Dielectric and Impedance Studies of (Ba,Ca)TiO3 Ceramics Obtained from Mechanically Synthesized Powders
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. SEM and EDS
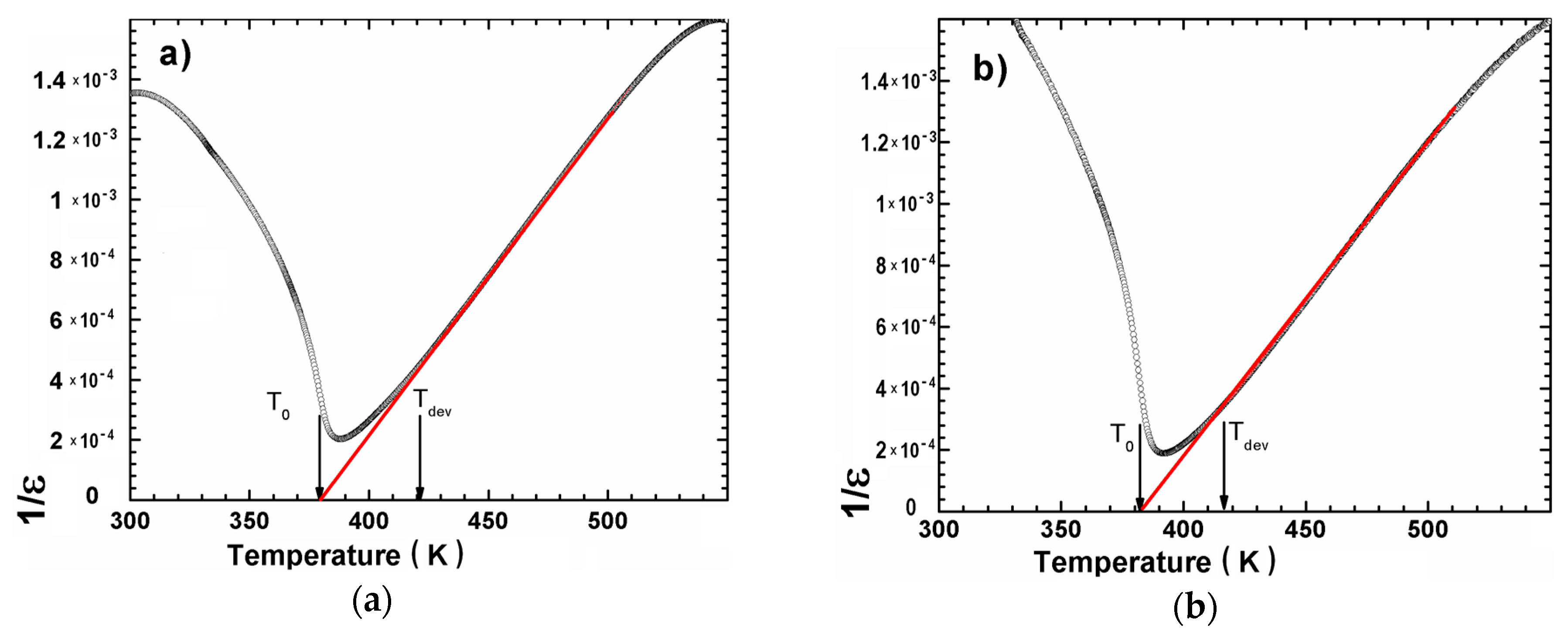
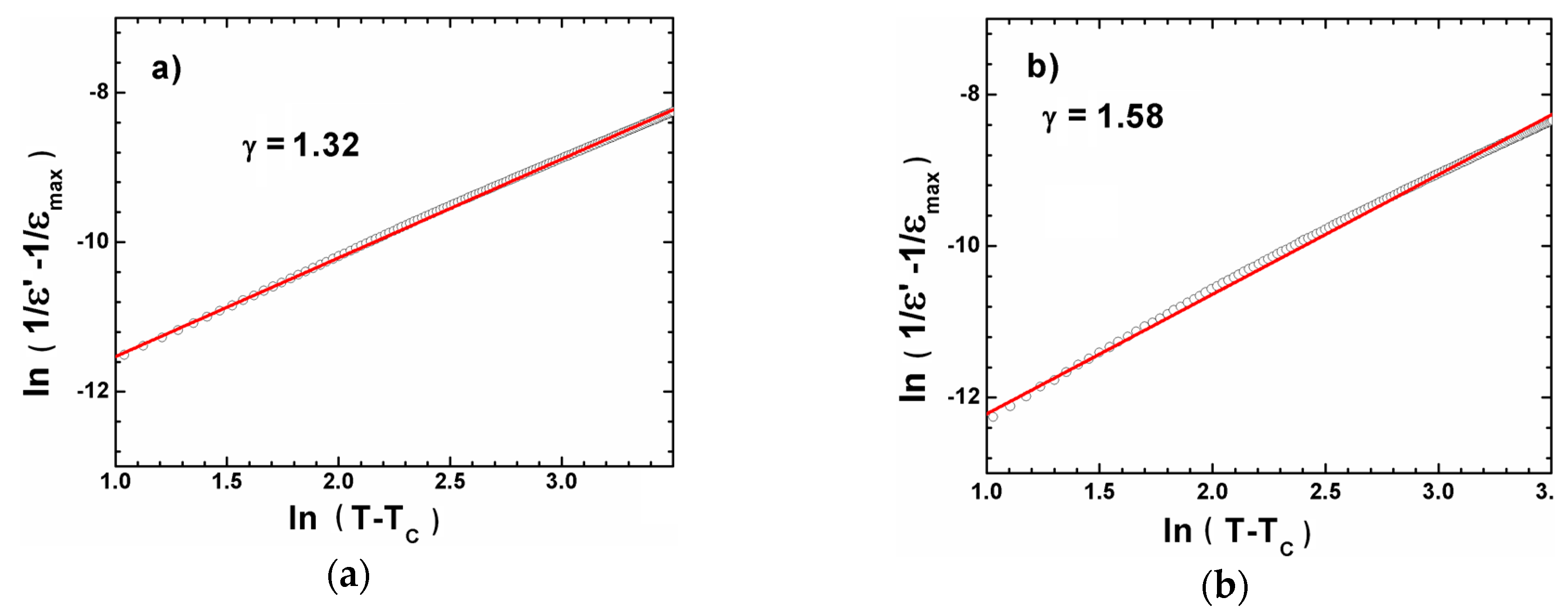
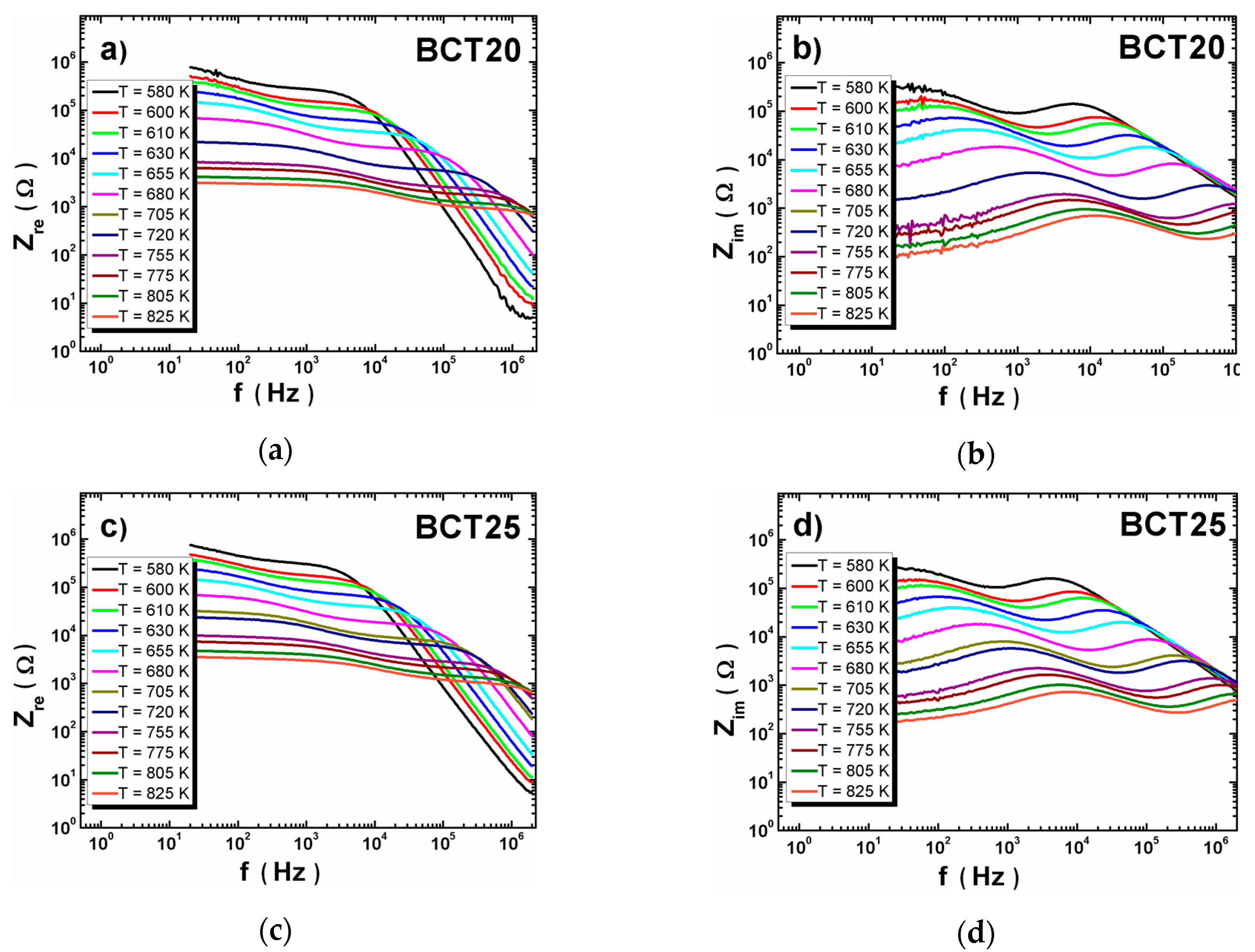
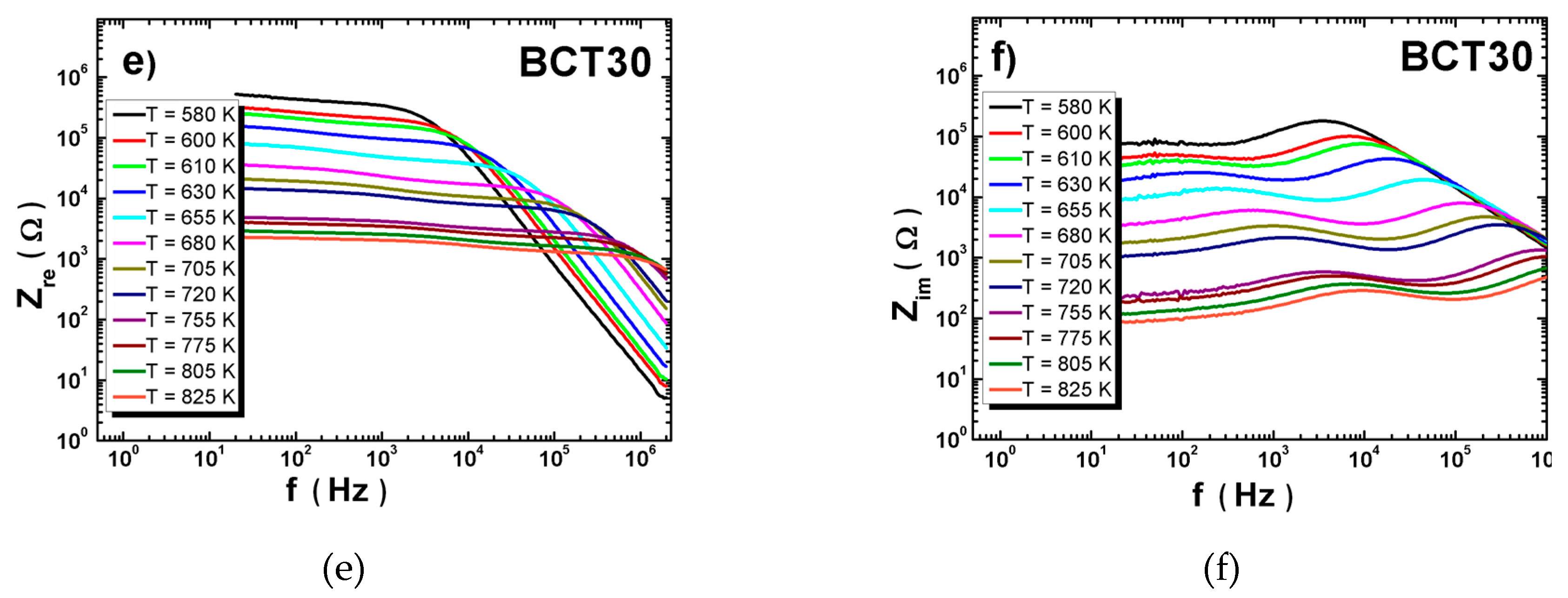
3.2. XRD Measurements and Dielectric Spectroscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jayanthi, S.; Kutty, T.R.N. Extended phase homogeneity and electrical properties of barium calcium titanate prepared by the wet chemical methods. Mater. Sci. Eng. B 2004, 110, 202–212. [Google Scholar] [CrossRef]
- Puli, V.S.; Kumar, A.; Chrisey, D.B.; Tomozawa, M.; Scott, J.F.; Katiyar, R.S. Barium zirconate-titanate/barium calcium-titanate ceramics via sol–gel process: Novel high-energy-density capacitors. J. Phys. D Appl. Phys. 2011, 44, 395403–395413. [Google Scholar] [CrossRef]
