In Situ Determination of pH at Nanostructured Carbon Electrodes Using IR Spectroscopy
Abstract
1. Introduction
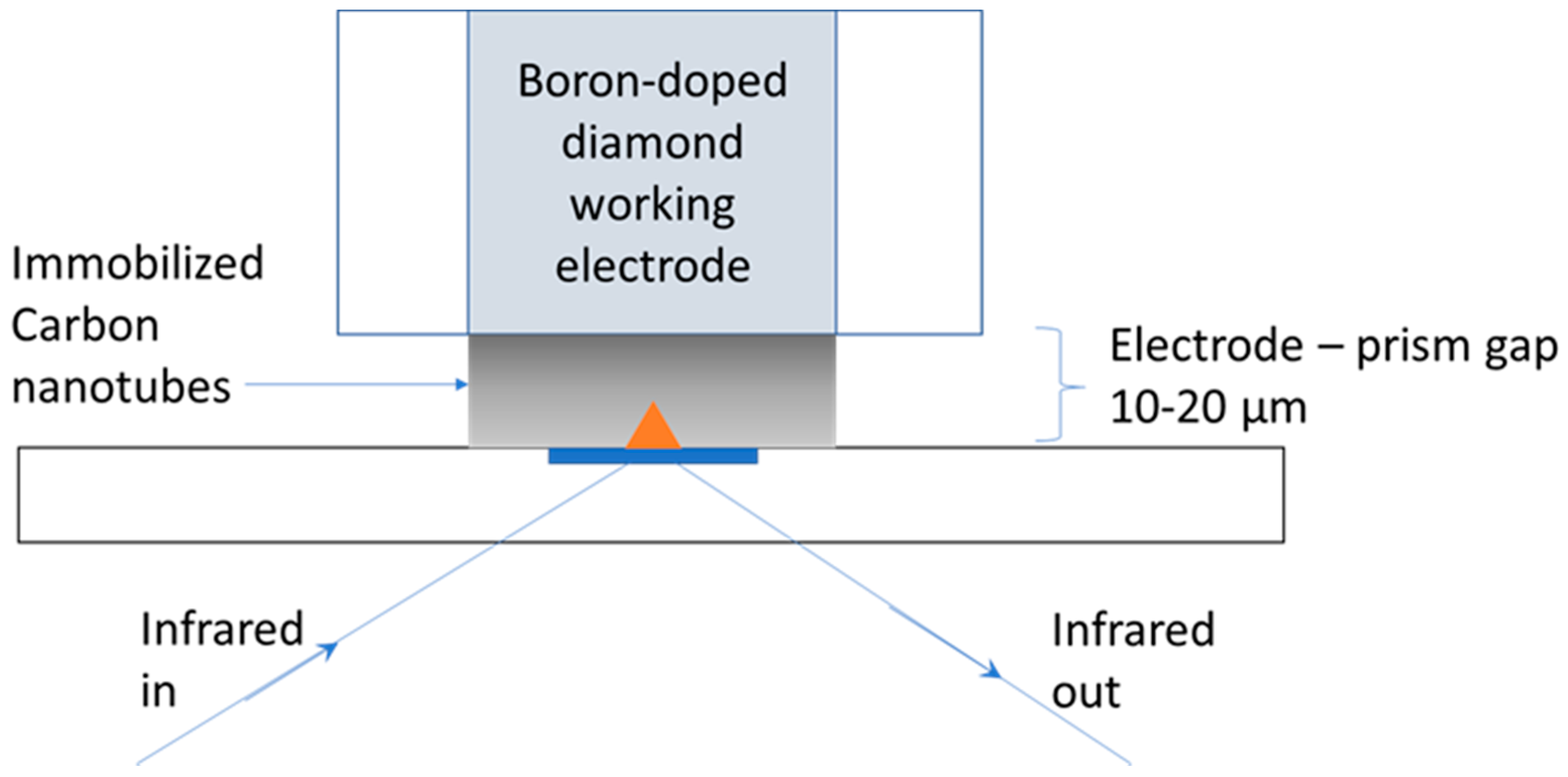
2. Materials and Methods
3. Results
3.1. IR Spectra of Aqueous Phosphate Solutions of Known pH
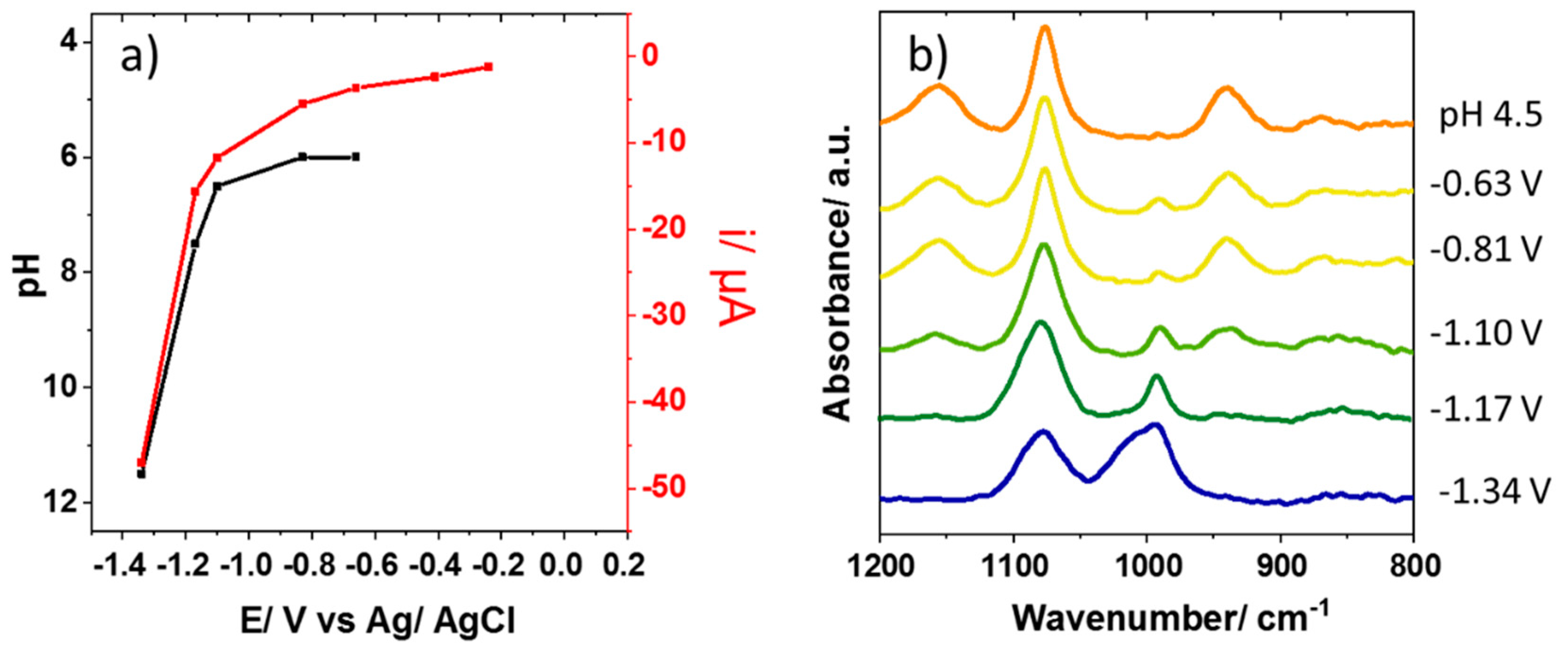
3.2. Interfacial pH Changes During the Water Reduction Reaction
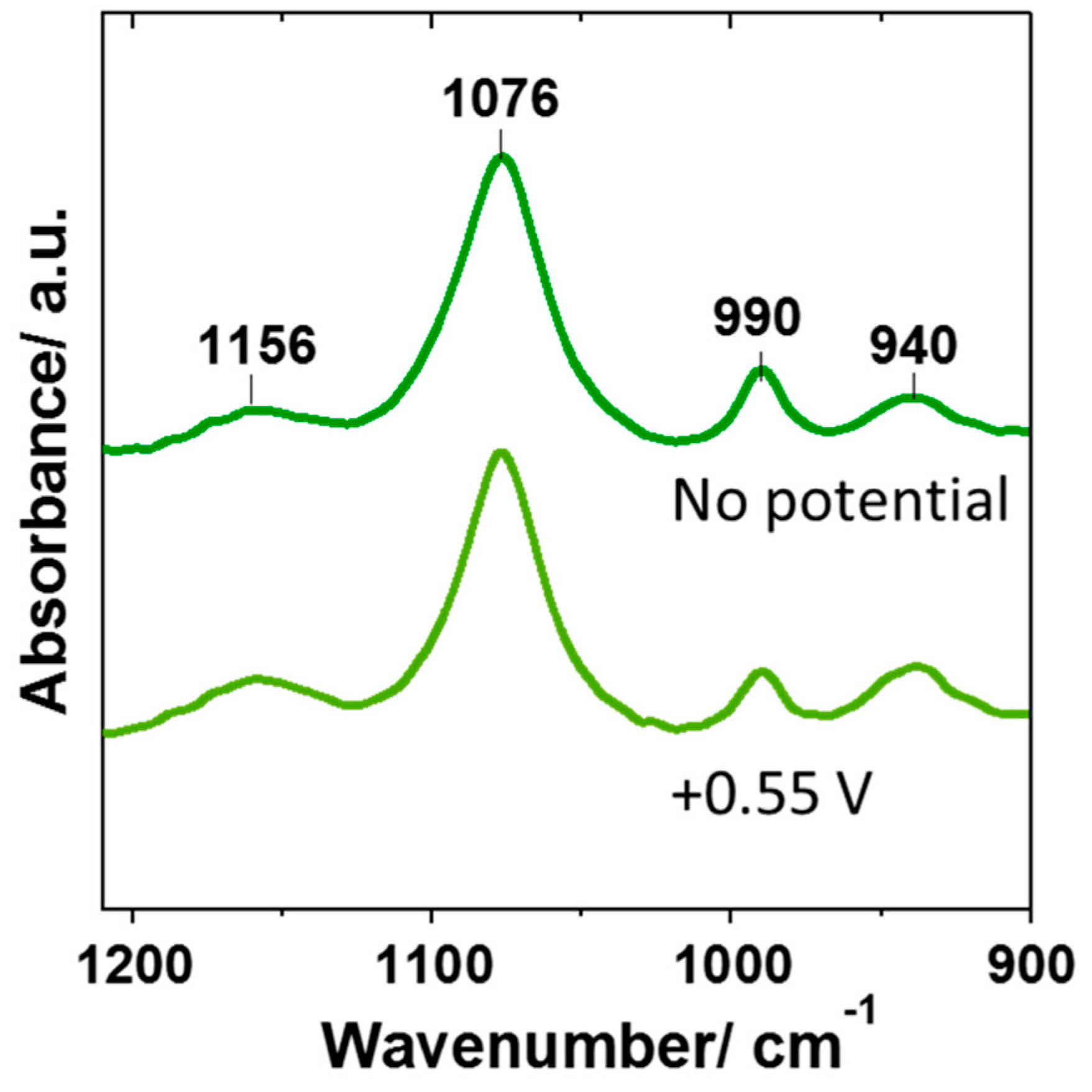
3.3. Improvement of Sensitivity Using Difference Spectroscopy
3.4. In Situ Determination of pH Change During the Oxidation of Hydroquinone
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fukuzumi, S.; Lee, Y.M.; Nam, W. Immobilization of Molecular Catalysts for Enhanced Redox Catalysis. ChemCatChem 2018, 10, 1686–1702. [Google Scholar] [CrossRef]
- Yang, Z.F.; Tian, J.R.; Yin, Z.F.; Cui, C.J.; Qian, W.Z.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.F.; Zhang, H.M.; Chen, J.Z. Progress on the electrode materials towards vanadium flow batteries (VFBs) with improved power density. J. Energy Chem. 2018, 27, 1292–1303. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Niu, Q.Y.; Gu, X.T.; Yang, N.; Zhoa, G.H. Recent progress on carbon nanomaterials for the electrochemical detection and removal of environmental pollutants. Nanoscale 2019, 11, 11992–12014. [Google Scholar] [CrossRef]
- Beitollah, H.; Movahedifar, F.; Tajik, S.; Jahini, S. A Review on the Effects of Introducing CNTs in the Modification Process of Electrochemical Sensors. Electroanalysis 2019, 31, 1195–1203. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Tarakolian-Ardakani, Z.; Hosu, O.; Cristea, C.; Mazloum-Ardakani, M.; Marrazza, G. Latest Trends in Electrochemical Sensors for Neurotransmitters: A Review. Sensors 2019, 19, 2037. [Google Scholar] [CrossRef]
