soc. 2005, 127, 10388–10395. [Google Scholar] [CrossRef]
- Barber, P.; Balasubramanian, S.; Anguchamy, Y.; Gong, S.; Wibowo, A.; Gao, H.; Ploehn, H.J.; Zur Loye, H.-C. Polymer Composite and Nanocomposite Dielectric Materials for Pulse Power Energy Storage. Materials 2009, 2, 1697–1733. [Google Scholar] [CrossRef]
- Su, Y.R.; Wang, C.L.; Xie, W.G.; Xie, F.Y.; Chen, J.; Zhao, N.; Xu, J.B. Low-Voltage Organic Field-Effect Transistors (OFETs) with Solution-Processed Metal-Oxide as Gate Dielectric. ACS Appl. Mater. Interfaces 2011, 3, 4662–4667. [Google Scholar] [CrossRef] [PubMed]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent Surface Modification of Oxide Surfaces. Angew. Chem. Int. Edit. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites-A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Gan, Y.; Cai, Q.J.; Li, C.M.; Yang, H.B.; Lu, Z.S.; Gong, C.; Chan-Park, M.B. Solution-Prepared Hybrid-Nanoparticle Dielectrics for High-Performance Low-Voltage Organic Thin-Film Transistors. ACS Appl. Mater. Interfaces 2009, 1, 2230–2236. [Google Scholar] [CrossRef]
- Kim, J.; Lim, S.H.; Kim, Y.S. Solution-Based TiO2−Polymer Composite Dielectric for Low Operating Voltage OTFTs. J. Am. Chem. Soc. 2010, 132, 14721–14723. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Wang, H.-Y.; Xu, H.-P. Influence of silane coupling agent on morphology and dielectric property in BaTiO3/polyvinylidene fluoride composites. Appl. Phys. Lett. 2006, 89, 112902. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Tummala, R.R. Next generation integral passives: materials, processes, and integration of resistors and capacitors on PWB substrates. J. Mater. Sci. Mater. Electron. 2000, 11, 253–268. [Google Scholar] [CrossRef]
- Zakhidov, A.A.; Lee, J.-K.; DeFranco, J.A.; Fong, H.H.; Taylor, P.G.; Chatzichristidi, M.; Ober, C.K.; Malliaras, G.G. Orthogonal processing: A new strategy for organic electronics. Chem. Sci. 2011, 2, 1178–1182. [Google Scholar] [CrossRef]
- Yang, K.; Huang, X.; Huang, Y.; Xie, L.; Jiang, P. Fluoro-Polymer@BaTiO3 Hybrid Nanoparticles Prepared via RAFT Polymerization: Toward Ferroelectric Polymer Nanocomposites with High Dielectric Constant and Low Dielectric Loss for Energy Storage Application. Chem. Mater. 2013, 25, 2327–2338. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.H.; Lee, A.; Kim, M.-S.; Yoo, B.; Lee, J.-K. Highly Fluorinated Polymer-Inorganic Nanoparticle Composites Processable with Fluorous Solvents. J. Nanosci. Nanotechnol. 2017, 17, 5510–5514. [Google Scholar] [CrossRef]
- Cho, S.-D.; Lee, J.-Y.; Hyun, J.-G.; Paik, K.-W. Study on epoxy/BaTiO3 composite embedded capacitor films (ECFs) for organic substrate applications. Mater. Sci. Eng. B 2004, 110, 233–239. [Google Scholar] [CrossRef]
- Uchino, K.; Sadanaga, E.; Hirose, T. Dependence of the Crystal Structure on Particle Size in Barium Titanate. J. Am. Ceram. Soc. 1989, 72, 1555–1558. [Google Scholar] [CrossRef]
- Waser, R. Dielectric analysis of intergrated ceramic thin film capacitors. Integr. Ferroelectr. 1997, 15, 39–51. [Google Scholar] [CrossRef]
- Arya, P.R.; Jha, P.; Subbanna, G.N.; Ganguli, A.K. Polymeric citrate precursor route to the synthesis of nano-sized barium lead titanates. Mater. Res. Bull. 2003, 38, 617–628. [Google Scholar] [CrossRef]