- Kong, L.B.; Zhang, T.S.; Ma, J.; Boey, F. Progress in Synthesis of Ferroelectric Ceramic Materials via High-Energy Mechanochemical Technique. Prog. Mater. Sci. 2008, 53, 207–322. [Google Scholar] [CrossRef]
- Beyer, M.K.; Clausen-Schaumann, H. Mechanochemistry: The Mechanical Activation of Covalent Bonds. Chem. Rev. 2004, 105, 2921–2948. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Chem. Rev. 2006, 75, 177–187. [Google Scholar] [CrossRef]
- Liu, W.F.; Ren, X.B. Large Piezoelectric Effect in Pb-Free Ceramics. Phys. Rev. Lett. 2009, 103, 257602–257604. [Google Scholar] [CrossRef]
- Tanaka, D.; Yamazaki, J.; Furukawa, M.; Tsukada, T. High Power Characteristics of (Ca, Ba) TiO3 Piezoelectric Ceramics with High Mechanical Quality Factor. Jpn. J. Appl. Phys. 2010, 49, 09MD03–09MD08. [Google Scholar] [CrossRef]
- Pullar, R.C.; Zhang, Y.; Chen, L.; Yang, S.; Evans, J.R.G.; Salak, A.N.; Kiselev, D.A.; Kholkin, A.L.; Ferreira, V.M.; Alford, N. Dielectric measurements on a novel Ba1−xCaxTiO3 (BCT) bulk ceramic combinatorial library. J. Electroceramics 2009, 22, 245–251. [Google Scholar] [CrossRef]
