Research on the Energy Release Characteristics of Six Kinds of Reactive Materials
Abstract
:1. Introduction
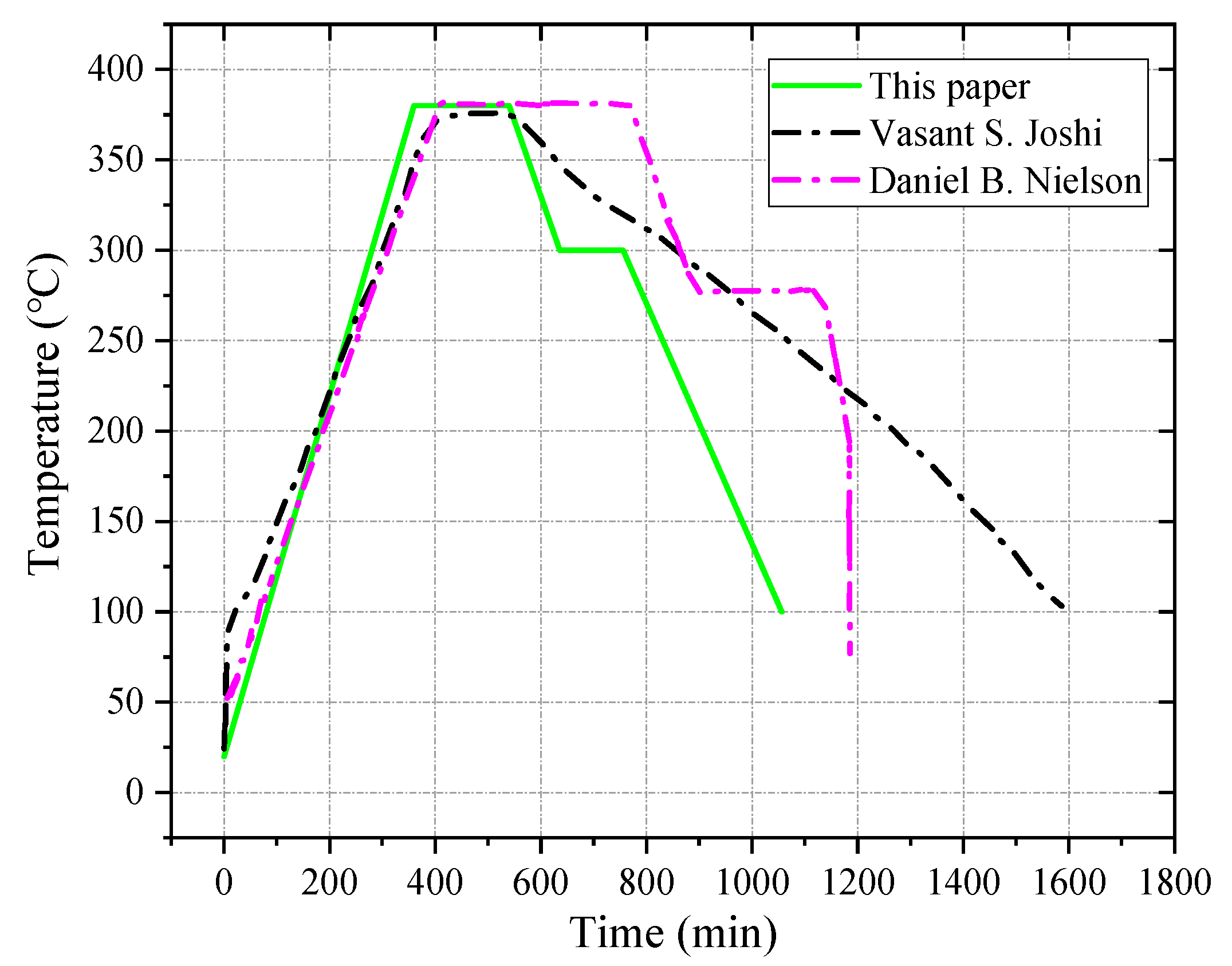
2. Formulation Design and Sample Preparation of Reactive Materials
2.1. Formulation Design of Reactive Materials
2.2. Formulation Design of Reactive Materials
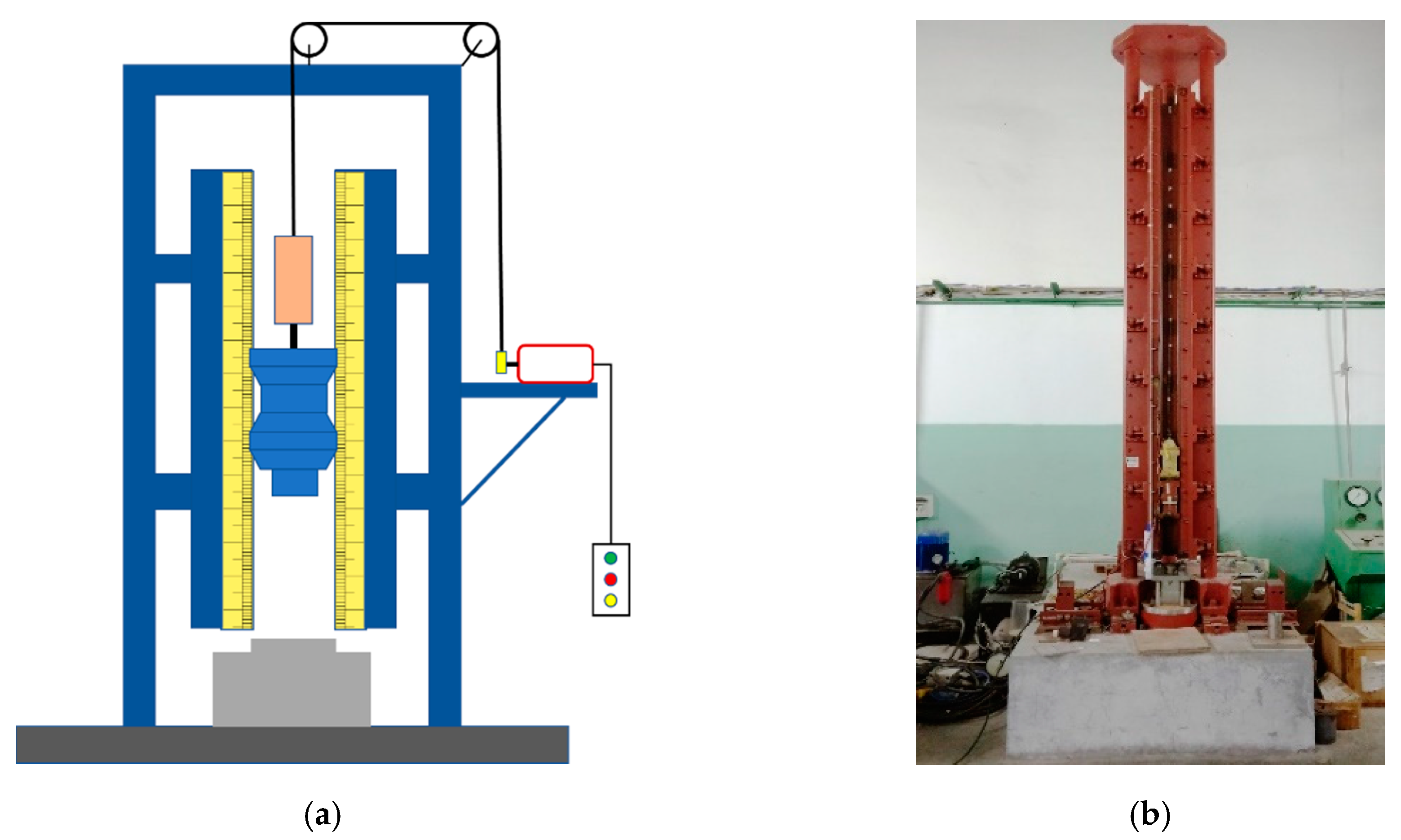
3. Energy Release Ability Test of Reactive Materials