- Boulos, M.; Guillemet-Fritsch, S.; Mathieu, F.; Durand, B.; Lebey, T.; Bley, V. Hydrothermal synthesis of nanosized BaTiO3 powders and dielectric properties of corresponding ceramics. Solid State Ion. 2005, 176, 1301–1309. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; He, J.; Zhu, Y.; O’Brien, S. New nonhydrolytic route to synthesize crystalline BaTiO3 nanocrystals with surface capping ligands. J. Mater. Res. 2011, 21, 3187–3195. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G. Organic reaction pathways in the nonaqueous synthesis of metal oxide nanoparticles. Chem. Eur. J. 2006, 12, 7282–7302. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Ma, Y.; Zhao, C.; Yang, W. Preparation and dielectric properties of core–shell structural composites of poly(1H,1H,2H,2H-perfluorooctyl methacrylate)@BaTiO3 nanoparticles. Appl. Surf. Sci. 2013, 277, 121–127. [Google Scholar] [CrossRef]
- Kim, P.; Jones, S.C.; Hotchkiss, P.J.; Haddock, J.N.; Kippelen, B.; Marder, S.R.; Perry, J.W. Phosphonic Acid-Modified Barium Titanate Polymer Nanocomposites with High Permittivity and Dielectric Strength. Adv. Mater. 2007, 19, 1001–1005. [Google Scholar] [CrossRef]
- Cai, Q.J.; Gan, Y.; Chan-Park, M.B.; Yang, H.B.; Lu, Z.S.; Li, C.M.; Guo, J.; Dong, Z.L. Solution-Processable Barium Titanate and Strontium Titanate Nanoparticle Dielectrics for Low-Voltage Organic Thin-Film Transistors. Chem. Mater. 2009, 21, 3153–3161. [Google Scholar] [CrossRef]
- De Roo, J.; Justo, Y.; De Keukeleere, K.; Van den Broeck, F.; Martins, J.C.; Van Driessche, I.; Hens, Z. Carboxylic-Acid-Passivated Metal Oxide Nanocrystals: Ligand Exchange Characteristics of a New Binding Motif. Angew. Chem. Int. Ed. 2015, 54, 6488–6491. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jo, S.H.; Jee, J.-H.; Han, W.; Kim, Y.; Park, H.-H.; Jin, H.-J.; Yoo, B.; Lee, J.-K. Fluorous-inorganic hybrid dielectric materials for solution-processed electronic devices. New J. Chem. 2015, 39, 836–842. [Google Scholar] [CrossRef]
- Studart, A.R.; Amstad, E.; Gauckler, L.J. Colloidal Stabilization of Nanoparticles in Concentrated Suspensions. Langmuir 2007, 23, 1081–1090. [Google Scholar] [CrossRef]
- Mao, Y.; Felix, N.M.; Nguyen, P.T.; Ober, C.K.; Gleason, K.K. Positive- and Negative-Tone CVD Polyacrylic Electron-Beam Resists Developable by Supercritical CO2. Chem. Vap. Depos. 2006, 12, 259–262. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, Z.; Zhu, J.; Zhou, S.; Liu, Z.; Ming, N. Atomic-Scale Characterization of Barium Titanate Powders Formed by the Hydrothermal Process. J. Am. Ceram. Soc. 2008, 91, 1002–1008. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G.; Pinna, N.; Antonietti, M. Nonaqueous and Halide-Free Route to Crystalline BaTiO3, SrTiO3, and (Ba,Sr)TiO3 Nanoparticles via a Mechanism Involving C−C Bond Formation. J. Am. Chem. Soc. 2004, 126, 9120–9126. [Google Scholar] [CrossRef]
- Takeuchi, T.; Tabuchi, M.; Ado, K.; Honjo, K.; Nakamura, O.; Kageyama, H.; Suyama, Y.; Ohtori, N.; Nagasawa, M. Grain size dependence of dielectric properties of ultrafine BaTiO3 prepared by a sol-crystal method. J. Mater. Sci. 1997, 32, 4053–4060. [Google Scholar] [CrossRef]
- Ashiri, R. Obtaining a novel crystalline/amorphous core/shell structure in barium titanate nanocrystals by an innovative one-step approach. RSC Adv. 2015, 5, 48281–48289. [Google Scholar] [CrossRef]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef]
- Takami, S.; Sato, T.; Mousavand, T.; Ohara, S.; Umetsu, M.; Adschiri, T. Hydrothermal synthesis of surface-modified iron oxide nanoparticles. Mater. Lett. 2007, 61, 4769–4772. [Google Scholar] [CrossRef]