- Stojanovic, B.D.; Jovalekic, C.; Vukotic, V.; Simoes, A.Z.; Varela, J.A. Ferroelectric Properties of Mechanically Synthesized Nanosized Barium Titanate. Ferroelectrics 2005, 319, 65–73. [Google Scholar] [CrossRef]
- Stojanovic, B.D.; Simoes, A.Z.; Paiva-Santos, C.O.; Jovalekic, C.; Mitic, V.V.; Varela, J.A. Mechanochemical synthesis of barium titanate. J. Eur. Ceram. Soc. 2005, 25, 1985–1989. [Google Scholar] [CrossRef]
- Szafraniak-Wiza, I.; Kozielski, L.; Sebastian, T. Preparation and properties of Ba1-xCaxTiO3 nanopowders obtained by mechanochemical synthesis. Phase Transit. 2016, 89, 803–807. [Google Scholar] [CrossRef]
- Cheng, X.; Shen, M. Different microstructure and dielectric properties of Ba1-xCaxTiO3 ceramics and pulsed-laser-ablated films. Mat. Res. Bull. 2007, 42, 1662–1668. [Google Scholar] [CrossRef]
- Li, L.Y.; Tang, X.G. Effect of electric field on the dielectric properties and ferroelectric phase transition of sol-gel derived (Ba0.99Ca0.10) TiO3 ceramics. Mater. Chem. Phys. 2009, 115, 507–511. [Google Scholar] [CrossRef]