- Scaini, D.; Ballerini, L. Nanomaterials at the neural interface. Curr. Opin. Neurobiol. 2018, 50, 50–55. [Google Scholar] [CrossRef]
- Reta, N.; Saint, S.P.; Michelmore, A.; Prieto-Simon, B.; Voelcker, N.H. Nanostructured Electrochemical Biosensors for Label-Free Detection of Water- and Food-Borne Pathogens. ACS Appl. Mater. Interfaces 2018, 10, 6055–6072. [Google Scholar] [CrossRef]
- Nasar, A.; Perveen, R. Applications of enzymatic biofuel cells in bioelectronic devices—A review. Int. J. Hydrog. Energy 2019, 44, 15287–15312. [Google Scholar] [CrossRef]
- Schindler, S.; Bechtold, T. Mechanistic insights into the electrochemical oxidation of dopamine by cyclic voltammetry. J. Electroanal. Chem. 2019, 836, 94–101. [Google Scholar] [CrossRef]
- Kuhn, A.T.; Chan, C.Y. pH changes at near-electrode surfaces. J. Appl. Electrochem. 1983, 13, 189–207. [Google Scholar] [CrossRef]
- Flatt, R.K.; Wood, R.W.; Brook, R.W. The concentration profiles in solution at dissolving anode surfaces (1) zinc, copper and brasses by the freezing techniques. J. Appl. Electrochem. 1971, 1, 35–39. [Google Scholar] [CrossRef]
- Ji, J.; Cooper, W.C.; Dreisinger, D.B.; Peters, E. Surface pH measurements during nickel electrodeposition. J. Appl. Electrochem. 1995, 25, 642–650. [Google Scholar] [CrossRef]