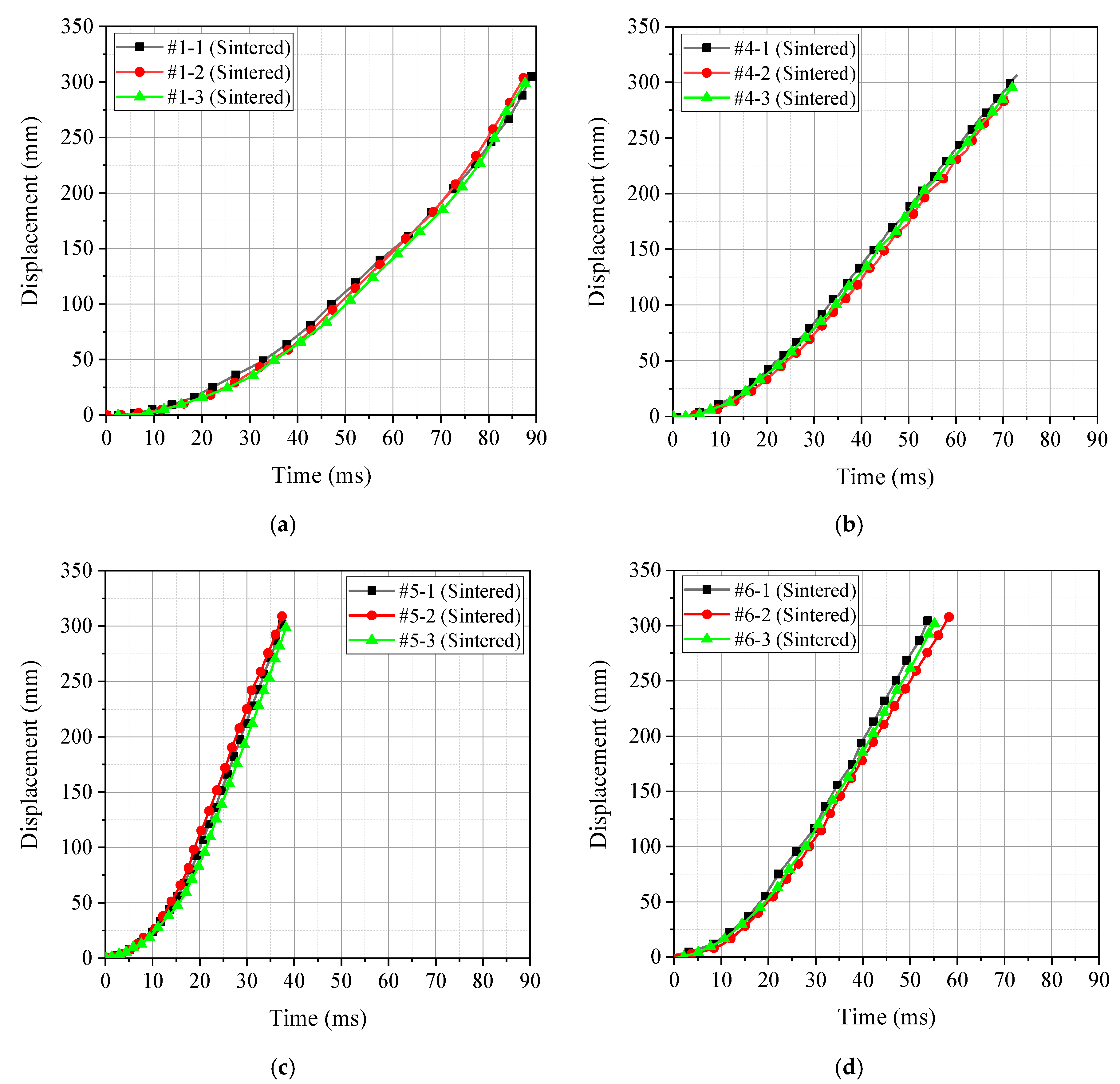
3.1. Qualitative Energy Release Test
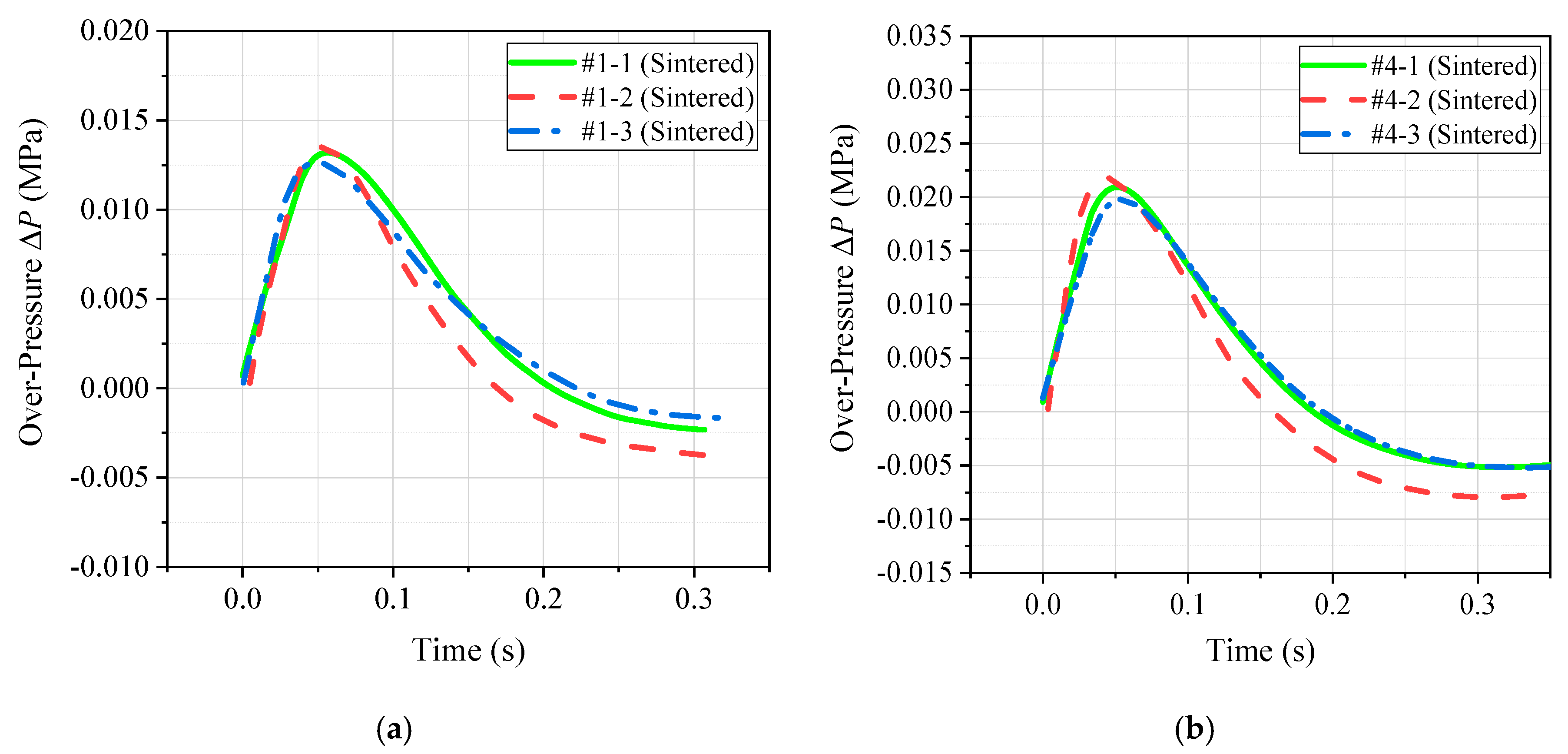
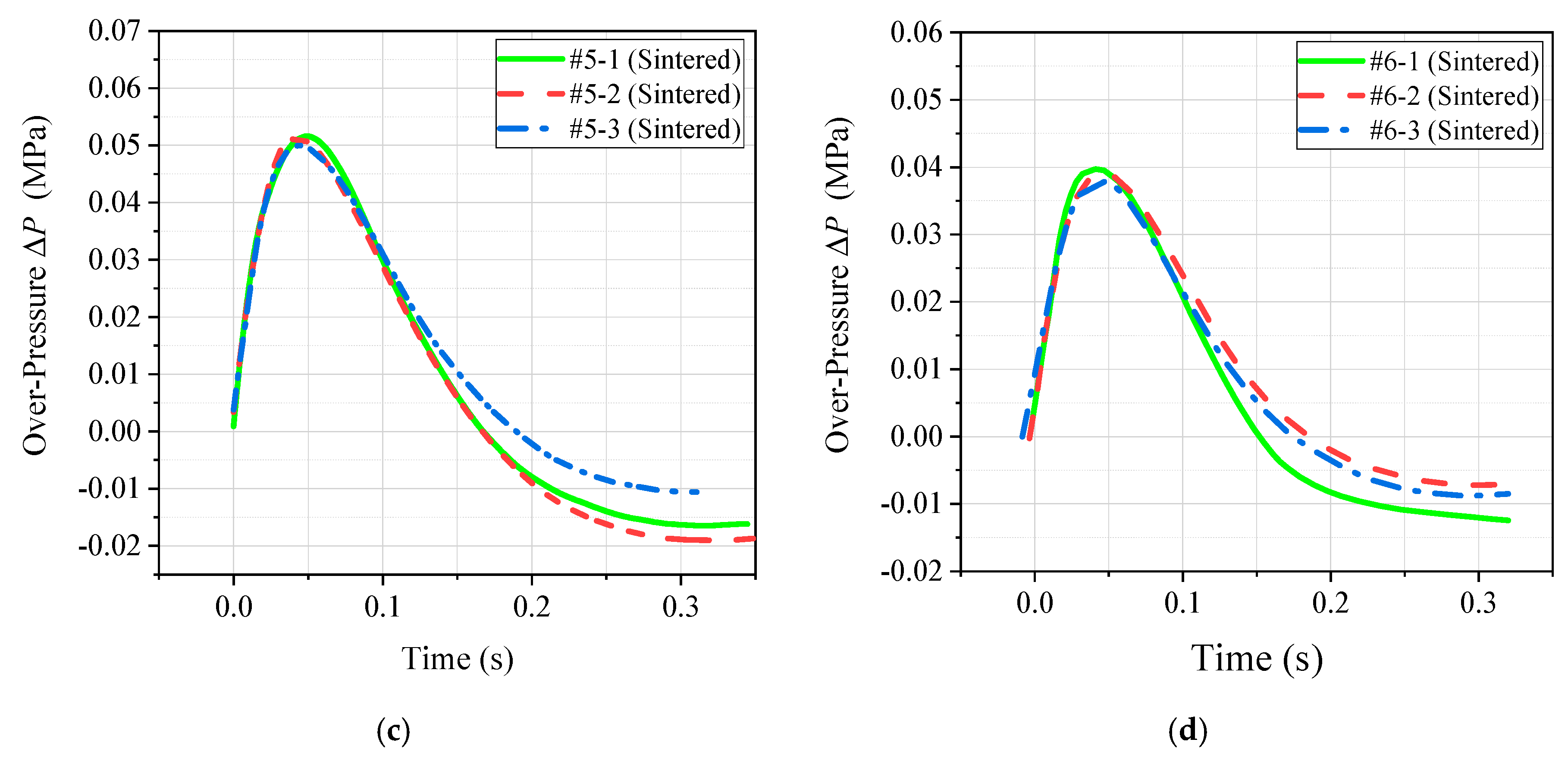
3.2. Quantitative Energy Release Test
4. Conclusions
- (1)
- The drop weight test was carried out on the six reactive material formulations, and the energy release ability of the six reactive material formulations was qualitatively compared. The results show that the reaction processes of #2 (PTFE/B) and #3 (PTFE/Si) were weak with only a very weak flare generated, while the impact reaction processes corresponding to #1 (PTFE/Al), #4 (PTFE/Al/B), #5 (PTFE/Al/Si), and #6 (PTFE/Al/CuO) were intense. However, the difference in the energy release ability among the formulations could not be specifically determined from the fire alone, thus it was necessary to select the best formulation through further quantitative comparison.
- (2)
- Based on the conventional drop weight system, a new type of energy release testing device was self-designed, which can quantitatively compare and analyze the energy release ability of reactive materials. The results show that the peak pressure of the chamber corresponding to the PTFE/Al/Si formulation was the largest, and the corresponding piston movement time was the smallest. Therefore, the above two phenomena can well prove that the energy release ability of the PTFE/Al/Si formulation was the strongest, and the energy release ability of the four groups of formulations could be obtained as following order: PTFE/Al/Si > PTFE/Al/ CuO > PTFE/Al/B > PTFE/Al.