- Victor, P.; Ranjith, R.; Krupanidhi, S.B. Normal ferroelectric to relaxor behavior in laser ablated Ca-doped barium titanate thin films. J. Appl. Phys. 2003, 94, 7702–7709. [Google Scholar] [CrossRef]
- Han, Y.H.; Appleby, J.B.; Smyth, D.M. Calcium as an Acceptor Impurity in BaTiO3. J. Am. Ceram. Soc. 1987, 70, 96–100. [Google Scholar] [CrossRef]
- Zhang, X.W.; Han, Y.H.; Lal, M.; Smyth, D.M. Defect Chemistry of BaTiO3 with Additions of CaTiO3. J. Am. Ceram. Soc. 1987, 70, 100–103. [Google Scholar] [CrossRef]
- Martirena, H.T.; Burfoot, J.C. Grain-size effects on properties of some ferroelectric ceramics. J. Phys. C 1974, 7, 3182–3192. [Google Scholar] [CrossRef]
- Chul Chang, M.Y.; Yu, S.C. Raman study for (Ba1−xCax) TiO3 and Ba(Ti1−yCay) O3 crystalline ceramics. J. Mater. Sci. Lett. 2000, 19, 1323–1325. [Google Scholar] [CrossRef]
- Tiwari, V.S.; Phandey, D.; Krishna, P.S.R.; Chakravarthy, R.; Dasananacharya, B.A. Neutron diffraction study of ferroelectric Ba0.88Ca0.12TiO3. Phys. B Condens. Matter 1991, 174, 112–116. [Google Scholar] [CrossRef]
- Rachna, S.; Gupta, S.M.; Bhattacharyya, S. Impedance analysis of Bi3.25La0.75Ti3O12 ferroelectric ceramic. Indian Acad. Sci. 2008, 71, 599–610. [Google Scholar] [CrossRef]
- Thirumal Reddy, N.; Madhavi, K.; Prasad, N.V.; Kumar, G.S.; Prasad, G. Impedance spectroscopic studies on Zr-modified Bi3.25La0.75Ti3O12ceramics. Int. J. Res. Eng. Technol. 2015, 4, 397–401. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, B.C.; Chung, J.W.; Jeong, J.H. Low-Frequency Dielectric Property and Impedance Spectroscopy of Bismuth-Lanthanum-Titanate Ceramics with NbDoping. J. Korean Phys. Soc. 2008, 52, 410–414. [Google Scholar] [CrossRef]
- Jaffe, B. Piezoelectric Ceramics. J. Am. Ceram. Soc. 1958, 41, 494–498. [Google Scholar] [CrossRef]