- Zimer, A.M.; Medina da Silva, M.; Machado, E.G.; Varela, H.; Mascaro, L.H.; Pereira, E.C. Development of a versatile rotating ring-disc electrode for in situ pH measurements. Anal. Chim. Acta 2015, 897, 17–23. [Google Scholar] [CrossRef]
- Wipf, D.O.; Ge, F.; Spaine, T.W.; Baur, J.E. Microscopic Measurement of pH with Iridium Oxide Microelectrodes. Anal. Chem. 2000, 72, 4921–4927. [Google Scholar] [CrossRef]
- Rudd, N.C.; Cannan, S.; Bitziou, E.; Ciani, I.; Whitworth, A.L.; Unwin, P.R. Fluorescence Confocal Laser Scanning Microscopy as a Probe of pH Gradients in Electrode Reactions and Surface Activity. Anal. Chem. 2005, 77, 6205–6621. [Google Scholar] [CrossRef]
- Chen, D.-J.; Tong, Y.Y.J. In situ Raman spectroscopic measurement of near-surface proton concentration changes during electrochemical reactions. Chem. Commun. 2015, 51, 5683–5686. [Google Scholar] [CrossRef]
- Erné, B.H.; Maroun, F.; Ozanam, F.; Chazalviel, J.-N. Local pH Change during Diffusion-Limited Proton Reduction Determined by In Situ Infrared Spectroscopy. Electrochem. Solid State Lett. 1999, 2, 231–232. [Google Scholar] [CrossRef]
- Maroun, F.; Ozanam, F.; Chazalviel, J.-N. Infrared determination of small variations of local pH. J. Electroanal. Chem. 1997, 435, 225–228. [Google Scholar] [CrossRef]
- Zakaria, S.N.A.; Hollingsworth, N.; Islam, H.U.; Roffey, R.; Santos-Carballal, D.; Roldan, R.; Bras, W.; Sankar, G.; Hogarth, G.; Holt, K.B.; et al. Insight into the Nature of Iron Sulfide Surfaces During the Electrochemical Hydrogen Evolution and CO2 Reduction Reactions. ACS Appl. Mater. Interfaces 2018, 10, 32078–32085. [Google Scholar] [CrossRef] [PubMed]