Author Contributions
Funding
Conflicts of Interest
References
- Mock, W., Jr.; Drotar, J.T. Effect of aluminum particle size on the impact initiation of pressed ptfe/al composite rods. In Proceedings of the Shock Compression of Condensed Matter, Waikoloa, HA, USA, 20 December 2007; pp. 971–974. [Google Scholar]
- Zhang, Y.; Li, H. Fluorine-Containing Functional Materials; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- De Technologies, Inc. Reactive Material Enhanced Warheads. Available online: http://www.detk.com/recent-projects/reactive-material-enhanced-warheads/ (accessed on 24 September 2019).
- Lee, R.J.; Mock, W.; Carney, J.R.; How, W.H.; Pangilinan, G.I.; Gamache, R.M.; Boteler, J.M.; Bohl, D.G.; Drotar, J.; Lawrence, G.W. Reactive materials studies. In Shock Compression of Condensed Matter-2005, Pts 1 and 2; AIP Publishing LLC.: Melville, NY, USA, 9 August 2006; pp. 169–174. [Google Scholar]
- Shi-qing, Y.; Song-lin, X.; Tong, Z. Preparation and Performance of PTEF Al Reactive Materials. J. Natl. Univ. Def. Technol. 2008, 30, 39–42. [Google Scholar]
- Ames, R. Energy release characteristics of impact-initiated energetic materials. MRS Online Proc. Libr. Arch. 2005, 896. [Google Scholar] [CrossRef]
- Ames, R. Vented Chamber Calorimetry for Impact-Initiated Energetic Materials. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, Nevada, 10–13 January 2005. [Google Scholar]
- Cai, J.; Jiang, F.; Vecchio, K.S.; Meyers, M.A.; Nesterenko, V.F. Mechanical and microstructural properties of PTFE/Al/W system. In Proceedings of the AIP Conf. Proc., Waikoloa, HA, USA, 20 December 2007; pp. 723–726. [Google Scholar]
- Cai, J.; Walley, S.M.; Hunt, R.J.A.; Proud, W.G.; Nesterenko, V.F.; Meyers, M.A. High-strain, high-strain-rate flow and failure in PTFE/Al/W granular composites. Mater. Sci. Eng. 2008, 472, 308–315. [Google Scholar] [CrossRef]
- Osborne, D.T. The Effects of Fuel Particle Size on the Reaction of Al/Teflon Mixtures; Texas Tech University: Lubbock, TX, USA, 2006. [Google Scholar]
- Mock, W., Jr.; Holt, W.H. Impact initiation of rods of pressed polytetrafluoroethylene (PTFE) and aluminum powders. In Proceedings of the AIP Conference Proceeding, Baltimore, MD, USA, 9 August 2006; pp. 1097–1100. [Google Scholar]
- Ames, R.G.; Waggener, S.S. Reaction Efficiencies for Impact-Initiated Energetic Materials. In Proceedings of the Energetic Materials: Performance and Safety 36th and 32nd, Karlsruhe, Germany, 1–28 June 2005. [Google Scholar]
- David, H. In Next-Gen Bullets and Bombs, Even the Casing Explodes. Available online: http://blog.wired.com/defense/2008/07/reactive-revolu.html (accessed on 24 September 2019).