- Rani, J.; Yadav, K.L.; Prakash, S. Structural, dielectric and optical properties of sol–gel synthesized 0.55Ba(Zr0.2Ti0.8) O3–0.45(Ba0.7Ca0.3) TiO3ceramic. Appl. Phys. A 2014, 117, 1131–1137. [Google Scholar] [CrossRef]
- Hungria, T.; Alguero, M.; Ana Hungrıa, B.; Castro, A. Dense, Fine-Grained Ba1-xSrxTiO3 Ceramics Prepared by the Combination of Mechanosynthesized Nanopowders and Spark Plasma Sintering. Chem. Mater. 2005, 17, 6205–6212. [Google Scholar] [CrossRef]
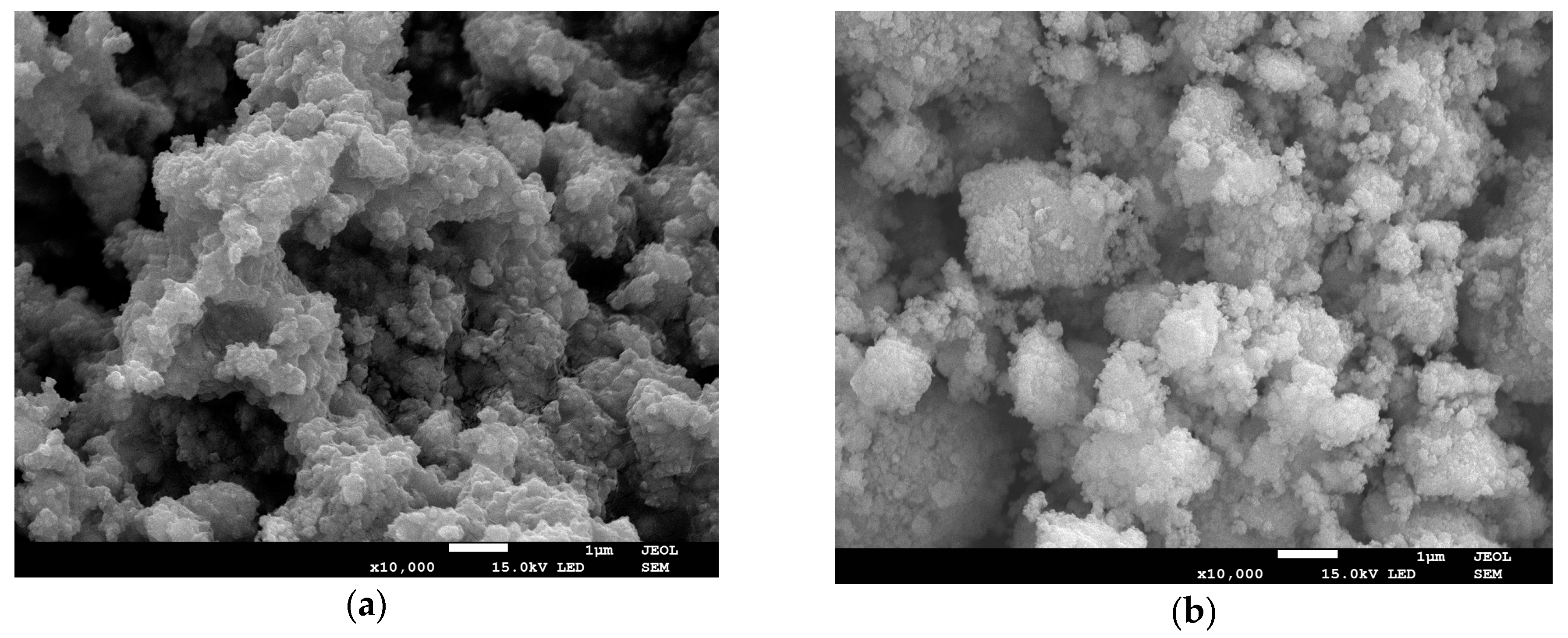
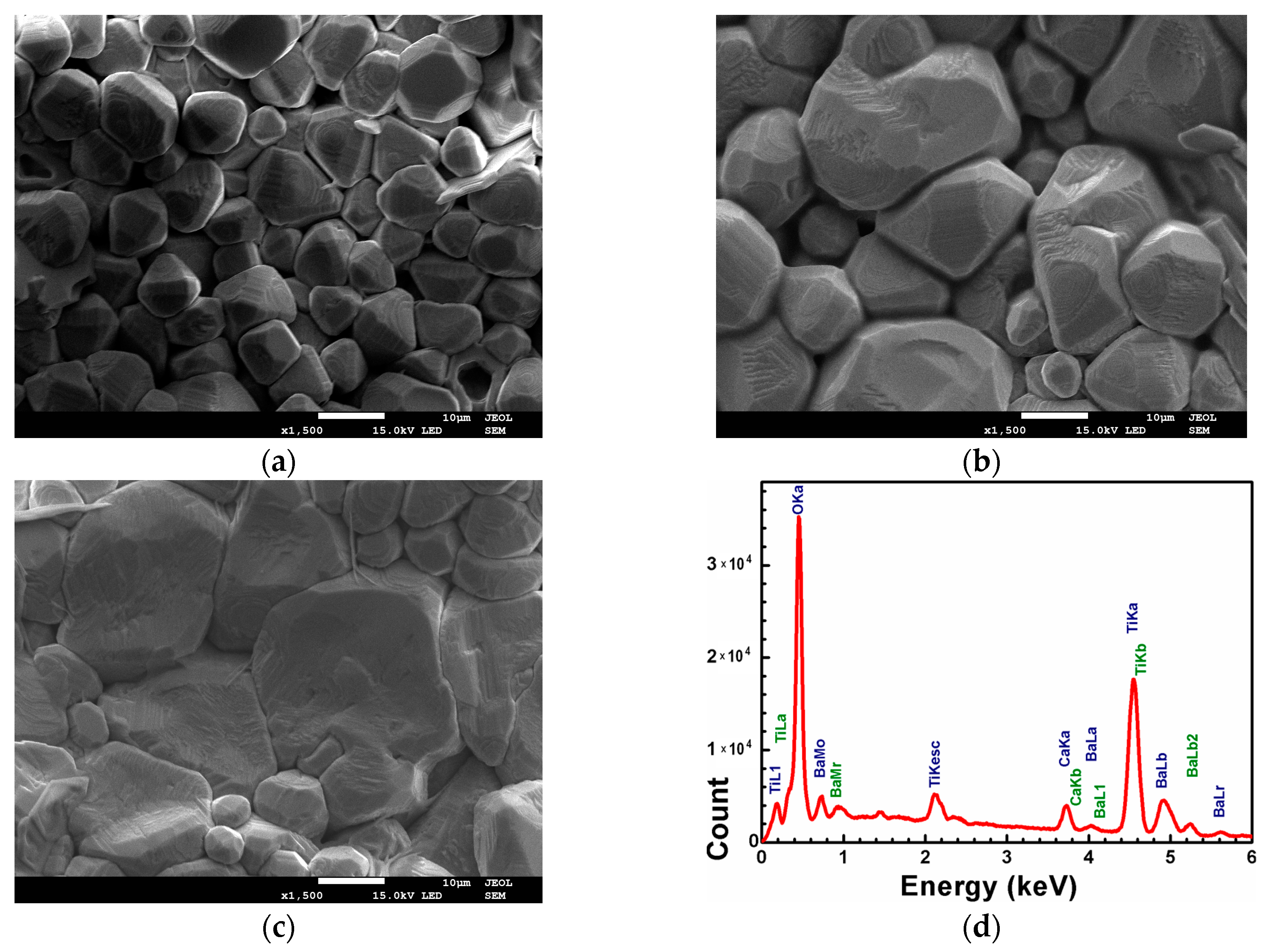
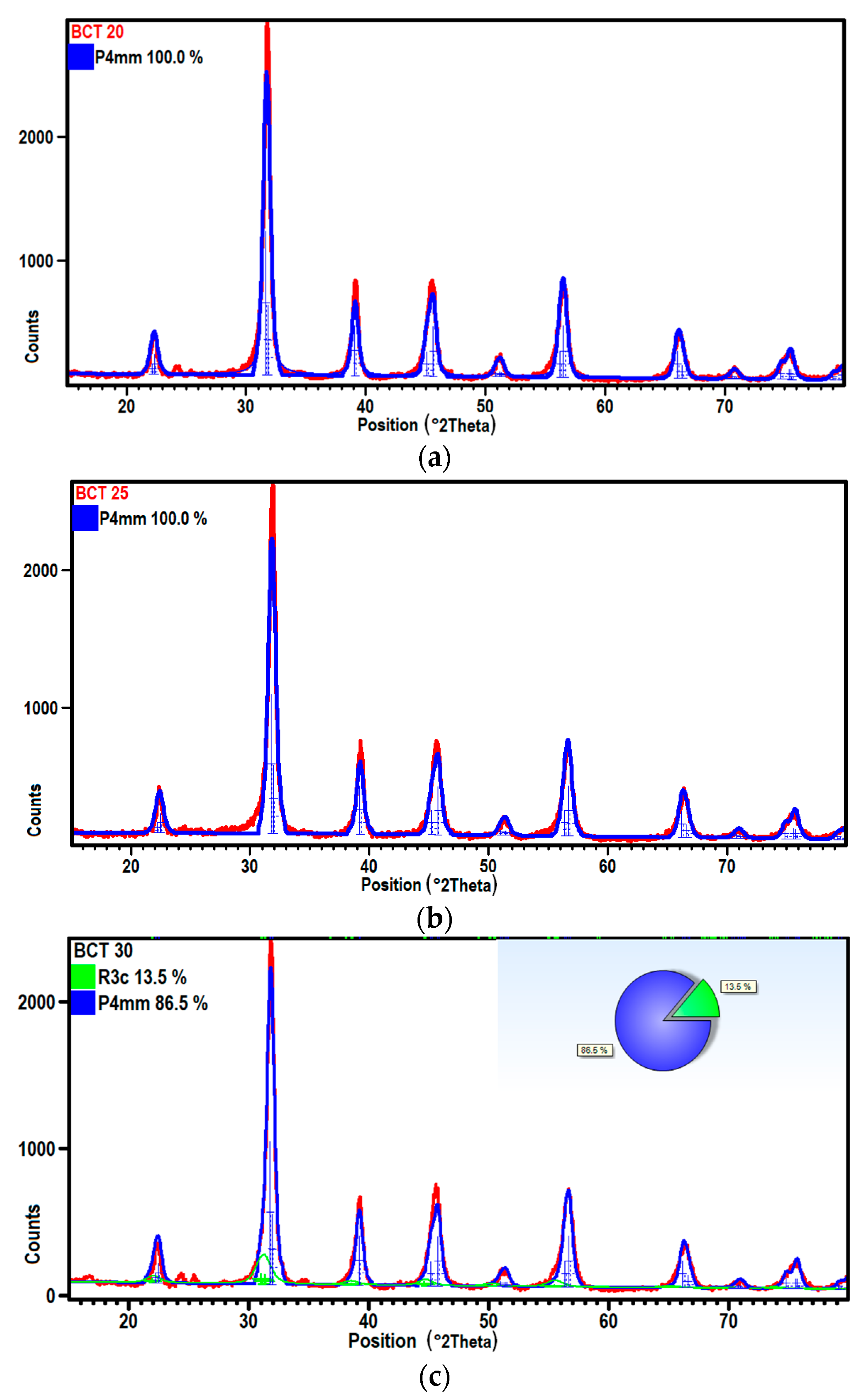
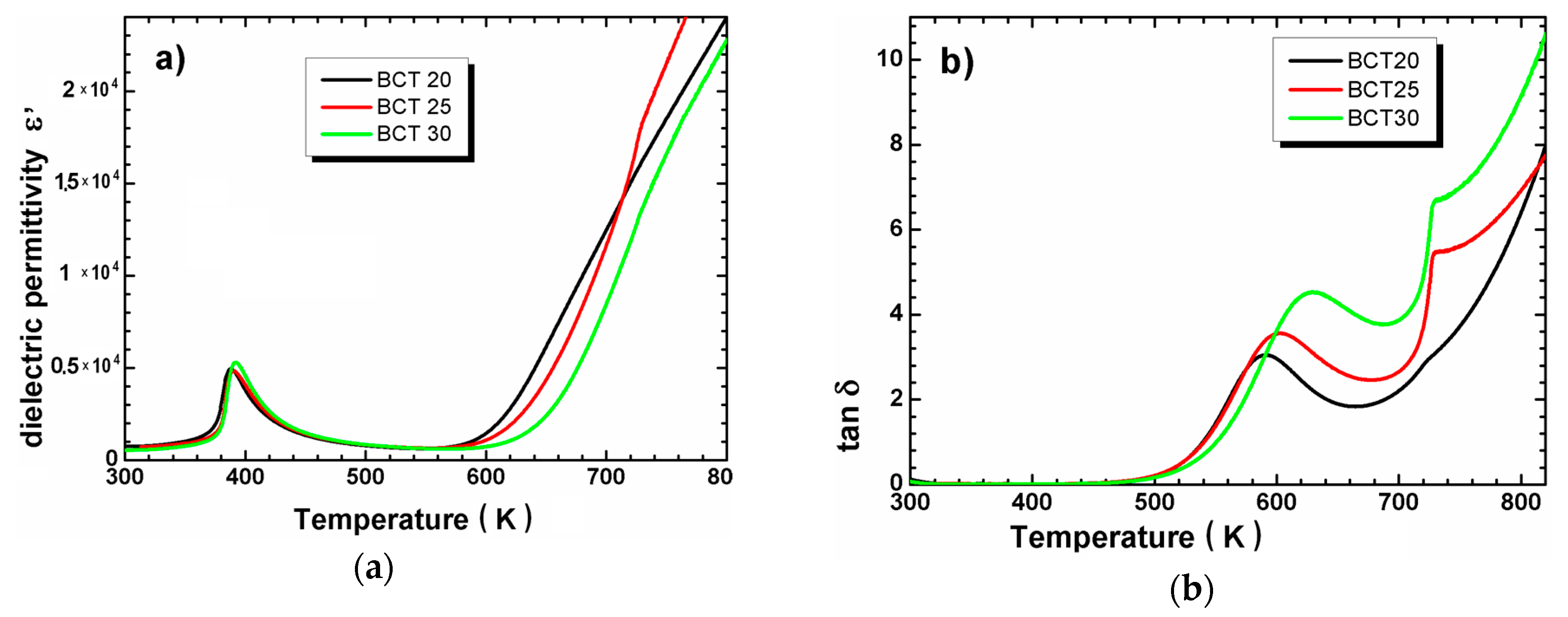

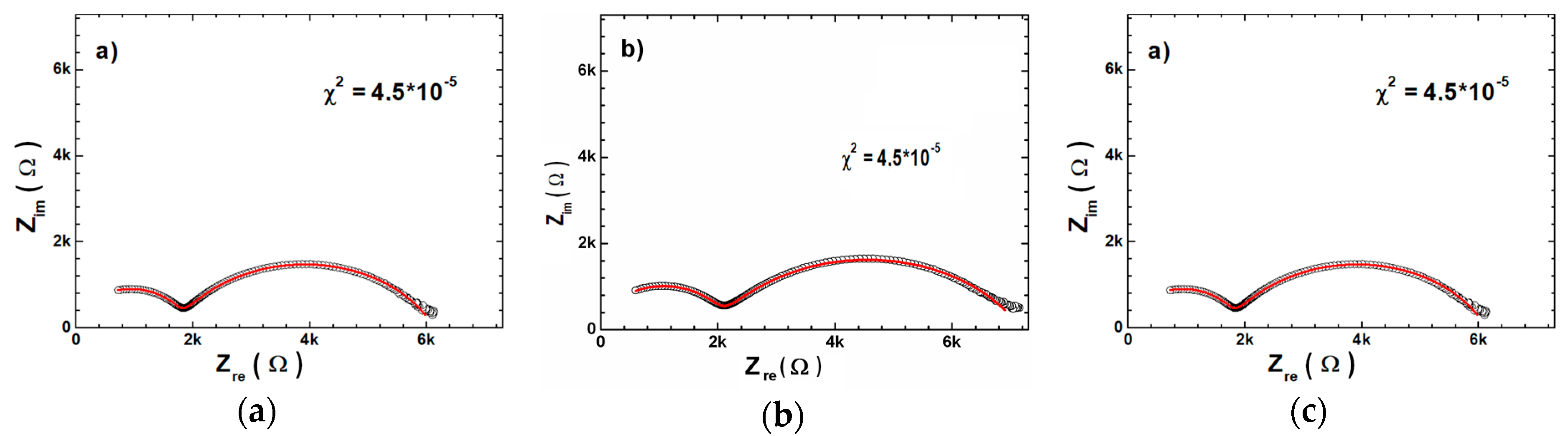
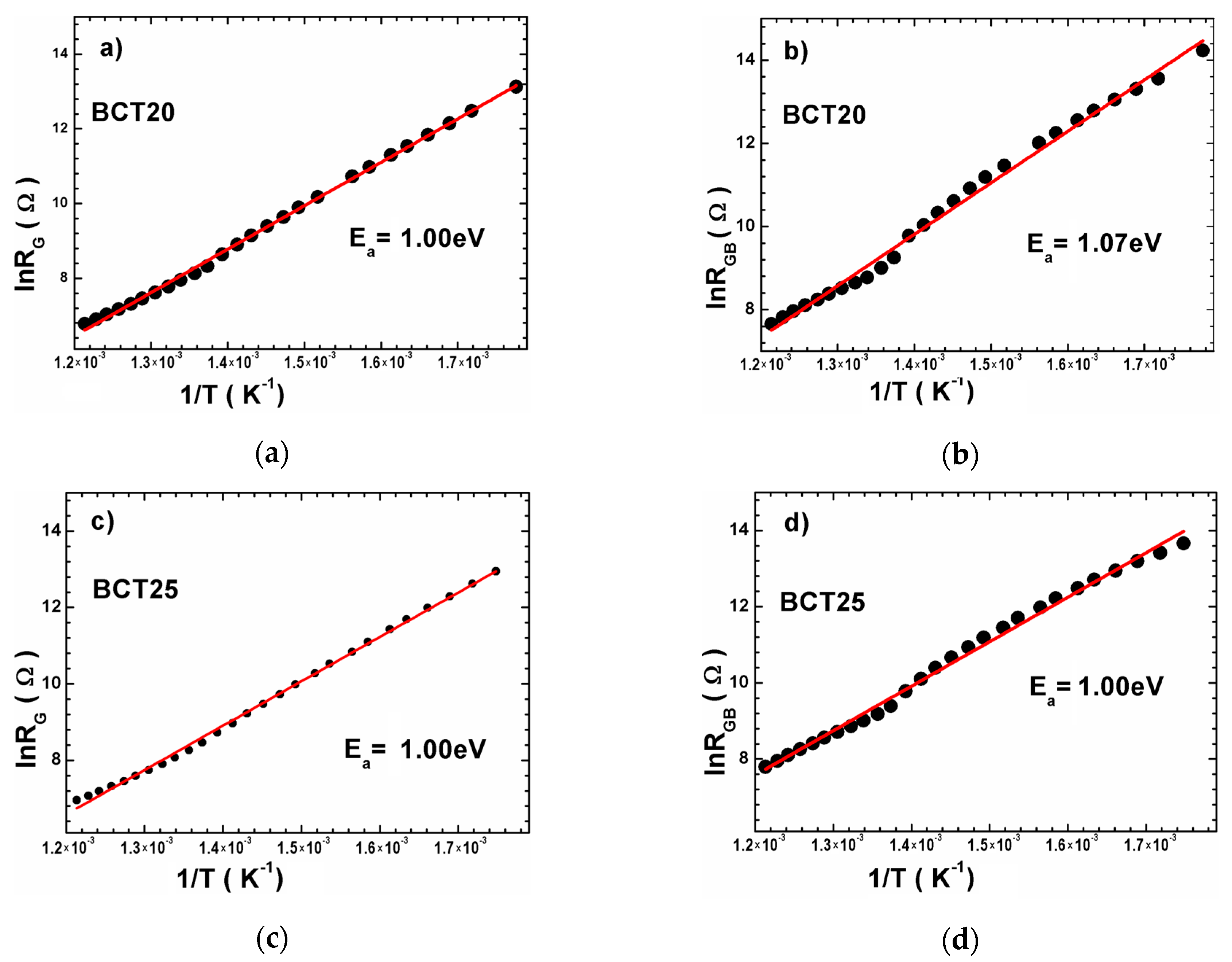
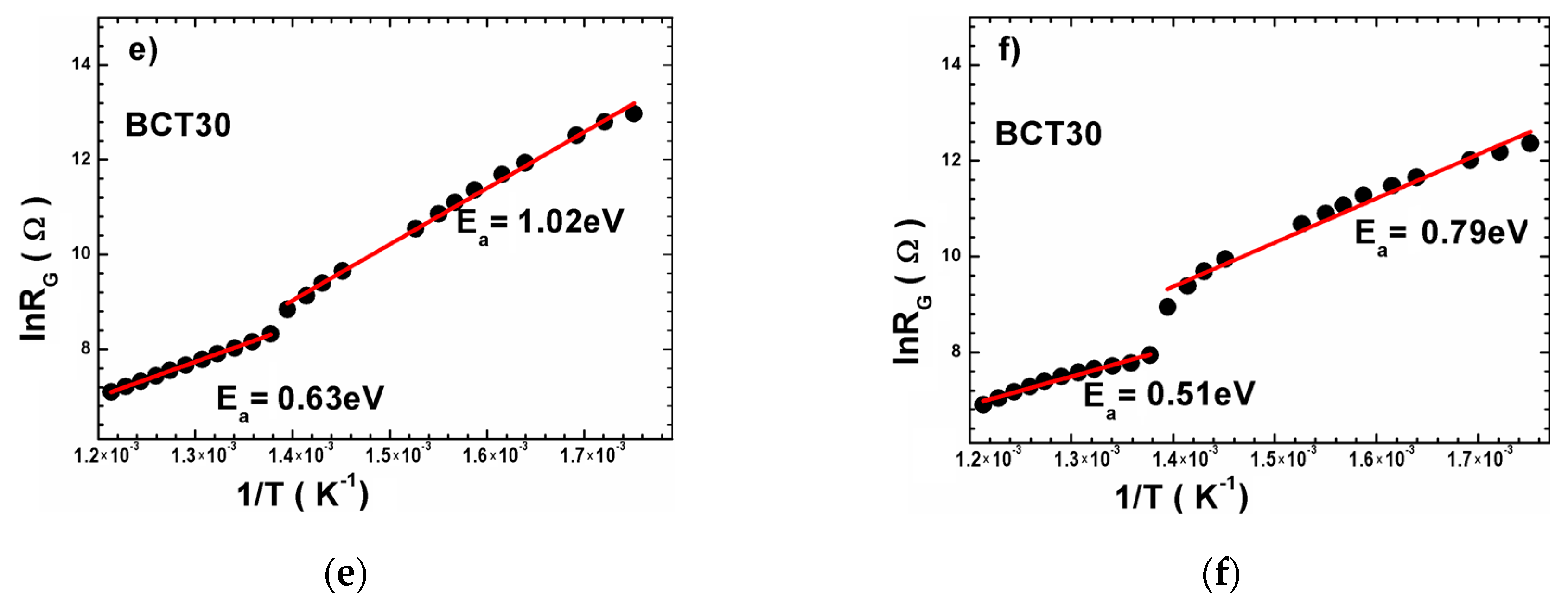
| Sample | Tsd (°C) | Tmd (°C) | Shrinkage (%) | Relative Density (%) |
|---|---|---|---|---|
| BCT20 | 1135 | 1358 | 14.12 | 0.98 |
| BCT25 | 1127 | 1377 | 13.84 | 0.97 |
| BCT30 | 1113 | 1379 | 13.55 | 0.95 |
| Ceramics | Ba (%) | Ca (%) | Ti (%) | Ba + Ca/Ti |
|---|---|---|---|---|
| BCT20 | 39 | 10 | 51 | 0.96 |