- Lounasvuori, M.M.; Holt, K.B. Acid deprotonation driven by cation migration at biased graphene nanoflake electrodes. Chem. Commun. 2017, 53, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, E.J.; Sparks, D.L. Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. J. Colloid Interface Sci. 2007, 308, 53–70. [Google Scholar] [CrossRef]


| Species | Wavenumber/cm−1 ν3 | ν3 | ν3 | ν1 |
|---|---|---|---|---|
| H3PO4 | 1172 | 1005 | 889 | |
| H2PO4− | 1159 | 1077 | 940 | 875 |
| HPO42− | 1078 | 990 | 850 | |
| PO43− | 1010 |
| Phosphate Concentration/mol.dm−3 | pH | [H+]/mol.dm−3 |
|---|---|---|
| 0.100 | 6.6 | 2.5 × 10−7 |
| 0.075 | 6.5 | 3.2 × 10−7 |
| 0.050 | 6.4 | 4.0 × 10−7 |
| 0.025 | 6.1 | 7.9 × 10−7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamgbelu, L.; Holt, K.B. In Situ Determination of pH at Nanostructured Carbon Electrodes Using IR Spectroscopy. Materials 2019, 12, 4044. https://doi.org/10.3390/ma12244044
Bamgbelu L, Holt KB. In Situ Determination of pH at Nanostructured Carbon Electrodes Using IR Spectroscopy. Materials. 2019; 12(24):4044. https://doi.org/10.3390/ma12244044
Chicago/Turabian StyleBamgbelu, Lolade, and Katherine B Holt. 2019. "In Situ Determination of pH at Nanostructured Carbon Electrodes Using IR Spectroscopy" Materials 12, no. 24: 4044. https://doi.org/10.3390/ma12244044
APA StyleBamgbelu, L., & Holt, K. B. (2019). In Situ Determination of pH at Nanostructured Carbon Electrodes Using IR Spectroscopy. Materials, 12(24), 4044. https://doi.org/10.3390/ma12244044