- Ledwa, K.A.; Kępiński, L. Dispersion of ceria nanoparticles on γ-alumina surface functionalized using long chain carboxylic acids. Appl. Surf. Sci. 2017, 400, 212–219. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Kim, S. Preparation of Fluorous Solvent-Dispersed Fe3O4 Nanocrystals: Role of Oxygen in Ligand Exchange. Langmuir 2016, 32, 3348–3353. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Troughton, E.B.; Tao, Y.T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Funaki, Y.; Hirokawa, Y.; Hashimoto, T. Selective Incorporation of Palladium Nanoparticles into Microphase-Separated Domains of Poly(2-vinylpyridine)-block-polyisoprene. Langmuir 1999, 15, 5200–5203. [Google Scholar] [CrossRef]
- Devaraju, N.G.; Kim, E.S.; Lee, B.I. The synthesis and dielectric study of BaTiO3/polyimide nanocomposite films. Microelectron. Eng. 2005, 82, 71–83. [Google Scholar] [CrossRef]
- Arlt, G.; Hennings, D.; With, G.d. Dielectric properties of fine-grained barium titanate ceramics. J. Appl. Phys. 1985, 58, 1619–1625. [Google Scholar] [CrossRef]
- Horikawa, T.; Mikami, N.; Makita, T.; Tanimura, J.; Kataoka, M.; Sato, K.; Nunoshita, M. Dielectric-Properties of (Ba, Sr)TiO3 Thin-Films Deposited by Rf-Sputtering. Jpn. J. Appl. Phys. 1993, 32, 4126–4130. [Google Scholar] [CrossRef]
- Jayasundere, N.; Smith, B.V. Dielectric-Constant for Binary Piezoelectric 0-3 Composites. J. Appl. Phys. 1993, 73, 2462–2466. [Google Scholar] [CrossRef]
- Wada, S.; Yazawa, A.; Hoshina, T.; Kameshima, Y.; Kakemoto, H.; Tsurumi, T.; Kuroiwa, Y. Preparation of barium titanate nanoparticle sphere arrays and their dielectric properties. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2008, 55, 1895–1899. [Google Scholar] [CrossRef]
- Todd, M.G.; Shi, F.G. Complex permittivity of composite systems: a comprehensive interphase approach. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 601–611. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, L.; Ducharme, S. Electric energy density of dielectric nanocomposites. Appl. Phys. Lett. 2007, 90, 132901. [Google Scholar] [CrossRef]
- Jung, S.-H.; Chuluunbandi, K.; Kim, Y.; Son, J.; Oh, H.-T.; Lee, J.H.; Lee, J.-K.; Beom-Hoan, O. Investigation of degradation pathways of poly(semiperfluoroalkyl methacrylate) thin films induced by electron-beam irradiation. J. Polym. Sci. A Polym. Chem. 2018, 56, 2672–2680. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, H.; Oh, H.-T.; Kim, S.; Lee, J.-K. Highly Fluorinated Barium Titanate Nanoparticle Dispersion for Fabrication of Lithographically Patterned Thin Films. Materials 2019, 12, 4045. https://doi.org/10.3390/ma12244045
Kim Y, Kim H, Oh H-T, Kim S, Lee J-K. Highly Fluorinated Barium Titanate Nanoparticle Dispersion for Fabrication of Lithographically Patterned Thin Films. Materials. 2019; 12(24):4045. https://doi.org/10.3390/ma12244045
Chicago/Turabian StyleKim, Youngtae, Heejin Kim, Hyun-Taek Oh, Sangwon Kim, and Jin-Kyun Lee. 2019. "Highly Fluorinated Barium Titanate Nanoparticle Dispersion for Fabrication of Lithographically Patterned Thin Films" Materials 12, no. 24: 4045. https://doi.org/10.3390/ma12244045
APA StyleKim, Y., Kim, H., Oh, H.-T., Kim, S., & Lee, J.-K. (2019). Highly Fluorinated Barium Titanate Nanoparticle Dispersion for Fabrication of Lithographically Patterned Thin Films. Materials, 12(24), 4045. https://doi.org/10.3390/ma12244045