| BCT25 | 38 | 13 | 49 | 1.04 |
| BCT30 | 37 | 15 | 48 | 1.08 |
| Parameter | Unit | BCT20 | BCT25 | BCT30 |
|---|---|---|---|---|
| Density calculated (XRD) | (g/cm3) | 5.7612 | 5.7899 | 8.001 |
| Tetragonality (c/a) | (−) | 1.0106 | 1.0111 | 1.0118 |
| Lattice parameter a | (Å) | 3.9827(9) | 3.9756(9) | 3.977(1) |
| Lattice parameter b | (Å) | 3.9827(9) | 3.9756(9) | 3.977(1) |
| Lattice parameter c | (Å) | 4.025(1) | 4.020(1) | 4.024(2) |
| Unit cell volume V | (106 pm3) | 63.8 | 63.5 | 63.6 |
| Space group | (−) | P4 mm | P4 mm | P4 mm |
| Ceramics | TC (K) | ε′max | Tdev (K) | C × 105 (K) | ΔT (K) | T0 (K) | γ | TC (K) |
|---|---|---|---|---|---|---|---|---|
| BCT 20 | 388 | 4936 | 424 | 0.95 | 36 | 379 | 1.32 | 388 |
| BCT 25 | 390 | 4833 | 420 | 1.00 | 30 | 380 | 1.39 | 390 |
| BCT 30 | 392 | 5299 | 419 | 0.96 | 27 | 384 | 1.58 | 392 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliksik, K.; Kozielski, L.; Szafraniak-Wiza, I.; Goryczka, T.; Adamczyk-Habrajska, M. Dielectric