| Element Component | Relative Molecular Mass | Density (g/cm3) |
|---|---|---|
| PTFE | 100 | 2.1 |
| Al | 27 | 2.7 |
| B | 11 | 2.46 |
| Si | 28 | 2.33 |
| CuO | 80 | 6.4 |
| Number | Formulation | Component Percentage (%) | Remark |
|---|---|---|---|
| #1 | PTFE/Al | PTFE:Al = 73.5:26.5 | - |
| #2 | PTFE/B | PTFE:B = 87.2:12.8 | - |
| #3 | PTFE/Si | PTFE:Si = 78.1:21.9 | - |
| #4 | PTFE/Al/B | PTFE:Al:B = 76.2:21.2:2.6 | (PTFE/Al):(PTFE/B) = 4:1 |
| #5 | PTFE/Al/Si | PTFE:Al:Si = 74.4:21.2:4.4 | (PTFE/Al):(PTFE/Si) = 4:1 |
| #6 | PTFE/Al/CuO | PTFE:Al:CuO = 58.8:24.9:16.3 | (PTFE/Al):(Al/CuO) = 4:1 |
| Number | Formulation | Mass (g) | Diameter (mm) | Thickness (mm) |
|---|---|---|---|---|
| #1-1 | PTFE/Al | 0.184 | 6.04 | 3.10 |
| #1-2 | 0.181 | 3.04 | ||
| #1-3 | 0.182 | 3.08 | ||
| #1-4 | 0.189 | 3.10 | ||
| #2-1 | PTFE/B | 0.175 | 2.90 | |
| #2-2 | 0.170 | 2.90 | ||
| #2-3 | 0.180 | 3.10 | ||
| #2-4 | 0.182 | 3.12 | ||
| #3-1 | PTFE/Si | 0.183 | 3.10 | |
| #3-2 | 0.187 | 3.14 | ||
| #3-3 | 0.187 | 3.14 | ||
| #3-4 | 0.174 | 3.00 | ||
| #4-1 | PTFE/Al/B | 0.194 | 3.14 | |
| #4-2 | 0.191 | 3.14 | ||
| #4-3 | 0.190 | 3.08 | ||
| #4-4 | 0.192 | 3.10 | ||
| #5-1 | PTFE/Al/Si | 0.185 | 2.96 | |
| #5-2 | 0.195 | 3.10 | ||
| #5-3 | 0.197 | 3.16 | ||
| #5-4 | 0.198 | 3.22 | ||
| #6-1 | PTFE/Al/CuO | 0.204 | 3.08 | |
| #6-2 | 0.210 | 3.12 | ||
| #6-3 | 0.208 | 3.02 | ||
| #6-4 | 0.209 | 3.10 |
| Number | Formulation | Mass (g) | Diameter (mm) | Thickness (mm) |
|---|---|---|---|---|
| #1-1 | PTFE/Al | 0.175 | 6.04 | 2.94 |
| #1-2 | 0.175 | 2.98 | ||
| #1-3 | 0.174 | 3.02 | ||
| #4-1 | PTFE/Al/B | 0.196 | 3.12 | |
| #4-2 | 0.196 | 3.18 | ||
| #4-3 | 0.197 | 3.14 | ||
| #5-1 | PTFE/Al/Si | 0.191 | 3.04 | |
| #5-2 | 0.191 | 2.98 | ||
| #5-3 | 0.191 | 3.02 | ||
| #6-1 | PTFE/Al/CuO | 0.206 | 2.96 | |
| #6-2 | 0.206 | 2.98 | ||
| #6-3 | 0.205 | 3.03 |
| Formulation | Peak Pressure ∆P (MPa) | Piston Motion Time t (ms) |
|---|---|---|
| PTFE/Al | 0.013 | 88 |
| PTFE/Al/B | 0.021 | 72 |
| PTFE/Al/Si | 0.051 | 37 |
| PTFE/Al/CuO | 0.039 | 55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, X.; Ding, L.; Zhou, J.; Tang, W. Research on the Energy Release Characteristics of Six Kinds of Reactive Materials. Materials 2019, 12, 3940. https://doi.org/10.3390/ma12233940
Ran X, Ding L, Zhou J, Tang W. Research on the Energy Release Characteristics of Six Kinds of Reactive Materials. Materials. 2019; 12(23):3940. https://doi.org/10.3390/ma12233940
Chicago/Turabian StyleRan, Xianwen, Liangliang Ding, Jingyuan Zhou, and Wenhui Tang. 2019. "Research on the Energy Release Characteristics of Six Kinds of Reactive Materials" Materials 12, no. 23: 3940. https://doi.org/10.3390/ma12233940
APA StyleRan, X., Ding, L., Zhou, J., & Tang, W. (2019). Research on the Energy Release Characteristics of Six Kinds of Reactive Materials. Materials, 12(23), 3940. https://doi.org/10.3390/ma12233940