and Impedance Studies of (Ba,Ca)TiO3 Ceramics Obtained from Mechanically Synthesized Powders. Materials 2019, 12, 4036. https://doi.org/10.3390/ma12244036
Feliksik K, Kozielski L, Szafraniak-Wiza I, Goryczka T, Adamczyk-Habrajska M. Dielectric and Impedance Studies of (Ba,Ca)TiO3 Ceramics Obtained from Mechanically Synthesized Powders. Materials. 2019; 12(24):4036. https://doi.org/10.3390/ma12244036
Chicago/Turabian StyleFeliksik, Kamil, Lucjan Kozielski, Izabela Szafraniak-Wiza, Tomasz Goryczka, and Małgorzata Adamczyk-Habrajska. 2019. "Dielectric and Impedance Studies of (Ba,Ca)TiO3 Ceramics Obtained from Mechanically Synthesized Powders" Materials 12, no. 24: 4036. https://doi.org/10.3390/ma12244036
APA StyleFeliksik, K., Kozielski, L., Szafraniak-Wiza, I., Goryczka, T., & Adamczyk-Habrajska, M. (2019). Dielectric and Impedance Studies of (Ba,Ca)TiO3 Ceramics Obtained from Mechanically Synthesized Powders. Materials, 12(24), 4036. https://doi.org/10.3390/ma12244036








